Abstract
The ErbB2 protein is a member of the tyrosine kinase family of growth factor receptors that is overexpressed in cancers of the breast, ovary, stomach, kidney, colon, and lung, and therefore represents an attractive candidate antigen for targeted cancer immunotherapy. Cytotoxic T lymphocytes specific for various immunogenic ErbB2 peptides have been described, but they often exhibit both poor functional avidity and tumor reactivity. In order to generate potent CD8+ T cells with specificity for the ErbB2369–377 peptide, we performed one round of in vitro peptide stimulation of CD8+ T cells isolated from an HLA-A2+ patient who was previously vaccinated with autologous dendritic cells pulsed with HLA class I ErbB2 peptides. Using this approach, we enriched highly avid ErbB2-reactive T cells with strong ErbB2-specific, antitumor effector functions. We then stimulated these ErbB2-reactive T cells with ErbB2+ HLA-A2+ tumor cells in vitro and sorted tumor-activated ErbB2369–377 peptide T cells, which allowed for the isolation of a novel T-cell receptor (TCR) with ErbB2369–377 peptide specificity. Primary human CD8+ T cells genetically modified to express this ErbB2-specific TCR specifically bound ErbB2369–377 peptide containing HLA-A2 tetramers, and efficiently recognized target cells pulsed with low nanomolar concentrations of ErbB2369–377 peptide as well as nonpulsed ErbB2+ HLA-A2+ tumor cell lines in vitro. In a novel xenograft model, ErbB2-redirected T cells also significantly delayed progression of ErbB2+ HLA-A2+ human tumor in vivo. Together, these results support the notion that redirection of normal T-cell specificity by TCR gene transfer can have potential applications in the adoptive immunotherapy of ErbB2-expressing malignancies.
Introduction
The ERBB2 (Her-2/neu) proto-oncogene encodes a member of a group of epithelial tyrosine kinase receptors involved in the initiation and progression of diverse malignancies, including breast, ovarian, and gastric cancers (Wong et al., 1995; Engel and Kaklamani, 2007). ErbB2 gene amplification and overexpression leads to uncontrolled cell growth and survival, increased colony formation (Bartsch et al., 2007), and impaired DNA repair (Pietras et al., 1994). Several different immunotherapeutic approaches directed against ErbB2-expressing breast and ovarian tumors have been developed to date. Anti-ErbB2 antibody-based immunotherapies, such as the monoclonal antibody trastuzumab, can treat breast cancer patients with ErbB2 overexpression, but this approach has not been as efficacious in ovarian cancer patients (Bookman et al., 2003). Additionally, cancer vaccines have been utilized to induce specific antitumor immunity, but produced only weak T-cell responses and did not induce objective tumor regression (Knutson et al., 2002; Disis et al., 2004; Peoples et al., 2005).
T-cell receptor (TCR) gene transfer has been developed over the last decade as a reliable method to generate large numbers of T cells of a given antigen specificity for adoptive cellular therapy of viral infectious diseases, virus-associated malignancies, and cancer (Engels and Uckert, 2007). The clinical feasibility of TCR gene therapy was first demonstrated in melanoma using a TCR specific for MART1, a commonly expressed melanoma antigen (Morgan et al., 2006). Adoptive transfer of MART1 TCR-transduced CD8+ T cells in 15 patients resulted in durable engraftment of the transferred population and significant tumor regression in two patients, demonstrating a proof of concept of adoptive T-cell transfer (Morgan et al., 2006). We later identified a higher affinity MART-1-specific TCR that conferred improved functional avidity and clinical efficacy in melanoma, although with greater incidence of vitiligo, uveitis, and hearing loss resulting from collateral destruction of normal melanocytes (Johnson et al., 2006, 2009). ErbB2-directed TCR gene therapy would appear to hold significant promise for common epithelial cancers; however, isolation of highly avid ErbB2-specific TCRs directly from cancer patients has been challenging and not clinically tested.
One promising strategy to generate ErbB2-specific T cells relies on vaccination of patients bearing ErbB2+ tumors with powerful immune regimens that can overcome immunological ErbB2 self-tolerance and prime preexisting T-cell immunity. We previously demonstrated that administration of an autologous, matured dendritic cell (DC) vaccine pulsed with ErbB2-derived HLA class I and II peptides to HLA-A2+ patients with ErbB2+ breast tumors efficiently primed ErbB2-specific T cells, increased their frequency, and resulted in measurable tumor regression in some patients in an ErbB2/DC vaccine study (Czerniecki et al., 2007). In this report, we characterize a novel ErbB2-specific TCR that was isolated from ErbB2369–377-specific CD8+ T cells expanded from an HLA-A2+ patient who was previously vaccinated on the ErbB2/DC study. The isolated TCR conferred transduced CD8+ T cells with high specificity and avidity for the HLA-A2-restricted ErbB2369–377 epitope, demonstrating reactivity against peptide-loaded targets and tumor cells expressing endogenous antigen.
Materials and Methods
Cells
Retroviral packaging was performed in immortalized normal fetal renal 293GP cells kindly provided by Dr. Paul Robbins (Center of Cancer Research, National Cancer Institute, Bethesda, MD). Human cell lines used in immune-based assays include the ovarian cancer cell lines SKOV3, OVCAR3, OVCAR-2, and OV55-2; the human breast cancer cell line MDA231; the human melanoma cell lines 624 and 938 (Marincola et al., 1994; Rivoltini et al., 1995); the human T-cell lymphoblastic lymphoma cell line SupT1; and the T2 lymphoblastoid cell line. Cell lines were maintained in RPMI-1640 (Invitrogen) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 100 μg/ml penicillin, and 100 U/ml streptomycin. All cell lines were routinely tested for mycoplasma contamination.
Preparation of ErbB2 peptide-loaded monocyte-derived DCs
All patients underwent pretreatment leukapheresis on a Baxter CS3000 using monocyte enrichment settings in the Apheresis Unit at the Hospital of the University of Pennsylvania under an Institutional Review Board (IRB)–approved protocol (Czerniecki et al., 2007). Patient peripheral blood monocytes were enriched from the leukapheresis product by elutriation. Monocytes were washed, counted, and cultured at 3×106/ml in sterile 24-well plates in RPMI medium supplemented with 10% FBS, 500 IU/ml of recombinant research-grade human granulocyte-macrophage colony stimulating factor (GM-CSF), and 250 IU/ml of interleukin-4 (IL-4) for 4 days. On day 5, about 1,000 units/ml of IFN-γ was added in the culture followed by overnight incubation at 37°C. On day 6, lipopolysaccharide (LPS) was added at 10 ng/ml for 6 hr to complete maturation of the DCs. The dendritic cells' DC1 phenotype was analyzed by flow cytometry using monoclonal antibodies against CD80, CD86, CD83, and CD40. The dendritic cells matured to the DC1 phenotype were subsequently pulsed with HLA-A2 class I-binding ErbB2369–377-specific peptide at 10 μg/ml, for 2 hr as described previously (Czerniecki et al., 2007). DCs were harvested 2 hr later, washed, counted, and assessed for viability before coculture with CD8+ T cells.
In vitro CD8+ T-cell priming with ErbB2 peptide-pulsed DCs (DC1)
For collection of vaccine-primed T cells, patients underwent posttreatment leukapheresis on a Baxter CS3000 under an IRB-approved protocol for human subjects research 2 weeks after last vaccine dose (Czerniecki et al., 2007). Autologous ErbB2 peptide-loaded DCs were cocultured with column-purified postvaccination CD8+ T cells at a ratio of 10:1 in 48-well plates. IL-2 (50 IU/ml) was added to the cultures on day 2. After 10 days of sensitization, the CD8+ T cells were harvested and restimulated with T2 cells pulsed with either relevant or irrelevant peptides or tumor cell lines. Supernatants were harvested after 24 hr and analyzed by enzyme-linked immunosorbent assay (ELISA).
Cytokine release assays
Cytokine release assays were carried out by coculture of 1×105 T cells with 1×105 tumor cells or peptide-loaded T2 cells per well in triplicate in 96-well round-bottom plates in 200 μl complete medium. For the preparation of peptide-loaded T2 antigen presenting cells (APCs), the latter were resuspended at 1×107/ml and loaded with ErbB2 or MART1 peptides at various peptide concentrations (1 ng/ml to 10 μg/ml) at 37°C for 2 hr. T2 cells were then washed twice with phosphate buffered saline (PBS) and resuspended at 1×106/ml with RPMI-1640 supplemented with 10% heat-inactivated FBS. After 20–24 hr, cell-free supernatants were assayed for the presence of IFN-γ using the BioLegend ELISA MAX Deluxe kit.
Construction of retroviral vectors
To identify the sequences of the TCR genes, a 5′-RACE-PCR (Kit) amplifying the variable regions of the TCRα and TCRβ chains including CDR3 was performed with RNA isolated from the T-cell clones. RACE-PCR products were sequenced. TCRα and TCRβ chains were linked by 2A peptide linker (TCRb-P2A-TCRa), and the complete constructs were cloned into the retroviral vector plasmid pMSGV1 vector backbone, a derivative of the vector pMSGV (murine stem cell virus [MSCV]-based splice-gag vector) that utilizes an MSCV long terminal repeat (LTR) (Cohen et al., 2005).
Recombinant retrovirus production
Replication-defective retroviral vectors were produced as previously described (Wargo et al., 2009). Briefly, 1×106 of 293-GP cells (transient viral producer cells) in a 6-well plate were cotransfected with 1.5 μg of retroviral vector DNA from each of the constructs and 0.5 μg of envelope DNA (RD114) using the Lipofectamine 2000 reagent (Invitrogen) and Optimem medium (BD Biosciences). The medium was changed to Dulbecco's modified Eagle's medium with 10% FBS after 18 hr, and viral supernatants were harvested at the 48-hr time point.
Human T-cell transduction
Primary human CD8+ T cells were purchased from the Human Immunology Core at University of Pennsylvania and were isolated from healthy volunteer donors following leukapheresis by negative selection. All specimens were collected under a University IRB–approved protocol, and written informed consent was obtained from each donor. T cells were plated at 1×106/ml in 24-well plates (Costar) in the complete medium (RPMI 1640 supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, and 10 mM HEPES) and stimulated with beads coated with anti-CD3 and anti-CD28-mAbs as described by the manufacturer (Invitrogen) (Levine et al., 1997) for 18–24 hr before transduction. For retroviral transduction, non–tissue culture-treated 12-well plates (Becton Dickinson Labware) were treated with 25 μg/ml of recombinant retronectin at 4°C as directed by the manufacturer (RetroNectin; Takara). After an overnight incubation, the retronectin was removed and wells were blocked with 2% bovine serum albumin in PBS at room temperature for 30 min. The retroviral vector supernatant (2–3 ml) was then applied by centrifugation (2000×g for 2 hr) and removed by aspiration. About 5×105 of stimulated T cells were added to each well in a final volume of 1 ml RMPI growth medium. Plates were centrifuged for 10 min at 1000×g and incubated overnight. The transduction process was repeated the following day. After transduction, the cells were grown in RPMI with 10% FBS, and human recombinant interleukin-2 (IL-2) (Novartis) was added every other day to 100 IU/ml final concentration. Cell density of 0.5–1×106 cells/ml was maintained.
Flow cytometry
To determine T-cell antigen specificity, CD8+ T cells were stained with anti-CD8-FITC and APC-labeled ErbB2369–377 or MART127–35 tetramer (Becton Dickinson). To assess T-cell activation phenotype, T cells were stained with the above reagents plus a PerCPCy5.5-labeled anti-human CD69 mAb. DC phenotype was assessed using CD14-PerCPCy5.5, CD11c-APC, HLA-DR-PE, CD80-FITC, CD86-FITC, CD83-FITC, and CD40-FITC. All antibodies were purchased from BD Biosciences.
Real-time PCR
Real-time PCR (RT-PCR) was used to analyze the expression of human TAP1, TAP2, tapasin, and LMP2 (antigen processing machinery [APM] components) in tumor cell lines. RNA was first isolated from tumor cells using the RNA easy kit (Qiagen). cDNA was then generated from 1 μg of RNA using First Strand Ready-To-Go beads (GE Healthcare). RT-PCR was then performed in triplicates using Applied Biosystem's TaqMan primers specific for TAP1, TAP2, tapasin, LMP2, and β-actin. mRNA levels were normalized to β-actin and compared with mRNA levels of APM-deficient T2 cells. Data are presented as fold mRNA level.
Xenograft model of breast cancer
All animals were obtained from the Stem Cell and Xenograft Core of the Abramson Cancer Center, University of Pennsylvania. Mice were bred, treated, and maintained under pathogen-free conditions in-house under University of Pennsylvania's Institutional Animal Care and Use Committee–approved protocols. For in vivo T-cell functional assessment, 6–12-week-old female NSG mice were subcutaneously injected on the flank with 1×106 MDA231 cells previously mixed with 1×106 ErbB2-specific T cells in 0.2 ml PBS. Control mice were injected with MDA231 tumor cells mixed with 1×106 MART1-specific T cells. Each group consisted of five mice. Tumor growth was determined by caliper measurement over time and tumor volumes calculated using the formula V=½ (length×width2), where length is the greatest longitudinal diameter and width is the greatest transverse diameter. Mice were terminated after 40 days or earlier if they became distressed and moribund. Following termination, tumors were resected, photographed, and weighted.
Statistical analysis
GraphPad Prism 4.0 (GraphPad Software) was used for statistical analysis.
Results
Induction of ErbB2-specific CD8+ T cells with ErbB2 peptide-loaded DCs
Peripheral blood monocytes and peripheral blood T cells were obtained from an HLA-A2+ patient (M10) who had previously been vaccinated with autologous DCs pulsed with a cocktail of HLA class I and class II peptides, including the HLA class I-restricted ErbB2369–377 peptide (Czerniecki et al., 2007). This patient's postvaccination CD8+ T cells demonstrated a robust IFN-γ response (>350 ng/ml) against autologous DCs pulsed with ErbB2369–377 peptide following one round of in vitro stimulation. Furthermore, postvaccination CD8+ T cells secreted 73-fold higher IFN-γ levels in response to the HLA-A2+/ErbB2+ breast cancer cell line MDA231 compared with IFN-γ secreted against control cell lines (Czerniecki et al., 2007; Koski et al., 2012). Of note, the patient's prevaccination CD8+ T cells showed low levels of IFN-γ production in response to either target, establishing evidence of a strong, vaccine-induced anti-ErbB2 response.
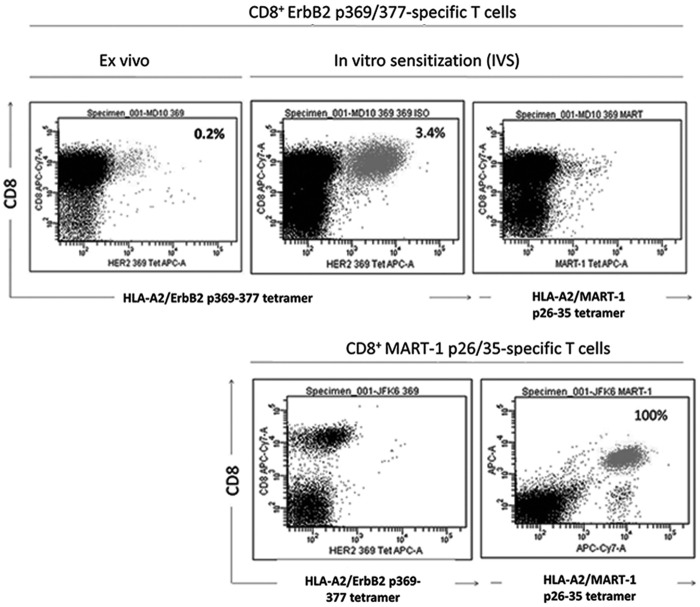
The patient's peripheral blood monocytes were matured into DCs utilizing an in vitro protocol and showed relatively high expression levels of CD80, CD86, CD83, and CD40 (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hum). The matured DCs were then pulsed with ErbB2369–377 peptide and used for the in vitro stimulation of CD8+ T cells purified from the patient's postvaccination peripheral blood. Following 7 days of in vitro stimulation, nearly 3% of the viable CD8+ T cell population recognized the stimulating ErbB2369–377 peptide as assessed by binding of an HLA-A2/ErbB2369–377 tetramer (Fig. 1). This represented a 17-fold increase over 1 week, relative to the starting percentage of ErbB2-specific T cells observed in the blood of the postvaccinated patient. ErbB2-specific T cells did not bind to MART-126–35 tetramer complexes, demonstrating their specificity for ErbB2369–377 peptide. In contrast, MART-1 TCR-transduced T cells did not bind to the ErbB2369–377 tetramer complex, but exhibited strong binding to MART-126–35 tetramer complexes (Fig. 1). Collectively, ErbB2 peptide-loaded DCs were capable of boosting the frequency of ErbB2369–377 peptide-specific T cells.
FIG. 1.
ErbB2-pulsed DC1 increase the frequency of ErbB2-directed T cells. CD8+ T cells were purified from a patient with ductal carcinoma in situ (DCIS) postadministration of the ErbB2-pulsed-DC1 vaccine and cocultured for 7 days with ErbB2369–377 peptide-pulsed autologous dendritic cells. After 1 week, CD8+ T cells were harvested and analyzed via flow cytometry with labeled tetramer bound to ErbB2369–377 or MART126–35. MART1 T cells served as negative control effector cells. The percentage of positive cells for CD8 and ErbB2 are indicated on the dot plot.
ErbB2-specific CD8+ T cells exert potent effector functions against ErbB2 peptide-loaded target cellsand ErbB2-expressing cancer cells
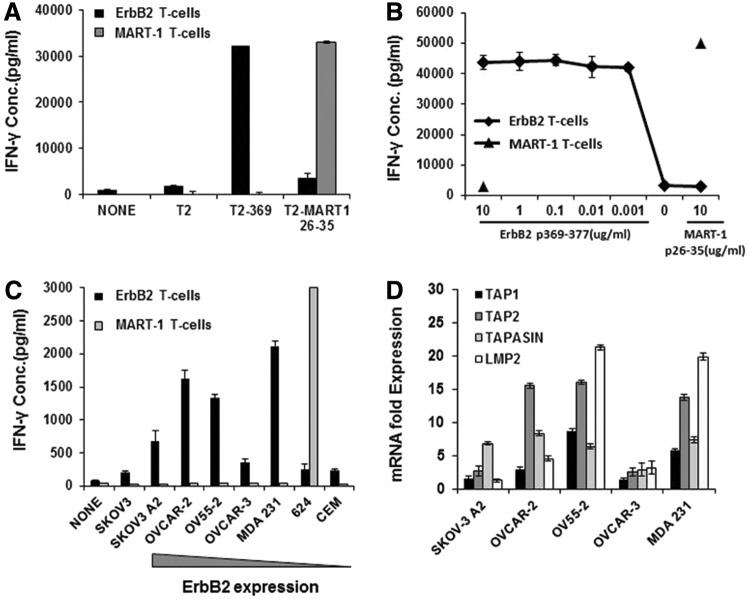
To evaluate their effector functions, ErbB2-specific T cells were initially exposed to HLA-A2+ T2 cells preloaded with the ErbB2369–377 peptide. ErbB2-specific T cells displayed high peptide-specific IFN-γ production upon coculture with antigen presenting cells (T2 cells) loaded with the relevant ErbB2 peptide. As expected, no IFN-γ was produced upon exposure to T2 cells pulsed with the irrelevant MART-126–35 peptide. As a positive control for functionality, MART-1-specific T cells recognized and reacted against MART-126–35 peptide-loaded T2 cells (Fig. 2A).
FIG. 2.
ErbB2369–377-specific T cells strongly recognize peptide-pulsed T2 cells and differentially recognize HLA-A2-restricted ErbB2-expressing tumor cells. (A) IFN-γ production of ErbB2369–377-specific T cells in response to peptide-pulsed targets. ErbB2- or MART1-specific T cells were cocultured with T2 cells loaded with HLA-A2-restricted ErbB2369–377 or MART126–35 peptide for 18 hr. (B) ErbB2369–377-specific T cells exhibit high avidity against the relevant peptide. ErbB2369–377-specific T cells were incubated for 18 hr with T2 cells pulsed with a range of concentrations of ErbB2369–377 peptide or 10 ug/mL control (MART-1) peptide. MART1 T cells served as negative control effector T cells. (C) ErbB2 or MART1-specific T cells were cultured alone (none) or stimulated overnight with human HLA-A2-restricted ErbB2+-established cancer cell lines. SKOV-3 (HLA-A2− ErbB2+) and CEM (HLA-A2− ErbB2−) served as negative control tumor targets. (D) APM expression of HLA-A2-restricted ErbB2-expressing tumor cell lines. The mRNA levels of human TAP1, TAP2, TAPASIN, and TAP2 were quantified by real-time PCR. mRNA levels are expressed as fold increase over the APM-negative T2 cell line. β-Actin was used as an endogenous gene control. Results depict the mean±SD of triplicate wells. For all assays, IFN-γ was quantified from cell-free supernatants by ELISA and is reported as the mean concentration (pg/ml)±SEM of duplicate wells. APM, antigen processing machinery; ELISA, enzyme-linked immunosorbent assay.
We further evaluated the functional avidity of these T cells by analyzing the production of IFN-γ in response to incubation with T2 target cells pulsed with titered amounts of the ErbB2369–377 peptide. ErbB2369–377-specific T cells exerted high functional avidity, as they were capable of secreting high amounts of IFN-γ even at low concentrations (1 nM) of the specific peptide (Fig. 2B). We therefore investigated if the ErbB2-specific T cells were able to recognize endogenously processed and presented ErbB2369–377 peptide. Coculture assays were performed utilizing ErbB2-specific T cells with HLA-A2-matched or HLA-A2-mismatched ovarian, breast, and melanoma cancer cells that express different levels of the ErbB2 protein (Lanitis et al., 2012). ErbB2369–377-specific CD8+ T cells specifically recognized and secreted IFN-γ upon interaction with ErbB2+ HLA-A2+ ovarian or breast cancer cells, while no recognition of HLA-A2− or ErbB2− tumors was observed (Fig. 2C). There was no correlation between the intensity of ErbB2 surface expression by tumor cell lines and the IFN-γ secretion by T cells (data not shown). To this end, we investigated the expression of various components of APM by tumor cells, including TAP1, TAP2, tapasin, and LMP2, via RT-PCR to determine if deficiencies existed in the peptide-processing pathway of these tumor cells. ErbB2+ tumor cell lines that were recognized to a lesser extent by the ErbB2369–377-specific T cells (SKOV-3 and OVCAR-3) (Fig. 2C) displayed a reduced mRNA expression of tested APM molecules (Fig. 2D). Tumor cell lines that were well recognized by the ErbB2369–377-specific T cells (OVCAR-2, OV55-2, and MDA231) (Fig. 2C) displayed a higher level of expression in most of the APM molecules investigated (Fig. 2D). Therefore, lack of recognition of some ovarian tumors by ErbB2369–377-specific T cells may be attributed, in part, to a lack of necessary APM components in the tumor cells, as observed elsewhere (Han et al., 2008). This observation highlights that both ErbB2 and HLA-A2 molecules are required, but not sufficient, for optimal immune recognition. Together, we conclude that vaccine-primed ErbB2369–377-specific T cells exert potent effector functions against peptide-loaded targets and HLA-A2-matched ErbB2-expressing tumor cells.
Identification and isolation of ErbB2-specific TCR α/β genes
Tumor recognition by T cells is often accompanied with specific upregulation of T-cell activation surface antigens such as the early activation marker CD69. In order to capture ErbB2369–377-specific T cells with high avidity for the tumor-presented ErbB2369–377 peptide, we cocultured the ErbB2-specific T cells with HLA-A2+ ErbB2+ MDA231 cells for 24 hr. ErbB2-specific T cells that upregulated CD69 (Supplementary Fig. S2) and bound HLA-A2/ErbB2369–377 tetramer were then isolated via fluorescence-activated flow sorting. In order to determine the TCR variable (TCRV) α-chain and TCRVβ-chain repertoire of the captured ErbB2-specific T cells, total RNA was isolated from the sorted cells and subjected to 5′ RACE. Twenty-three individual α-chain cDNA clones and 14 individual β-chain cDNA clones were fully sequenced from 2 independent PCRs. Sequence data demonstrated two relatively dominant sequences in the TCRVβ repertoire that belonged to the BV3-1(9S1) family of β chains. More heterogeneity was observed in the TCRVα repertoire, with two repeats each for the AV3 and the AV12-1 α chains (Table 1).
Table 1.
T Cell Receptor α and β DNA Constructs
| TCR α and β chain sequencing resultsa | TCR α/β retroviral constructsb | ||||
|---|---|---|---|---|---|
| TRAV | Number of clones | TRBV | Number of clones | Construct number | TCR construct |
| AV1-1 | 1 | BV2(22s1) | 1 | 1 | AV12-1 BV3-1(CB1) |
| AV1-2 | 1 | BV3-1(9S1) | 2,3 | 2 | AV3 BV3-1(CB-1) |
| AV2 | 1 | BV4-1(7S1) | 1 | 3 | AV12-2a BV3-1(CB-1) |
| AV3 | 2 | BV4-3(7S2) | 1 | 4 | AV12-2b BV3-1(CB-1) |
| AV10 | 1 | BV5-1(5S1) | 1 | 5 | AV12-2a BV3-1(CB-2) |
| AV12-1 | 2,1 | BV5-4(5S6) | 1 | 6 | AV12-2b BV3-1(CB-2) |
| AV12-2 | 1,1,1,1 | BV5-6(5S2) | 1 | 7 | AV3 BV3-1(CB-2) |
| AV17 | 1 | BV20-1(2S1) | 1,1,1 | 8 | AV12-1 BV3-1(CB-2) |
| AV21 | 1,1 | ||||
| AV38-1 | 1 | ||||
| AV38-2 | 1,1,1,1,1 | ||||
TCR V α/β usage of HLA-A2/ErbB2 multimer+ CD69+ CD8+ T-cells. Twenty-three TCR α chain clones and 14 TCR β chain clones were isolated from ErbB2-specific CD8+ T-cells. The TRAV and TRBV repertoire was determined by sequencing. The number of repeats for each clone is shown on the right side of the table.
Eight different retroviral backbones encoding eight different TCR α/β combinations were constructed for the propagation of retroviral particles. TCR α and β chains that were presented more than once in the TCR repertoire were subcloned into the MSGV-1 retroviral backbones.
TCR, T-cell receptor; TRAV, T cell receptor α chain variable; TRBV, T cell receptor β chain variable.
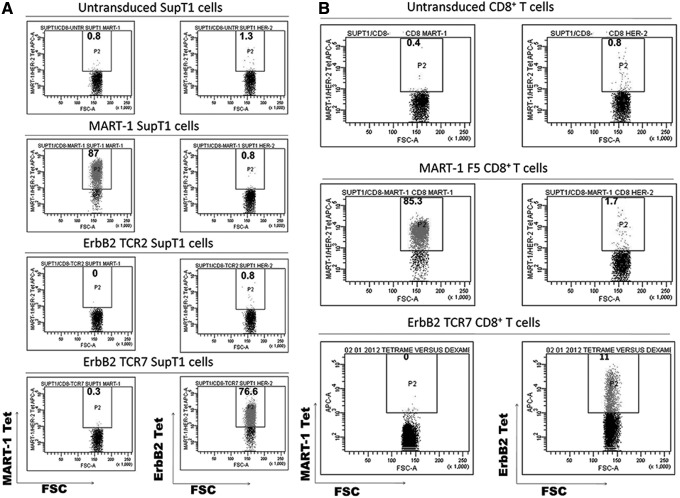
TCR α and β chains that presented more than once in the TCR repertoire were subcloned into the MSGV-1 retroviral backbone. A total of eight retroviral vectors harboring the α- and β-chain cDNAs were constructed (Table 1). Retroviruses encoding the eight different TCR α/β combinations were produced and utilized for the transduction of SupT1 cells. Subsequently, the genetically modified SupT1 cells were stained with HLA-A2/ErbB2369–377 tetramer and assessed via flow cytometry to identify TCRs with specificity for the ErbB2369–377 peptide. One out of eight TCR combinations exhibited specific and strong binding to the HLA-A2/ErbB2369–377 tetramer (Fig. 3A). Hence, this paired TCR harboring the AV3 α chain and the BV3-1 β chain was chosen for further characterization (herein referred to as ErbB2369-377-specific TCR7 or ErbB2369-377 TCR).
FIG. 3.
Expression of the ErbB2 TCR on retrovirally transduced SupT1 cells and CD8+ T cells. (A) Screening of TCR α/β pairs by retroviral transduction of SupT1 cells. Retroviruses encoding eight different TCR combinations were screened for ErbB2369–377 specificity by transduction of SupT1 cells. HLA-A2/ErbB2369–377 tetramer staining of the genetically modified SupT1 cells was performed 5 days after transduction and analyzed by flow cytometry. Two representative SupT1 populations are shown, each bearing different TCRs whose α and β chains were isolated from the ErbB2-specific polyclonal CD8+ T cells. Untransduced (NV) and MART1 SupT1 cells served as negative controls for HLA-A2/ErbB2369–377 tetramer binding. (B) HLA-A2/ErbB2369–377 tetramer staining of primary TCR-transduced CD8+ T cells. CD8+ T cells transduced with either the ErbB2 TCR7 or the MART1 TCR and untransduced CD8+ T cells (NV) were stained with the indicated HLA-A2/peptide tetramers. Numbers represent the percentage of tetramer+ cells. TCR, T-cell receptor.
Retroviral transfer of ErbB2369–377-specific TCR7 into CD8+ T cells confers antigen specificity
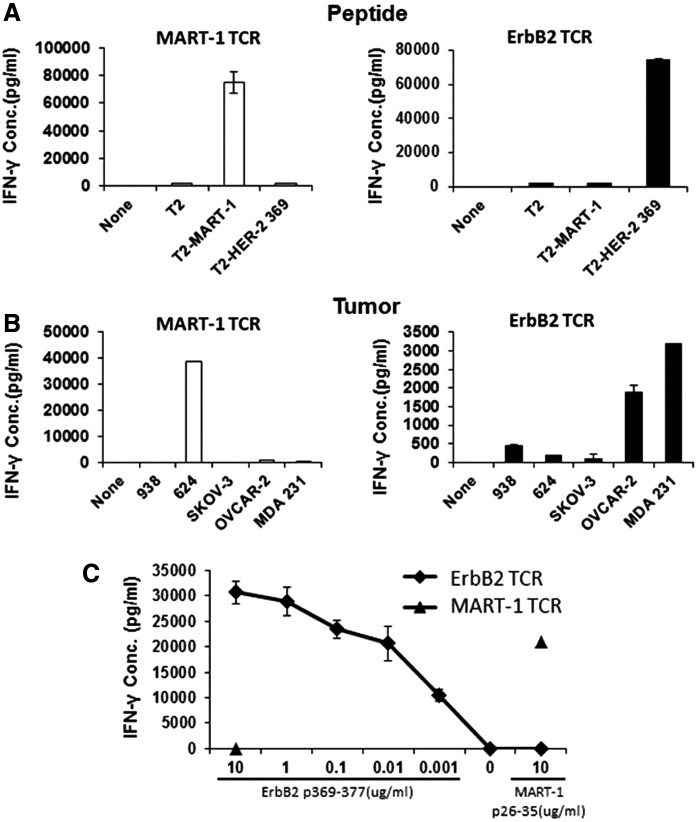
We next investigated the functional properties that TCR7 confers upon expression in primary human T cells. Retroviral TCR gene transfer into CD8+ T cells resulted in specific HLA-A2/ErbB2369–377 tetramer binding (Fig. 3B). However, the percentage of tetramer+ cells was low (∼10%) when compared with SupT1 cells, suggesting that transduced TCRs may not be assembled well enough to be detected or that mispairing with endogenous α chains may occur. Importantly, however, even at low tetramer binding frequencies, the ErbB2 TCR7-transduced T cells demonstrated specific, robust reactivity against peptide-pulsed APC targets (Fig. 4A). ErbB2 TCR T cells demonstrated high peptide avidity, as they secreted high IFN-γ levels at peptide concentrations as low as 1 ng/ml (Fig. 4C). Upon analyzing the tumor reactivity of the ErbB2 TCR CD8+ T cells, we observed IFN-γ secretion in response to HLA-A2+ ErbB2+ OVCAR-2 and MDA231 tumor cells at levels similar to that produced by the initial ErbB2 polyclonal T-cell population (Fig. 4B/Fig. 2C). No reactivity was observed against tumors lacking HLA-A2 or ErbB2 expression or HLA-A2+ 624 melanoma cells expressing very low levels of ErbB2 (Fig. 4B).
FIG. 4.
ErbB2369–377-specific T cells show potent IFN-γ production in response to ErbB2-peptide-loaded targets and ErbB2-expressing cancer cell lines in vitro. (A) ErbB2 or MART1 TCR-transduced T cells were cocultured with T2 cells loaded with HLA-A2-restricted ErbB2369–377 or with MART126–35 for 18 hr. (B) ErbB2 or MART1 TCR-transduced T cells were cultured alone (none) or stimulated overnight with human HLA-A2-restricted ErbB2+-established cancer cell lines. SKOV-3 (HLA-A2− ErbB2+) and CEM (HLA-A2− ErbB2−) served as negative control tumor targets. (C) CD8+ T cells transduced with the ErbB2369–377-specific TCR as well as the control MART1 TCR were incubated 11 days after transduction for 18 hr with T2 cells pulsed with a range of titrated concentrations of ErbB2369–377 peptide. T2 pulsed with MART126–35 peptide served as negative control target T cells. For all assays, IFN-γ was quantified from cell-free supernatants by ELISA and is reported as the mean concentration (pg/ml)±SEM of duplicate wells.
T cells expressing ErbB2369–377-specific TCR7 delay tumor growth in vivo
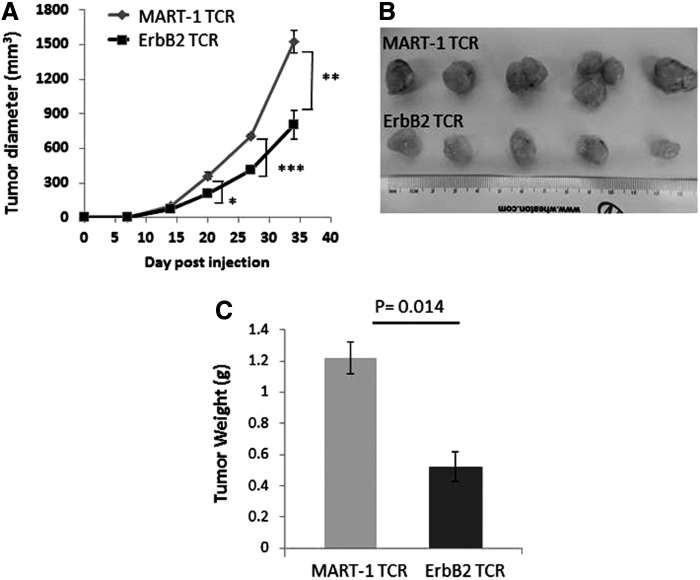
To determine the antitumor efficacy of T cells expressing ErbB2369–377-specific TCR7 in vivo, we subcutaneously co-injected equal numbers of TCR7- or control MART-126–35 TCR-transduced CD8+ T cells and MDA231 tumor cells into NOD/SCID/IL2-γcnull (NSG) mice and monitored tumor outgrowth. MDA231 tumors grew aggressively with palpable tumors evident 14 days after injection. Compared with MART-1 TCR-specific T cells, ErbB2 TCR7-transduced T cells were capable of significantly delaying tumor burden over time (Fig. 5A). At the termination of the study, mice were euthanized and tumors were excised. Consistent with the measured tumor volume (Fig. 5A), resected tumors from the ErbB2 TCR7 group were visibly smaller (Fig. 5B) and weighed significantly less compared with those in mice treated with the MART-1 TCR (Fig. 5C).
FIG. 5.
T cells expressing ErbB2369–377-specific TCR7 delay tumor growth in vivo. T cells expressing ErbB2369–377-specific TCR7 delay tumor growth in vivo. Retrovirally transduced ErbB2 TCR7 CD8+ T cells and the breast cancer cell line MDA231 were co-injected subcutaneously into the flank of NSG mice on day 0. MART1-specific F5 TCR-transduced T cells co-injected with MDA231 were used as controls. (A) Tumor growth was determined by caliper measurement over time. Results are expressed as mean tumor volume (mm3±SEM) with n=5 for all groups. Statistical significance of p<0.05 is reported as *p=0.0495, **p=0.0075, and ***p=0.0029. After 35 days, tumors were resected, photographed (B), and measured for tumor weight (C). NSG, NOD/SCID/γ-chain−/−.
Discussion
Introduction of tumor-specific TCR genes has been proposed as a method to produce de novo antitumor lymphocytes for cancer immunotherapy without the need to isolate tumor-reactive T cells (Cordaro et al., 2002; Schumacher, 2002; Sadelain et al., 2003; Willemsen et al., 2003). This proposition requires the existence of tumor antigens common to divergent human cancers and the isolation of a tumor-reactive TCR from the appropriate T-cell population that recognizes these natural tumor antigens.
Since its discovery, the synthetic ErbB2369–377 peptide has been widely investigated for the ex vivo and in vivo generation of ErbB2-specific cytotoxic T-lymphocytes following stimulation in vitro (Brossart et al., 1998; Rongcun et al., 1999; Anderson et al., 2000; Seliger et al., 2000; Keogh et al., 2001; zum Buschenfelde et al., 2002; Liu et al., 2004) or vaccination (Zaks and Rosenberg, 1998; Brossart et al., 2000; Knutson et al., 2002; Murray et al., 2002; Peoples et al., 2005). Some ErbB2-specific T cells exert high reactivity against the ErbB2 peptide, but fail to recognize endogenously processed peptide presented by ErbB2+ tumors (Zaks and Rosenberg, 1998; Conrad et al., 2008). Recent work demonstrates that ErbB2369–377-specific T cells cross react with the overlapping HLA class I-restricted ErbB2373–382 peptide (Henle et al., 2013). Importantly, ErbB2373–382 is naturally processed and ErbB2373–382-specific T cells also cross react with the ErbB2369–377 peptide (Henle et al., 2013), suggesting continued clinical importance for the ErbB2369–377 peptide though controversy of its natural processing exists.
We sought to isolate and test ErbB2-reactive T cells from HLA-A2+ patients with ErbB2+ breast tumors who had been vaccinated with autologous preconditioned DCs (DC1) pulsed with ErbB2 HLA class I and II peptides (Czerniecki et al., 2007). DCs polarized toward the DC1 phenotype produce cytokines and chemokines critical for maximizing antitumor immunity (Xu et al., 2003) and therefore may enhance the efficacy of antitumor vaccines and offer a strong approach to induce and expand tumor-reactive T cells in vivo and ex vivo. After one round of ex vivo stimulation with DC1 cells loaded with ErbB2369–377 peptide, the frequency of ErbB2369–377 peptide-specific T cells increased to a level (∼3.4%) sufficient for robust downstream functional analysis. Of note, these T cells were capable of recognizing peptide loaded onto T2 cells at nanomolar levels, but also HLA-A2+ ErbB2-expressing tumors. Fluorescence-activated cell sorting allowed us to maximize the purity of ErbB2-specific T cells (∼95%), and molecular analysis of the TCR repertoire and subsequent testing of various TCR α and β combinations led us to identify and isolate a novel ErbB2369–377-specific TCR (TCR7 AV3/BV3-1).
Retroviral particles encoding the ErbB2 TCR were propagated and utilized for the genetic engineering of primary T cells. We routinely observed nearly a 10% TCR expression efficiency by transduced T cells, as measured by binding to ErbB2369–377 multimers. Although the percentage of multimer+ cells was low in primary human T cells, we observed high expression of ErbB2 TCR in SupT1 cells (∼80%) that lack endogenous TCR α and β chains, suggesting the possibility that mispairing with endogenous TCR α/β chains impairs proper paired assembly of the exogenous TCR chains on the surface of the transduced T cells. Nevertheless, transduced T cells demonstrated HLA-A2-restricted, ErbB2-specific effector T cells functions, as measured by cytokine release against peptide-pulsed targets and HLA-A2+ ErbB2+ ovarian and breast cancer tumor cells lines. Similar to the starting ErbB2-specific T-cell population, high functional avidity of the ErbB2369–377 TCR-transduced T cells was demonstrated by their ability to recognize T2 cells pulsed with very low amounts of the cognate peptide (1 ng/ml) and their ability to significantly delay tumor outgrowth in a human breast cancer xenograft model.
Our finding that tumor growth was only moderately delayed in vivo suggests that further preclinical refinement of this TCR gene approach is warranted. Proper pairing of exogenous TCR α/β chains may represent one maneuver to augment TCR-transduced T cell function in vivo. Multiple approaches exist that lessen mixed dimer formation and increase the expression of properly dimerized exogenous TCR on the T-cell surface. This can be achieved by replacing the constant region of the human TCR chains by their murine counterparts (Cohen et al., 2006), the introduction of additional cysteine residues within the constant region of the TCR α and β chains (Cohen et al., 2007; Voss et al., 2008), the provision of exogenous CD3 molecules (Ahmadi et al., 2011), and/or the inclusion of small interfering RNA (siRNA) to specifically downregulate the endogenous TCR (Okamoto et al., 2009). We anticipate that the application of the above maneuvers will augment the expression of the ErbB2369–377 TCR on the surface of transduced T cells. In addition, we expect that the refinement of ErbB2369-377 TCR expression will lead to enhanced antitumor efficacy in vivo and robust tumor eradication. Alternatively, tumor intrinsic effects may also limit T-cell activity. For instance, the reactivity of ErbB2 TCR T cells can be potentiated by immune checkpoint blockade via the co-administration of recombinant human antibodies specific for negative immunoregulatory molecules, such as B7-H4, which is often expressed by tumor cells (Dangaj et al., 2013).
In summary, the ErbB2369–377-specific TCR described here represents an “off-the-shelf” reagent that can be utilized to generate autologous tumor antigen-specific T cells without the need to identify antitumor T cells unique for each patient. This approach can yield sufficient numbers of T cells with high avidity and specificity for the ErbB2369–377 peptide for the treatment of a variety of common epithelial malignancies.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the Ovarian Cancer Research Fund, Sandy Rollman Ovarian Cancer Foundation, NIH (1R21CA152540), the Joint Fox Chase Cancer Center and University of Pennsylvania Ovarian Cancer SPORE (P50 CA083638), and the Immunobiology of Normal and Neoplastic Lymphocytes T32 training grant (5T32CA009140-37).
Author Disclosure Statement
No competing financial interests exist.
References
- Ahmadi M., King J.W., Xue S.A., et al. (2011). CD3 limits the efficacy of TCR gene therapy in vivo. Blood 118, 3528–3537 [DOI] [PubMed] [Google Scholar]
- Anderson B.W., Peoples G.E., Murray J.L., et al. (2000). Peptide priming of cytolytic activity to HER-2 epitope 369–377 in healthy individuals. Clin. Cancer Res. 6, 4192–4200 [PubMed] [Google Scholar]
- Bartsch R., Wenzel C., Zielinski C.C., and Steger G.G. (2007). HER-2-positive breast cancer: hope beyond trastuzumab. BioDrugs 21, 69–77 [DOI] [PubMed] [Google Scholar]
- Bookman M.A., Darcy K.M., Clarke-Pearson D., et al. (2003). Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J. Clin. Oncol. 21, 283–290 [DOI] [PubMed] [Google Scholar]
- Brossart P., Stuhler G., Flad T., et al. (1998). Her-2/neu-derived peptides are tumor-associated antigens expressed by human renal cell and colon carcinoma lines and are recognized by in vitro induced specific cytotoxic T lymphocytes. Cancer Res. 58, 732–736 [PubMed] [Google Scholar]
- Brossart P., Wirths S., Stuhler G., et al. (2000). Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood 96, 3102–3108 [PubMed] [Google Scholar]
- Cohen C.J., Zheng Z., Bray R., et al. (2005). Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J. Immunol. 175, 5799–5808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C.J., Zhao Y., Zheng Z., et al. (2006). Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 66, 8878–8886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C.J., Li Y.F., El-Gamil M., et al. (2007). Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 67, 3898–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad H., Gebhard K., Kronig H., et al. (2008). CTLs directed against HER2 specifically cross-react with HER3 and HER4. J. Immunol. 180, 8135–8145 [DOI] [PubMed] [Google Scholar]
- Cordaro T.A., de Visser K.E., Tirion F.H., et al. (2002). Can the low-avidity self-specific T cell repertoire be exploited for tumor rejection? J. Immunol. 168, 651–660 [DOI] [PubMed] [Google Scholar]
- Czerniecki B.J., Koski G.K., Koldovsky U., et al. (2007). Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 67, 1842–1852 [DOI] [PubMed] [Google Scholar]
- Dangaj D., Lanitis E., Zhao A., et al. (2013). Novel recombinant human B7-H4 antibodies overcome tumoral immune escape to potentiate T cell anti-tumor responses. Cancer Res. 73, 4820–4829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disis M.L., Schiffman K., Guthrie K., et al. (2004). Effect of dose on immune response in patients vaccinated with an her-2/neu intracellular domain protein—based vaccine. J. Clin. Oncol. 22, 1916–1925 [DOI] [PubMed] [Google Scholar]
- Engel R.H., and Kaklamani V.G. (2007). HER2-positive breast cancer: current and future treatment strategies. Drugs 67, 1329–1341 [DOI] [PubMed] [Google Scholar]
- Engels B., and Uckert W. (2007). Redirecting T lymphocyte specificity by T cell receptor gene transfer—a new era for immunotherapy. Mol. Aspects Med. 28, 115–142 [DOI] [PubMed] [Google Scholar]
- Han L.Y., Fletcher M.S., Urbauer D.L., et al. (2008). HLA class I antigen processing machinery component expression and intratumoral T-Cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin. Cancer Res. 14, 3372–3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle A.M., Erskine C.L., Benson L.M., et al. (2013). Enzymatic discovery of a HER-2/neu epitope that generates cross-reactive T cells. J. Immunol. 190, 479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L.A., Heemskerk B., Powell D.J., Jr, et al (2006). Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J. Immunol. 177, 6548–6559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L.A., Morgan R.A., Dudley M.E., et al. (2009). Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114, 535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh E., Fikes J., Southwood S., et al. (2001). Identification of new epitopes from four different tumor-associated antigens: recognition of naturally processed epitopes correlates with HLA-A*0201-binding affinity. J. Immunol. 167, 787–796 [DOI] [PubMed] [Google Scholar]
- Knutson K.L., Schiffman K., Cheever M.A., and Disis M.L. (2002). Immunization of cancer patients with a HER-2/neu, HLA-A2 peptide, p369–377, results in short-lived peptide-specific immunity. Clin. Cancer Res. 8, 1014–1018 [PubMed] [Google Scholar]
- Koski G.K., Koldovsky U., Xu S., et al. (2012). A novel dendritic cell-based immunization approach for the induction of durable Th1-polarized anti-HER-2/neu responses in women with early breast cancer. J. Immunother. 35, 54–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanitis E., Dangaj D., Hagemann I.S., et al. (2012). Primary human ovarian epithelial cancer cells broadly express HER2 at immunologically-detectable levels. PLoS ONE 7, e49829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B.L., Bernstein W.B., Connors M., et al. (1997). Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J. Immunol. 159, 5921–5930 [PubMed] [Google Scholar]
- Liu G., Ying H., Zeng G., et al. (2004). HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer Res. 64, 4980–4986 [DOI] [PubMed] [Google Scholar]
- Marincola F.M., Shamamian P., Simonis T.B., et al. (1994). Locus-specific analysis of human leukocyte antigen class I expression in melanoma cell lines. J. Immunother. Emphasis Tumor Immunol. 16, 13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.A., Dudley M.E., Wunderlich J.R., et al. (2006). Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314, 126–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.L., Gillogly M.E., Przepiorka D., et al. (2002). Toxicity, immunogenicity, and induction of E75-specific tumor-lytic CTLs by HER-2 peptide E75 (369–377) combined with granulocyte macrophage colony-stimulating factor in HLA-A2+ patients with metastatic breast and ovarian cancer. Clin. Cancer Res. 8, 3407–3418 [PubMed] [Google Scholar]
- Okamoto S., Mineno J., Ikeda H., et al. (2009). Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR. Cancer Res. 69, 9003–9011 [DOI] [PubMed] [Google Scholar]
- Peoples G.E., Gurney J.M., Hueman M.T., et al. (2005). Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J. Clin. Oncol. 23, 7536–7545 [DOI] [PubMed] [Google Scholar]
- Pietras R.J., Fendly B.M., Chazin V.R., et al. (1994). Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene 9, 1829–1838 [PubMed] [Google Scholar]
- Rivoltini L., Barracchini K.C., Viggiano V., et al. (1995). Quantitative correlation between HLA class I allele expression and recognition of melanoma cells by antigen-specific cytotoxic T lymphocytes. Cancer Res. 55, 3149–3157 [PMC free article] [PubMed] [Google Scholar]
- Rongcun Y., Salazar-Onfray F., Charo J., et al. (1999). Identification of new HER2/neu-derived peptide epitopes that can elicit specific CTL against autologous and allogeneic carcinomas and melanomas. J. Immunol. 163, 1037–1044 [PubMed] [Google Scholar]
- Sadelain M., Riviere I., and Brentjens R. (2003). Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer 3, 35–45 [DOI] [PubMed] [Google Scholar]
- Schumacher T.N. (2002). T-cell-receptor gene therapy. Nat. Rev. Immunol. 2, 512–519 [DOI] [PubMed] [Google Scholar]
- Seliger B., Rongcun Y., Atkins D., et al. (2000). HER-2/neu is expressed in human renal cell carcinoma at heterogeneous levels independently of tumor grading and staging and can be recognized by HLA-A2.1-restricted cytotoxic T lymphocytes. Int. J. Cancer 87, 349–359 [PubMed] [Google Scholar]
- Voss R.H., Willemsen R.A., Kuball J., et al. (2008). Molecular design of the Calphabeta interface favors specific pairing of introduced TCRalphabeta in human T cells. J. Immunol. 180, 391–401 [DOI] [PubMed] [Google Scholar]
- Wargo J.A., Robbins P.F., Li Y., et al. (2009). Recognition of NY-ESO-1+ tumor cells by engineered lymphocytes is enhanced by improved vector design and epigenetic modulation of tumor antigen expression. Cancer Immunol. Immunother. 58, 383–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen R.A., Debets R., Chames P., and Bolhuis R.L. (2003). Genetic engineering of T cell specificity for immunotherapy of cancer. Hum. Immunol. 64, 56–68 [DOI] [PubMed] [Google Scholar]
- Wong Y.F., Cheung T.H., Lam S.K., et al. (1995). Prevalence and significance of HER-2/neu amplification in epithelial ovarian cancer. Gynecol. Obstet. Invest. 40, 209–212 [DOI] [PubMed] [Google Scholar]
- Xu S., Koski G.K., Faries M., et al. (2003). Rapid high efficiency sensitization of CD8+ T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. J. Immunol. 171, 2251–2261 [DOI] [PubMed] [Google Scholar]
- Zaks T.Z., and Rosenberg S.A. (1998). Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res. 58, 4902–4908 [PubMed] [Google Scholar]
- zum Buschenfelde C.M., Hermann C., Schmidt B., et al. (2002). Antihuman epidermal growth factor receptor 2 (HER2) monoclonal antibody trastuzumab enhances cytolytic activity of class I-restricted HER2-specific T lymphocytes against HER2-overexpressing tumor cells. Cancer Res. 62, 2244–2247 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.