Abstract
Objective: A randomized, double-blind, placebo-controlled flexible-dose, parallel group trial was conducted at 26 clinical investigational sites in the United States to examine the safety and efficacy of the selegiline transdermal system (STS) (EMSAM®) in adolescents (ages 12–17 years) meeting American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) criteria for moderate to severe major depressive disorder (MDD) without psychotic features.
Methods: Adolescents (n=308) with moderate to severe MDD were randomized to either STS (n=152) or placebo (n=156). Two hundred and fifteen (69.8%) subjects completed the study and 17 (5.5%) reported discontinuation because of adverse events (AEs). The primary efficacy outcome measure was the mean change from baseline to end of study (week 12 last observation carried forward [LOCF]) in the Children's Depression Rating Scale-Revised (CDRS-R) total score. Secondary outcome measures included end-point Clinical Global Impressions – Severity (CGI-S) and Clinical Global Impressions – Improvement (CGI-I).
Results: Patients on STS or placebo had a significant decline from baseline (p<0.001) on their CDRS-R total score with mean reductions±SD as follows: STS 21.4±16.6; placebo 21.5±16.5. Both groups had similar response rates (58.6% vs. 59.3%) defined as CGI-I of 1 or 2 at study end. However, these between-group efficacy findings were without statistical significance. The overall incidence of reported AEs was 62.5% for STS-treated patients and 57.7% for placebo-treated patients. Most commonly reported AEs in STS or placebo groups were application site reactions (STS=24.3%; placebo=21.8%), headache (STS=17.1%; placebo=16.7%), and nausea (STS=7.2%; placebo=7.7%). Treatment groups did not differ on any laboratory parameters, vital signs, or electrocardiogram (ECG) findings. No suspected hypertensive crises were reported in the trial.
Conclusions: These data demonstrated that the STS was safe and well tolerated in this adolescent sample. However, both STS-treated and placebo-treated subjects demonstrated a decline from baseline in depressive symptoms (CDRS-R total score) over the length of the study, without statistical superiority by either group.
Introduction
Monoamine oxidase inhibitors (MAOIs) were the first antidepressant drugs to consistently demonstrate efficacy in patients with major depressive disorder (MDD), especially those with atypical and treatment-resistant depression (Thase et al. 1995; Robinson 2002). These medications are thought to act by inhibiting monoamine oxidase A (MAO-A) in brain tissue and thus increasing the pharmacodynamic half-life of transmitter amines norepinephrine, serotonin, and dopamine (Stahl 1998).
The selegiline transdermal system (STS) (EMSAM®) is an MAOI formulation that was developed to reduce the dietary tyramine safety issue (“cheese reaction”) of orally administered MAOIs (Blackwell et al. 1967). In 2006, the United States Food and Drug Administration approved the STS (doses of 6, 9, and 12 mg/24 hours) for the treatment of MDD in adults. Its efficacy and safety were established in three short-term studies of 6–8 weeks (Bodkin and Amsterdam 2002; Amsterdam 2003; Feiger et al. 2006) and a relapse-prevention trial of 52 weeks (Amsterdam and Bodkin 2006). Oral tyramine challenge studies conducted with EMSAM in volunteers demonstrated that STS reduced the risk of dietary tyramine-induced hypertension when compared with traditional MAOIs (Azzaro et al. 2006). Accordingly, product labeling states that no dietary modifications are required for the 6 mg/24 hour dose; however, at the higher doses of 9 mg/24 hours and 12 mg/24 hours, dietary modifications are still required.
Depression affects ∼2–8% of children and adolescents in the United States (Fleming and Oxford 1990; Findling et al. 1999; Emslie et al. 2002; Bostic et al. 2005; Hazell 2009). Suicide-related behaviors, especially among adolescents, are a serious health concern in the United States (Hill et al. 2011), and these suicides are generally associated with symptoms of depression. There is evidence that pediatric depression may have unique features when compared with the adult form of the illness. Biederman et al. (1995) showed that the predominant mood in juvenile depression is dysphoria and irritability rather than sadness and melancholia. Childhood depression also commonly presents with “mood reactivity,” that is, improved mood in response to positive events, which is more commonly seen in atypical forms of adult depression (Nierenberg et al. 1998). Casper et al. (1985) reported that symptoms of atypical depression were frequent in young adults, which has been subsequently supported by others (Kaminski et al. 1995; Bostic et al. 2005). Furthermore, depression in children and adolescents exhibits selective efficacy with agents such as selective serotonin reuptake inhibitor drugs (SSRIs) (Emslie et al. 1997, 2002; Braconnier et al. 2003) and a lack of response to tricyclic antidepressants (TCAs) (Hazell et al. 1995; Weller and Weller 2000; Bostic et al. 2005).
Controlled trials of many available antidepressants have been conducted in youth with depression, with few studies demonstrating efficacy compared with placebo. The lack of efficacy in some studies is because of the large placebo response (Bostic et al. 2005; Bridge et al. 2009). To date, little is known about the safety and efficacy of MAOIs in children and adolescents with MDD (Findling et al. 1999; Bostic et al. 2005). Fear of acute dietary tyramine-induced hypertension associated with traditional MAOIs, such as tranylcypromine or phenelzine has discouraged the study of these agents in youth. However, the STS has a reduced risk of dietary tyramine-induced hypertension and, therefore, may be a good option for youth with MDD (Azzaro et al. 2006). Accordingly, the current controlled clinical trial was conducted to examine the efficacy and safety of the STS, in adolescents with moderate to severe MDD. This study was conducted as a post-marketing commitment to the United States Food and Drug Administration (US FDA) under the Pediatric Research Equity Act (PREA).
Methods
A randomized, double-blind, placebo-controlled, flexible-dose, parallel group trial was conducted at 26 clinical investigational sites in the United States to examine the efficacy and safety of the STS in youth with moderate or severe MDD. The trial was conducted following Good Clinical Practice Guidelines and in accordance with the Declaration of Helsinki (2002). Approval was obtained by central or local independent institutional review boards (IRBs), and prior to enrollment, an IRB-approved written informed consent was obtained from each subject's guardian, and assent was obtained from each study participant.
Outpatients, 12–17 years of age, who met the criteria for moderate to severe MDD, based upon the Kiddie Schedule for Affective Disorders and Schizophrenia for School Aged Children (K-SADS) semistructured psychiatric interview, and a Children's Depression Rating Scale-Revised (CDRS-R) score of ≥45, were eligible for enrollment. Subjects were included who had a current episode of MDD of at least 2 months' duration, but not more than 2 years' duration. Patients were excluded if they had psychotic features, another primary American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR) Axis-I or Axis-II diagnosis, a diagnosis of conduct disorder, or a history of a substance use disorder, as defined by DSM-IV-TR criteria (American Psychiatric Association 2000). In addition, patients were excluded if they had a medical illness that could compromise their safety or the interpretation of the results. Girls who were pregnant or breast-feeding were excluded, and all other girls were required to use a reliable method of contraception to remain in the trial. Finally, all subjects were required to be free of other psychoactive medications, sympathomimetics, or agents contraindicated with MAOIs, for five half-lives prior to baseline. These agents were also prohibited during the course of the study.
Clinical, pharmacokinetic, and statistical procedures
Screening procedures were completed during a period of up to 14 days. Eligible subjects were randomized 1:1 to STS or matching placebo patches. Selegiline transdermal patches were applied daily and removed after 24 hours. Active treatment consisted of STS at a dose of 6 mg/24 hours (20 cm2), 9 mg/24 hours (30 cm2), or 12 mg/24 hours (40 cm2). Placebo treatment consisted of patches devoid of selegiline but the same size as each of the active doses. During a 24 hour application, a loose or dislodged patch could be held in place with dermal tape, if needed.
Subjects started treatment at the 6 mg/24 hour dose, active or matching placebo, for 4 weeks. Those subjects responding to the 6 mg/24 hour dose (or matching placebo) continued treatment for an additional 8 weeks. Subjects who did not show a significant response (based on a Clinical Global Impressions – Improvement [CGI-I] score of >2 relative to baseline) after the initial 4 weeks of treatment were eligible for a dose increase to 9 mg/24 hour (or matching placebo). Subjects who responded continued treatment for the duration of the study. Subjects showing inadequate improvement (based upon the CGI-I score) after 4 weeks of treatment with the 9 mg/24 hour patch (or placebo) were eligible for a dose increase to the 12 mg/24 hour patch (or matching placebo) for the final 4 weeks of the study. Dose-dependent dietary-tyramine modifications were required with the 9 mg/24 hour and 12 mg/24 hour active or matching placebo treatments. To avoid the potential for a hypertensive adverse event, these subjects/parents were instructed to avoid foods rich in tyramine (e.g., aged/fermented meats, poultry, or fish; fava bean pods; aged cheeses; tap or unpasteurized beer; marmite, sauerkraut, soy sauce, and tofu) according to guidelines in the EMSAM label. Subjects experiencing dose-limiting adverse events after a dose increase had their dose decreased by one level and completed the study at the highest tolerated dose. However, if subjects were titrated down to the 6 mg / 24 hour dose, a dietary-tyramine restriction was to be maintained for 2 weeks following the dose change. All subjects (6 mg/24 hour, 9 mg/24 hour, 12 mg/24 hour, and placebo) were instructed to maintain a food diary that was collected at each visit.
Both the subjects/parents and the clinical sites were instructed to report the possible occurrence of a hypertensive adverse event, characterized by some or all of the following symptoms: occipital headache, which may radiate frontally; cardiac arrhythmias (including palpitations, tachycardia, or bradycardia); neck stiffness or soreness; nausea; vomiting; sweating (sometimes with fever and sometimes with cold, clammy skin); dilated pupils; or constricting chest pain. In those subjects identified for a possible occurrence of a hypertensive adverse event, vital signs were to be measured to determine whether or not a pressor response was present, and sound clinical judgment was to be exercised regarding discontinuation of the study medication and the initiation of treatment for the hypertensive event.
Efficacy was evaluated using the CDRS-R and the investigator's Clinical Global Impressions – Severity and Improvement measures (CGI-S and CGI-I, respectively). Experienced, trained practitioners performed all ratings. Efforts were made to have individual subjects assessed by the same rater at each visit. Rater training was conducted on each of the outcome measures with instruction, video practice sessions, and grading. The training and competence of each rater was documented in writing. Patients were evaluated at 1, 2, 4, 5, 6, 8, 9, 10, and 12 weeks after randomization.
Safety was assessed by physical examination, a 12 lead electrocardiogram, respiration rate, temperature, supine and standing blood pressure and heart rate, application site assessments, AEs, and clinical laboratory tests at various time points from screening to the end of study. More frequent monitoring of vital signs could be obtained at the investigator's discretion. Subjects were queried at each clinic visit concerning the use of concomitant medications. The use of all medications taken during the study, including medication taken for the treatment of AEs, was recorded. Patch application sites were observed for the presence or absence of local irritation at all scheduled and interim clinic visits, including early termination. If the application site reaction required treatment for an inflammatory response, a topical corticosteroid (0.5% hydrocortisone ointment) was applied. The need for additional intervention was at the discretion of the investigator.
Pharmacokinetic blood samples were obtained from each subject at screening and after 6 and 10 weeks of treatment. At least one blood sample was drawn, and an attempt was made to obtain two or three blood samples over a period of 4–6 hours at visits for weeks 6 and 10. Steady-state plasma concentrations of selegiline, N-desmethylselegiline, phenylethylamine, R(-) amphetamine and R(-) methamphetamine were determined using a validated high-performance liquid chromatography (HPLC)/ tandem mass spectrometric (MS/MS) method. The assay limit of quantitation (LOQ) was 0.01 ng/mL for selegiline and ranged from 0.01 ng/mL to 0.05 ng/mL for the metabolites.
Plasma concentrations of selegiline and its metabolites were summarized by group, dose, visit, and time after patch application. Analyses were performed to describe the effects of covariates (e.g., age, gender, body weight) on the steady-state plasma concentrations of selegiline and its major metabolites. Concentrations that were reported as being below the assay LOQ were assigned a value of 0.0 for the pharmacokinetic analysis.
The primary efficacy variable was the change from baseline to end-point (last observation carried forward [LOCF]) on the CDRS-R total score. A linear analysis of covariance (ANCOVA) was used to examine the efficacy variable, including terms for baseline as covariate, treatment effect, and center as a blocking factors. A sample size of 150 evaluable subjects/group was calculated as necessary to detect a between-group difference of ∼5.5 on the CDRS-R total score, with 90% power.
The primary efficacy analysis was conducted with the modified intent-to-treat (m-ITT) population (i.e., all subjects who were randomized to STS or placebo, took any study drug, and had a baseline and at least one posttreatment CDRS-R assessment; n=304 subjects) and consisted of a two way ANCOVA model fitted using the LOCF CDRS-R change from baseline to end of study (week 12) as the response, and treatment groups (active vs. placebo) and center as the main effects, with baseline scores as covariates. Treatment-by-center interaction was tested using a three way analysis of variance (ANOVA) model, including treatment, center, and treatment-by-center interaction as main effects. If this interaction term was not significant at α=0.05, no further investigation was undertaken. If it was significant at α=0.05, then further investigation was undertaken to determine if the treatment effects varied by center in terms of their magnitude or in terms of their direction.
The CGI-S and CGI-I scores were collected at baseline (only CGI-S) and weeks 1, 2, 4, 5, 6, 8, 9, 10, and 12. A Cochran–Mantel–Haenszel (CMH) Type 2 (ANOVA mean score) statistic using pseudocenter as stratum was used for treatment comparison. Testing was at the 0.05 α level. In the responder analysis, a CGI-I responder was defined by having a score of 1 or 2 at the end of the study. A CMH Type 1 statistic using pseudocenter as stratum was used for treatment comparisons.
The safety analysis included all patients who received at least one dose of study drug (n=308 subjects). All treatment emergent AEs were summarized by system organ class/preferred term (MedDRA) for each treatment group. The Fisher's exact test was used to test for differences between groups. Each vital sign observed value and change from baseline were summarized using descriptive statistics (mean, median, SD, minimum, maximum, and number of subjects) for each treatment group at each measured time point. Ninety percent confidence intervals (CIs) were constructed for within treatment group change from baseline. Laboratory values were collected at screening and week 12 for hematology, blood chemistry, and urinalysis with urine drug screen. All laboratory test results that were outside the reference ranges were reviewed by the Investigator. Clinically significant laboratory test results were reported as AEs. Summaries of the change from screening to end-point were obtained for hematology, blood chemistry, and urinalysis data, and analyzed with shift tables. All results presented were considered statistically significant at the α level ≤0.05. All statistical analyses were performed using SAS software, Version 6.12 (SAS Institute, Cary, NC).
Results
Clinical and pharmacokinetic findings
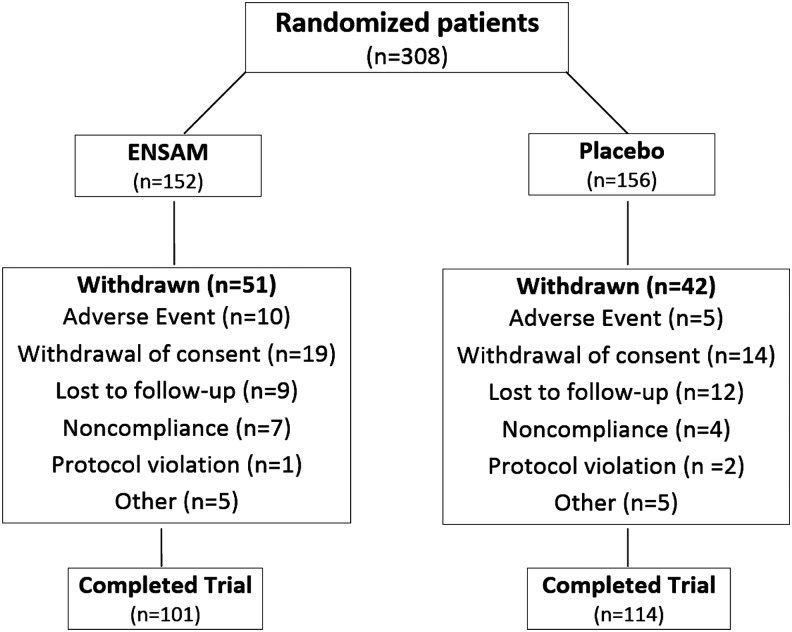
A total of 308 adolescent subjects with MDD were randomly assigned to STS (n=152) or matching placebo (n=156). Of the initial 308 who were randomized, 215 (70%) completed the trial. Fifty-one (34%) patients receiving STS and 42 (27%) receiving placebo dropped out of the study before week 12. The primary reasons for discontinuation were: AEs, withdrawn consent, noncompliance, protocol violation, or loss to follow-up (see Fig. 1 for the number of subjects in each category). The AEs that led to withdrawal in the STS group included suicidal ideations (n=3), and single reports of headache, application site reaction, anxiety, medication-induced agitation, nightmare, agitation, and panic attack. The AEs that led to withdrawal in the placebo group included suicidal ideation, migraines, nausea, rash, and increased depression.
FIG. 1.
Patient disposition.
Demographic characteristics of all study participants are listed in Table 1. There were no meaningful treatment group differences in demographic characteristics or study participation. Patients who were randomized to STS or placebo were treated for a mean of 62 days and 63 days, respectively. Treatment compliance was also equally balanced between the two groups. Patients randomized to STS or placebo used a mean of 74 and 75 patches, respectively, which included patches dislodged or those removed on the same day.
Table 1.
Demographic Characteristics of 308 Adolescent Patients with MDD Randomly Assigned to Selegiline Transdermal System or Matching Placebo
| Placebo (n=156) | EMSAM® (n=152) | Overall (n=308) | |
|---|---|---|---|
| Age (years) | |||
| Mean | 14.7 | 14.8 | 14.8 |
| SD | 1.60 | 1.62 | 1.61 |
| Sex, | |||
| Female, n (%) | 104 (66.7%) | 93 (61.2%) | 197 (64.0%) |
| Race | |||
| Caucasian | 77 (49.4%) | 69 (45.4%) | 146 (47.4%) |
| Black | 34 (21.8%) | 46 (30.3%) | 80 (26.0%) |
| Hispanic | 37 (23.7%) | 32 (21.1%) | 69 (22.4%) |
| Asian | 2 (1.3%) | 1 (0.7%) | 3 (1.0%) |
| Native American | 1 (0.6%) | 1 (0.7%) | 2 (0.6%) |
| Mixed race | 5 (3.2%) | 3 (2.0%) | 8 (2.6%) |
| Weight (kg) | |||
| Mean | 68.4 | 70.6 | 69.5 |
| SD | 18.8 | 23.9 | 21.5 |
| Height (cm) | |||
| Mean | 163.6 | 164.6 | 164.1 |
| SD | 9.3 | 9.2 | 9.2 |
MDD, major depressive disorder.
The m-ITT efficacy population consisted of 304 subjects rather than the 308 subjects randomized (i.e., 4 subjects did not have a posttreatment assessment); 150 subjects receiving STS and 154 subjects receiving placebo (Table 2).
Table 2.
CDRS-R Total Score (Child): Baseline and Week 12 (Study End-Point)
| Placebo n=154 | EMSAM® n=150 | Overall n=304 | |
|---|---|---|---|
| Baseline | |||
| Arithmetic mean (SD) | 57.9 (12.57) | 56.7 (12.34) | 57.3 (12.45) |
| Week 12 | |||
| Arithmetic mean (SD) | 36.4 (15.91) | 35.4 (15.30) | 35.9 (15.60) |
| Week 12 - change from baseline | |||
| Arithmetic mean (SD) | −21.5 (16.47) | −21.4 (16.61) | −21.5 (16.51) |
| p value for treatment - overall ANCOVAa | 0.7159 | ||
ANCOVA: main effects of treatment and pseudocenter, baseline score as covariate.
ANCOVA; analysis of covariance; CDRS-R, Children Depression Rating Scale-Revised.
Baseline scores for STS and placebo groups were 56.7 (SD=12.3) and 57.9 (SD=12.6), respectively. STS and placebo groups both had significant reductions from baseline to end-of-study on their CDRS-R total score, with mean reductions±SD as follows: STS 21.4±16.6 (p<0.001) placebo 21.5±16.5 (p<0.001). The between group difference in change from baseline to end-of-study CDRS-R total score was not statistically significant (p=0.72). There was no treatment-by-center interaction. In addition, both groups had similar CGI-I response rates (58.6% vs. 59.3%) at study endpoint (Table 3). There was no difference between treatment groups in the CGI-S at baseline (4.49 vs. 4.53) or week 12 (3.00 vs.3.01).
Table 3.
CGI-I Responders at Week 12 (Study End-Point)
| Characteristic | Placebo (n=150) | EMSAM® (n=145) |
|---|---|---|
| Week 12 CGI-I respondera | ||
| No n (%) | 61 (40.7%) | 60 (41.4%) |
| Yes n (%) | 89 (59.3%) | 85 (58.6%) |
| p value* | 0.8992 |
Nine subjects in the m-ITT population failed to have a CGI-I score at week 12.
Responder defined as <3. Nonresponder defined as ≥3.
p value calculated using CMH test with pseudocenter as a stratum (general association p value).
CGI-I, Clinical Global Impressions – Improvement; CMH, Cochran–Mantel–Haenszel; m-ITT, modified intent-to-treat.
The safety analysis included 152 subjects treated with STS and 156 subjects treated with placebo. Overall, treatment was well tolerated. The overall incidence of AEs was 62.5% for STS and 57.7% for placebo. Only 15 subjects discontinued from the trial for AEs while receiving STS or placebo; 5 of the AEs were serious (SAEs) (Fig. 1). There were no differences in the number of occurrences of AEs between the two treatment groups (Table 4). The most commonly reported AEs were patch application site reactions (n=71, 23.1%), headache (n=52, 16.9%), and nausea (n=23, 7.5%). There were some AEs that were observed more frequently in STS-treated subjects. These included decreased appetite (3.3% vs. 1.3%), agitation (2.6% vs. 1.9%), anxiety (2.6% vs. 1.3%), insomnia (5.9% vs. 2.6%), somnolence (4.6% vs. 2.6%), upper respiratory tract infection (7.2% vs. 2.6%), and vomiting (4.6% vs. 2.6%). However, these events were generally reported as mild to moderate in intensity.
Table 4.
Summary of the Most Frequently Reported Treatment-Emergent Adverse Events (>2% of Subjects)
| Placebo (n=156) | EMSAM® (n=152) | |
|---|---|---|
| Any adverse event | 90 (57.7%) | 95 (62.5%) |
| Gastrointestinal disorders | 31 (19.9%) | 31 (20.4%) |
| Abdominal pain | 4 (2.6%) | 3 (2.0%) |
| Abdominal pain upper | 7 (4.5%) | 4 (2.6%) |
| Diarrhea | 7 (4.5%) | 5 (3.3%) |
| Nausea | 12 (7.7%) | 11 (7.2%) |
| Vomiting | 4 (2.6%) | 7 (4.6%) |
| General disorders & administration site conditions | 42 (26.9%) | 42 (27.6%) |
| Application site reaction | 34 (21.8%) | 37 (24.3%) |
| Fatigue | 4 (2.6%) | 4 (2.6%) |
| Infections and infestations | 18 (11.5%) | 18 (11.8%) |
| Nasopharyngitis | 7 (4.5%) | 6 (3.9%) |
| Metabolism and nutrition disorders | 4 (2.6%) | 6 (3.9%) |
| Decreased appetite | 2 (1.3%) | 5 (3.3%) |
| Musculoskeletal & connective tissue disorders | 9 (5.8%) | 13 (8.6%) |
| Back pain | 4 (2.6%) | 4 (2.6%) |
| Nervous system disorders | 38 (24.4%) | 41 (27.0%) |
| Dizziness | 7 (4.5%) | 8 (5.3%) |
| Headache | 26 (16.7%) | 26 (17.1%) |
| Somnolence | 4 (2.6%) | 7 (4.6%) |
| Psychiatric disorders | 17 (10.9%) | 24 (15.8%) |
| Agitation | 3 (1.9%) | 4 (2.6%) |
| Anxiety | 2 (1.3%) | 4 (2.6%) |
| Insomnia | 4 (2.6%) | 9 (5.9%) |
| Irritability | 4 (2.6%) | 2 (1.3%) |
| Suicidal ideation | 4 (2.6%) | 4 (2.6%) |
| Respiratory, thoracic & mediastinal disorders | 12 (7.7%) | 25 (16.4%) |
| Pharyngolaryngeal pain | 3 (1.9%) | 4 (2.6%) |
| Upper respiratory tract infection | 4 (2.6%) | 11 (7.2%) |
Primary system organ classes and preferred terms sorted alphabetically.
Subjects with more than one occurrence in a category only counted once.
There were 14 SAEs reported in 10 subjects (3.2% of subjects), 5 of whom discontinued from the study. Suicidal ideation resulted in discontinuation of one STS- treated subject and one placebo-treated subject. Both occurrences were considered SAEs. SAEs of medication-induced agitation, agitation, and uncontrollable screaming also resulted in discontinuations of three additional STS-treated subjects. SAEs not leading to discontinuation in the placebo group included vomiting, loss of consciousness, and hospitalization for mood swings. SAEs within the STS-treated subjects not leading to discontinuation included suicidal ideation, gastritis, anxiety, syncope, orthostatic hypotension, and psychotic break. No other significant AEs were identified in the adolescent subjects enrolled in this study, and no evidence of acute dietary-induced hypertension was observed at any dose of STS.
The majority of laboratory test results were within normal limits, and no STS-treated subjects had laboratory findings deemed clinically significant. No clinically meaningful treatment group differences were identified for any of the laboratory parameters. Two laboratory findings were deemed clinically significant in a placebo patient: elevated liver enzymes and low circulatory thyroid hormone free T4.
No clinically meaningful differences in supine heart rate or systolic blood pressue (SBP) or diastolic blood pressure (DBP) were identified during the study. Evaluation of the orthostatic change in vital signs at week 12, showed a slight increase from baseline in the orthostatic change in heart rate (+2.8 bpm) and a slight decrease in the orthostatic change in SBP (- 2.3) and DBP (-1.5) in the STS treatment group.
Investigators reviewed all ECG tracings prior to patient randomization and at the end of study. Mean change from baseline in PR interval, ventricular heart rate, QRS duration, QT interval, and QTc (Bazett and Fridericia corrections) interval were evaluated. No ECG findings were deemed clinically significant over the 12 week study period.
Two hundred and ninety-four subjects who were randomized and provided at least one pharmacokinetic sample were included in the pharmacokinetic analysis. A total of 862 plasma selegiline values were analyzed and reported. A total of 292 selegiline results were reported for samples obtained during treatment weeks 6 and 10 in the active group.
Selegiline transdermal patches delivering 6, 9, or 12 mg/24 hours of selegiline provided a consistent and dose-proportional exposure to selegiline over the 12 week period. Median plasma selegiline levels at week 10 were 1.31 ng/mL at the 6 mg/day dose, 2.38 ng/mL at the 9 mg/day dose, and 2.96 ng/mL at the 12 mg/day dose. Scatter plots of plasma concentrations versus the time after the most recent patch application in each group showed no significant relationship, suggesting that plasma selegiline levels remained relatively constant during daily STS administration. Plotting the median with 10th and 90th percentiles for the 6 mg and 9 mg treatment groups suggested that plasma selegiline levels did not change between week 6 and week 10 of the study, indicating that steady state had been achieved prior to week 6 of the study. The metabolite/selegiline ratios remained the same regardless of dose, indicating no change in metabolism with dose.
Discussion
This is the first randomized controlled trial of a MAOI inhibitor in adolescents with MDD. Previous use of MAOIs in adolescents has been limited, given concerns about this population following dietary restrictions. The advent of the STS patch with possibly decreased AEs, and less concern about dietary restrictions, was an important advance. However, although STS was generally well tolerated, the current study was unable to demonstrate greater efficacy of STS compared with placebo. Comparison of the results of this study with that of the Treatment for Adolescents with Depression Study (TADS) (March et al. 2004) revealed that the STS and placebo groups had changes in the CDRS-R total scores (21.4 and 21.5, respectively), which were similar to the TADS fluoxetine treatment group (-22.6). Similarly, CGI-I response rates for the STS and placebo groups were 58.6% and 59.3%, respectively, and 60.6% for the TADS fluoxetine group. However, the placebo response rates in the two studies were very different. Placebo response in TADS was only 35%, versus 59.3% for this selegiline trial.
Failure of the STS to separate from placebo was likely associated with the high response rate of the placebo group (59.3%). A review of other controlled trials by Bostic et al. (2005), conducted with other antidepressants in youth, revealed that the placebo response rate must be in the 20–30% range to demonstrate efficacy of the antidepressant. Interestingly, the placebo response rates were 20% (Bodkin and Amsterdam 2002) and 30% (Feiger et al. 2006) in the two acute pivotal STS trials conducted in adult patients with MDD.
The increasing placebo response in clinical trials of depression has been noted extensively (Bridge et al. 2009), although the reason for this has received little study in youth. Predictors of higher placebo response rates among depressed adults have included: younger age, male sex, longer trial length, shorter duration of depression, lower severity of illness, fewer prior episodes, and United States versus European study location (Khan et al. 1991; Wilcox et al. 1992; Charney et al. 2002; Stein et al. 2006; Posternak and Zimmerman 2007; Kirsch et al. 2008). Bridge et al. (2009) conducted a literature review of 12 studies, analyzing 2863 adolescents who were randomized in controlled clinical trials of antidepressants. The results of their study demonstrated that the number of patients randomized, the number of study sites, severity of illness, and participants per site all had significant effects on the number of placebo responders. Large numbers randomized, large numbers of study sites, low severity of illness at baseline, and small numbers of participants per site were associated with an increase in the placebo response rate. Interestingly, the active treatment response rate was unaffected by each of these variables. The authors concluded that placebo response rate could best be reduced by limiting the number of study sites in the trial, thus improving the selection process of patient participants, quality assurance of the recruiting methods, and conduct of the essential elements of the trial. It is difficult to assign one or more of these variables as the cause for the high placebo rate in the current trial. However, the use of 26 study sites to enroll and complete the study was likely to have been a significant factor in the efficacy outcome variable.
On the positive side, the safety data for the STS was very favorable across all three doses administered. The overall incidence of adverse events was similar between STS-treated patients and patients randomized to placebo. Only 15 subjects discontinued because of AEs, including 5 subjects reporting SAEs. Of the 10 subjects discontinuing because of nonserious AEs, 6 had received STS and 4 had received placebo treatment. In addition, there were no reports of acute hypertension or hypertensive crisis, dietary or otherwise, no differences in resting or orthostatic vital signs, and no abnormalities in cardiac function over the course of the study. Finally, laboratory test results for STS-treated patients were within normal limits.
Conclusions
This multicenter, double-blind, placebo-controlled, randomized, flexible-dose efficacy and safety study of the STS (EMSAM) in adolescents with moderate to severe MDD failed to show superiority of EMSAM over placebo on any of the efficacy measures. The large number of investigational clinical sites (26 sites) required to enroll and randomize the 308 subjects may have played a significant role in this outcome. However, the STS was safe and well tolerated in this adolescent sample, as evidenced by the lack of significant vital sign, ECG, and laboratory findings, and the similar incidence of SAEs across treatment groups. There were no signs or reports of dietary-induced hypertension over the course of the study.
Clinical Significance
The safety profile seen in adolescent subjects was consistent with the current STS (EMSAM) prescribing information. However, EMSAM is only approved by the US FDA for treatment of MDD in adults (see Introduction) not children or adolescents. This study in youth was conducted as a post-marking commitment to the US FDA under the PREA.
Acknowledgments
The authors acknowledge Eva Kemper, David McLaughlin, and Deborah Lees, at Cognitive Research Corporation, for their assistance in the final preparation of this manuscript. The authors also acknowledge Melvin Sharoky, MD, Lawrence Blob, MD, and Melissa Goodhead (Somerset Pharmaceuticals, Inc.), for the design and completion of this trial.
Disclosures
Dr. DelBello has received research support from each of the following pharmaceutical companies: AstraZeneca, Brain & Behavior Research Foundation, Eli Lilly, GlaxoSmithKline, Janssen, Johnson and Johnson, Lundbeck, Merck, National Institute for Mental Health (NIMH), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Drug Abuse (NIDA), Novartis, Otsuka, Pfizer, Shire, Somerset, and Sumitomo. She also serves on the lecture bureau for Bristol-Myers Squibb, Merck, and Otsuka, and as a consultant at Dey, Eli Lilly, Lundbeck, Merck, Pfizer, Schering-Plough, and Sunovion. Dr. Hochadel is Chief Operating Officer for Cognitive Research Corporation, a Contract Research Organization. Cognitive Research Corporation was compensated by Mylan Specialty L.P. for operational oversight, data management, and analysis of this study. Dr. Portland is employee of Mylan Specialty, L.P. Dr. Azzaro was the former Chief Scientific Officer and member of the new drug application (NDA) submission team for EMSAM at Somerset Pharmaceuticals, Inc. Dr. Katic received grant support from Abbott, Arbor, Eli Lilly, Forest, Noven, Shire, and Targacept. Dr. Khan, principal investigator of >340 clinical trials sponsored by >65 pharmaceutical companies and 30 contract research organizations (CROs), has done no compensated consulting or speaking on their behalf, nor does he own stock in any of these or other pharmaceutical companies. Dr. Khan is not compensated for his role as author of medical manuscripts. The Northwest Clinical Research Center randomized 26 patients into the selegiline transdermal system (EMSAM) for treatment of adolescent depression clinical trial. In 2009, Dr. Khan founded Columbia Northwest Pharmaceuticals LLC, and is Medical Director of the company. Columbia Northwest Pharmaceuticals owns intellectual property rights for potential therapies for central nervous system disorders and other medical conditions. Dr. Emslie has received research support from BioMarin, Duke University, Eli Lilly, Forest Laboratories, GlaxoSmithKline, Mylan, and Somerset; has served as a consultant for Allergan, Biobehavioral Diagnostics Inc., Bristol-Myers Squibb, Eli Lilly, Forest Laboratories, GlaxoSmithKline, INC Research Inc., Lundbeck, Pfizer, Seaside Therapeutics, Shire, Texas Department of State Health Services, University of Miami, Valeant, and Wyeth Pharmaceuticals, and has been on the Speaker's Bureau for Forest Laboratories.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- Amsterdam JD: A double-blind, placebo-controlled trial of the safety and efficacy of selegiline transdermal system without dietary restrictions in patients with major depressive disorder. J Clin Psychiatry 64:208–214, 2003 [DOI] [PubMed] [Google Scholar]
- Amsterdam JD, Bodkin JA: Selegiline transdermal system in the prevention of relapse of major depressive disorder: A 52-week double-blind, placebo-substitution, parallel-group clinical trial. J Clin Psychopharmacol 26:579–586, 2006 [DOI] [PubMed] [Google Scholar]
- Azzaro AJ, VanDenBerg CM, Blob LF, Kemper EM, Sharoky M, Oren DA, Campbell BJ: Tyramine pressor sensitivity during treatment with selegiline transdermal system 6mg/24hrs in healthy subjects. J Clin Pharmacol 46:933–944, 2006 [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone S, Mick E, Lelon E: Psychiatric comorbidity among referred juveniles with major depression: Fact or artifact? J Am Acad Child Adolesc Psychiatry 34:579–590, 1995 [DOI] [PubMed] [Google Scholar]
- Blackwell B, Marley E, Price J, Taylor D: Hypertensive interactions between monoamine oxidase inhibitors and foodstuffs. Br J Psychiatry 113:349–365, 1967 [DOI] [PubMed] [Google Scholar]
- Bodkin JA, Amsterdam JD: Transdermal selegiline in major depression: a double-blind, placebo-controlled, parallel-group study in outpatients. Am J Psychiatry 159:1869–1875, 2002 [DOI] [PubMed] [Google Scholar]
- Bostic JQ, Rubin DH, Prince J, Schlozman S: Treatment of depression in children and adolescents. J Psychiatr Pract 11:141–154, 2005 [DOI] [PubMed] [Google Scholar]
- Braconnier A, LeCoent R, Cohen D: Paroxetine versus clomipramine in adolescents with severemajor depression: A double-blind, randomized, multicenter trial. J Am Acad Child Adolesc Psychiatry 42:22–29, 2003 [DOI] [PubMed] [Google Scholar]
- Bridge JA, Birmaher B, Lyengar S, Barbe RP, Brent DA: Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry 166:42–49, 2009 [DOI] [PubMed] [Google Scholar]
- Casper RC, Redmond DE, Katz MM, Schaffer CB, Davis JM, Koslow SH: Somatic symptoms in primary affective disorder: Presence and relationship to the classification of depression. Arch Gen Psychiatry 42:1098–1104, 1985 [DOI] [PubMed] [Google Scholar]
- Charney DS, Nemeroff CB, Lewis L, Laden SK, Gorman JM, Laska EM, Borenstein M, Bowden CL, Caplan A, Emslie GJ, Evans DL, Geller B, Grabowski LE, Herson J, Kalin NH, Keck PE, Jr, Kirsch I, Kirshnan KR, Kupfer DL, Makuch RW, Miller FG, Pardes H, Post R, Reynolds MM, Roberts L, Rosenbaum JF, Rosenstein DL, Rubinow DR, Rush AJ, Ryan ND, Sachs GS, Schatzberg AF, Soloman S: Consensus development panel: National depressive and manic-depressive association consensus statement on the use of placebo in clinical trails of mood disorder. Gen Arch Psychiatry 59:262–270, 2002 [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Heiligenstein JH, Wagner KD, Hoog SI, Ernest DE, Brown E, Nilsson M, Jacobson JG: Fluoxetine for acute treatment of depression in children and adolescents: A placebo-controlled, randomized clinical trial. J Amer Acad Child Adolesc Psychiatry 41:1205–1215, 2002 [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Rush AJ, Weinberd WA, Kowatch RA, Hughes CW, Carmody T, Rintelmann J: A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry 54:1031–1037, 1997 [DOI] [PubMed] [Google Scholar]
- Feiger AD, Rickels K, Moira RA, Zimbroff DL, Robinson DS: Selegiline transdermal system for the treatment of major depressive disorder: an8-week, double-blind, placebo-controlled, flexible-dose titration study. J Clin Psychiatry 67:1354–1361, 2006 [DOI] [PubMed] [Google Scholar]
- Findling RL, Reed MD, Blumer JL: Pharmacological treatment of depression in children and adolescents. Paediatr Drugs 1:161–182, 1999 [DOI] [PubMed] [Google Scholar]
- Fleming JE, Offord DR: Epidemiology of childhood depressive disorders: a critical review. J Am Acad Child Adolesc Psychiatry 29:571–580, 1990 [DOI] [PubMed] [Google Scholar]
- Hazell P: Depression in children and adolescents. Clin Evid (online) 2009:1008–1039, 2009. 19445770 [Google Scholar]
- Hazell P, O'Connell D, Heathcote D, Robertson J, Henry D: Efficacy of tricyclic drugs in treating child and adolescent depression: a meta-analysis. BMJ 310:897–901, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RM, Castellanos D, Petit JW.2011. Suicide-related behaviors and anxiety in children and adolescencents: A review. Clin Psychol Rev 31:1133–1144, 1995 [DOI] [PubMed] [Google Scholar]
- Kaminski KM, Naylor MW, King CA, Ghaziuddin N: Reactive mood and atypical depression in psychiatrically hospitalized adolescents. Depression 3:176–181, 1995 [Google Scholar]
- Khan A, Dager SR, Cohen S, Avery DH, Scherzo B, Dunner DL: Chronicity of depressive episodes in relation to antidepressant placebo response. Neuropsychopharmacol 4:125–130, 1991 [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo–Medina TB, Moore TJ, Johnson BT: Initial severity and antidepressant benefits: A meta-analysis of data submitted to the Food and Drug Administration. Plos Med 5:e45,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S: Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for adolescents with depression study (TADS) randomized controlled trial. J Am Med Assoc 292:807–820, 2004 [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Alpert JE, Pava J, Rosenbaum JF, Fava M: Course and treatment of atypical depression. J Clin Psychiatry 59:5–9, 1998 [PubMed] [Google Scholar]
- Posternak MA, Zimmerman M: Therapeutic effects of follow-up assessments on antidepressant and placebo response rates in antidepressant efficacy trials; meta-analysis. Br J Psychiatry 190:287–292, 2007 [DOI] [PubMed] [Google Scholar]
- Robinson DS: Monoamine oxidase inhibitors: A new generation. Psychopharmacol Bull 36:124–138, 2002 [PubMed] [Google Scholar]
- Stahl SM: Basic psychopharmacology of antidepressants: Pt 1: antidepressants have seven distinct mechanisms of action. J Clin Psychiatry 59 (Suppl 4):5–14, 1998 [PubMed] [Google Scholar]
- Stein DJ, Baldwin DS, Bolberg OT, Despiegel N, Bandelow B: Which factors predict placebo response in anxiety disorders and major depression? An analysis of placebo-controlled studies of escitalopram. J Clin Psychiatry 67:1741–1746, 2006 [DOI] [PubMed] [Google Scholar]
- Thase ME, Trivedi MH, Rush AJ: MAOIs in contemporary treatment of depression. Neuropsychopharmacol 12:185–219, 1995 [DOI] [PubMed] [Google Scholar]
- Weller EB, Weller RA: Treatment options in the management of adolescent depression. J Affective Disord 61:S23–S28, 2000 [DOI] [PubMed] [Google Scholar]
- Wilcox CS, Cohn JB, Linden RD, Heiser JF, Lucas PB, Morgan DL, DeFrancisco D: Predictors of placebo response: A retrospective analysis. Psychopharmacol Bull 28:157–162, 1992 [PubMed] [Google Scholar]