Abstract
A major challenge to the success of cell-based implants for tissue regeneration is an insufficient supply of oxygen before host vasculature is integrated into the implants, resulting in premature cell death and dysfunction. Whereas increasing oxygenation to the implants has been a major focus in the field, our strategy is aimed at lowering oxygen consumption by downregulating cellular metabolism of cell-based implants. Adenosine, which is a purine nucleoside that functions as an energy transferring molecule, has been reported to increase under hypoxia, resulting in reducing the adenosine triphosphate (ATP) demands of the Na+/K+ ATPase. In the present study, we investigated whether adenosine could be used to downregulate cellular metabolism to achieve prolonged survival under hypoxic conditions. Murine myoblasts (C2C12) lacking a self-survival mechanism were treated with adenosine under 0.1% hypoxic stress. The cells, cultured in the presence of 5 mM adenosine, maintained their viability under hypoxia, and regained their normal growth and function of forming myotubes when transferred to normoxic conditions at day 11 without further supply of adenosine, whereas nontreated cells failed to survive. An increase in adenosine concentrations shortened the onset of reproliferation after transfer to normoxic conditions. This increase correlated with an increase in metabolic downregulation during the early phase of hypoxia. A higher intracellular ATP level was observed in adenosine-treated cells throughout the duration of hypoxia. This strategy of increasing cell survival under hypoxic conditions through downregulating cellular metabolism may be utilized for cell-based tissue regeneration applications as well as protecting tissues against hypoxic injuries.
Introduction
One of the primary challenges encountered in building volumetric tissues for cell-based human applications is inadequate supply of oxygen.1 This is mainly due to the delay of vasculogenesis and integration of vessels into the tissue constructs after implantation. Insufficient oxygenation limits normal cellular metabolism, resulting in ischemia within the tissue implants leading to cellular dysfunction and premature cell death. Consequently, the implanted cells will not survive and tissue regeneration will not occur.
It is well known that cells can only survive within 200 μm from the outer boundaries of an implant due to diffusion limitations.2–4 As a consequence, tissue implants greater than 1 cm3 are likely to become ischemic and eventually necrotic.5–7 Such necrosis is likely to occur in the central region of the tissue implant because oxygen tension becomes too low to support viable cells. The diffusion distance is estimated to have an inverse square relationship with the maximum concentration of cells. This is why large tissue constructs implanted in vivo often fail, while successful in smaller implants.8
Given the challenges associated with inadequate supply of oxygen for many cell-based tissue constructs, a number of strategies have been explored. These include the use of synthetic oxygen carriers such as perfluorocarbons9,10 and oxygen-generating biomaterials,3,11,12 and the incorporation of angiogenic factors such as vascular endothelial growth factor and endothelial cells to enhance neovascularization into the matrix.13,14 Another approach is the design of a microcirculation network within matrices that allows enhanced oxygen diffusion.15 Facilitating oxygenation to the implants at the time of implantation is the common focus of these current strategies, however, none has been successful to date in achieving survival of a clinically applicable volumeteric tissue mass.3,11,16–18
In this study, we tested the hypothesis that it is possible to maintain cell viability without facilitating oxygenation. Our strategy is to downregulate cellular metabolism to a new hypometabolic steady state, resulting in lowering oxygen consumption. Adenosine, a purine nucleoside that functions as an energy transferring molecule, is known to be a key regulator in controlling the metabolic activity.19 It has been reported to increase in hypoxia-tolerant cells under hypoxic stress and reduce the adenosine triphosphate (ATP) demands of the Na+/K+ ATPase, the dominant ATP consuming cellular process, especially under severe oxygen limitations.20 By exploiting this protective property of adenosine under hypoxic conditions, we showed in vitro that, exogenously supplied adenosine promotes survival and maintains function under hypoxic conditions of the murine myoblasts (C2C12), which lack the self-survival mechanism observed in hypoxia-tolerant cells.
Materials and Methods
Cell culture
C2C12 myoblasts were selected for their relatively high proliferation rate, ∼12–16 h of doubling time,21 which we predicted would enable us to detect more sensitive cellular responses to adenosine. C2C12 cells (ATCC) were cultured in the Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal bovine serum, 500 U/mL penicillin, and 500 μg/mL streptomycin.
Hypoxic treatment
At 60–80% confluency under normal conditions, a 100 μL sample of a cell suspension containing 2500 cells was plated into each well of a 96-well plate. Cells were incubated for 24 h in normoxic conditions (21% O2, 37°C) before placement in a hypoxic chamber to allow time for attachment to the culture plates. The hypoxic condition was maintained with a gas mixture containing 0.1% O2, 5% CO2, and 94.9% N2 at 37°C and full humidity in an X-Vivo System (Biospherix). The 0.1% hypoxic level, defined as extreme pathological hypoxia,22 was selected to avoid partial saturation of oxygen (between 1% and 10%) found in the mature skeletal muscle.23 At day 0, each group was supplemented with a fresh medium, where adenosine groups received various concentrations of adenosine (0.05, 1, 2, and 5 mM, Sigma-Aldrich) dissolved in the medium. The hypoxic groups were then placed in the hypoxic chamber and incubated up to day 11, whereas the normoxic group continued to be incubated in the regular incubator. No medium change was made for both normoxic and hypoxic groups up to day 11. After transfer to normoxic conditions, cells were cultured in the regular incubator with an exchange of no adenosine-containing, fresh medium every third day.
Cell counts
Cell proliferation of each group was determined by a Quanti-iT PicoGreen double-stranded DNA (dsDNA) Kit (Invitrogen). After washed with phosphate-buffered saline (PBS), cells were lysed with 55 μL of RIPA buffer (Sigma) on ice for 5 min, and then the supernatants were mixed with an equal volume of PicoGreen dsDNA quantitation reagent, a fluorescent DNA dye (excitation at 480 nm; emission at 520 nm). The DNA content was quantified using a SpectraMax M5 microplate reader (Molecular Devices). The number of cells was calculated using a standard curve plotted from fluorescent readings of serial dilutions of a known concentration of cells.
Viable cell imaging
Cell viability was visualized using a calcein AM (Invitrogen) where viable cells fluoresce green through the reaction of calcein AM. Cells were rinsed with PBS and incubated for 30 min in a PBS composed of 2 μM calcein AM. Then, the cells were observed under an inverted fluorescent microscope (Leica).
Histological analysis for myotube formation
After C2C12 cells that survived the hypoxic stress in the presence of adenosine were transferred to normoxic conditions without further supply of adenosine, they were cultured continuously for 3 days in the growth medium followed by a 2% horse serum-containing medium for another 6 days. They were then fixed with methanol and immunostained with a monoclonal antibody directed against the muscle sarcomeric myosin (MF-20, 1:25; abcam) followed by an exposure to a secondary antibody (anti-mouse alexa 594, 1:500; abcam). Nuclei were counterstained with DAPI (Vector). Photomicrographs were obtained using a Leica inverted fluorescence microscope. Myotube formation was also observed by Giemsa staining (1:20; Sigma-Aldrich) using a Zeiss upright light microscope.
MTS metabolic assay
The mitochondrial metabolic activity of viable cells was assessed using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Promega). Cells were rinsed with PBS three times followed by the addition of 120 μL of MTS reagent to each well. After an hour of incubation, the optical density (OD) of a brown formazan product by dehydrogenase enzymes in metabolically active cells was measured with a microplate reader at 490 nm. The mean OD value obtained from media blanks was standardized as 0% metabolic inhibition. For some analyses, the metabolic activity measured at each time point was normalized by the cell number obtained from PicoGreen assay as previously described. The total metabolic activity represented by the area under the curve over time was computed using Origin Pro v. 8 (Origin Lab Corporation).
Measurement of intracellular ATP level
Intracellular ATP was measured by using the ATP Bioluminescence Assay Kit HS II (Roche Applied Science). Cells were lysed with 50 μL of lysis buffer and transferred into a well, and then 50 μL of luciferase reagent was added to it. After mixing, the light emitted was measured and integrated for 10 s by using a SpectraMax M5 luminometer (Molecular Devices). The blank value (from a well containing no ATP) was subtracted from each sample's raw data. ATP concentrations were calculated from the linear part of the standard curve prepared with serial dilutions of a known concentration of ATP and expressed as moles per cell. This, in turn, was recalculated to a percent ATP of that expressed in the cells grown under normoxic conditions.
Survival of muscle tissue under hypoxia
All animal work was approved by the Animal Care and Use Committee of Wake Forest University (protocol number: A10–233). Soleus muscle tissues dissected immediately after sacrifice of Sprague–Dawley rats (17 weeks old, 380–400 g; Harlan) were assigned to one of the three groups in the growth medium: (1) native tissue group, (2) no adenosine-treated hypoxic group, and (3) hypoxic group with 5 mM adenosine supplemented in 2 mL of medium. The media were changed every third day. At day 10, a half of the tissue sample was fixed in 10% neutral buffered formalin (Sigma-Aldrich), 6 μm sections were generated by a cryotome (Leica), and stained with hematoxylin and eosin (H&E). Microscopic analysis was performed using a light microscope (Zeiss). Dead assay was assessed on the remaining tissues using 4 μM ethidium homodimer-1 (EthD-1; Invitrogen) to stain dead cells with damaged cell membranes. The stained sections were observed using a fluorescent microscope (Leica). The number of dead cells was quantitated using the analyze particle method with ImageJ software (U.S. National Institute of Health) on the fluorescence images, and its percentage was calculated based on the number of DAPI-stained cells on each section.
Statistical analysis
Statistical analysis was performed using a single-tailed Student's t-test and one-way analysis of variance with Tukey's post hoc tests (Origin Pro v.8; Origin Lab Corporation). A p-value <0.05 was considered significant. All values are reported as the mean and standard deviation of the mean.
Results
Adenosine enhances cell survival under hypoxia
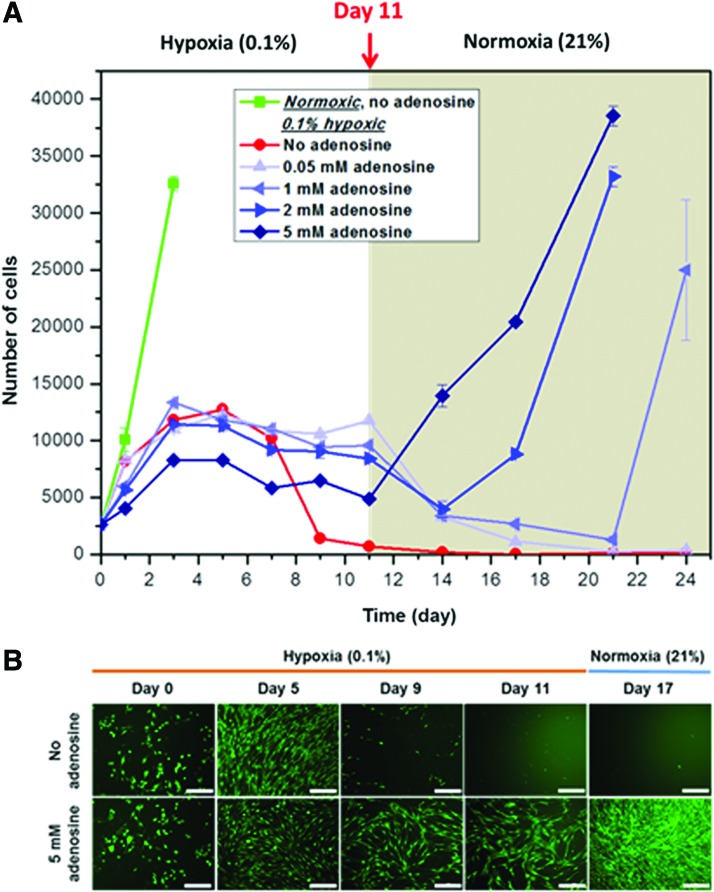
The effect of adenosine on C2C12 cell survival under hypoxia was investigated by culturing cells under 0.1% hypoxic conditions for 11 days followed by normoxic conditions without further supply of adenosine. Adenosine was found to be stable up to 7 days, the longest duration tested (data not shown). The normoxic cells became fully confluent at day 3 (Fig. 1A), and then their number declined. The growth of all hypoxic groups was substantially limited under 0.1% oxygen conditions. Hypoxic cells not treated with adenosine showed an increase in number up to day 5, but then continued to decline. This resulted in cell death at day 11 with only 5.5% of the initial number of cells remaining viable. Growth of these cells was never recovered even after transfer to normoxic conditions as most of them died. This was also observed in 0.05 mM adenosine-treated cells, however, cells exposed to 1, 2, and 5 mM adenosine survived 11 days of hypoxic stress, and still maintained approximately two to four times the initial number of cells. These observations were also supported by the fluorescent images of live C2C12 cells stained with calcein AM (Fig. 1B). These surviving cells resumed their proliferation at a growth rate comparable to that of normoxic cells until they became fully confluent after transfer to normal oxygen tension (Fig. 1A). The time for onset of reproliferation was found to be concentration dependent: the higher the adenosine concentration the shorter the time to initiation of cell growth. An effect of concentration on cell numbers was also revealed under hypoxia, however, no substantial differences in number of cells were observed among the groups except for the 5 mM adenosine-treated group. Since the most effective adenosine concentration was 5 mM, this concentration was used for further experiments.
FIG. 1.
(A) Adenosine enhances C2C12 cell survival under hypoxia. C2C12 cells (2500 cells per well) were cultured for 11 days under 0.1% hypoxic conditions followed by normoxic conditions without further supply of adenosine. The number of cells was assessed using the dsDNA content. All the experimental groups with various concentrations of adenosine (1, 2, and 5 mM) survived hypoxic stress (n=4). After a transfer to normoxic conditions, these cells reproliferated with a growth rate comparable to that of normoxic cells, whereas the control cells did not reproliferate. The onset of reproliferation was concentration dependent. An earlier onset was observed with an increase of concentration. (B) Representative fluorescent images of live C2C12 cells stained with calcein AM (green), without (Upper) and with 5 mM adenosine (Lower) (scale bars=200 μm). dsDNA, double-stranded DNA. Color images available online at www.liebertpub.com/tea
C2C12 cells surviving in the presence of adenosine retain their differentiating property
It is critical that cells retain their normal function after adenosine removal. As shown in Figure 1, exposure to adenosine did not affect the proliferative capability of C2C12 cells. Another important function of cells especially for cell-based tissue applications is their differentiating capability. C2C12 cells possess the unique property of differentiating into myotubes in the 2% horse serum-containing medium. Using this property, the differentiating capability was qualitatively evaluated on C2C12 cells that underwent 11 days of exposure to adenosine under 0.1% hypoxic conditions. C2C12 cells cultured under normoxic conditions were used as a control. As shown in Figure 2, adenosine-treated C2C12 cells showed capability of fusing into myotubes that align along their elongated cytoplasmic extensions. Moreover, they displayed a typical culture of multicellular myotubes compared with those obtained from the control cells in terms of the number, length, and thickness. In the cells not treated with adenosine, only cellular debris was stained with Giemsa without myotubes formed.
FIG. 2.
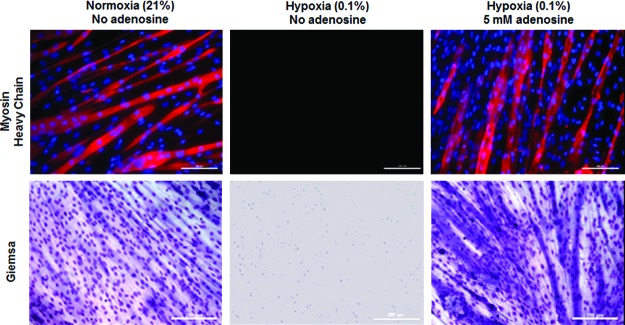
Representative images of myotubes formed only in hypoxia-survived C2C12 cells in the presence of adenosine. C2C12 cells underwent identical testing conditions, as described in Figure 1, except that these cells were cultured in the complete growth medium for 3 days after transfer to normoxic conditions followed by a 2% horse serum-containing differentiating medium for 6 days. C2C12 cells were immunostained (Upper) with MF-20 (myosin heavy chain, red) and DAPI (nuclei, blue), and stained with Giemsa (Lower) (scale bars=200 μm). Color images available online at www.liebertpub.com/tea
Mechanism behind cell survival: adenosine maintains hypometabolic steady state of hypoxic C2C12 cells
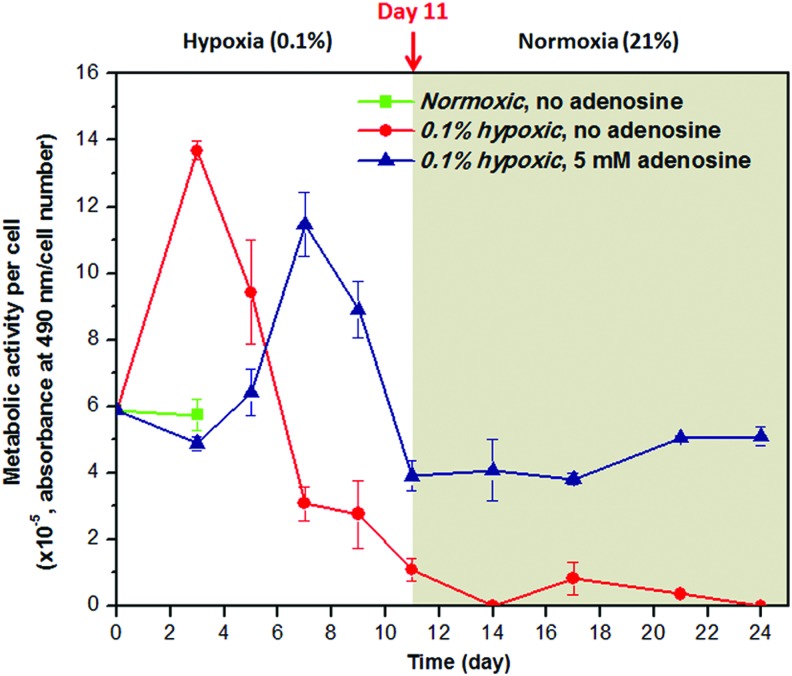
We investigated whether downregulation of metabolic activity is observed in the surviving C2C12 cells under hypoxic conditions by the presence of adenosine. The metabolic activity was presented as MTS absorbance normalized by the number of cells at each corresponding time point. As shown in Figure 3, the metabolic activity of the hypoxic cells without adenosine increased initially, but then decreased and never recovered even after transfer to normoxic conditions due to the fact that the untreated cells died. In the cells treated with 5 mM adenosine, however, the metabolic activity was suppressed initially up to day 5, and showed a transient increase followed by decrease, still maintaining ∼68% of the initial metabolic activity at day 11. After transfer to normoxic conditions, it was restored to a level similar to that in normoxic cells.
FIG. 3.
Effect of adenosine on metabolic activity of a single C2C12 cell assessed through the MTS metabolic activity and dsDNA content (n=4). When treated with 5 mM adenosine under 0.1% hypoxic conditions, the metabolic activity was shifted to the right of the control group, resulting in its downregulated states approximately for the first 5 days. When these cells were transferred to normoxic conditions, the metabolic activity was restored equivalent to a normal level measured in normoxic cells. A normal level is shown only up to day 3 because the C2C12 cells became fully confluent at this time. MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium. Color images available online at www.liebertpub.com/tea
A higher intracellular ATP level is observed in the adenosine-treated cells
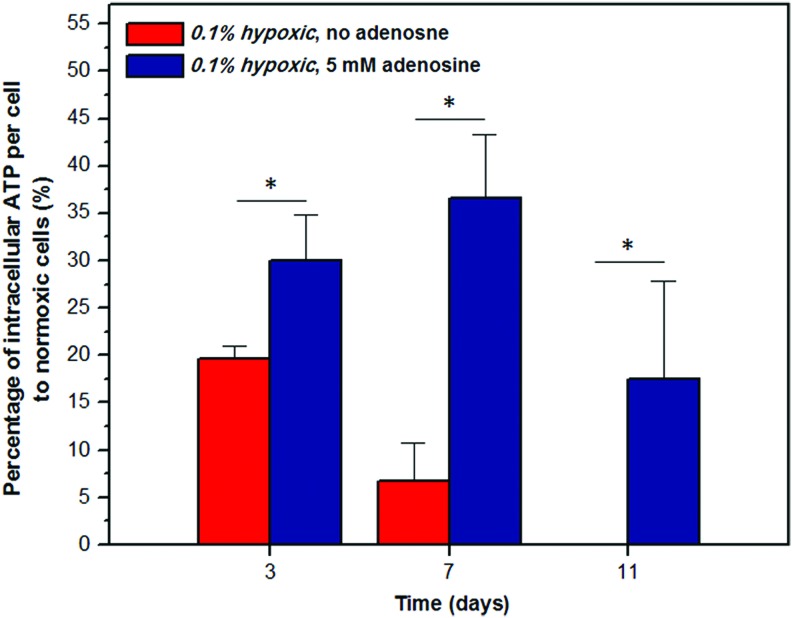
The cytosolic ATP level was assessed in C2C12 cells during the hypoxic phase to investigate the effect of adenosine on mitochondrial metabolism. The ATP level was normalized by the number of cells, and this, in turn, was expressed as a percentage of the ATP level measured in normoxic cells (Fig. 4). In the no adenosine-treated control cells, an 81% reduction in the ATP level was observed by day 3. This decrease continued to 93% by day 7, and ATP was no longer detected by day 11. In contrast, a consistently higher ATP level (p<0.05) was observed throughout the hypoxic duration in the cells treated with adenosine than the control cells, with 18% ATP still detected at day 11.
FIG. 4.
Effect of adenosine on intracellular ATP level of a single C2C12 cell during the 0.1% hypoxic phase. Intracellular ATP of hypoxic cells was expressed as a percentage of that measured in normoxic cells at day 0. With only 19% remained at day 3, ATP of the control group continued to decline until no ATP was detected at day 11. Throughout the hypoxic duration, a higher ATP level was observed in the cells treated with 5 mM adenosine, still maintaining 18% of ATP by day 11 (n=4, Student t-test, *p<0.05). ATP, adenosine triphosphate. Color images available online at www.liebertpub.com/tea
Adenosine provides tissue protection under hypoxia
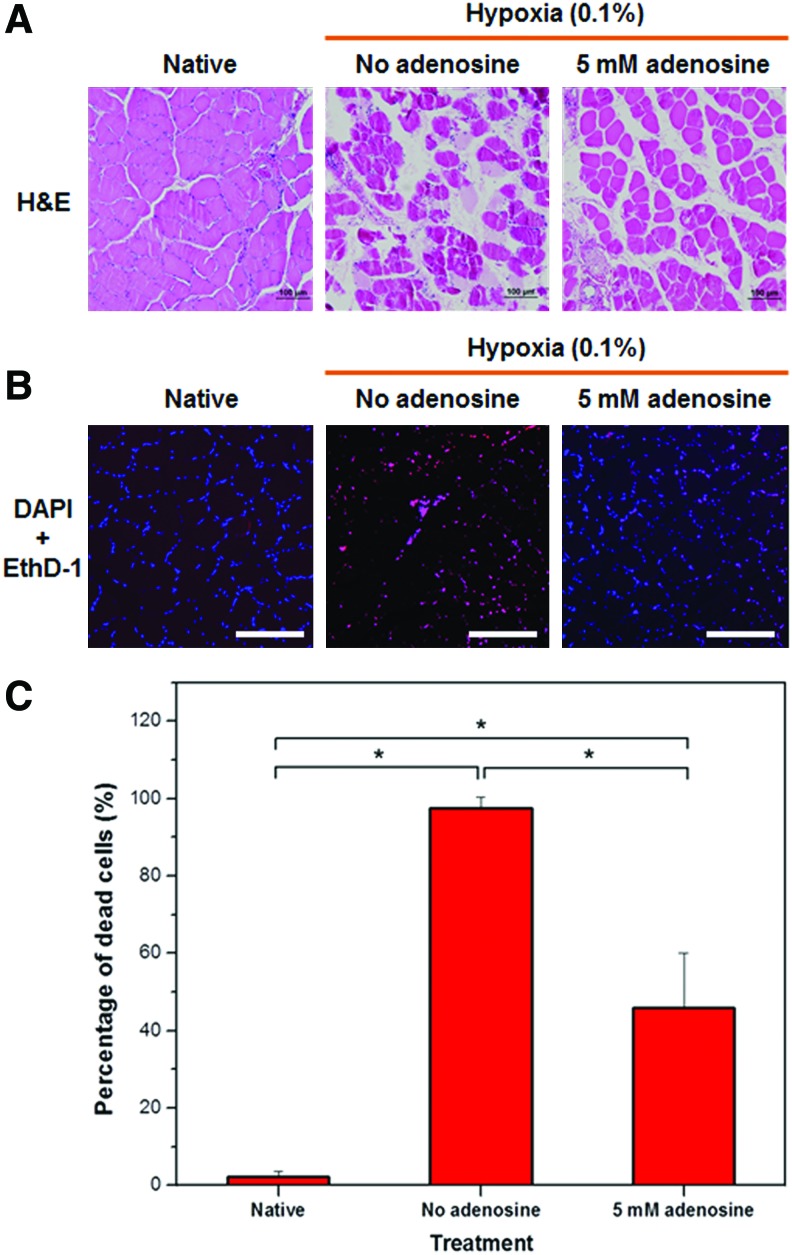
The concept of prolonging cell survival proposed in this study could also be beneficial for protecting against hypoxic injury. We evaluated such effects of adenosine on soleus muscles, which are primarily aerobic, by culturing these muscles under hypoxic conditions for 10 days. The degree of muscle damage with H&E was assessed as shown in Figure 5A. Native muscle tissues had no histologic evidence of injury. The tissues cultured without adenosine showed more damage than those treated with 5 mM adenosine, indicated by degenerated myofibers and loss of connective tissues. We then evaluated whether the degree of damage correlates with the number of dead cells. Fluorescent images of cross-sectioned muscle tissues immunostained with EthD-1 and DAPI showed that most of the cells died in the no adenosine-treated tissues, whereas in the presence of adenosine, a pronounced reduction in cell death was observed (Fig. 5B). This observation was more evident when the number of dead cells was quantified and expressed as a percentage of number of DAPI-stained cells (Fig. 5C). The no adenosine-treated group revealed 97% cell death, while only 46% of dead cells was shown in the tissues supplied with adenosine (p=0.0007). This result suggests that adenosine can also be effective in protecting hypoxic muscles by reducing cell death.
FIG. 5.
Adenosine protects hypoxic tissues. (A) Representative H&E images of soleus muscle tissues after 10 days of culture in hypoxic conditions (scale bar=100 μm). The tissues cultured without adenosine show more damage compared with tissues treated with 5 mM adenosine, indicated by degenerated myofibers and loss of connective tissues. (B) Representative fluorescent images of tissues stained with EthD-1 (dead cells, red) and DAPI (nuclei, blue) (scale bar=200 μm). (C) Quantitative analysis on a number of dead cells expressed as a percentage of total number of cells stained with DAPI based on images in panel B. The 5 mM adenosine- treated tissues showed a significantly fewer dead cells compared with the controls (n=3, analysis of variance with Tukey's post hoc test, *p<0.005). EthD-1, ethidium homodimer-1; H&E, hematoxylin and eosin. Color images available online at www.liebertpub.com/tea
Discussion
Building a clinically relevant sized tissue or organ using cell-based tissue constructs requires maintenance of cell viability and function during the early engraftment period. In this study, we demonstrated that metabolic rate depression by exogenously applied adenosine can be utilized to maintain cell viability and function under hypoxic conditions.
A continued supply of oxygen is essential to maintain adequate ATP production for ensuring normal function and survival of mammalian cells. In case of oxygen limitation, the cellular ATP demands tend to remain constant without concomitant reduction in its utilization. This will lead to events associated with uncontrolled cellular swelling, and ultimately, cell necrosis.24 Interestingly, certain marine species are capable of surviving prolonged periods of oxygen deprivation.25,26 The survival mechanism of such hypoxic adaptation was mainly based on lowering the ATP demand to a level that can be supplied by anaerobic ATP production alone, a term known as metabolic downregulation.
A mechanism of metabolic downregulation has been utilized in a number of strategies to protect cells.27–31 For example, induced hypothermia has been routinely used in surgery since the early 1950s.30 It is a well-established means of cytoprotection in which, lowering temperature slows metabolism and renders cells in a suspended state to maintain cell survival. However, only a few clinical studies have convincingly demonstrated a role in humans. As an alternative, we sought a pharmacologic approach to induce cytoprotection.
Adenosine is an excellent pharmacological candidate that could modulate ATP utilization in hypoxia-sensitive cells that lack the self-survival mechanism. It has been shown to increase in hypoxia-tolerant cells under hypoxic stress and downregulates the Na+/K+ ATPase activity in an autocrine manner.19,32 Na+/K+ ATPase is one of the prominent ATP consumers accounting for up to 75% of the ATP demands under hypoxia.33 When the cells initially encounter hypoxic episodes, a dephosphorylation of ATP to adenosine occurs, resulting in accumulation within cells, as ATP produced by glycolysis alone becomes insufficient to meet the cellular needs. Adenosine then exits the cell and binds to its receptors, resulting in induction of yet unknown G-coupled second messenger pathways followed by eventual reduction in the Na+/K+ ATPase activity.20 For a potential clinical utilization of adenosine in humans, however, the presence of adenosine receptors is a prerequisite. All four adenosine receptors have been cloned in humans and are expressed in most organs.34
In the present study, we first tested whether the use of adenosine prolonged cell survival under hypoxic conditions. In this setting, the C2C12 cell line was selected for its relatively high proliferation rate, ∼12–16 h of doubling time,21 with which more sensitive cellular responses to adenosine were predicted. The 0.1% hypoxic level, defined as extreme pathological hypoxia,22 was also selected to avoid the partial saturation of oxygen found in mature skeletal muscle between 1% and 10%,23 which may be a normal physiologic range of oxygen for C2C12 cells.
We demonstrated that cells survived throughout the duration of hypoxia in the presence of adenosine (Fig. 1). In addition, these hypoxic cells regained their normal growth after transfer to normoxic conditions without further supplements of adenosine, suggesting that exposure to adenosine did not affect their growing capability. Such transfer was made to simulate a clinical condition when an enriched vascular network has started oxygen transport to the transplanted cells. In the absence of adenosine, most of the hypoxic cells became necrotic by day 11, which was the decisive factor for transferring cells to normoxic conditions at this time point. A higher number of cells up to day 7 was maintained compared to that found in the 5 mM adenosine-treated group. This may be due to the downregulated metabolic activity at this concentration of adenosine interfering with the cell growth. It is interesting to note that the effect of adenosine concentration on cell survival under hypoxic and normoxic phases were found to be the opposite. The number of cells on the last day of hypoxia was lowest with the highest concentration of adenosine, whereas the reproliferating capability after transfer to normoxic conditions was the most rapid. A precise mechanism for this observation remains to be elucidated.
Maintaining the cellular function as well as the viability is important for tissue regeneration applications due to the therapeutic potential of cells in creating a functional tissue. Surviving cells not only regained their normal growth after transfer to normoxic conditions but they also retained their inherent ability to form myotubes (Fig. 2). Myotube formation is a complex process that requires a precisely coordinated cascade event, which includes alignment of myoblasts, cell to cell recognition, and contact guidance followed by membrane fusion. If cells are not healthy enough and any of those processes fails, myotubes will not form. Yun et al.35 showed that myogenic differentiation capacity is limited under hypoxia. In their study, differentiation of C2C12 cells was recovered only to a limited extent when the cells were transferred to normoxic conditions after 3 days of culture under extreme hypoxic conditions. Considering our study with 11 days of culture under similar extreme conditions, this suggests that exposure to adenosine might even protect the differentiating capability of cells.
Studies seeking to confirm the underlying mechanism of cell survival in the presence of adenosine under hypoxic conditions revealed its correlation with metabolic downregulation in terms of MTS metabolic activity (Fig. 3) and intracellular ATP content (Fig. 4). A lower MTS metabolic activity of the cells treated with 5 mM adenosine was observed during the early hypoxic phase compared to that in controls, suggesting a sustained metabolic downregulation as a mechanism for prolonged cell survival. The restoration of normal metabolic function is noteworthy as it implies the maintenance of the therapeutic potential of cells that survived the hypoxic stress. The ATP level in the adenosine-treated cells remained significantly higher throughout the hypoxic period (p<0.05), whereas no ATP was detected in the control cells at day 11. This observation is in agreement with other studies,36,37 and this supports our hypothesis that the prolonged cell survival achieved was attributed to the reduced ATP demand caused by adenosine. However, adenosine has been reported to affect cell cycle arrest at G138 as well as total protein synthesis39 and apoptosis40 by binding to various combinations of adenosine receptors. Since all these alternatives could be a potential mechanism for cell survival demonstrated in this study, they need to be further investigated.
For the concept demonstrated in this study (i.e., improving cell viability under hypoxia via metabolic downregulation) to be successfully utilized in tissue-engineered constructs, adenosine should possess certain kinetic properties when incorporated into a construct. We have demonstrated a dose-dependent release of adenosine released from a porous PLGA scaffold fabricated by a salt-leaching method and agarose sheet (unpublished data). In addition, cells cultured together with these adenosine-containing scaffolds revealed metabolic suppression, while normal growth was observed in the scaffold-only groups, demonstrating the retention of the biological activity of adenosine released from these scaffolds. We are currently investigating the survival of cells when they are incorporated directly to the adenosine-containing scaffolds.
Another important aspect for this concept to be successful is the later removal of adenosine once the construct is vascularized because a continuous metabolic suppression could interfere with the therapeutic function of transplanted cells. One of the interesting properties of adenosine is its enzymatic degradation by adenosine deaminase,34 the key enzyme of purine metabolism. Adenosine deaminase catalyzes the irreversible hydrolytic deamination of adenosine to inosine, which can be further deribosylated to hypoxanthine,41 the effect of which on metabolic downregulation has not been reported to the best of our knowledge. It is noteworthy that adenosine deaminase is present in human blood plasma.42 As such, we predict that an enzymatic activity of endogenous adenosine deaminase released from newly formed vessels that are integrated near or within an implanted construct would degrade adenosine remaining in the construct in a spontaneous way. Such strategy could also be combined with the current drug delivery technology so as to fine tune a near full release of adenosine by the time vessels have integrated.
There are potential benefits of the concept of metabolic downregulation for other clinical problems, especially hypoxic injuries. We demonstrated in vitro that hypoxic muscle tissues were protected in the presence of adenosine, and showed a significantly reduced number of dead cells compared with controls (p=0.0007, Fig. 5C). Dissected tissues are considered to be hypoxic inherently because they instantly loose blood supply, however, muscles designated as hypoxic groups were still cultured in the hypoxic medium to prevent or minimize the exposure of tissues in the medium to oxygen in the air, even though its effect might not be significant.
Most mammalian cells, including humans, possess little inherent tolerance to severe hypoxia.19 Using the survival mechanisms of hypoxia-tolerant cells found in nature, we have shown that exogenously applied adenosine can function as a suppressant of metabolism, and that this suppression has a favorable effect on the survival and function of hypoxia-sensitive cells under hypoxic stress. This novel strategy may be applied for cell-based tissue regeneration applications as well as protecting against hypoxic tissue injuries.
Acknowledgments
The authors would like to thank Dr. Delrae Eckman for helpful comments and suggestions. This study was supported by the Telemedicine and Advanced Technology Research Center (TATRC) at the U.S. Army Medical Research and Material Command (USAMRMC) through award W81XWH-07-1-0718 and the Mary K. Chapman Foundation.
Disclosure Statement
No competing financial interests exist.
References
- 1.Khademhosseini A., Langer R., Borenstein J., and Vacanti J.P.Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A 103,2480, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malda J., Woodfield T.B., van der Vloodt F., Kooy F.K., Martens D.E., Tramper J., van Blitterswijk C.A., and Riesle J.The effect of PEGT/PBT scaffold architecture on oxygen gradients in tissue engineered cartilaginous constructs. Biomaterials 25,5773, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Oh S.H., Ward C.L., Atala A., Yoo J.J., and Harrison B.S.Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials 30,757, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Radisic M., Malda J., Epping E., Geng W., Langer R., and Vunjak-Novakovic G.Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol Bioeng 93,332, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Davis B.H., Schroeder T., Yarmolenko P.S., Guilak F., Dewhirst M.W., and Taylor D.A.An In vitro system to evaluate the effects of ischemia on survival of cells used for cell therapy. Ann Biomed Eng 35,1414, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Griffith C.K., Miller C., Sainson R.C., Calvert J.W., Jeon N.L., Hughes C.C., and George S.C.Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng 11,257, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Ishaug-Riley S.L., Crane-Kruger G.M., Yaszemski M.J., and Mikos A.G.Three-dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials 19,1405, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Muschler G.F., Nakamoto C., and Griffith L.G.Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am 86-A,1541, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Iyer R.K., Radisic M., Cannizzaro C., and Vunjak-Novakovic G.Synthetic oxygen carriers in cardiac tissue engineering. Artif Cells Blood Substit Immobil Biotechnol 35,135, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Tan Q., El-Badry A.M., Contaldo C., Steiner R., Hillinger S., Welti M., Hilbe M., Spahn D.R., Jaussi R., Higuera G., van Blitterswijk C.A., Luo Q., and Weder W.The effect of perfluorocarbon-based artificial oxygen carriers on tissue-engineered trachea. Tissue Eng Part A 15,2471, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Harrison B.S., Eberli D., Lee S.J., Atala A., and Yoo J.J.Oxygen producing biomaterials for tissue regeneration. Biomaterials 28,4628, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Pedraza E., Coronel M.M., Fraker C.A., Ricordi C., and Stabler C.L.Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc Natl Acad Sci U S A 109,4245, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grunewald M., Avraham I., Dor Y., Bachar-Lustig E., Itin A., Jung S., Chimenti S., Landsman L., Abramovitch R., and Keshet E.VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124,175, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Kalka C., Masuda H., Takahashi T., Kalka-Moll W.M., Silver M., Kearney M., Li T., Isner J.M., and Asahara T.Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A 97,3422, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J., Shi G., Bei J., Wang S., Cao Y., Shang Q., Yang G., and Wang W.Fabrication and surface modification of macroporous poly(L-lactic acid) and poly(L-lactic-co-glycolic acid) (70/30) cell scaffolds for human skin fibroblast cell culture. J Biomed Mater Res 62,438, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Ness P.M., and Cushing M.M.Oxygen therapeutics: pursuit of an alternative to the donor red blood cell. Arch Pathol Lab Med 131,734, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kim H.W., and Greenburg A.G.Artificial oxygen carriers as red blood cell substitutes: a selected review and current status. Artif Organs 28,813, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Stowell C.P., Levin J., Spiess B.D., and Winslow R.M.Progress in the development of RBC substitutes. Transfusion 41,287, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Boutilier R.G.Mechanisms of cell survival in hypoxia and hypothermia. J Exp Biol 204,3171, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Buck L.T.Adenosine as a signal for ion channel arrest in anoxia-tolerant organisms. Comp Biochem Physiol B Biochem Mol Biol 139,401, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Toy M.F., Richard S., Kuhn J., Franco-Obregon A., Egli M., and Depeursinge C.Enhanced robustness digital holographic microscopy for demanding environment of space biology. Biomed Opt Express 3,313, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kook S.H., Son Y.O., Lee K.Y., Lee H.J., Chung W.T., Choi K.C., and Lee J.C.Hypoxia affects positively the proliferation of bovine satellite cells and their myogenic differentiation through up-regulation of MyoD. Cell Biol Int 32,871, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Li X., Zhu L.L., Chen X.P., and Fan M.Effects of hypoxia on proliferation and differentiation of myoblasts. Med Hypotheses 69,629, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Hochachka P.W.Defense strategies against hypoxia and hypothermia. Science 231,234, 1986 [DOI] [PubMed] [Google Scholar]
- 25.Krumschnabel G., Schwarzbaum P.J., and Wieser W.Coupling of energy supply and energy demand in isolated goldfish hepatocytes. Physiol Zool 67,438, 1994 [Google Scholar]
- 26.Lutz P.L., and Kabler S.Release of adenosine and ATP in the brain of the freshwater turtle (Trachemys scripta) during long-term anoxia. Brain Res 769,281, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Kiss A.J., Muir T.J., Lee R.E., Jr., and Costanzo J.P.Seasonal variation in the hepatoproteome of the dehydration and freeze-tolerant wood frog, Rana sylvatica. Int J Mol Sci 12,8406, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maia J., Dekkers B.J., Provart N.J., Ligterink W., and Hilhorst H.W.The re-establishment of desiccation tolerance in germinated Arabidopsis thaliana seeds and its associated transcriptome. PLoS One 6,e29123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Southard J.H., van Gulik T.M., Ametani M.S., Vreugdenhil P.K., Lindell S.L., Pienaar B.L., and Belzer F.O.Important components of the UW solution. Transplantation 49,251, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Tang X.N., and Yenari M.A.Hypothermia as a cytoprotective strategy in ischemic tissue injury. Ageing Res Rev 9,61, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yenari M.A., and Hemmen T.M.Therapeutic hypothermia for brain ischemia: where have we come and where do we go? Stroke 41,S72, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newby A.C.Adenosine and the concept of retaliatory metabolites. Trends Biochem Sci 9,42, 1984 [Google Scholar]
- 33.Buck L.T., and Hochachka P.W.Anoxic suppression of Na(+)-K(+)-ATPase and constant membrane potential in hepatocytes: support for channel arrest. Am J Physiol 265,R1020, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Manjunath S., and Sakhare P.M.Adenosine and adenosine receptors: Newer therapeutic perspective. Indian J Pharmacol 41,97, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yun Z., Lin Q., and Giaccia A.J.Adaptive myogenesis under hypoxia. Mol Cell Biol 25,3040, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jurkowitz M.S., Litsky M.L., Browning M.J., and Hohl C.M.Adenosine, inosine, and guanosine protect glial cells during glucose deprivation and mitochondrial inhibition: correlation between protection and ATP preservation. J Neurochem 71,535, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Krumschnabel G., Biasi C., and Wieser W.Action of adenosine on energetics, protein synthesis and K(+) homeostasis in teleost hepatocytes. J Exp Biol 203,2657, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Shirali S., Aghaei M., Shabani M., Fathi M., Sohrabi M., and Moeinifard M.Adenosine induces cell cycle arrest and apoptosis via cyclinD1/Cdk4 and Bcl-2/Bax pathways in human ovarian cancer cell line OVCAR-3. Tumour Biol 34,1085, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Dubey R.K., Gillespie D.G., and Jackson E.K.Adenosine inhibits collagen and total protein synthesis in vascular smooth muscle cells. Hypertension 33,190, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Aghaei M., Karami-Tehrani F., Panjehpour M., Salami S., and Fallahian F.Adenosine induces cell-cycle arrest and apoptosis in androgen-dependent and -independent prostate cancer cell lines, LNcap-FGC-10, DU-145, and PC3. Prostate 72,361, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Wen J., and Xia Y.Adenosine signaling: good or bad in erectile function? Arterioscler Thromb Vasc Biol 32,845, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Aghaei M., Karami-Tehrani F., Salami S., and Atri M.Adenosine deaminase activity in the serum and malignant tumors of breast cancer: the assessment of isoenzyme ADA1 and ADA2 activities. Clin Biochem 38,887, 2005 [DOI] [PubMed] [Google Scholar]