Abstract
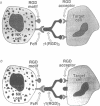
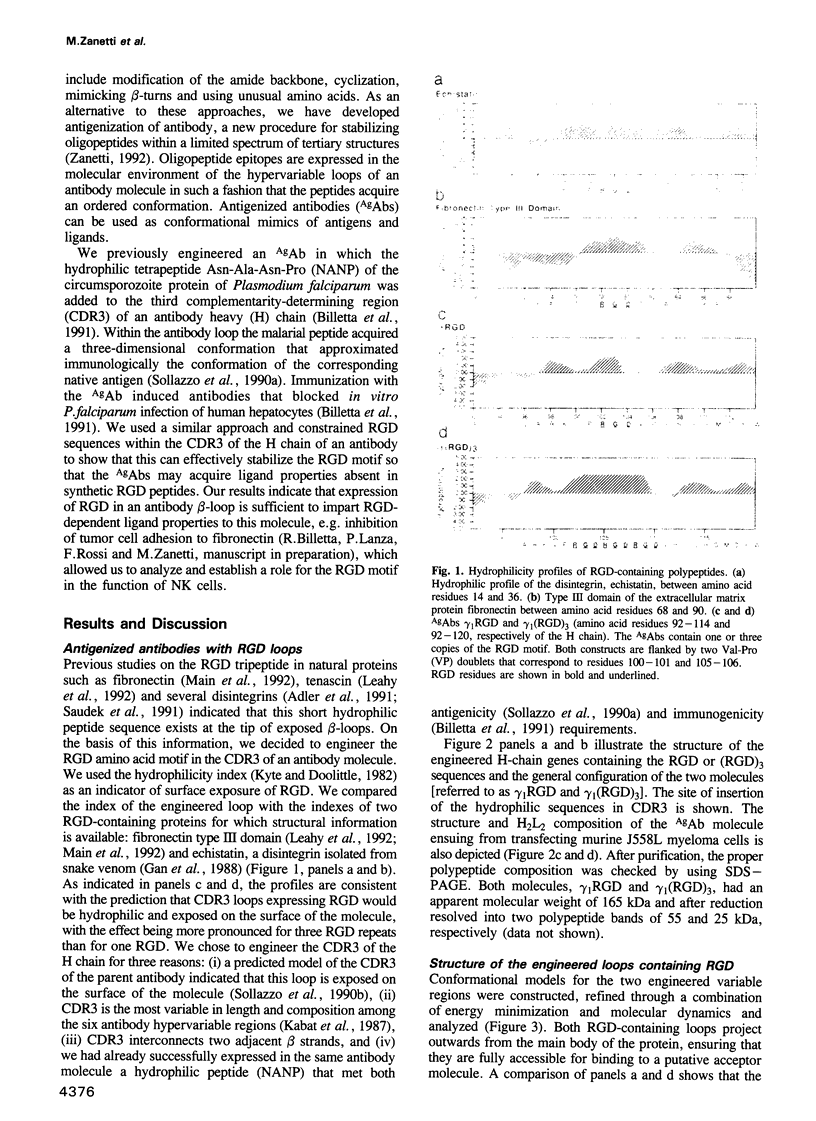
We report that an antibody engineered to express three Arg-Gly-Asp (RGD) repeats in the third complementarity-determining region of the heavy chain (antigenized antibody) efficiently inhibits the lysis of human erythroleukemia K-562 cells by natural killer (NK) cells. Synthetic peptides containing RGD did not inhibit. Inhibition was specific for the (RGD)3-containing loop and required simultaneous occupancy of the Fc receptor (CD16) on effector cells. The antigenized antibody inhibited other forms of cytotoxicity mediated by NK cells but not cytotoxicity mediated by major histocompatibility complex-restricted cytotoxic T lymphocytes (CTL). A three-dimensional model of the engineered antibody loop shows the structure and physicochemical characteristics probably required for the ligand activity. The results indicate that an RGD motif is involved in the productive interaction between NK and target cells. Moreover, they show that peptide expression in the hypervariable loops of an antibody molecule is an efficient procedure for stabilizing oligopeptides within a limited spectrum of tertiary structures. This is a new approach towards imparting ligand properties to antibody molecules and can be used to study the biological function and specificity of short peptide motifs, including those involved in cell adhesion.
Full text
PDF

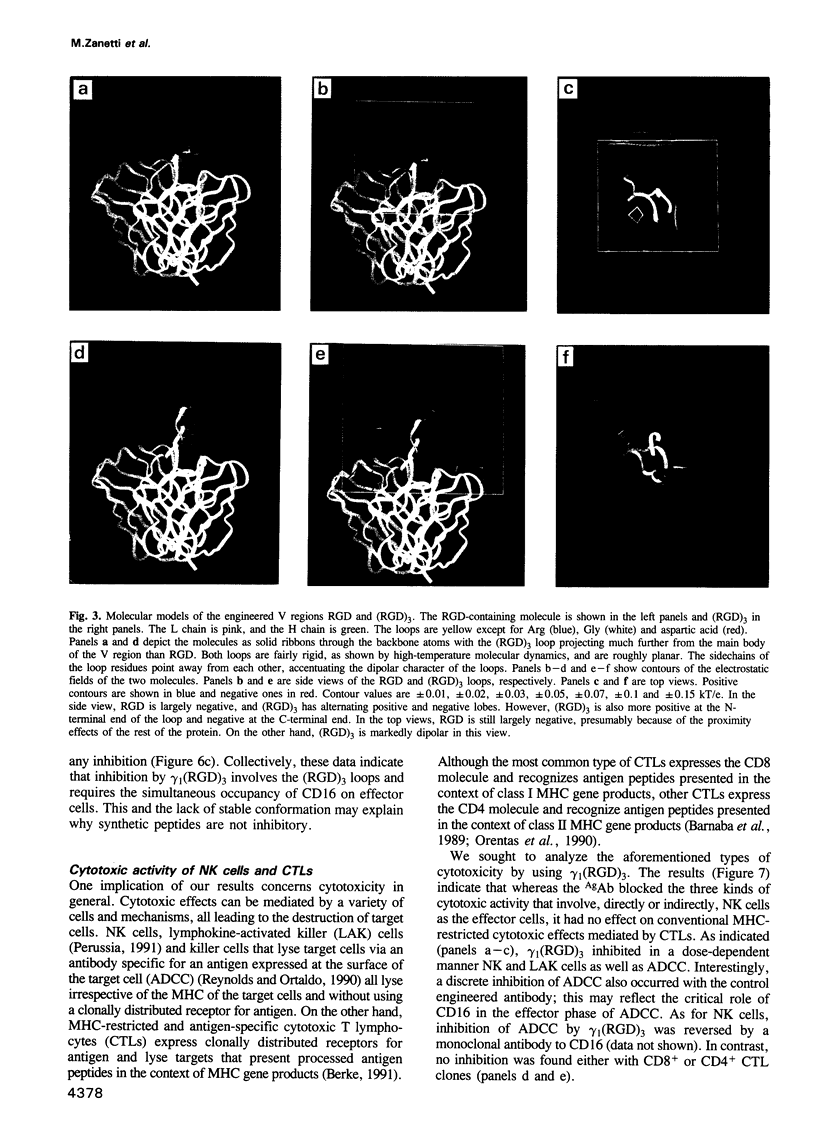




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler M., Lazarus R. A., Dennis M. S., Wagner G. Solution structure of kistrin, a potent platelet aggregation inhibitor and GP IIb-IIIa antagonist. Science. 1991 Jul 26;253(5018):445–448. doi: 10.1126/science.1862345. [DOI] [PubMed] [Google Scholar]
- Anderson P., Caligiuri M., O'Brien C., Manley T., Ritz J., Schlossman S. F. Fc gamma receptor type III (CD16) is included in the zeta NK receptor complex expressed by human natural killer cells. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2274–2278. doi: 10.1073/pnas.87.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z. Precise determination of protein antigenic structures has unravelled the molecular immune recognition of proteins and provided a prototype for synthetic mimicking of other protein binding sites. Mol Cell Biochem. 1980 Aug 29;32(1):21–43. doi: 10.1007/BF00421293. [DOI] [PubMed] [Google Scholar]
- Axberg I., Ramstedt U., Patarroyo M., Beatty P., Wigzell H. Inhibition of natural killer cell cytotoxicity by a monoclonal antibody directed against adhesion-mediating protein gp 90 (CD18). Scand J Immunol. 1987 Nov;26(5):547–554. doi: 10.1111/j.1365-3083.1987.tb02288.x. [DOI] [PubMed] [Google Scholar]
- Bacchetta R., Vandekerckhove B. A., Touraine J. L., Bigler M., Martino S., Gebuhrer L., de Vries J. E., Spits H., Roncarolo M. G. Chimerism and tolerance to host and donor in severe combined immunodeficiencies transplanted with fetal liver stem cells. J Clin Invest. 1993 Mar;91(3):1067–1078. doi: 10.1172/JCI116264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnaba V., Franco A., Alberti A., Balsano C., Benvenuto R., Balsano F. Recognition of hepatitis B virus envelope proteins by liver-infiltrating T lymphocytes in chronic HBV infection. J Immunol. 1989 Oct 15;143(8):2650–2655. [PubMed] [Google Scholar]
- Barnaba V., Franco A., Alberti A., Benvenuto R., Balsano F. Selective killing of hepatitis B envelope antigen-specific B cells by class I-restricted, exogenous antigen-specific T lymphocytes. Nature. 1990 May 17;345(6272):258–260. doi: 10.1038/345258a0. [DOI] [PubMed] [Google Scholar]
- Barton C. H., Dickson G., Gower H. J., Rowett L. H., Putt W., Elsom V., Moore S. E., Goridis C., Walsh F. S. Complete sequence and in vitro expression of a tissue-specific phosphatidylinositol-linked N-CAM isoform from skeletal muscle. Development. 1988 Sep;104(1):165–173. doi: 10.1242/dev.104.1.165. [DOI] [PubMed] [Google Scholar]
- Bergelson L. D., Dyatlovitskaya E. V., Klyuchareva T. E., Kryukova E. V., Lemenovskaya A. F., Matveeva V. A., Sinitsyna E. V. The role of glycosphingolipids in natural immunity. Gangliosides modulate the cytotoxicity of natural killer cells. Eur J Immunol. 1989 Nov;19(11):1979–1983. doi: 10.1002/eji.1830191102. [DOI] [PubMed] [Google Scholar]
- Berke G. T-cell-mediated cytotoxicity. Curr Opin Immunol. 1991 Jun;3(3):320–325. doi: 10.1016/0952-7915(91)90031-u. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Billetta R., Hollingdale M. R., Zanetti M. Immunogenicity of an engineered internal image antibody. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4713–4717. doi: 10.1073/pnas.88.11.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix M., Liao N. S., Zijlstra M., Loring J., Jaenisch R., Raulet D. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991 Jan 24;349(6307):329–331. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- Blobel C. P., Wolfsberg T. G., Turck C. W., Myles D. G., Primakoff P., White J. M. A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature. 1992 Mar 19;356(6366):248–252. doi: 10.1038/356248a0. [DOI] [PubMed] [Google Scholar]
- Bronson R. A., Fusi F. Evidence that an Arg-Gly-Asp adhesion sequence plays a role in mammalian fertilization. Biol Reprod. 1990 Dec;43(6):1019–1025. doi: 10.1095/biolreprod43.6.1019. [DOI] [PubMed] [Google Scholar]
- Buckley R. H., Schiff S. E., Sampson H. A., Schiff R. I., Markert M. L., Knutsen A. P., Hershfield M. S., Huang A. T., Mickey G. H., Ward F. E. Development of immunity in human severe primary T cell deficiency following haploidentical bone marrow stem cell transplantation. J Immunol. 1986 Apr 1;136(7):2398–2407. [PubMed] [Google Scholar]
- Chothia C., Novotný J., Bruccoleri R., Karplus M. Domain association in immunoglobulin molecules. The packing of variable domains. J Mol Biol. 1985 Dec 5;186(3):651–663. doi: 10.1016/0022-2836(85)90137-8. [DOI] [PubMed] [Google Scholar]
- Dahl C. A., Schall R. P., He H. L., Cairns J. S. Identification of a novel gene expressed in activated natural killer cells and T cells. J Immunol. 1992 Jan 15;148(2):597–603. [PubMed] [Google Scholar]
- Dearman R. J., Stevenson F. K., Wrightham M., Hamblin T. J., Glennie M. J., Stevenson G. T. Lymphokine-activated killer cells from normal and lymphoma subjects are cytotoxic for cells coated with antibody derivatives displaying human Fc gamma. Blood. 1988 Dec;72(6):1985–1991. [PubMed] [Google Scholar]
- Dongworth D. W., Gotch F. M., Hildreth J. E., Morris A., McMichael A. J. Effects of monoclonal antibodies to the alpha and beta chains of the human lymphocyte function-associated (H-LFA-1) antigen on T lymphocyte functions. Eur J Immunol. 1985 Sep;15(9):888–892. doi: 10.1002/eji.1830150905. [DOI] [PubMed] [Google Scholar]
- Drobyski W. R., Piaskowski V., Ash R. C., Casper J. T., Truitt R. L. Preservation of lymphokine-activated killer activity following T cell depletion of human bone marrow. Transplantation. 1990 Oct;50(4):625–632. doi: 10.1097/00007890-199010000-00020. [DOI] [PubMed] [Google Scholar]
- Fischer A., Landais P., Friedrich W., Morgan G., Gerritsen B., Fasth A., Porta F., Griscelli C., Goldman S. F., Levinsky R. European experience of bone-marrow transplantation for severe combined immunodeficiency. Lancet. 1990 Oct 6;336(8719):850–854. doi: 10.1016/0140-6736(90)92348-l. [DOI] [PubMed] [Google Scholar]
- Franco A., Paroli M., Testa U., Benvenuto R., Peschle C., Balsano F., Barnaba V. Transferrin receptor mediates uptake and presentation of hepatitis B envelope antigen by T lymphocytes. J Exp Med. 1992 May 1;175(5):1195–1205. doi: 10.1084/jem.175.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Z. R., Gould R. J., Jacobs J. W., Friedman P. A., Polokoff M. A. Echistatin. A potent platelet aggregation inhibitor from the venom of the viper, Echis carinatus. J Biol Chem. 1988 Dec 25;263(36):19827–19832. [PubMed] [Google Scholar]
- Ghayur T., Seemayer T. A., Kongshavn P. A., Gartner J. G., Lapp W. S. Graft-versus-host reactions in the beige mouse. An investigation of the role of host and donor natural killer cells in the pathogenesis of graft-versus-host disease. Transplantation. 1987 Aug;44(2):261–267. doi: 10.1097/00007890-198708000-00017. [DOI] [PubMed] [Google Scholar]
- Gilson M. K., Honig B. Calculation of the total electrostatic energy of a macromolecular system: solvation energies, binding energies, and conformational analysis. Proteins. 1988;4(1):7–18. doi: 10.1002/prot.340040104. [DOI] [PubMed] [Google Scholar]
- Ginsberg M., Pierschbacher M. D., Ruoslahti E., Marguerie G., Plow E. Inhibition of fibronectin binding to platelets by proteolytic fragments and synthetic peptides which support fibroblast adhesion. J Biol Chem. 1985 Apr 10;260(7):3931–3936. [PubMed] [Google Scholar]
- Grayson G., Ladisch S. Immunosuppression by human gangliosides. II. Carbohydrate structure and inhibition of human NK activity. Cell Immunol. 1992 Jan;139(1):18–29. doi: 10.1016/0008-8749(92)90096-8. [DOI] [PubMed] [Google Scholar]
- Hayman E. G., Pierschbacher M. D., Ruoslahti E. Detachment of cells from culture substrate by soluble fibronectin peptides. J Cell Biol. 1985 Jun;100(6):1948–1954. doi: 10.1083/jcb.100.6.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrand K., Kjellson B., Hermodsson S. Monocyte-induced down-modulation of CD16 and CD56 antigens on human natural killer cells and its regulation by histamine H2-receptors. Cell Immunol. 1991 Nov;138(1):44–54. doi: 10.1016/0008-8749(91)90131-t. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hemperly J. J., DeGuglielmo J. K., Reid R. A. Characterization of cDNA clones defining variant forms of human neural cell adhesion molecule N-CAM. J Mol Neurosci. 1990;2(2):71–78. doi: 10.1007/BF02876913. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Thirup S. Using known substructures in protein model building and crystallography. EMBO J. 1986 Apr;5(4):819–822. doi: 10.1002/j.1460-2075.1986.tb04287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Chang C., Azuma M., Ruitenberg J. J., Hemperly J. J., Phillips J. H. Molecular and functional analysis of human natural killer cell-associated neural cell adhesion molecule (N-CAM/CD56). J Immunol. 1991 Jun 15;146(12):4421–4426. [PubMed] [Google Scholar]
- Lanier L. L., Phillips J. H. Natural killer cells. Curr Opin Immunol. 1992 Feb;4(1):38–42. doi: 10.1016/0952-7915(92)90121-t. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Yu G., Phillips J. H. Co-association of CD3 zeta with a receptor (CD16) for IgG Fc on human natural killer cells. Nature. 1989 Dec 14;342(6251):803–805. doi: 10.1038/342803a0. [DOI] [PubMed] [Google Scholar]
- Leahy D. J., Hendrickson W. A., Aukhil I., Erickson H. P. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Science. 1992 Nov 6;258(5084):987–991. doi: 10.1126/science.1279805. [DOI] [PubMed] [Google Scholar]
- Lerner R. A. Synthetic vaccines. Sci Am. 1983 Feb;248(2):66–74. doi: 10.1038/scientificamerican0283-66. [DOI] [PubMed] [Google Scholar]
- Liao N. S., Bix M., Zijlstra M., Jaenisch R., Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991 Jul 12;253(5016):199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- Lopez C., Kirkpatrick D., Livnat S., Storb R. Natural killer cells in bone marrow transplantation. Lancet. 1980 Nov 8;2(8202):1025–1025. doi: 10.1016/s0140-6736(80)92177-7. [DOI] [PubMed] [Google Scholar]
- Main A. L., Harvey T. S., Baron M., Boyd J., Campbell I. D. The three-dimensional structure of the tenth type III module of fibronectin: an insight into RGD-mediated interactions. Cell. 1992 Nov 13;71(4):671–678. doi: 10.1016/0092-8674(92)90600-h. [DOI] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Gugel E. A., Dustin M. L., Springer T. A., Shaw S. Functional evidence that intercellular adhesion molecule-1 (ICAM-1) is a ligand for LFA-1-dependent adhesion in T cell-mediated cytotoxicity. Eur J Immunol. 1988 Apr;18(4):637–640. doi: 10.1002/eji.1830180423. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Krensky A. M., Burakoff S. J. Mapping functional epitopes of the human LFA-1 glycoprotein: monoclonal antibody inhibition of NK and CTL effectors. Hum Immunol. 1986 Nov;17(3):288–296. doi: 10.1016/0198-8859(86)90280-6. [DOI] [PubMed] [Google Scholar]
- Orentas R. J., Hildreth J. E., Obah E., Polydefkis M., Smith G. E., Clements M. L., Siliciano R. F. Induction of CD4+ human cytolytic T cells specific for HIV-infected cells by a gp160 subunit vaccine. Science. 1990 Jun 8;248(4960):1234–1237. doi: 10.1126/science.2190315. [DOI] [PubMed] [Google Scholar]
- Perussia B. Lymphokine-activated killer cells, natural killer cells and cytokines. Curr Opin Immunol. 1991 Feb;3(1):49–55. doi: 10.1016/0952-7915(91)90076-d. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., McKinney L., Azuma M., Spits H., Lanier L. L. A novel beta 4, alpha 6 integrin-associated epithelial cell antigen involved in natural killer cell and antigen-specific cytotoxic T lymphocyte cytotoxicity. J Exp Med. 1991 Dec 1;174(6):1571–1581. doi: 10.1084/jem.174.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N. E., Parker D. C. Cross-linking of B lymphocyte Fc gamma receptors and membrane immunoglobulin inhibits anti-immunoglobulin-induced blastogenesis. J Immunol. 1984 Feb;132(2):627–632. [PubMed] [Google Scholar]
- Phillips N. E., Parker D. C. Fc-dependent inhibition of mouse B cell activation by whole anti-mu antibodies. J Immunol. 1983 Feb;130(2):602–606. [PubMed] [Google Scholar]
- Qiu W. Q., de Bruin D., Brownstein B. H., Pearse R., Ravetch J. V. Organization of the human and mouse low-affinity Fc gamma R genes: duplication and recombination. Science. 1990 May 11;248(4956):732–735. doi: 10.1126/science.2139735. [DOI] [PubMed] [Google Scholar]
- Rini J. M., Schulze-Gahmen U., Wilson I. A. Structural evidence for induced fit as a mechanism for antibody-antigen recognition. Science. 1992 Feb 21;255(5047):959–965. doi: 10.1126/science.1546293. [DOI] [PubMed] [Google Scholar]
- Rixon M. W., Chan W. Y., Davie E. W., Chung D. W. Characterization of a complementary deoxyribonucleic acid coding for the alpha chain of human fibrinogen. Biochemistry. 1983 Jun 21;22(13):3237–3244. doi: 10.1021/bi00282a031. [DOI] [PubMed] [Google Scholar]
- Robertson M. J., Caligiuri M. A., Manley T. J., Levine H., Ritz J. Human natural killer cell adhesion molecules. Differential expression after activation and participation in cytolysis. J Immunol. 1990 Nov 15;145(10):3194–3201. [PubMed] [Google Scholar]
- Robertson M. J., Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990 Dec 15;76(12):2421–2438. [PubMed] [Google Scholar]
- Rose G. D., Geselowitz A. R., Lesser G. J., Lee R. H., Zehfus M. H. Hydrophobicity of amino acid residues in globular proteins. Science. 1985 Aug 30;229(4716):834–838. doi: 10.1126/science.4023714. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986 Feb 28;44(4):517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Ryu S. E., Kwong P. D., Truneh A., Porter T. G., Arthos J., Rosenberg M., Dai X. P., Xuong N. H., Axel R., Sweet R. W. Crystal structure of an HIV-binding recombinant fragment of human CD4. Nature. 1990 Nov 29;348(6300):419–426. doi: 10.1038/348419a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni A., Gismondi A., Morrone S., Procopio A., Modesti A., Scarpa S., D'Orazi G., Piccoli M., Frati L. Rat natural killer cells synthesize fibronectin. Possible involvement in the cytotoxic function. J Immunol. 1989 Oct 1;143(7):2415–2421. [PubMed] [Google Scholar]
- Saudek V., Atkinson R. A., Pelton J. T. Three-dimensional structure of echistatin, the smallest active RGD protein. Biochemistry. 1991 Jul 30;30(30):7369–7372. doi: 10.1021/bi00244a003. [DOI] [PubMed] [Google Scholar]
- Schwarz R. E., Hiserodt J. C. The expression and functional involvement of laminin-like molecules in non-MHC restricted cytotoxicity by human Leu-19+/CD3- natural killer lymphocytes. J Immunol. 1988 Nov 15;141(10):3318–3323. [PubMed] [Google Scholar]
- Siliciano R. F., Pratt J. C., Schmidt R. E., Ritz J., Reinherz E. L. Activation of cytolytic T lymphocyte and natural killer cell function through the T11 sheep erythrocyte binding protein. Nature. 1985 Oct 3;317(6036):428–430. doi: 10.1038/317428a0. [DOI] [PubMed] [Google Scholar]
- Sollazzo M., Billetta R., Zanetti M. Expression of an exogenous peptide epitope genetically engineered in the variable domain of an immunoglobulin: implications for antibody and peptide folding. Protein Eng. 1990 Dec;4(2):215–220. doi: 10.1093/protein/4.2.215. [DOI] [PubMed] [Google Scholar]
- Sollazzo M., Castiglia D., Billetta R., Tramontano A., Zanetti M. Structural definition by antibody engineering of an idiotypic determinant. Protein Eng. 1990 May;3(6):531–539. doi: 10.1093/protein/3.6.531. [DOI] [PubMed] [Google Scholar]
- Sollazzo M., Hasemann C. A., Meek K. D., Glotz D., Capra J. D., Zanetti M. Molecular characterization of the VH region of murine autoantibodies from neonatal and adult BALB/c mice. Eur J Immunol. 1989 Mar;19(3):453–457. doi: 10.1002/eji.1830190307. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Suh S. W., Bhat T. N., Navia M. A., Cohen G. H., Rao D. N., Rudikoff S., Davies D. R. The galactan-binding immunoglobulin Fab J539: an X-ray diffraction study at 2.6-A resolution. Proteins. 1986 Sep;1(1):74–80. doi: 10.1002/prot.340010112. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Suzuki T., Engleman E. G. Evidence for the involvement of CD56 molecules in alloantigen-specific recognition by human natural killer cells. J Exp Med. 1991 Jun 1;173(6):1451–1461. doi: 10.1084/jem.173.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timonen T., Gahmberg C. G., Patarroyo M. Participation of CD11a-c/CD18, CD2 and RGD-binding receptors in endogenous and interleukin-2-stimulated NK activity of CD3-negative large granular lymphocytes. Int J Cancer. 1990 Dec 15;46(6):1035–1040. doi: 10.1002/ijc.2910460615. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. H., Yan Y. W., Garrett T. P., Liu J. H., Rodgers D. W., Garlick R. L., Tarr G. E., Husain Y., Reinherz E. L., Harrison S. C. Atomic structure of a fragment of human CD4 containing two immunoglobulin-like domains. Nature. 1990 Nov 29;348(6300):411–418. doi: 10.1038/348411a0. [DOI] [PubMed] [Google Scholar]
- Yokoyama W. M., Jacobs L. B., Kanagawa O., Shevach E. M., Cohen D. I. A murine T lymphocyte antigen belongs to a supergene family of type II integral membrane proteins. J Immunol. 1989 Aug 15;143(4):1379–1386. [PubMed] [Google Scholar]