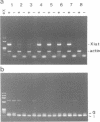
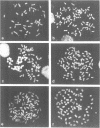
Abstract
It is unknown how and why the genetically inactivated mammalian X chromosome replicates late in S phase. There are also occasional inactive X chromosomes characterized by an opposite behavior replicating early in S phase. Two clonal cell lines, MTLB3 and MTLH8, isolated from a cultured murine T-cell lymphoma have an allocyclic X chromosome of the latter type. This precociously replicating X chromosome was judged to be genetically inactive as the late replicating one. Immediately after fusion with another cell line, the precociously replicating X chromosome from these cells starts to replicate late in S phase. This finding seems to suggest that late replication characterizing the inactive X chromosome is actively maintained by a trans-acting factor in female somatic cells, and that its lack entails a switch from late replication to precocious replication. It remains unknown whether this presumptive factor also modifies the autosomal replication pattern.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borsani G., Tonlorenzi R., Simmler M. C., Dandolo L., Arnaud D., Capra V., Grompe M., Pizzuti A., Muzny D., Lawrence C. Characterization of a murine gene expressed from the inactive X chromosome. Nature. 1991 May 23;351(6324):325–329. doi: 10.1038/351325a0. [DOI] [PubMed] [Google Scholar]
- Brockdorff N., Ashworth A., Kay G. F., Cooper P., Smith S., McCabe V. M., Norris D. P., Penny G. D., Patel D., Rastan S. Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature. 1991 May 23;351(6324):329–331. doi: 10.1038/351329a0. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Ballabio A., Rupert J. L., Lafreniere R. G., Grompe M., Tonlorenzi R., Willard H. F. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991 Jan 3;349(6304):38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- FORD D. K., YERGANIAN G. Observations on the chromosomes of Chinese hamster cells in tissue culture. J Natl Cancer Inst. 1958 Aug;21(2):393–425. [PubMed] [Google Scholar]
- Fangman W. L., Brewer B. J. A question of time: replication origins of eukaryotic chromosomes. Cell. 1992 Oct 30;71(3):363–366. doi: 10.1016/0092-8674(92)90505-7. [DOI] [PubMed] [Google Scholar]
- Hales A. A procedure for the fusion of cells in suspension by means of polyethylene glycol. Somatic Cell Genet. 1977 Mar;3(2):227–230. doi: 10.1007/BF01551817. [DOI] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978 Oct;15(2):317–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Holmquist G. P. Role of replication time in the control of tissue-specific gene expression. Am J Hum Genet. 1987 Feb;40(2):151–173. [PMC free article] [PubMed] [Google Scholar]
- Holmquist G., Gray M., Porter T., Jordan J. Characterization of Giemsa dark- and light-band DNA. Cell. 1982 Nov;31(1):121–129. doi: 10.1016/0092-8674(82)90411-1. [DOI] [PubMed] [Google Scholar]
- Kanda N. A new differential technique for staining the heteropycnotic X-chromosome in female mice. Exp Cell Res. 1973 Aug;80(2):463–467. doi: 10.1016/0014-4827(73)90324-8. [DOI] [PubMed] [Google Scholar]
- Konecki D. S., Brennand J., Fuscoe J. C., Caskey C. T., Chinault A. C. Hypoxanthine-guanine phosphoribosyltransferase genes of mouse and Chinese hamster: construction and sequence analysis of cDNA recombinants. Nucleic Acids Res. 1982 Nov 11;10(21):6763–6775. doi: 10.1093/nar/10.21.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock L. F., Melton D. W., Caskey C. T., Martin G. R. Methylation of the mouse hprt gene differs on the active and inactive X chromosomes. Mol Cell Biol. 1986 Mar;6(3):914–924. doi: 10.1128/mcb.6.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock L. F., Takagi N., Martin G. R. Methylation of the Hprt gene on the inactive X occurs after chromosome inactivation. Cell. 1987 Jan 16;48(1):39–46. doi: 10.1016/0092-8674(87)90353-9. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Epstein C. J., Travis B., Tucker G., Yatziv S., Martin D. W., Jr, Clift S., Cohen S. X-chromosome inactivation during differentiation of female teratocarcinoma stem cells in vitro. Nature. 1978 Jan 26;271(5643):329–333. doi: 10.1038/271329a0. [DOI] [PubMed] [Google Scholar]
- McClelland M., Hanish J., Nelson M., Patel Y. KGB: a single buffer for all restriction endonucleases. Nucleic Acids Res. 1988 Jan 11;16(1):364–364. doi: 10.1093/nar/16.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Markham A. F., Orkin S. H. Isolation and DNA sequence of a full-length cDNA clone for human X chromosome-encoded phosphoglycerate kinase. Proc Natl Acad Sci U S A. 1983 Jan;80(2):472–476. doi: 10.1073/pnas.80.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon B. R., Schmidt M., Axelman J., Cullen C. R. Complete reactivation of X chromosomes from human chorionic villi with a switch to early DNA replication. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2182–2186. doi: 10.1073/pnas.83.7.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe R. T., Henderson S. C., Spector D. L. Dynamic organization of DNA replication in mammalian cell nuclei: spatially and temporally defined replication of chromosome-specific alpha-satellite DNA sequences. J Cell Biol. 1992 Mar;116(5):1095–1110. doi: 10.1083/jcb.116.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstraus M. J., Levine A. J. Alterations in the developmental potential of embryonal carcinoma cells in mixed aggregates of nullipotent and pluripotent cells. Cell. 1979 Jun;17(2):337–346. doi: 10.1016/0092-8674(79)90160-0. [DOI] [PubMed] [Google Scholar]
- Selig S., Ariel M., Goitein R., Marcus M., Cedar H. Regulation of mouse satellite DNA replication time. EMBO J. 1988 Feb;7(2):419–426. doi: 10.1002/j.1460-2075.1988.tb02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Sam J., Grant M., LeBon J. M., Okuyama K., Chapman V., Monk M., Riggs A. D. Use of a HpaII-polymerase chain reaction assay to study DNA methylation in the Pgk-1 CpG island of mouse embryos at the time of X-chromosome inactivation. Mol Cell Biol. 1990 Sep;10(9):4987–4989. doi: 10.1128/mcb.10.9.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Sam J., LeBon J. M., Tanguay R. L., Riggs A. D. A quantitative HpaII-PCR assay to measure methylation of DNA from a small number of cells. Nucleic Acids Res. 1990 Feb 11;18(3):687–687. doi: 10.1093/nar/18.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Sam J., Robinson M. O., Bellvé A. R., Simon M. I., Riggs A. D. Measurement by quantitative PCR of changes in HPRT, PGK-1, PGK-2, APRT, MTase, and Zfy gene transcripts during mouse spermatogenesis. Nucleic Acids Res. 1990 Mar 11;18(5):1255–1259. doi: 10.1093/nar/18.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somssich I. E., Spira J., Hameister H., Klein G. Correlation between tumorigenicity and banding pattern of chromosome 15 in murine T-cell leukemia cells and hybrids of normal and malignant cells. Chromosoma. 1984;91(1):39–45. doi: 10.1007/BF00286483. [DOI] [PubMed] [Google Scholar]
- Somssich I. E., Spira J., Hameister H., Klein G. Cytogenetic replication studies on murine T-cell leukemias with special consideration to chromosome 15. Chromosoma. 1982;86(2):197–208. doi: 10.1007/BF00288676. [DOI] [PubMed] [Google Scholar]
- Sugawara O., Takagi N., Sasaki M. Allocyclic early replicating X chromosome in mice: genetic inactivity and shift into a late replicator in early embrogenesis. Chromosoma. 1983;88(2):133–138. doi: 10.1007/BF00327333. [DOI] [PubMed] [Google Scholar]
- Takagi N. Differentiation of X chromosomes in early female mouse embryos. Exp Cell Res. 1974 May;86(1):127–135. doi: 10.1016/0014-4827(74)90657-0. [DOI] [PubMed] [Google Scholar]
- Takagi N., Endo S., Sugawara O. X chromosome inactivation in bone marrow cells of adult mice carrying Searle's X-autosome translocation: occurrence of the early-replicating inactive X chromosome. Cytogenet Cell Genet. 1984;38(1):62–69. doi: 10.1159/000132031. [DOI] [PubMed] [Google Scholar]
- Takagi N., Ootsuyama A., Tanooka H. Cytological evidence for single-cell origin of tumors induced with 3-methylcholanthrene in female mice carrying Cattanach's translocation. Jpn J Cancer Res. 1986 Apr;77(4):376–384. [PubMed] [Google Scholar]
- Takagi N., Sugawara O., Sasaki M. Regional and temporal changes in the pattern of X-chromosome replication during the early post-implantation development of the female mouse. Chromosoma. 1982;85(2):275–286. doi: 10.1007/BF00294971. [DOI] [PubMed] [Google Scholar]
- Takagi N., Yoshida M. A., Sugawara O., Sasaki M. Reversal of X-inactivation in female mouse somatic cells hybridized with murine teratocarcinoma stem cells in vitro. Cell. 1983 Oct;34(3):1053–1062. doi: 10.1016/0092-8674(83)90563-9. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel W., Autenrieth M., Speit G. Detection of bromodeoxyuridine-incorporation in mammalian chromosomes by a bromodeoxyuridine-antibody. I. Demonstration of replication patterns. Hum Genet. 1986 Feb;72(2):129–132. doi: 10.1007/BF00283930. [DOI] [PubMed] [Google Scholar]
- WALEN K. H. SPATIAL RELATIONSHIPS IN THE REPLICATION OF CHROMOSOMAL DNA. Genetics. 1965 Jun;51:915–929. doi: 10.1093/genetics/51.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis J. J. Mid-prophase human chromosomes. The attainment of 2000 bands. Hum Genet. 1981;56(3):293–298. doi: 10.1007/BF00274682. [DOI] [PubMed] [Google Scholar]