Abstract
Vascular surgery for atherosclerosis is confronted by the lack of a suitable bypass material. Tissue engineering strives to produce bio-artificial conduits to provide resistance to thrombosis. The objectives of our study were to culture endothelial cells (EC) on composite assemblies of extracellular matrix proteins, and to evaluate the cellular phenotype under flow. Cell-adhesive assemblies were fabricated on glass slides as combinations of collagen (Co), laminin (LM), and fibronectin (FN), resulting in three samples: Co, Co/LM, and Co/FN. Surface topography, roughness, and wettability were determined. Human saphenous vein EC were harvested from cardiac patients, cultured on the assemblies and submitted to laminar shear stress (SS) of 12 dyn/cm2 for 40, 80, and 120 min. Cell retention was assessed and qRT-PCR of adhesion genes (VE-cadherin, vinculin, KDR, CD-31 or PECAM-1, β1-integrins) and metabolic genes (t-PA, NF-κB, eNOS and MMP-1) was performed. Quantitative immunofluorescence of VE cadherin, vinculin, KDR, and vonWillebrand factor was performed after 2 and 6 h of flow. Static samples were excluded from shearing. The cells reached confluence with similar growth curves. The cells on Co/LM and Co/FN were resistant to flow up to 120 min but minor desquamation occurred on Co corresponding with temporary downregulation of VE cadherin and vinculin-mRNA and decreased fluorescence of vinculin. The cells seeded on Co/LM initially more upregulated vinculin-mRNA and also the inflammatory factor NF-κB, and the cells plated on Co/FN changed the expression profile minimally in comparison with the static control. Fluorescence of VE cadherin and vonWillebrand factor was enhanced on Co/FN. The cells cultured on Co/LM and Co/FN increased the vinculin fluorescence and expressed more VE cadherin and KDR-mRNA than the cells on Co. The cells plated on Co/FN upregulated the mRNA of VE cadherin, CD-31, and MMP 1 to a greater extent than the cells on Co/LM and they enhanced the fluorescence of VE cadherin, KDR, and vonWillebrand factor. Some of these changes sustained up to 6 h of flow, as confirmed by immunofluorescence. Combined matrices Co/LM and Co/FN seem to be more suitable for EC seeding and retention under flow. Moreover, Co/FN matrix promoted slightly more favorable cellular phenotype than Co/LM under SS of 2–6 h.
Introduction
Atherosclerosis of coronary and peripheral arteries has become a major disease in industrialized countries. The annual number of lower extremity interventions totals more than 120 bypass procedures per 100,000 inhabitants in the United States.1 Cardiovascular surgery, however, confronts a shortage of available material for bypass grafting, since the gold-standard patients' own artery or vein is frequently lacking. Currently used synthetic blood vessel prostheses, namely polyethylene terephthalate (PET, Dacron) and expanded polytetrafluoroethylene (ePTFE), have achieved excellent results in bypassing large-diameter vessels, for example, the aorta and iliac arteries; however, they do not perform well in low-flow or small-diameter vessels (<6 mm), for example, in coronary, below the knee, or microvascular regions. One of the main reasons for this failure is the surface thrombogenicity and the development of intimal hyperplasia as a result of a mismatch in the viscoelastic properties of native tissue and the synthetic material.2
Large areas of cardiovascular implants, such as heart valves and vascular prostheses, do not spontaneously cover with endothelial cells (EC) in humans, and it has been shown that preimplant seeding and preconditioning of the patient's autologous EC improves the long-term patency and performance of synthetic grafts.3 Tissue engineering of vascular grafts has become a meaningful objective of research in the last three decades, and advances in materials science4 together with culturing and seeding living cells has proven the concept of a bio-artificial vascular bypass to be practicable. To fabricate a vascular graft, some of the major drawbacks that need to be overcome in cell seeding are as follows: first, the attachment of EC to the adhesive substrate, second, their retention under flow and, third, maintaining the complex physiological functions of the endothelial lining, for example, synthesis of molecules important for cell-matrix adhesion, vasodilation, fibrinolysis, and reduced immune responses, which are essential in normal vascular homeostasis and remodeling.5
We assume that the protein composition of the extracellular matrix (ECM) should have a potential role in the following physiological processes of EC: adhesion and proliferation in the culture, resistance to the flow in a parallel-plate flow chamber, and finally shear stress (SS)-mediated gene expression of EC. We therefore studied the flow-dependent response of EC to three different assemblies of adhesive matrix proteins: collagen type I gel (Co), collagen I with attached laminin (Co/LM), and collagen I with attached fibronectin (Co/FN). Protein assemblies of this type have been recognized to be practicable in coating scaffolds for tissue engineering.6 In the flow experiment, we chose to explore a set of five genes that are involved in cell–cell (vascular endothelial cadherin [VE-cadherin]) and cell–matrix (vinculin, β1-integrins) adhesion and also in mechanical force sensing (kinase insert domain receptor [KDR], cluster of differentiation molecule-31 [CD-31]), or platelet/endothelial cell adhesion molecule-1 [PECAM-1]), and a set of four genes involved in cell metabolism related to coagulation (tissue plasminogen activator [t-PA]), vasodilation (endothelial nitric oxide synthase [eNOS]), immune response (nuclear factor of kappa light polypeptide gene enhancer in B cells [NF-κB]), and vascular remodeling (matrix metalloproteinase-1 [MMP-1]). The protein assemblies were characterized in terms of their surface topography, roughness, and wettability. In addition, the growth curves, SS resistance (12 dyn/cm2 up to 2 h) and gene expression profiles were determined in human saphenous vein EC with a view to tissue engineering of blood vessels.
Materials and Methods
Preparation and characterization of the protein assemblies
The assemblies were prepared from collagen type I (rat tail, CN-354236; Bioscience), LM (Engelbreth-Holm-Swarm murine sarcoma basement membrane, CN-L2020; Sigma-Aldrich), and FN (human plasma, CN-10688851001; Roche). A stock solution of Co was diluted by 0.02M acetic acid to a concentration of 75 μg/mL. A standard microscopic glass slide (4.5×2.5 cm2) was coated with 2 mL of the solution to obtain a solution layer containing 5 μg/cm2. The solution layer was converted into a Co gel by exposing the sample to ammonia vapor for 5 min and rinsing it with phosphate-buffered saline (PBS) diluted with water to 10%. LM was attached to the Co film by incubation with 2 mL PBS solutions at a LM concentration of 40 μg/mL, or FN at a concentration of 50 μg/mL overnight. Samples coated only with collagen gel were rinsed with water, and the samples Co/LM and Co/FN were rinsed with 10% PBS. All the samples were finally air dried and UV sterilized for 30 min.
The attachment of LM and FN from PBS to a Co layer prepared on the gold surface of a surface plasmon resonance (SPR) chip was observed in situ using an SPR instrument custom-built at the Institute of Photonics and Electronics, Academy of Sciences of the Czech Republic, Prague. A four-channel flow cell was pressed on the collagen-coated SPR chip and the SPR responses to LM and FN solutions driven by a peristaltic pump simultaneously through independent channels were recorded.
The surface wettability of the assemblies was characterized by measuring the sessile drop contact angles using the Laplace-Young method (OCA 20; Dataphysics). Millipore Q water droplets 2 μL in volume were put on the surface and the average contact angle values were obtained by measuring the contact angles of individual droplets.
The surface topography was observed using a multimode AFM (Nanoscope IIIa; Digital Instruments). The images were recorded using the tapping mode and silicon tips with a spring constant of 42 N/m and a nominal radius of curvature of 7 nm (OTESPA; Bruker AFM Probes). Average roughness (Ra) was calculated for all scanned areas based on the program provided with the instrument.
EC seeding, shearing, and evaluation
Human saphenous vein endothelial cells (HSVEC) were harvested from human patients after an aorto-coronary bypass procedure as a primary cell culture according to methods described elsewhere.7 Briefly, saphenous vein graft was cannulated, washed with PBS, and filled with collagenase type 2 (Sigma Aldrich) at 37°C for 15 min. EC were recovered, pooled, and amplified in plastic flasks (Falcon, BD Biosciences) in an M199 culture medium (Invitrogen) supplemented with 20% of fetal calf serum (FCS; PAA), heparin 50 IU/mL (Choay), basic fibroblast growth factor 10 ng/mL (Promocell), and a mixture solution of penicillin (10×103 IU/mL) and streptomycin (10 μg/mL) 1:100 (Sigma). For the proliferation assay, the HSVEC of passage number P5 were seeded in a lower density of 3×104 cells/cm2 onto each of the surfaces. The adhering cells were observed 6, 24, 48, 72, and 144 h after seeding (Olympus IX50 light microscope); six randomly chosen microscopic fields were photographed (Olympus DP70 digital camera, magnification 20×) and the cell numbers were evaluated by counting the cell nuclei.
For the flow experiment, the HSVEC were seeded at a higher density of 5×104 cells/cm2. When reaching confluence at 48 h, the HSVEC were exposed to a parallel-plate flow chamber, which simulated the blood stream. The circulating medium (37°C) was composed of M199, 10% FCS, and heparin 50 IU/mL. The tested surfaces were submitted to a flow of 20 mL/min and the laminar SS=12 dyn/cm2 was applied for 40, 80, and 120 min. For the purposes of immunofluorescence imaging and quantification, the flow was applied for 2 h and then extended up to 6 h. Static control samples were kept without shearing. After SS administration, the adhering cells were photographed and counted to assess their resistance to desquamation during flow.
Molecular biology
Immediately after flow exposure, the samples were immersed into cold (4°C) Hanks solution (HBSS; Invitrogen) and the cells were trypsinized from the support with Trypsin+EDTA (Sigma) solution (TE) and frozen. The total messenger ribonucleic acid (mRNA) was then extracted and treated according to Fernandez et al.7 A quantitative real-time polymerase chain reaction of the following nine genes was performed: VE-cadherin (VE-cad.), vinculin, KDR or vascular endothelial growth factor receptor-2 (VEGFR-2) or Flk-1, CD-31 or PECAM-1, β1-integrin chain, t-PA, NF-κB, eNOS, and MMP-1. The up- or downregulation of the mRNA expression is shown as a relative value in relation to the housekeeping gene P0 that encodes for a ribosomal protein and is not influenced by the experimental conditions. The primer, temperatures, and length of polymerase chain reaction products are listed elsewhere.8,9 The normalized mRNA level in cells under static conditions was arbitrarily set at “1,” and the data are presented as fold values that are compared either to the static control or to another sample.
Immunofluorescence analysis
To evaluate whether the differential expression of mRNA was translated into protein, the samples were fixed with 70% ethanol, rinsed with PBS, and stained with anti-human antibodies: rabbit anti-VE-cadherin IgG (AHP628Z; AbD Serotec), mouse anti-Vinculin IgG1 (V9131; Sigma), mouse anti-KDR/VEGFR2 IgG1 (LS-C109100; LS Biosciences), and rabbit anti-von Willebrand factor (vWF, F3520; Sigma). Anti-rabbit or anti-mouse Alexa Fluor 488-conjugated goat IgG (A11070, A11017; Invitrogen) was used as a secondary antibody. The cell nuclei were counterstained with Hoechst 33258 (861405; Sigma). The cells were observed using an Olympus IX50 light microscope. Nine randomly chosen microscopic fields were photographed with the same exposure settings (Olympus DP70 digital camera, magnification 20× for VE-cadherin, vinculin, and 100× for KDR and vWF). A threshold was set on every image to remove the nonprotein area from the image data. The threshold setting was the same for each image for a given protein. Then, the cumulative sum of all pixel intensities was computed and the background intensity of the negative staining control was subtracted. The total immunofluorescence intensity of the protein was expressed in relative values and normalized to the number of cells in the microscopic field. The intensity of the static samples was arbitrarily set to “1.”
Statistical analysis
The data within one experiment were expressed as mean and standard deviation. However, an effort was made to perform each step of the experiment in triplicate (n=3). These data were pooled and expressed as mean and standard error of mean. In some data groups n=2 or n=1, since some of the measurements had to be erased, either due to repeated technical difficulties (e.g., cell desquamation on Co) or because some of the values were numerically distant outliers and thus unlikely. The data were compared using one-way analysis of variance (Holm-Sidak test) for multiple comparison (SigmaStat 3.1 2004; Systat Software, Inc.). The difference between groups was considered significant at p<0.05.
Results
Surface composition
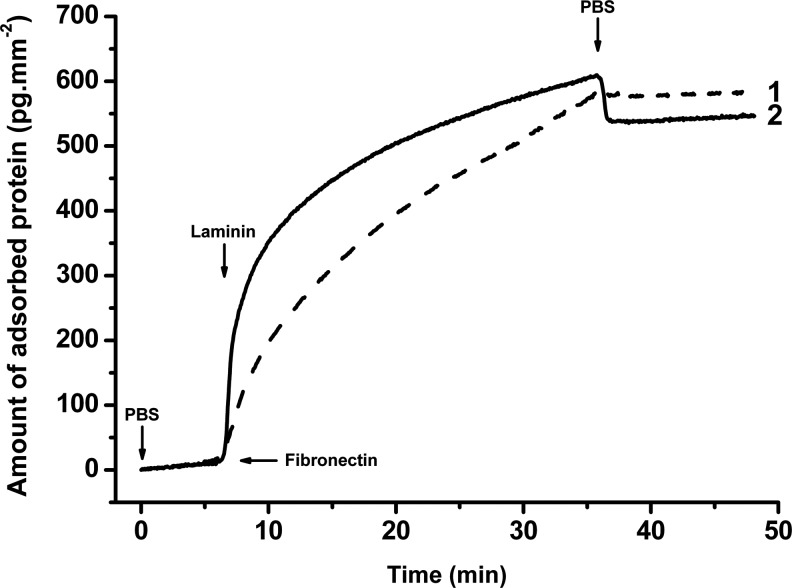
Figure 1 shows the attachment of LM and FN from PBS to a Co layer prepared on the gold surface of a SPR chip. The SPR response was observed in situ simultaneously on the same surface located in different flow channels through which the LM or FN solutions were pumped. The proteins remained attached after replacing their solutions with PBS.
FIG. 1.
Attachment of fibronectin (curve 1) and laminin (curve 2) to a collagen type I layer observed by surface plasmon resonance (SPR). Arrows indicate replacement of the solutions: phosphate-buffered saline (PBS), fibronectin (50 μg/mL), and laminin (40 μg/mL) in PBS.
Surface wettability and roughness
While FN is capable of binding to Co type I via specific binding sites, LM binds in this way only to collagen type IV. The electrostatic interaction between the Co of isoelectric point pI=7.8 and FN (pI=5.4), or LM (pI=4.8), positively and negatively charged in PBS (pH=7.4), respectively, might provide the driving forces for LM and FN adsorption to the Co I coating prepared in this work. The water sessile drop contact angles, θ, measured on the Co, Co/LM, and Co/FN surfaces are shown in Table 1. An increase in the contact angle from 30°±3° on Co to 63°±1° and 64°±1° on Co/LM and Co/FN, respectively, indicated a decrease in the wettability of the Co coating. The wettability probably decreased by a drop in the number of strongly hydrated positively charged Co groups due to the formation of ionic bonds between some of them, and negatively charged groups on the adsorbed FN or LM. In addition, some nonpolar amino acid residues of adsorbed proteins could be exposed to water, thus increasing the free coating/water interfacial energy. The formation of polar bonds, probably ionic, with the Co surface overcomes the increase in interfacial energy.
Table 1.
Surface Wettability (Sessile Water Drop Contact Angle θ) and Surface Roughness of Protein Assemblies on a Glass Substrate
| (A) Glass | (B) Co | (C) Co/LM | (D) Co/FN | |
|---|---|---|---|---|
| θ (deg) | 37±2 | 30±3 | 63±1 | 64±1 |
| p<0.001 | vs. B, C, D | vs. A, C, D | vs. A, B | vs. A, B |
| Ra (nm) | 0.18±0.02 | 0.54±0.03 | 0.87±0.08 | 0.86±0.09 |
| p<0.001 | vs. B, C, D | vs. A, C, D | vs. A, B | vs. A, B |
Mean±SD, θ n=4.
Ra n=5; deg., degrees; vs., versus; Co, collagen type I; Co/LM, collagen/laminin; Co/FN, collagen/fibronectin; Ra, average roughness.
Figure 2 shows a representative surface morphology of the protein assemblies prepared on microscopic glass slides. Similar collagen fibers are visible on all the samples. Even if the individual fibers extend more from the Co/FN surface than from the Co/LM surface, the average roughness values measured on the surfaces were nearly the same on the Co/FN and Co/LM surfaces (Table 1). The marked difference between the high wettability of the Co surface and the low wettability of the Co/FN and Co/LM surfaces (Table 1) evidently does not correspond with the similar topography of these surfaces. Assuming Co density of about 1.2 mg/cm3 and thickness of the deposited collagen film containing 0.005 mg/cm2, film thickness of 2.4 μm can be estimated. No defects deeper than 20 nm were observed in the coatings that could indicate areas of uncoated glass surface.
FIG. 2.
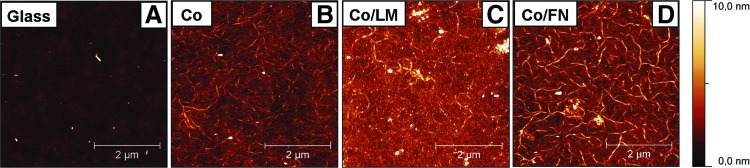
Surface topography of a microscopic glass slide (A), collagen type I (B), collagen/laminin (C), and collagen/fibronectin (D) surfaces visualized by atomic force microscopy of dry samples. Scanning area 5×5 μm. Color images available online at www.liebertpub.com/tea
Cell adhesion, proliferation, and flow experiment
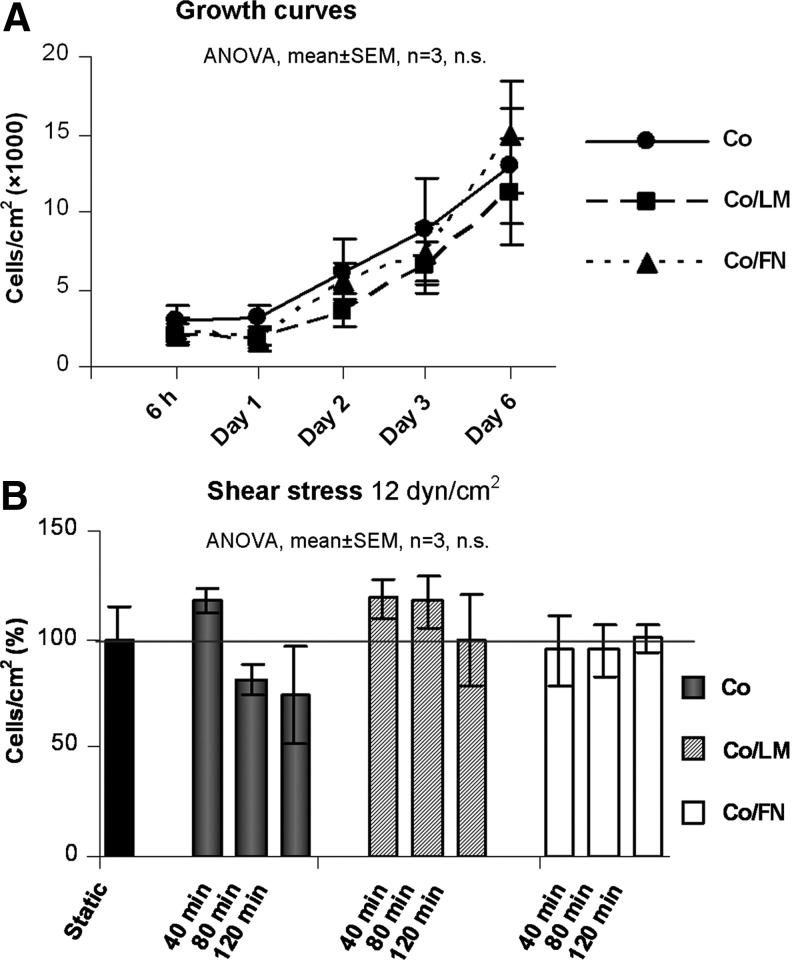
The growth curves of the HSVEC seeded at lower density over a period of 6 days are shown in Figure 3A. Though a statistical difference in contact angle and in surface roughness was detected between Co and Co/LM or Co/FN, (p<0.001) (Table 1), no difference in proliferation rate was noted among the surfaces. The cells seeded at higher density reached confluence 48 h after seeding on all tested surfaces and were subsequently submitted to flow. The cell densities on Co, Co/LM, and Co/FN after 40, 80, and 120 min SS of 12 dyn/cm2 are displayed in Figure 3B. Postflow detachment of some of the cells was observed mainly on the Co surface; however, this difference was not statistically significant.
FIG. 3.
Growth curves (A) and flow resistance (B) of human saphenous vein endothelial cells (HSVEC) on collagen type I (Co), collagen/laminin (Co/LM), and collagen/fibronectin (Co/FN) assemblies. The seeding density was 3×104 cells/cm2 and the culture period was 6 days (A) and seeding density 5×104 cells/cm2 and culture period 48 h, static control=100% (B). ANOVA, analysis of variance; n.s., nonsignificant; SEM, standard error of mean.
Molecular biology
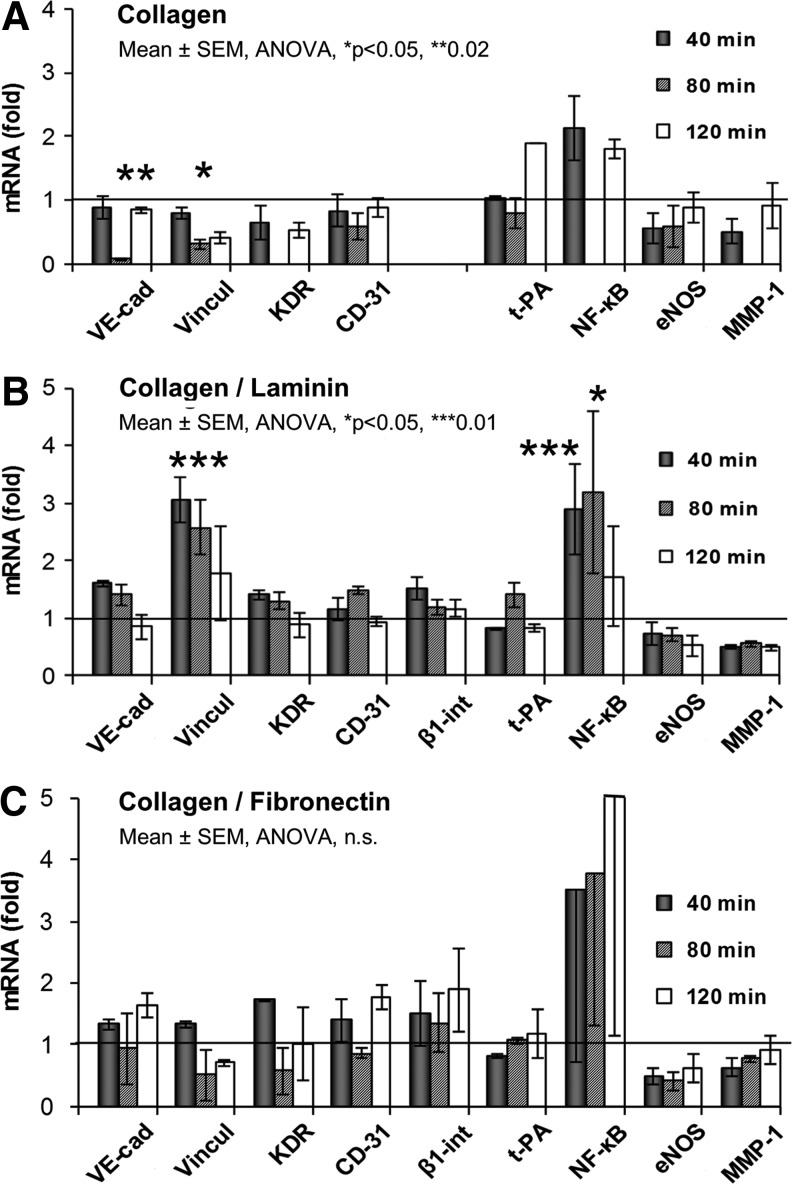
The relative amount of mRNA of nine selected genes extracted from the cells at 40, 80, and 120 min intervals and the corresponding gene expression profiles on Co, Co/LM and Co/FN are displayed in Figure 4A–C, respectively. The data were compared with the corresponding static control. On the collagen sample, downregulation of the expression of some of the adhesion genes was observed, and this was statistically significant at the 80-min interval for VE-cadherin (p<0.02) and vinculin (p<0.05).
FIG. 4.
Gene expression profiles of HSVEC under shear stress on collagen type I (Co) (A), Co/LM (B), and Co/FN (C) assemblies. Fold values of mRNA obtained by qRT-PCR. Static control=1 was measured separately for each of the surfaces. See Introduction/Discussion for the gene description. mRNA, messenger ribonucleic acid; qRT-PCR, quantitative real-time polymerase chain reaction.
As for Co/LM, most of the adhesive genes were upregulated at 40 min with a gradual return to a static value at 80 and 120 min. However, this upregulation was considered significant only in vinculin at 40-min (p<0.01). Metabolic genes were mostly downregulated, although nonsignificantly, with the exception of NF-κB, which was increased at both 40 min (p<0.01) and 80 min (p<0.05).
The effect of SS on the cells on Co/FN was as follows: most of the adhesive genes were also upregulated, but with temporary attenuation at 80 min. A decreased amount of mRNA was detected in metabolic genes, with the exception of NF-κB, although a slight trend to return to a static level was observed. However, none of these changes was statistically significant.
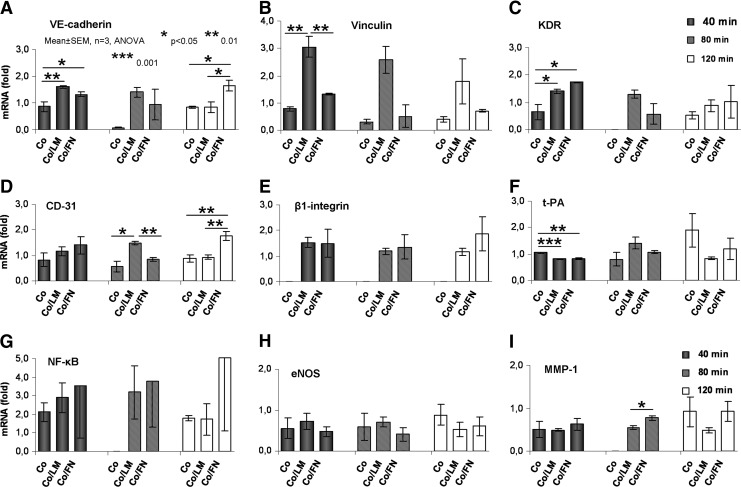
A statistical comparison of the gene expression in cells cultured on Co, Co/LM, and Co/FN substrata at 40, 80, and 120-min intervals of SS is visualized in Figure 5. Adding LM onto Co generally resulted in enhanced expression of cell adhesion genes, which was significant in VE-cadherin, vinculin (p<0.01) and KDR (p<0.05) at 40 min and in CD-31 (p<0.05) at 80 min, respectively. However, this significant increase became less marked after 120-min exposure to flow. Significant changes were not demonstrated in the expression of the metabolic genes in the course of the experiment. Only the amount of mRNA of t-PA decreased (p<0.01) temporarily at the 40-min point.
FIG. 5.
Comparison of gene expression in HSVEC on collagen type I (Co), Co/LM, and Co/FN surfaces. Fold values of mRNA obtained by qRT-PCR. See Introduction/Discussion for the gene description.
Supplementing FN on to Co also led to augmented expression of cell adhesion genes, significantly in VE-cadherin and KDR (p<0.05) at 40 min. The elevation of mRNA was nonsignificant at the 80-min interval, and it was significant again in VE-cadherin (p<0.05) and CD-31 (p<0.01) at 120 min. As in the case of Co/LM, the expression of metabolic genes was not remarkably changed over time, except for temporary attenuation of t-PA at 40 min (p<0.01).
A comparison of Co/LM versus Co/FN showed that the mRNA of cell adhesion genes at 40 min was expressed variously. Co/LM provided significantly more expression of vinculin (p<0.01) at 40 min and CD-31 (p<0.01) at 80 min. However, after 120 min the adhesive genes were more elevated on Co/FN (with the exception of vinculin), VE-cadherin (p<0.05), and CD-31 (p<0.01) being significant. The synthesis of the mRNA of metabolic genes was comparable at the 40-min and 80-min time points, with the exception of augmented MMP-1 (p<0.05) on Co/FN at 80 min. Co/FN support generated upregulation of all of the metabolic genes at the 120-min interval, although this was not statistically significant.
Immunofluorescence
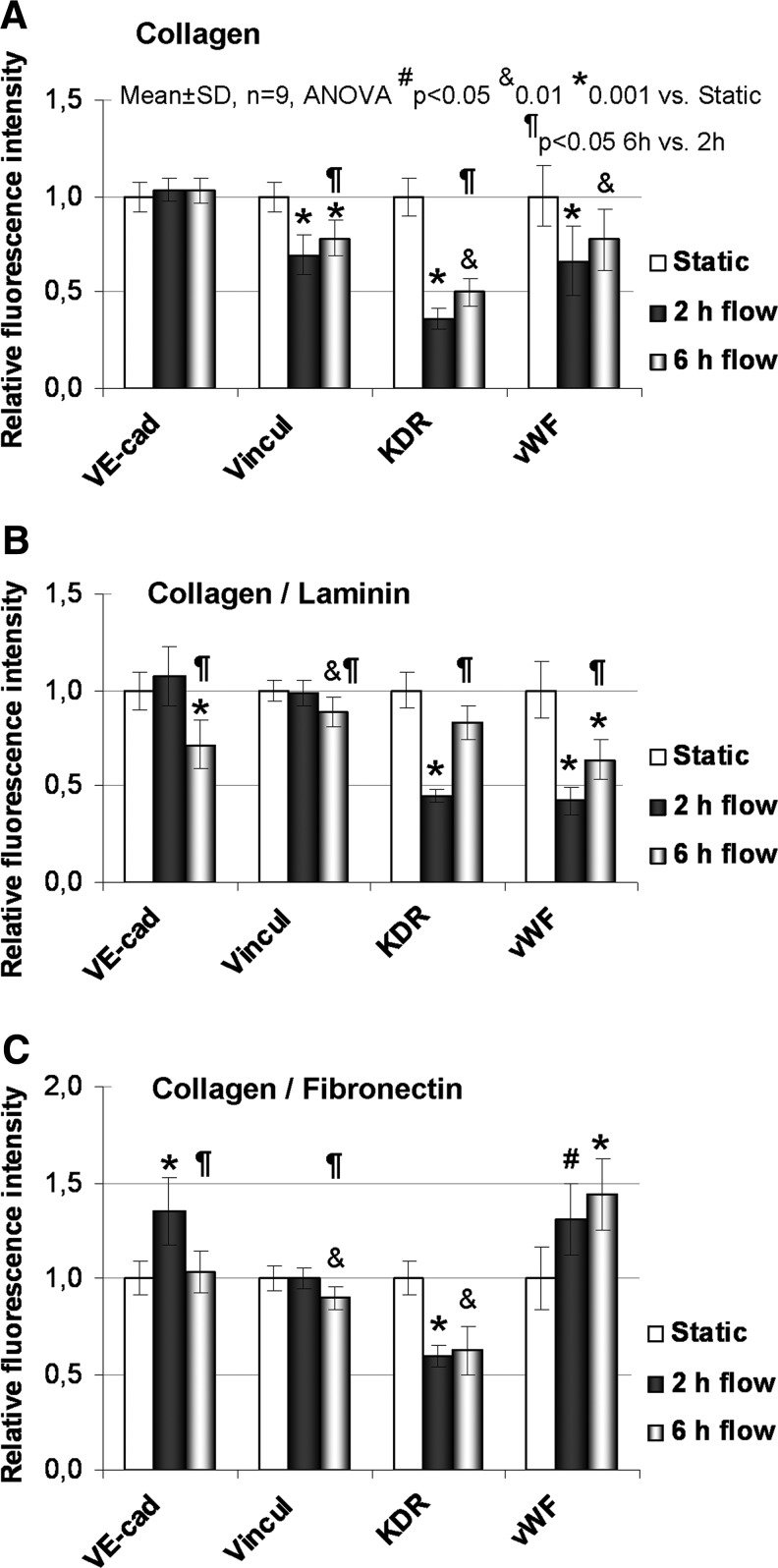
The relative immunofluorescence intensities of VE-cadherin, vinculin, KDR, and vWF in static, 2 and 6 h flow samples are shown in Figure 6, including the levels of statistical significance. On the collagen surface, vinculin, KDR, and vWF were significantly decreased at 2 h compared with static. This decrease was still significant at 6 h; however, vinculin and KDR increased their intensities between 2 and 6 h. VE-cad. remained unchanged. VE-cad. and vinculin were temporarily downregulated at mRNA level—significantly at 80 min but insignificantly at 2 h. The decrease of KDR mRNA was not significant (Fig. 4A).
FIG. 6.
Relative immunofluorescence intensity of VE-cadherin (VE-cad), vinculin (Vincul), kinase domain receptor (KDR), and von Willebrand factor (vWF) in HSVEC cultured on collagen (A), Co/LM (B), and Co/FN (C) assemblies. A comparison of static, 2 and 6 h flow samples is presented.
On the Co/LM surface, the intensities of VE-cad. and vinculin were unchanged at 2 h but significantly decreased at 6 h. The intensities of KDR and vWF significantly dropped at 2 h and vWF also at 6 h. The intensities of KDR and vWF were augmented between 2 and 6 h; however, vWF did not reach a static level. The unchanged intensities of VE-cad. and vinculin correspond with the mRNA levels. The decrease of KDR mRNA was not significant (Fig. 4B).
As for Co/FN, the intensities of VE-cad. and vWF increased significantly, vinculin was unchanged, and KDR significantly dropped after 2 h flow. At 6 h, VE-cad. decreased to a static level, vinculin dropped, KDR remained decreased, and vWF remained increased. VE-cad. and vinculin decreased and KDR and vWF did not change in intensity between 2 and 6 h. VE-cad. mRNA also increased (nonsignificantly) after 2 h, vinculin was similarly unchanged, and KDR mRNA was also unchanged (Fig. 4C).
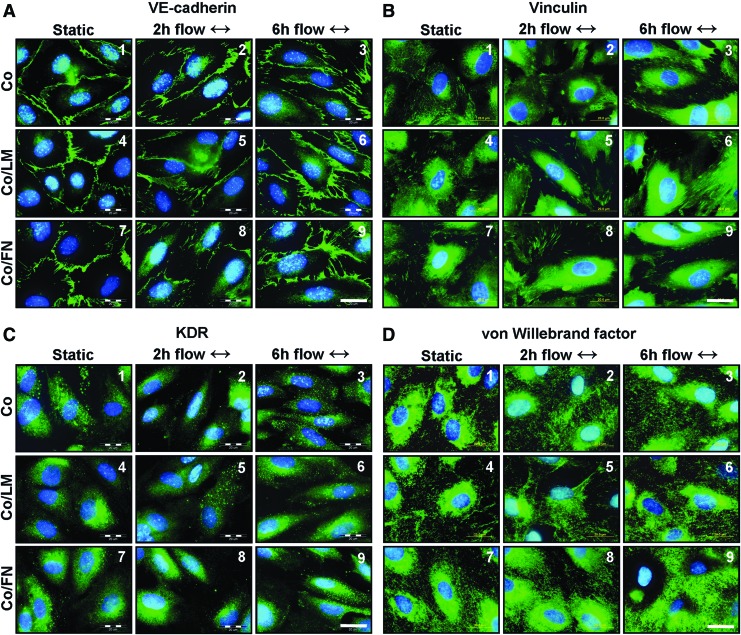
The results of the immunofluorescence staining are presented in Figure 7. The cells are confluent and display a cobble-stone morphology in static cultures (Fig. 7A–D, 1, 4, 7). They become elongated and oriented with the direction of flow, slightly after 2 h of flow (Fig. 7A–D, 2, 5, 8) and more after 6 h of flow (Fig. 7A–D, 3, 6, 9). VE-cadherin stains continuously at the cell–cell interface under static (Fig. 7A, 1, 4, 7) and becomes slightly disintegrated under flow (Fig. 7A, 2–3, 5–6, 8–9). Vinculin-containing focal adhesions are somewhat better developed under flow on Co/LM (Fig. 7B, 5–6) and on Co/FN (Fig. 7B, 8–9) than on Co (Fig. 7B, 2–3). The KDR stain becomes more fine-grained under flow (Fig. 7C, 2–3, 5–6, 8–9) compared with the more dispersed distribution under static (Fig. 7C, 1, 4, 7). vWF staining is localized intracellular in Weibel–Palade bodies under static (Fig. 7D, 1, 4, 7) and appears also extracellular under flow (Fig. 7D, 2–3, 5–6, 8–9).
FIG. 7.
Morphology of HSVEC on collagen (Co), Co/LM, and Co/FN assemblies. Immunofluorescence of VE-cadherin (A1–9), Vinculin (B1–9), KDR (C1–9), and vWF (D1–9) (green), cell nuclei counterstained with Hoechst (blue). The flow direction is from left to right, as indicated by the two-way arrows. Epifluorescence microscope Olympus IX50, Olympus DP70 digital camera, magnification 100×, scale bar=20 μm. Color images available online at www.liebertpub.com/tea
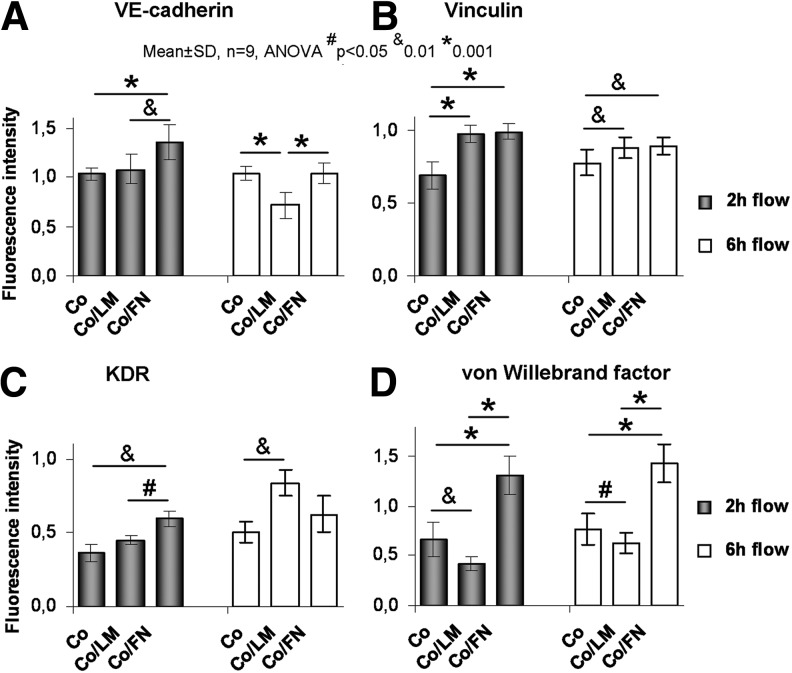
A comparison of the fluorescence intensities of Co, Co/LM, and Co/FN is given in Figure 8, including the statistical significances. Comparing Co/LM to Co at 2 h, vinculin increased (the increase in vinculin mRNA was nonsignificant, Fig. 5B), vWF decreased, and VE-cad. and KDR were unchanged (similar to the VE-cad. and KDR mRNA levels, Fig. 5A, C). Most importantly, the intensity of all four proteins (VE-cad., vinculin, KDR, and vWF) was significantly higher on Co/FN than on both Co and Co/LM at the 2 h time-point (except for vinculin on Co/LM, which was similar to Co/FN). This closely corresponds with the amount of VE-cad. mRNA (Fig. 5A); however, the changes in vinculin and KDR mRNA were nonsignificant (Fig. 5B, C).
FIG. 8.
Relative immunofluorescence intensity of VE-cadherin (A), vinculin (B), KDR (C), and vWF (D) in HSVEC. A comparison of collagen (Co), Co/LM, and Co/FN assemblies is presented.
At 6 h, VE-cad. and vWF on Co/FN were still increased, while vinculin and KDR were comparable to Co/LM. Vinculin and vWF on Co/FN were increased, VE-cad. and KDR were comparable to Co at 6 h. Vinculin and KDR on Co/LM increased, VE-cad. and vWF on Co/LM decreased compared with Co at 6 h.
Discussion
The concept of autologous EC seeding on to the lumen of a vascular prosthesis was introduced in the early 1980s.10 A healthy normal endothelial lining ensures a blood-compatible surface,11 and the combination of polymeric artificial material and seeding of living cells onto a protein-based scaffold is still a prevailing option in vascular tissue engineering.12–14
The surface topography and the wettability of biomaterials have a great impact on the adhesion and growth of cultured cells.15 In our study, glass coated with Co alone was a more hydrophilic surface, while Co/LM and Co/FN bi-layers were shown to be more hydrophobic. However, EC’ adhesion and proliferation followed a similar growth curve on all three samples. This can be explained by the similar surface topography, by the relatively high seeding density and by the capability of primary endothelium with a low passage number (P5) to reach rapidly confluence, in particular on an ECM matrix support of any kind.16 However, under dynamic conditions, the cell adhesion genes were slightly downregulated on Co, which may correspond with their relatively high hydrophilicity (contact angle ∼30°). Cell adhesion is optimal on moderately hydrophilic surfaces, that is, those with a contact angle of 50–90°, while on highly hydrophilic or highly hydrophobic surfaces the cell adhesion is lower (for a review, see Bacakova et al.4). In accordance with this, the adhesion genes tended to be upregulated in cells on Co/LM and Co/FN, that is, on surfaces having contact angles on an average of 63° and 64°, respectively. Moreover, cell retention under flow showed a tendency to be better (although nonsignificantly) on these bi-layers. This confirms our concept that cells can grow,17 and particularly resist flow, better on composite matrices18 resembling the natural ECM, and show more physiological gene expression under SS, depending on the underlying matrix.
Balcells and Edelman19 seeded bovine aortic endothelial cells (BAEC) in serum-free media on tissue culture polystyrene coated with FN or LM or gelatin. They did not find significant differences in cell adhesion and proliferation. They suggest that preadsorbed proteins nonspecifically enhance cell adhesion, but they specifically influence cell function, as was shown by the increased prostacyclin production on FN or LM compared to gelatin. The authors explain this observation by the existence of different types and/or affinity of the integrins that regulate cell function in dependence on adhesive support. These findings are in full agreement with our study, where the functional gene profile of the EC under flow proved to be better on the Co/LM samples, and mainly on Co/FN.
The main challenge for current vascular tissue engineering is to maintain the retention and the function of the endothelial layer. Exposing EC to physiological long-term laminar SS (10–20 dyn/cm2) can provide an analogy with the native endothelial lining in the straight part of arteries.20,21 Physiological laminar SS produces atheroprotective endothelial phenotype, while turbulent flow and low levels of SS produce atherothrombosis-susceptible phenotype both in vitro22 and in vivo.23,24 We are aware that a 2-h-gene profile may represent an “acute onset” response to SS that may transiently be pro-inflammatory, and that chronic effects of normal SS (cell alignment and steady state of gene expression) would have been achieved several hours later.25,26 To confirm the cell phenotype, we extended the flow up to 6 h for the immunofluorescence study. Last but not least, 2 h interval may produce some of the early changes in mRNA levels related to endothelial dysfunction27 and it is theoretically an acceptable time for potential seeding and preconditioning of human cells on body implants during surgery.28
We investigated nine genes and four proteins, which were previously shown to be differentially expressed in cultured EC after submitting to flow. Although they play a clear role in vascular health, the reference data for comparison regarding the expression profiles of EC on engineered surfaces typically contain some contradictory results.29,30 VE-cadherin is a cell–cell adhesion and permeability-associated glycoprotein, which also serves as an adaptor in blood flow sensing. Increased endothelial permeability is associated with localization of atherosclerosis. Disintegration of VE-cad. stain at 2 and 6 h under laminar flow is physiological and transient and neither the expression level of VE-cad., nor its distribution between membrane and cytosol are changed upon shearing of BAEC (12 dyn/cm2, 6–24 h) on pure glass23 or on type I collagen.31 Our experiment revealed transitory downregulation of VE-cad. on the Co sample at 80 min, suggesting a temporary disintegration of cell–cell junctions. However, VE-cad. mRNA was significantly more expressed at the 120-min point on Co/FN (1.6-fold) (protein 1.4-fold) than on both Co and Co/LM, indicating better reorganization of the cell–cell contacts. Parallel to this, Fernandez et al.8 found 1.6-fold augmentation on gelatin and 2.8-fold augmentation in cells on fibrin glue-coated slides after 4 h.
Vinculin is a membrane-cytoskeletal protein localized in focal adhesion plaques, and it employs the linkage of integrin adhesion molecules to the actin cytoskeleton.32,33 The onset of flow does not significantly change the mean remodeling rate of the adhesion sites in confluent cells, but it promotes a shift of adhesion displacement in the downstream direction.32 Cells under increased shear reinforce their adhesion to the substrate; however, the extent of the increased expression of vinculin and paxillin is not extremely high.34 In our experiment, EC significantly downregulated vinculin on the Co substratum—mRNA at 80 min and protein fluorescence at 2 h, suggesting disintegration of cell adhesion under flow. By contrast, cells on Co/LM and Co/FN increased the total fluorescence of vinculin compared with Co, and cells on Co/LM increased the amount of mRNA for vinculin (significantly at 40 min) possibly indicating improved cell attachment to combined matrices.
KDR (VEGFR-2, Flk-1) is a membrane-bound receptor for VEGF. It is involved in signaling transduction, angiogenesis, and cell survival. Long-term physiological SS (6–24 h) has been shown to selectively upregulate the expression of KDR.35 In our setting, testing KDR mRNA and protein gave mixed results, as the period of flow was probably too short to see clear activation of KDR similar to the activation in other studies.8,36,37
CD-31 (PECAM-1) is found in intercellular junctions, and plays an important role in the EC-leukocyte interaction. In the EC stimulated by fluid SS, CD-31 is directly involved in transmitting the flow mechanical forces.38 Moreover, PECAM-1, VE-cadherin, and KDR molecules together comprise a mechanosensory complex of EC.39 HSVEC exposed to 12 dyn/cm2 for 4 h significantly downregulated CD-31 when seeded on planar gelatin, but the level was unchanged on planar fibrin glue or inside tubular vascular grafts.8,40 In our study, CD-31 expression was significantly more stimulated at 80 min on Co/LM than on Co and Co/FN and, conversely, the mRNA level was significantly greater (1.77-fold) on Co/FN at 120 min than on Co and Co/LM.
β1-integrin is a part of the integrin super-family of adhesion molecules that directly bind to ECM proteins, and interact with signaling and cytoskeletal proteins (grouped in focal adhesions) to regulate cellular adherence, focal adhesion plasticity, cell motility, and survival. Integrins are also directly involved in mechanosensing of flow.41 HUVECs under physiologically high SS (15 dyn/cm2) enhance more than 2.5-fold the expression of integrin subunits α5 and β1, which are preferential receptors for FN. This increases cell-matrix attachment, migration, and survival, thus being an atheroprotective change.42 In our experiment, β1-integrin was insignificantly upregulated on both Co/LM and Co/FN at 40 min compared to static. At 80 min and mainly at 120 min, it was more increased on Co/FN (1.89-fold) than on Co/LM (1.16-fold), but this was not significant. We were unable to measure the mRNA for β1-intergrin on pure Co for major cell loss during flow. Increased upregulation of integrin on Co/FN compared with Co/LM may be in accordance with Urbich's study,42 since the preferential receptor for LM (α6) was only slightly upregulated by shear compared with FN receptor β1. Moreover, FN integrin receptors αvβ3 and α5β1 play a major role in the adaptation of EC to flow.43,44 This may explain the slightly better cellular response on Co/FN than on Co or Co/LM under SS in this study.45
t-PA is an important enzyme involved in blood clot lysis, and it is related to normal vascular health. Its recombinant form is widely used as a therapeutic agent. McCormick et al.46 found the DNA microarray ratios for t-PA 1.03 at 6 h and 1.28 at 24 h of SS (25 dyn/cm2). However, the Northern ratio (protein) for t-PA at 6 h was 2.23, twice that of the t-PA Northern ratio at 24 h. The authors conclude that this discrepancy, although not significant, may be due to the low expression level in HUVEC. However, HSVEC exposed to SS of 12 dyn/cm2 for 4 h increased the expression of t-PA 2.2-fold (p<0.05), 2.9-fold (nonsignificant), and 2.56-fold (p<0.05) on planar gelatin, planar fibrin-glue, and tubular fibrin glue-coated ePTFE grafts, respectively, suggesting regulation of EC under laminar SS in an anti-thrombotic way.8 In our setting, the expression levels of mRNA for t-PA were variable and nonsignificant among the samples (apart from temporary upregulation on Co at 40 min).
NF-κB is a protein complex controlling transcription of DNA. In almost all cell types, it is integrated in responses to stress, and plays a key role in inflammation. BAEC cultured on tissue culture plastic and exposed to 12 dyn/cm2 showed a transient increase (two to three-fold) in NF-κB at 30 and 60 min, and no further increase was observed at 120 min.47 In human aortic EC (HAEC) the activation of NF-κB was significantly elevated when exposed to prolonged (>2 h) steady low shear (2 dyn/cm2) compared with exposure to prolonged high (i.e., physiological) shear of 16 dyn/cm2.48 This is in accordance with our study, where EC on Co/LM and Co/FN upregulated NF-κB around three-fold, being significant on Co/LM at 40 min and 80 min and nonsignificant at 120 min, and never significant on Co/FN for any interval. We assume that prolonged stimulation (up to 24 h) would probably reverse this temporary augmentation.49 In addition, NF-κB expression under laminar SS potentially exerts dual functions by inducing both pro-inflammatory and cytoprotective transcripts.50 Thus, upregulation of NF-κB by physiologically high SS is only transitory, in contrast to disturbed flow, which can cause prolonged activation and early atherosclerotic changes.
Human cells in vivo are constantly exposed to multi-component matrices varying in composition. Flow-induced early signaling in BAEC plated on various ECM proteins was previously studied,51,52 and the authors found that SS-induced NF-κB activation is more pronounced in cells cultured on FN and fibrinogen, which are transitional ECM proteins deposited more in case of inflammation, injury, and angiogenesis. However, BAEC plated on Co and LM retained more quiescent phenotype under shear, consistent with low cell turnover. Thus, including collagen in mixed artificial basement membrane actively suppressed the FN-dependent activation of NF-κB by flow, as shown in cells on combined matrix Co/FN. However, the causal relationship in vivo between ECM remodeling and NF-κB activation remains to be elucidated.51 Moreover, mixed matrices in certain concentrations, such as Co/FN, may result in signaling co-operativity in EC.52 This is in agreement with our study, which was also performed on a two-component matrix, although the increased activation of some molecules on Co/FN was not statistically significant compared to Co and Co/LM, due to variation among the experiments. Another example of matrix-specific activation of the inflammatory signaling pathway under SS (45 min) is the activation of c-Jun NH2-terminal kinases in EC cultured on FN, but not on Co.53 However, this happens transiently after the acute onset of laminar SS or after chronic stimulation with oscillatory flow, and this pro-inflammatory pathway would be quiescent for cells under prolonged application of laminar shear.54
eNOS generates the production of NO in blood vessels, and is vital in cell signaling and in regulating normal vascular tone. SS is believed to be the most important stimulus for NO production. Porcine aortic EC were proved to enhance two-fold the production of eNOS upon 16 dyn/cm2 shear for 6 h when seeded on glass with LM support; however, culturing on Co or FN was noninductive for eNOS under SS thanks to additional cell-laminin interaction via nonintegrin proteins.55 eNOS is also clearly upregulated by SS in BAEC. However, the evidence is less convincing in HUVEC (25 dyn/cm2, 6 h), since the output of NO from EC is promoted by a number of other mechanisms for example, synthesis of NO precursor L-arginine.46 To support this, we did not observe significant changes in eNOS mRNA in HSVEC up to 2 h in any of our samples.
MMP-1 is a member of the family of enzymes involved in the breakdown of ECM in both physiological and pathological processes. Twenty-four hours of laminar SS of 12 dyn/cm2 significantly (2.8-fold) increases the amount of MMP-1 in HAEC as a manifestation of normal vascular remodeling and potential wound healing.35 1.7-fold induction was reported in HUVEC subjected to a similar experimental setting and, on the other hand, a 1.4-fold increase was noted under turbulent (athero-inductive) flow.56 We did not observe relevant changes, probably due to the short time of shearing; however, cells on Co/FN augmented MMP-1 more than on Co/LM (significantly at the 80 min point).
vWF is an adhesive glycoprotein involved in blood coagulation and serves as a marker of EC differentiation.57 SS induces physiological release of vWF from EC both into the medium and into basolateral ECM without enhanced vWF mRNA expression.58 However, we observed increased fluorescence of vWF protein (probably both intracellular+extracellular) on Co/FN compared with Co and Co/LM at 2 h and at 6 h. Since vWF mediates EC adhesion to the vessel wall in physiologic conditions, we regard this as improved cellular phenotype on Co/FN rather than a pathologic pro-thrombotic change.5,8,58
In conditions of tissue engineering, the goal is to achieve rapid surface endothelialization of vascular implants, possible preconditioning by laminar flow, and maintaining the function of the seeded cells, mainly their anti-thrombogenic properties.30 The contribution of our study consists of defining the short-term (up to 2 h) effect of physiological laminar SS on phenotypic modulation of human patient-derived EC in close dependence on precoating composite ECM matrices for the purposes of vascular tissue engineering.
Conclusion
Primary cultures of HSVEC rapidly formed a confluent monolayer on Co, Co/LM and Co/FN planar substrates. Despite the divergence in surface roughness and wettability, there were no differences in cell adhesion and growth. However, combined Co/LM and Co/FN matrices seemed to be more suitable for EC retention under flow than Co surface alone. Moreover, Co/FN matrix promoted slightly more favorable cellular phenotype than Co/LM, as shown by the upregulated expression of some of the adhesion and metabolic genes under SS up to 2 h and corresponding enhanced immunofluorescence of proteins under flow up to 6 h.
Acknowledgments
We are grateful for the support received from various grant agencies: Grant Agency of the Czech Republic (projects P108/10/1106, P108/11/1857, P305/08/0108, and P205/12/1702). Ministry of Education, Youth and Sports of the Czech Republic (Project Barrande 2005-06-036-1, BIOPOL EE2.3.30.0029, and BIOCEV CZ.1.05/1.1.00/02.0109). Grant Agency of the Ministry of Health CR (project No NT11270-4/2010). Grant Agency of Charles University in Prague, Czech Republic (grant GAUK-637712). Mr. Robin Healey (Czech Technical University, Prague, Czech Republic) is gratefully acknowledged for his language revision of the article.
Disclosure Statement
No competing financial interests exist.
References
- 1.Goodney P.P., Beck A.W., Nagle J., Welch H.G., and Zwolak R.M.National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg 50,54, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Chlupac J., Filova E., and Bacakova L.Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol Res 58(Suppl. 2), 119, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Meinhart J.G., Deutsch M., Fischlein T., Howanietz N., Fröschl A., and Zilla P.Clinical autologous in vitro endothelialization of 153 infrainguinal ePTFE grafts. Ann Thorac Surg 71(5 Suppl), 327, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Bacakova L., Filova E., Parizek M., Ruml T., and Svorcik V.Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol Adv 29,739, 2011 [DOI] [PubMed] [Google Scholar]
- 5.McGuigan A.P., and Sefton M.V.The influence of biomaterials on endothelial cell thrombogenicity. Biomaterials 28,2547, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brynda E., Pacherník J., Houska M., Pientka Z., and Dvořák P.Surface immobilized protein multilayers for cell seeding. Langmuir 21,7877, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Fernandez P., Daculsi R., Rémy-Zolghadri M., Bareille R., and Bordenave L.Endothelial cells cultured on engineered vascular grafts are able to transduce shear stress. Tissue Eng 12,1, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Fernandez P., Bourget C., Bareille R., Daculsi R., and Bordenave L.Gene response in endothelial cells cultured on engineered surfaces is regulated by shear stress. Tissue Eng 13,1607, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Rémy M., Bareille R., Rerat V., Bourget C., Marchand-Brynaert J., and Bordenave L.Polyethylene terephthalate membrane grafted with peptidomimetics: endothelial cell compatibility and retention under shear stress. J Biomater Sci Polym Ed 24,269, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Herring M., Gardner A., and Glover J.Seeding human arterial prostheses with mechanically derived endothelium. The detrimental effect of smoking. J Vasc Surg 1,279, 1984 [PubMed] [Google Scholar]
- 11.Deutsch M., Meinhart J., Zilla P., Howanietz N., Gorlitzer M., Froeschl A., Stuempflen A., Bezuidenhout D., and Grabenwoeger M.Long-term experience in autologous in vitro endothelialization of infrainguinal ePTFE grafts. J Vasc Surg 49,352, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Stegemann J.P., Kaszuba S.N., and Rowe S.L.Review: advances in vascular tissue engineering using protein-based biomaterials. Tissue Eng 13,2601, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Visscher G., Mesure L., Meuris B., Ivanova A., and Flameng W.Improved endothelialization and reduced thrombosis by coating a synthetic vascular graft with fibronectin and stem cell homing factor SDF-1α. Acta Biomater 8,1330, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Williams S.K., Kleinert L.B., and Patula-Steinbrenner V.Accelerated neovascularization and endothelialization of vascular grafts promoted by covalently bound laminin type 1. J Biomed Mater Res A 99,67, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandrovcová M., and Bačáková L.Adhesion, growth and differentiation of osteoblasts on surface-modified materials developed for bone implants. Physiol Res 60,403, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Prasad C.K., and Krishnan L.K.Effect of passage number and matrix characteristics on differentiation of endothelial cells cultured for tissue engineering. Biomaterials 26,5658, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Sreerekha P.R., and Krishnan L.K.Cultivation of endothelial progenitor cells on fibrin matrix and layering on dacron/polytetrafluoroethylene vascular grafts. Artif Organs 30,242, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Prasad C.K., and Krishan L.K.Regulation of endothelial cell phenotype by biomimetic matrix coated on biomaterials for cardiovascular tissue engineering. Acta Biomater 4,182, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Balcells M., and Edelman E.R.Effect of pre-adsorbed proteins on attachment, proliferation, and function of endothelial cells. J Cell Physiol 191,155, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Li Y.S., Haga. J.H., and Chien S.Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech 38,1949, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Chien S.Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol 292,H1209, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Brooks A.R., Lelkes P.I., and Rubanyi G.M.Gene expression profiling of vascular endothelial cells exposed to fluid mechanical forces: relevance for focal susceptibility to atherosclerosis. Endothelium 11,45, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Miao H., Hu Y.L., Shiu Y.T., Yuan S., Zhao Y., Kaunas R., Wang Y., Jin G., Usami S., and Chien S.Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: in vivo and in vitro investigations. J Vasc Res 42,77, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Davies P.F.Endothelial transcriptome profiles in vivo in complex arterial flow fields. Ann Biomed Eng 36,563, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Wasserman S.M., Mehraban F., Komuves L.G., Yang R.B., Tomlinson J.E., Zhang Y., Spriggs F., and Topper J.N.Gene expression profile of human endothelial cells exposed to sustained fluid shear stress. Physiol Genomics 12,13, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Wasserman S.M., and Topper J.N.Adaptation of the endothelium to fluid flow: in vitro analyses of gene expression and in vivo implications. Vasc Med 9,35, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Hsieh H.J., Li N.Q., and Frangos J.A.Shear stress increases endothelial platelet-derived growth factor mRNA levels. Am J Physiol 260(2 Pt 2),H642, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Bordenave L., Menu P., and Baquey C.Developments towards tissue-engineered, small-diameter arterial substitutes. Expert Rev Med Devices 5,337, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Punchard M.A., Stenson-Cox C., O'Cearbhaill E.D., Lyons E., Gundy S., Murphy L., Pandit A., McHugh P.E., and Barron V.Endothelial cell response to biomechanical forces under simulated vascular loading conditions. J Biomech 40,3146, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Vara D.S., Punshon G., Sales K.M., Hamilton G., and Seifalian A.M.Haemodynamic regulation of gene expression in vascular tissue engineering. Curr Vasc Pharmacol 9,167, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Kondapalli J., Flozak A.S., and Albuquerque M.L.Laminar shear stress differentially modulates gene expression of p120 catenin, Kaiso transcription factor, and vascular endothelial cadherin in human coronary artery endothelial cells. J Biol Chem 279,11417, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Mott R.E., and Helmke B.P.Mapping the dynamics of shear stress-induced structural changes in endothelial cells. Am J Physiol Cell Physiol 293,C1616, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albuquerque M.L., and Flozak A.S.Lamellipodial motility in wounded endothelial cells exposed to physiologic flow is associated with different patterns of beta1-integrin and vinculin localization. J Cell Physiol 195,50, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Kano Y., Katoh K., and Fujiwara K.Lateral zone of cell-cell adhesion as the major fluid shear stress-related signal transduction site. Circ Res 86,425, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Chen B.P., Li Y.S., Zhao Y., Chen K.D., Li S., Lao J., Yuan S., Shyy J.Y., and Chien S.DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol Genomics 7,55, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Staalesen T., Risberg B., and Mattsson E.The kinase insert domain-containing receptor (KDR) is regulated by shear stress. Scand Cardiovasc J 36,368, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Urbich C., Stein M., Reisinger K., Kaufmann R., Dimmeler S., and Gille J.Fluid shear stress-induced transcriptional activation of the vascular endothelial growth factor receptor-2 gene requires Sp1-dependent DNA binding. FEBS Lett 535,87, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Fujiwara K.Platelet endothelial cell adhesion molecule-1 and mechanotransduction in vascular endothelial cells. J Intern Med 259,373, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Tzima E., Irani-Tehrani M., Kiosses W.B., Dejana E., Schultz D.A., Engelhardt B., Cao G., DeLisser H., and Schwartz M.A.A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437,426, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Vara D.S., Punshon G., Sales K.M., Sarkar S., Hamilton G., and Seifalian A.M.Endothelial cell retention on a viscoelastic nanocomposite vascular conduit is improved by exposure to shear stress preconditioning prior to physiological flow. Artif Organs 32,977, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Shyy J.Y., and Chien S.Role of integrins in endothelial mechanosensing of shear stress. Circ Res 91,769, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Urbich C., Walter D.H., Zeiher A.M., and Dimmeler S.Laminar shear stress upregulates integrin expression: role in endothelial cell adhesion and apoptosis. Circ Res 87,683, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Hahn C., and Schwartz M.A.Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 10,53, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jalali S., del Pozo M.A., Chen K., Miao H., Li Y., Schwartz M.A., Shyy J.Y., and Chien S.Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci U S A 98,1042, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katsumi A., Orr A.W., Tzima E., and Schwartz M.A.Integrins in mechanotransduction. J Biol Chem 279,12001, 2004 [DOI] [PubMed] [Google Scholar]
- 46.McCormick S.M., Eskin S.G., McIntire L.V., Teng C.L., Lu C.M., Russell C.G., and Chittur K.K.DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci U S A 98,8955, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lan Q., Mercurius K.O., and Davies P.F.Stimulation of transcription factors NF kappa B and AP1 in endothelial cells subjected to shear stress. Biochem Biophys Res Commun 201,950, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Mohan S., Mohan N., and Sprague E.A.Differential activation of NF-kappa B in human aortic endothelial cells conditioned to specific flow environments. Am J Physiol 273(2 Pt 1),C572, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Lehoux S., Castier Y., and Tedgui A.Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med 259,381, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Partridge J., Carlsen H., Enesa K., Chaudhury H., Zakkar M., Luong L., Kinderlerer A., Johns M., Blomhoff R., Mason J.C., Haskard D.O., and Evans P.C.Laminar shear stress acts as a switch to regulate divergent functions of NF-kappaB in endothelial cells. FASEB J 21,3553, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Orr A.W., Sanders J.M., Bevard M., Coleman E., Sarembock I.J., and Schwartz M.A.The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J Cell Biol 169,191, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orr A.W., Ginsberg M.H., Shattil S.J., Deckmyn H., and Schwartz M.A.Matrix-specific suppression of integrin activation in shear stress signaling. Mol Biol Cell 17,4686, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hahn C., Orr A.W., Sanders J.M., Jhaveri K.A., and Schwartz M.A.The subendothelial extracellular matrix modulates JNK activation by flow. Circ Res 104,995, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shyy J.Y.Extracellular matrix differentiating good flow versus bad flow. Circ Res 104,931, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gloe T., Riedmayr S., Sohn H.Y., and Pohl U.The 67-kDa laminin-binding protein is involved in shear stress-dependent endothelial nitric-oxide synthase expression. J Biol Chem 274,15996, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Garcia-Cardeña G., Comander J., Anderson K.R., Blackman B.R., and Gimbrone M.A., Jr.Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci U S A 98,4478, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai H.M.von Willebrand factor, shear stress, and ADAMTS13 in hemostasis and thrombosis. ASAIO J 58,163, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Galbusera M., Zoja C., Donadelli R., Paris S., Morigi M., Benigni A., Figliuzzi M., Remuzzi G., and Remuzzi A.Fluid shear stress modulates von Willebrand factor release from human vascular endothelium. Blood 90,1558, 1997 [PubMed] [Google Scholar]