Abstract
ATP-binding cassette transporter A1 (ABCA1) is a key transporter and receptor in promoting cholesterol efflux, and increasing the expression level of ABCA1 is antiatherogenic. In our previous study, rutaecarpine (RUT) was found to protect ApoE–/– mice from developing atherosclerosis through preferentially up-regulating ABCA1 expression. In the present work, a series of RUT derivatives were synthesized and examined as ABCA1 expression up-regulators. Compounds CD1, CD6, and BCD1–2 were found to possess the most potential activity as antiatherosclerotic agents among all compounds tested.
Keywords: Rutaecarpine, ABCA1, RCT, atherosclerosis
Atherosclerosis is the main cause of cardiovascular disease (CVD). Statins are able to prevent only about one-third of CVD cases through lowering low-density lipoprotein (LDL) expression levels,1,2 and thus, additional therapeutic strategies are still required. The limitless uptakes of modified low density lipoprotein (mLDL) by scavenger receptor SR-A and CD36 in the macrophages are key events in the initiation and development of atherosclerosis.3−5 Removing excess cholesterol from foamed macrophage cells is considered to be one of the antiatherosclerotic therapeutic strategies.6 Reverse cholesterol transport (RCT) is an efficient means of lowering cholesterol in the extra-hepatic tissues. Through RCT, high-density lipoproteins (HDL) carry cholesterol from peripheral tissues to the liver, where it can be secreted directly into bile or converted to bile acids.7−9 ATP-binding cassette A1 (ABCA1), which is the key transporter in RCT,10,11 transports both free cholesterol (FC) and phospholipids (PL) to apolipoprotein A-I (ApoA-I).12,13 Therefore, it plays an important role in atherosclerosis.14 On the basis of this consideration, up-regulation of ABCA1 expression represents an attractive strategy for combating atherosclerosis.15,16
In our previous study, a high through-put screening (HTS) assay was performed to identify ABCA1 up-regulators using ABCA1p-LUC HepG2 cells.17 ABCA1p-LUC HepG2 cell line was a stably transformed clone from HepG2 cells, which were cotransfected with pGL3-ABCA1P and pcDNA3 (Invitrogen) plasmids. The constructed plasmid pGL3-ABCA1P contains human ABCA1 promoter region (−819 bp to +71 bp; nucleotide +1 corresponds to the transcription start site).17 Rutaecarpine (8,13-dihydroindolo[2′,3′:3,4]pyrido[2,1-b]quinazolin-5(7H)-one, RUT, Figure 1) was identified as a possible candidate, with an EC50 value of 0.27 μM and a maximum up-regulating value of 240% in ABCA1p-LUC HepG2 cells.18 We then demonstrated that RUT indeed suppressed atherosclerosis through up-regulating ABCA1 within the RCT process in ApoE–/– mice.18 Recent studies have also shown that RUT exhibits various pharmacological effects,19,20 including cardiovascular protection,21 antithrombotic activity, and anti-inflammatory properties.22,23 All these effects might be beneficial in terms of reducing atherosclerosis.24 Taken together, these data suggested that RUT might be of use in the treatment of atherosclerosis. In the present study, a series of compounds based on the optimization of RUT were designed with the aim of improving the ABCA1 up-regulatory activity and antiatherosclerotic efficacy. The structure–activity relationship (SAR) of these compounds was also explored.
Figure 1.
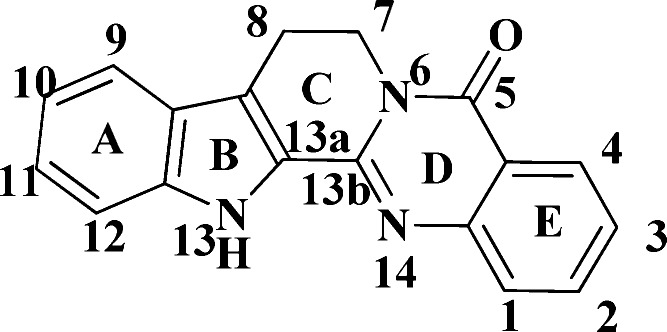
Structure of RUT.
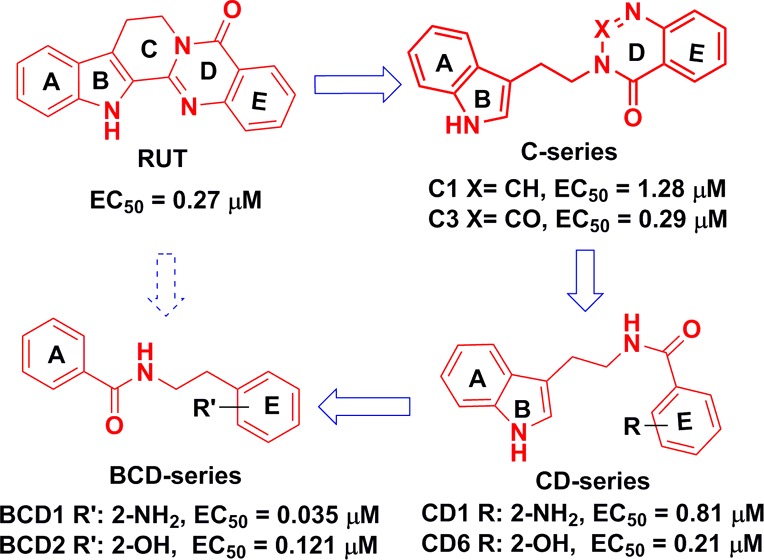
RUT exhibits both high structural rigidity and poor solubility. Therefore, a scaffold-opened structure was designed to improve the ABCA1 up-regulatory activity and enhance solubility and bioavailability at the same time. This process was also an effective way to examine novel structures with improved activity.
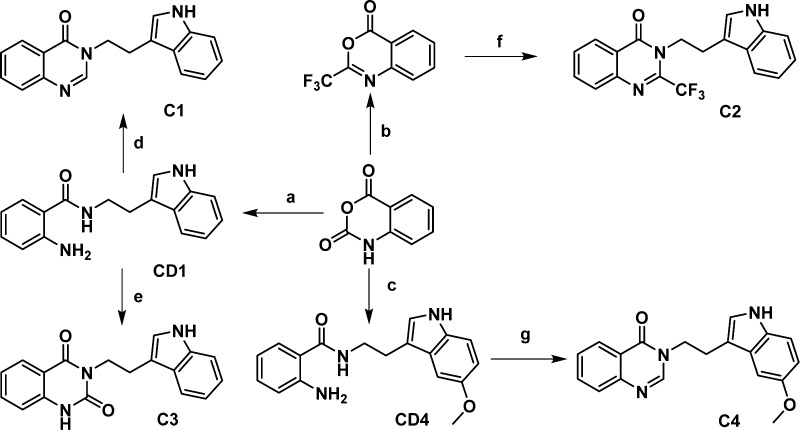
Initially, the structure of RUT was truncated at the C13a–C13b bond to investigate the contribution of the indole and quinalin-4-one moieties to the activity. Compounds C1–4 were synthesized based on the design presented in Scheme 1.25,26 CD1 was prepared by reflux of tryptamine and isatoic anhydride in alcohol. CD1 then refluxed in triethoxy orthoformate or ethyl chloroformate resulted in C1 and C3, respectively. C2 was obtained when isatoic anhydride was treated with trifluoroacetic anhydride in pyridine, and then refluxed with tryptamine. C4 was obtained by 5-methoxytryptamine using the same synthetic method as C1.
Scheme 1. Synthesis of C Series Compounds.
Reagents and conditions: (a) tryptamine, EtOH, reflux; (b) (CF3CO)2O, pyridine, 25 °C; (c) 5-methoxytryptamine, EtOH, reflux; (d, g) triethoxy orthoformate, reflux; (e) ethyl chloroformate, reflux; (f) tryptamine, pyridine, reflux.
It was noted that C1 (EC50 = 1.28 μM and maximum value = 176%) and C3 (EC50 = 0.29 μM and maximum value = 149%) showed moderate ABCA1 up-regulating activities in ABCA1 up-regulating assays, while C2 and C4 exhibited no activities at all. Although both trifluoromethyl and carbonyl are electron-withdrawing groups, their effects were evidently quite different. Thus, it could be concluded that the steric effect was likely more important than the electric effect at the 2 position of quinalin-4-one moiety. Small variation between C1 and C4 led to the activities being dramatically changed, which indicated that introducing electron-donating groups on the indole moiety was not good for improving the activity. These results indicated that the ring-opened structures successfully maintained fairly high activities similar to that of the original RUT molecule.
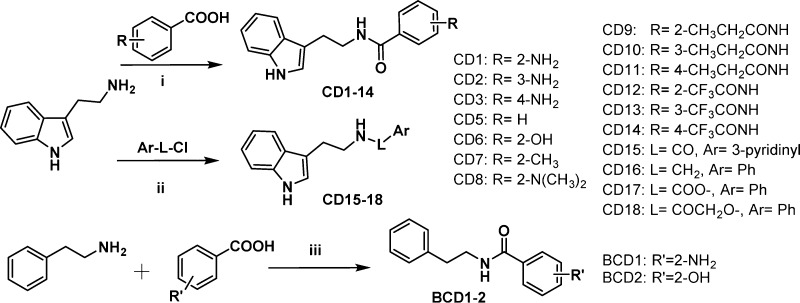
It was interesting to see that CD1, with both C and D rings opened and obtained as the intermediate to form C1, showed better activity than C1. It was concluded that modification of the C and D rings might be beneficial in terms of improving up-regulating ABCA1 activities. For this reason, both the C and D ring-isolated structures were designed for improving the up-regulating ABCA1 activities based on CD1. Thus, a further series of CD1 were synthesized by the condensation of tryptamine and substituted benzoic acid with EDCI hydrochloride salt in dichloromethane (DCM) or N,N-dimethylformamide (DMF) (Scheme 2).
Scheme 2. Synthesis of CD and BCD Series Compounds.
Reagents and conditions: (i, iii) EDCI HCl salt, DCM/DMF; (ii) CD16, CH3CN, Et3N; CD15, CD17–18 DCM, Et3N.
As an initial step, we changed the position of the amino group of CD1 to produce CD2–3. The activities of CD1–3 (Table 1) indicated that the effect of the amino position was greatest in the order of ortho > para > meta. The disappeared activity of CD4 was probably due to the introduction of 5-methoxy on the indole ring. Second, substitution of the amino group of CD1 with hydrogen or with hydroxyl, methyl, and N,N-dimethylamino groups resulted in compounds CD5–8 (Table 1) with the substituents maintained at the ortho position. According to Table 1, CD6 exhibited the best performance, CD5 and CD7 showed moderate activities, and CD8 completely lost its activity. Therefore, it appeared that the optimal activity occurred when the E ring had hydrogen-donating substituents. Third, the amino group on the E ring was acylated by trifluoroacetyl and n-propionyl to give compounds CD9–14 (Table 1). The up-regulating ABCA1 activity showed that the order of activity with regard to the acylation position remained ortho > para > meta.
Table 1. ABCA1 Activity of CD and BCD Series Compounds.
| compd | EC50 (μM)a | max (%)b | compd | EC50 (μM)a | max (%)b |
|---|---|---|---|---|---|
| CD1 | 0.81 ± 0.09 | 203 | CD10 | 1.28 ± 0.03 | 138 |
| CD2 | 10.72 ± 0.08 | 138 | CD11 | 33.24 ± 0.15 | 192 |
| CD3 | 6.55 ± 0.07 | 239 | CD12 | 1.56 ± 0.06 | 192 |
| CD4 | CD13 | 19.55 ± 0.12 | 169 | ||
| CD5 | 0.71 ± 0.10 | 193 | CD14 | 8.86 ± 0.06 | 175 |
| CD6 | 0.21 ± 0.10 | 182 | CD15 | 3.67 ± 0.09 | 180 |
| CD7 | 1.66 ± 0.07 | 166 | CD16 | ||
| CD8 | CD17 | 6.38 ± 0.05 | 160 | ||
| CD9 | 3.28 ± 0.10 | 204 | CD18 | ||
| BCD1 | 0.035 ± 0.08 | 187 | BCD2 | 0.121 ± 0.07 | 183 |
Data represent an average of multiple determinations (n ≥ 3) ± standard error of the mean (SEM).
Maximum up-regulating value (%).
For the most part, the CD series maintained good up-regulating ABCA1 activities. To determine whether the E ring of CD1 is important to maintaining the activity, the ring was exchanged with a pyridine ring to give CD15. CD15 also exhibited good ABCA1 activity (Table 1). This implied that the E ring (benzene) could be replaced with different aromatic rings without a loss in performance. The effects of changing the molecule at the L position were also investigated by synthesizing products CD16–18, in which the carbonyl group was replaced with methylene, ester, and 2-oxoacetyl groups, respectively (Table 1). CD16 was synthesized by the substitution of tryptamine with benzyl chloride in acetonitrile. CD15, CD17, and CD18 were obtained by the acylation of tryptamine with the corresponding acyl chloride in the presence of triethylamine in dichloromethane (Scheme 2). Among the L-changed compounds, CD16 lost its activity without the carbonyl, while CD17, with one oxygen atom inserted between the benzene and carbonyl groups, maintained its activity. CD18, with two atoms inserted between the benzene and carbonyl groups, lost its effectiveness (Table 1). These data indicated that the carbonyl group was critical with regard to maintaining activity, and the length of the linker was also important, based on a comparison of CD16–18. Finally, to examine whether the indole ring was important to maintaining activity, the indole rings of CD1 and CD6 were replaced with benzene rings to give compounds BCD1 and BCD2, respectively. Compounds BCD1–2 were synthesized simply by condensation of 2-phenylethanamine with a substituted benzoic acid (Scheme 2). Both BCD1 and BCD2 exhibited reasonably high activities, close to the performance of CD1 and CD6 (Table 1), which implied that the indole ring was not necessary to maintain the activity.
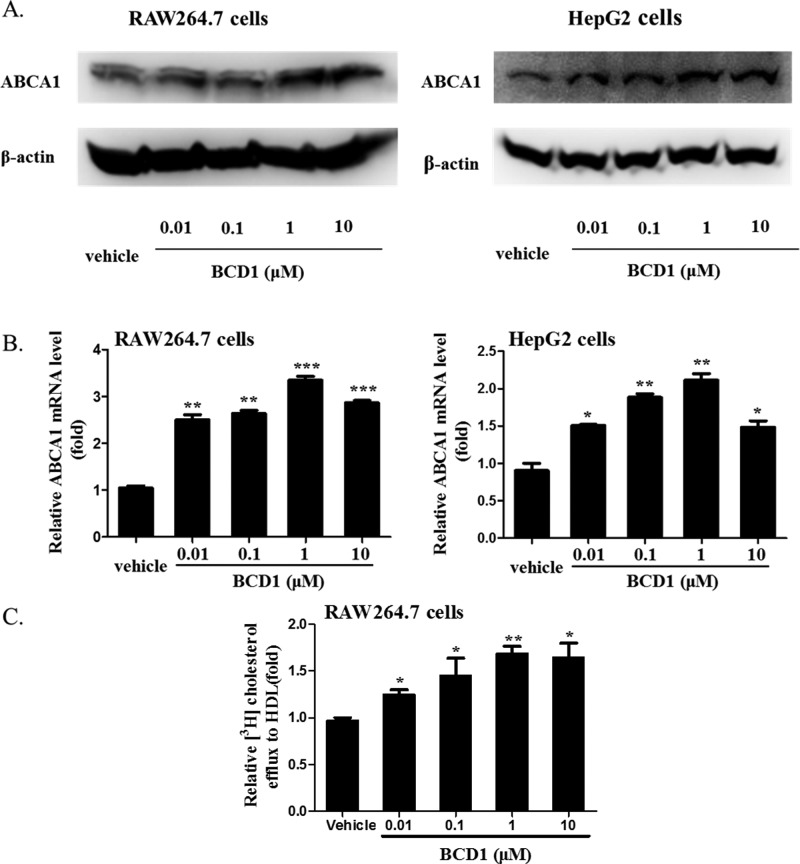
To confirm ABCA1 up-regulation by the RUT derivatives in the luciferase reporter assay, we examined the effect of BCD1 on ABCA1 mRNA and protein expression levels in HepG2 and RAW264.7 cells. Western blot analysis showed that the protein levels of ABCA1 significantly increased when incubated with 0.01, 0.1, 1.0, or 10.0 μM BCD1 (Figure 2A). Quantitative real-time PCR analysis showed that BCD1 stimulated the expression of ABCA1 at both mRNA and protein levels. The mRNA levels of ABCA1 were significantly up-regulated by BCD1 both in HepG2 (220% of control) and RAW264.7 cells (350% of control) (Figure 2B). Then the capability of promoting cholesterol efflux to HDL induced by BCD1 was then confirmed by the [3H]-cholesterol experiment. According to Figure 2C, BCD1 significantly increased the cholesterol efflux from RAW264.7 cells to HDL, which indicated that compound BCD1 had a good antiatherosclerotic effect in vitro.
Figure 2.
BCD1 induced ABCA1 expression in HepG2 and RAW264.7 cells and promoted [3H] cholesterol efflux from RAW264.7 cells to HDL. (A) Western blot analysis of ABCA1 expression level induced by BCD1 in HepG2 and RAW264.7 cells. A representative immunoblot of three separate experiments is shown. (B) Quantitative real-time PCR analysis of ABCA1 mRNA levels induced by BCD1 in HepG2 and RAW264.7 cells. The average copy numbers of ABCA1 were normalized to GAPDH. Data are expressed as means ± SEM of three independent experiments and are expressed relative to control. *P < 0.05 versus vehicle. (C) Potency of [3H] cholesterol efflux induced by BCD1 to HDL in RAW264.7 cells. Data are means ± SEM (*P < 0.05 versus vehicle).
To this point, various compounds in the C, CD, and BCD series had been obtained through varying the structure of RUT, and compounds CD1, CD6, and BCD1–2 exhibited good up-regulating ABCA1 activities. Considering the results obtained for all the compounds noted above, we could attempt to produce a preliminary structure–activity relationship. First, the C and CD series maintained up-regulating ABCA1 activity when the C and/or D ring of the RUT scaffold was truncated or removed. As well, the compounds in which E ring substituents were replaced with hydrogen-donating groups at the ortho position showed the most pronounced performance. C4 and CD4 both with a 5-methoxy group on the indole ring (AB) exhibited no activities, which was likely because electron-donating groups substituted on the indole ring were not helpful in terms of activity. Second, a carbonyl group (amide or ester) inserted between two phenyl rings (A and E) was critical for maintaining activity. The distance of the carbonyl from these rings was also important; the space from the carbonyl to E was limited to one atom, while the distance from the amide to A was restricted to two or three atoms. Third, the BCD series showed excellent activity when the indole ring (AB) was replaced with a benzene ring. It was also beneficial when hydrogen-donating groups (such as hydroxyl, amino, hydroxyl-alkyl, alkyl, and hydrogen) were introduced on the E ring. Therefore, the indole and quinazolin-4-one rings of RUT are not necessary for the ABCA1 up-regulating activity, and simplification of the structure could afford novel compounds with potent up-regulating activities.
So far, there are some ABCA1 up-regulators reported. According to the structures of these compounds, they mainly could be classified as two classes. Class 1 includes oxysterols,27 (iso)flavonoids, xanthone, aclarubicin, and pyrromycin,17 which can be classified as cyclic structures. Class 2 includes trichostatin (TSA),28 9-cis retinoic acid (9-CRA), curcumin,29 mycophenolic acid (MPA),30 oleic acids, and linoleic acids,31,32 which can be classified as aryl unsaturated aldehyde/ketone derivatives. Compared with these known compounds, N-aryl/alkyl-substituted aromatic amide derivatives based on the optimization of RUT were first synthesized and found as ABCA1 expression up-regulators in this study, and the up-regulating ABCA1 activities of compounds CD1, CD6, and BCD1–2 are comparable and even better than most known compounds.
ABCA1 gene expression is transcriptionally regulated.33 Recent studies indicate that peroxisome proliferator-activated receptor gamma (PPARγ) enhances cholesterol efflux by inducing transcription of the liver X receptor (LXRα) and thus ABCA1.34 Our results showed that some compounds optimized in this study could partly activate PPARα/γ or LXRα/β (data not shown), which might explain the up-regulating ABCA1 mechanism of these compounds and might reduce the side effect in vivo. However, the specific mechanism of up-regulating ABCA1 expression induced by these compounds will be further investigated in vitro and in vivo in future.
In summary, a series of N-aryl/alkyl-substituted aromatic amide derivatives were synthesized as ABCA1 expression up-regulators based on the optimization of RUT. The carbonyl group was identified critical for maintaining activity; the distance of the rings (A and E) from the carbonyl group and the position of the hydrogen-donating groups on the E ring were identified beneficial to the up-regulating ABCA1 activity. The C and D rings and the indole ring, however, could be removed without affecting the up-regulating activity. Compounds CD1, CD6, and BCD1–2 were found to be the best up-regulators, and all had simplified structures compared with RUT. BCD1 could significantly promote cholesterol efflux in RAW264.7 cells. These four compounds showed promise as potent compounds for the treatment of atherosclerosis.
Supporting Information Available
Cell culture, ABCA1 transcriptional activity assay, real-time quantitative PCR, Western blots assay, cholesterol efflux assay, and the synthesis and identification of compounds C1–C4, CD1–18, and BCD1–2. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was supported by the Key New Drug Creation and Manufacturing Program (2012ZX09301002-003 and 2012ZX09301002-001); the National Natural Science Foundation of China (81102443, 81273515, and 81321004); PUMC Youth Fund (3332013089); the Fundamental Research Funds for the Central Universities (3332013089); and the National Natural Science Foundation of China-Guangdong Provincial People’s Government of the Joint Natural Science Fund Projects (U1032007).
The authors declare no competing financial interest.
Supplementary Material
References
- Law M. R.; Wald N. J.; Rudnicka A. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. Br. Med. J. 2003, 32674041423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unit E. S. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [DOI] [PubMed] [Google Scholar]
- Martin C. A.; Longman E.; Wooding C.; Hoosdally S. J.; Ali S.; Aitman T. J.; Gutmann D. A.; Freemont P. S.; Byrne B.; Linton K. J. Cd36, a class B scavenger receptor, functions as a monomer to bind acetylated and oxidized low-density lipoproteins. Protein Sci. 2007, 16112531–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endemann G.; Stanton L. W.; Madden K. S.; Bryant C. M.; White R. T.; Protter A. A. CD36 is a receptor for oxidized low density lipoprotein. J. Biol. Chem. 1993, 2681611811–11816. [PubMed] [Google Scholar]
- Sun B.; Boyanovsky B. B.; Connelly M. A.; Shridas P.; van der Westhuyzen D. R.; Webb N. R. Distinct mechanisms for OxLDL uptake and cellular trafficking by class B scavenger receptors CD36 and SR-BI. J. Lipid Res. 2007, 48122560–2570. [DOI] [PubMed] [Google Scholar]
- Yvan-Charvet L.; Wang N.; Tall A. R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 2010, 302139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause B. R.; Auerbach B. J. Reverse cholesterol transport and future pharmacological approaches to the treatment of atherosclerosis. Curr. Opin. Invest. Drugs 2001, 23375–381. [PubMed] [Google Scholar]
- Nanjee M. N.; Cooke C. J.; Garvin R.; Semeria F.; Lewis G.; Olszewski W. L.; Miller N. E. Intravenous apoA-I/lecithin discs increase pre-beta-HDL concentration in tissue fluid and stimulate reverse cholesterol transport in humans. J. Lipid Res. 2001, 42101586–1593. [PubMed] [Google Scholar]
- von Eckardstein A.; Nofer J. R.; Assmann G. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 2001, 21113–27. [DOI] [PubMed] [Google Scholar]
- Wang X.; Collins H. L.; Ranalletta M.; Fuki I. V.; Billheimer J. T.; Rothblat G. H.; Tall A. R.; Rader D. J. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 2007, 11782216–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello R. J.; Brees D.; Bourassa P. A.; Royer L.; Lindsey S.; Coskran T.; Haghpassand M.; Francone O. L. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler. Thromb. Vasc. Biol. 2002, 224630–637. [DOI] [PubMed] [Google Scholar]
- Haidar B.; Denis M.; Marcil M.; Krimbou L.; Genest J. Jr. Apolipoprotein A-I activates cellular cAMP signaling through the ABCA1 transporter. J. Biol. Chem. 2004, 279119963–9969. [DOI] [PubMed] [Google Scholar]
- Frank P. G.; Marcel Y. L. Apolipoprotein A-I: structure–function relationships. J. Lipid Res. 2000, 416853–872. [PubMed] [Google Scholar]
- Francone O. L.; Aiello R. J. ABCA1: regulation, function and relationship to atherosclerosis. Curr. Opin. Invest. Drugs 2002, 33415–419. [PubMed] [Google Scholar]
- Attie A. D.; Kastelein J. P.; Hayden M. R. Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J. Lipid Res. 2001, 42111717–1726. [PubMed] [Google Scholar]
- Singaraja R. R.; Fievet C.; Castro G.; James E. R.; Hennuyer N.; Clee S. M.; Bissada N.; Choy J. C.; Fruchart J. C.; McManus B. M.; Staels B.; Hayden M. R. Increased ABCA1 activity protects against atherosclerosis. J. Clin. Invest. 2002, 110135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J.; Xu Y.; Yang Y.; Yang Y.; Zheng Z.; Jiang W.; Hong B.; Yan X.; Si S. Identification of upregulators of human ATP-binding cassette transporter A1 via high-throughput screening of a synthetic and natural compound library. J. Biomol. Screening 2008, 137648–656. [DOI] [PubMed] [Google Scholar]
- Xu Y. N.; Liu Q.; Xu Y.; Liu C.; Wang X.; He X. B.; Zhu N. Y.; Liu J. K.; Wu Y. X.; Li Y. Z.; Li N.; Feng T. T.; Lai F. F.; Zhang M. R.; Hong B.; Jiang J. D.; Si S. Y. Rutaecarpine suppresses atherosclerosis in ApoE–/–mice through up-regulating ABCA1 and SR-BI within RCT. J. Lipid Res. 2014, 10.1194/jlr.M044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S.; Hu C. Pharmacological effects of rutaecarpine as a cardiovascular protective agent. Molecules 2010, 1531873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H.; Son J. K.; Jeong B. S.; Jeong T. C.; Chang H. W.; Lee E. S.; Jahng Y. Progress in the studies on rutaecarpine. Molecules 2008, 132272–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.; Hu G.; Li D.; Chen J.; Li Y.; Zhou H.; Xie Y. Synthesis and vasodilator effects of rutaecarpine analogues which might be involved transient receptor potential vanilloid subfamily, member 1 (TRPV1). Bioorg. Med. Chem. 2009, 1762351–2359. [DOI] [PubMed] [Google Scholar]
- Lee C. M.; Gu J. A.; Rau T. G.; Yang C. H.; Yang W. C.; Huang S. H.; Lin F. Y.; Lin C. M.; Huang S. T. Low-cytotoxic synthetic bromorutaecarpine exhibits anti-inflammation and activation of transient receptor potential vanilloid type 1 activities. Biomed. Res. Int. 2013, 2013, 795095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo S. K.; Yun H. J.; Yi H. S.; Noh E. K.; Park S. D. Evodiamine and rutaecarpine inhibit migration by LIGHT via suppression of NADPH oxidase activation. J. Cell. Biochem. 2009, 1071123–133. [DOI] [PubMed] [Google Scholar]
- Chen Y. C.; Zeng X. Y.; He Y.; Liu H.; Wang B.; Zhou H.; Chen J. W.; Liu P. Q.; Gu L. Q.; Ye J. M.; Huang Z. S. Rutaecarpine analogues reduce lipid accumulation in adipocytes via inhibiting adipogenesis/lipogenesis with AMPK activation and UPR suppression. ACS Chem. Biol. 2013, 8102301–2311. [DOI] [PubMed] [Google Scholar]
- Bergman J.; Bergman S. Synthesis of rutaecarpine and related indole alkaloids. Heterocycles 1981, 163347–347. [Google Scholar]
- Bergman J.; Bergman S. Studies of rutaecarpine and related quinazolinocarboline alkaloids. J. Org. Chem. 1985, 5081246–1255. [Google Scholar]
- Koldamova R. P.; Lefterov I. M.; Ikonomovic M. D.; Skoko J.; Lefterov P. I.; Isanski B. A.; DeKosky S. T.; Lazo J. S. 22R-Hydroxycholesterol and 9-cis-retinoic acid induce ATP-binding cassette transporter A1 expression and cholesterol efflux in brain cells and decrease amyloid beta secretion. J. Biol. Chem. 2003, 2781513244–13256. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Xu Y.; Bao Y.; Hong B.; Si S. Identification of dehydroxytrichostatin A as a novel up-regulator of the ATP-binding cassette transporter A1 (ABCA1). Molecules 2011, 1697183–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S. Z.; Zhao S. P.; Wu Z. H.; Yang J.; Xie X. Z.; Yu B. L.; Nie S. Curcumin promotes cholesterol efflux from adipocytes related to PPARgamma-LXRalpha-ABCA1 passway. Mol. Cell. Biochem. 2011, 3581–2281–285. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Lai F.; Xu Y.; Wu Y.; Liu Q.; Li N.; Wei Y.; Feng T.; Zheng Z.; Jiang W.; Yu L.; Hong B.; Si S. Mycophenolic acid induces ATP-binding cassette transporter A1 (ABCA1) expression through the PPARgamma-LXRalpha-ABCA1 pathway. Biochem. Biophys. Res. Commun. 2011, 4144779–782. [DOI] [PubMed] [Google Scholar]
- Reyes-Quiroz M. E.; Alba G.; Saenz J.; Santa-Maria C.; Geniz I.; Jimenez J.; Ramirez R.; Martin-Nieto J.; Pintado E.; Sobrino F. Oleic acid modulates mRNA expression of liver X receptor (LXR) and its target genes ABCA1 and SREBP1c in human neutrophils. Eur. J. Nutr. 2014, 10.1007/s00394-014-0677-0. [DOI] [PubMed] [Google Scholar]
- Kammerer I.; Ringseis R.; Biemann R.; Wen G.; Eder K. 13-hydroxy linoleic acid increases expression of the cholesterol transporters ABCA1, ABCG1 and SR-BI and stimulates apoA-I-dependent cholesterol efflux in RAW264.7 macrophages. Lipids Health. Dis. 2011, 10, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M.; Hou M.; Zhu H.; Ma J.; Tang Z.; Wang Q.; Li Y.; Chi D.; Yu X.; Zhao T.; Han P.; Xia X.; Ling W. Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: the role of the peroxisome proliferator-activated receptor γ-liver X receptor α-ABCA1 pathway. J. Biol. Chem. 2005, 2804436792–36801. [DOI] [PubMed] [Google Scholar]
- Soumian S.; Albrecht C.; Davies A. H.; Gibbs R. G. ABCA1 and atherosclerosis. Vasc. Med. 2005, 102109–119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.