Abstract
Low aqueous solubility is a common challenge in drug discovery and development and can lead to inconclusive biological assay results. Attaching small, polar groups that do not interfere with the bioactivity of the pharmacophore often improves solubility, but there is a dearth of viable neutral moieties available for this purpose. We have modified several poorly soluble drugs or drug candidates with the oxetanyl sulfoxide moiety of the DMSO analog MMS-350 and noted in most cases a moderate to large improvement of aqueous solubility. Furthermore, the membrane permeability of a test sample was enhanced compared to the parent compound.
Keywords: Aqueous solubility, MMS-350, oxetane, sulfoxide, JP4-039
Improving the low aqueous solubility of many organic molecules remains a considerable challenge in drug discovery and development. The addition of ionizable groups that increase solubility is complicated by the need to balance this property with membrane permeability. In fact, drugs are classified based solely on these two properties to predict intestinal absorption in the biopharmaceutics classification system (BCS).1 A poorly soluble compound can yield misleading results in biological assays and suffer from low bioavailability in vivo.2,3 Formulation is frequently used to mitigate inadequate physicochemical properties in vivo, but this technique is less prevalent in in vitro screening. To increase the concentration of lipophilic small organic molecules in biochemical assays, DMSO is often added, but toxicity in cell-based screens or interference with the assay readout limits the percentage of DMSO that can be used. Ultimately, an improvement in the intrinsic water solubility of a compound would be desirable and requires the addition of polar functions or global structural changes such as disruption of symmetry or molecular planarity.4 Adding ionizable side chains such as dimethylamines, morpholines, piperidines, or carboxylic acids usually causes a decrease in lipophilicity, which consequently decreases cell permeability and hydrophobic compound–receptor interactions.5 Examples of nonionizable, covalent modifications that can improve solubility6 include glycolyl and glyceryl,7 polyethylene glycol,8 glycoside,9 and mesylpropoxy10 side chains.
Recent work has highlighted the utility of the oxetanyl group as a polar isostere of the popular gem-dimethyl group in drug design, as well as the concomitant enhancement of solubility and log P.11,12 Oxetanes are more polar and better metal ligands than dialkylethers,13 and they are superior hydrogen bond acceptors. They also have the added benefit of increasing steric bulk to potentially protect adjacent sites of chemical or metabolic instability.11,14−16
We have exploited the properties of the oxetane moiety in our recent search for new solvents.17 MMS-350 (Figure 1, 1), a symmetric oxetanyl sulfoxide, was successfully used as a solvent additive to increase the solubility of a number of low solubility drugs.18 In a comparison to the commonly used cosolvent DMSO, MMS-350 was superior in maintaining solute stability as an aqueous solubility enhancement. For example, MMS-350 increased the solubility of naproxen by 200 μg/mL over an equal weight percentage of DMSO as an additive. Furthermore, it was found to be stable19 and relatively nontoxic, and it did not influence in vitro screening results.18
Figure 1.
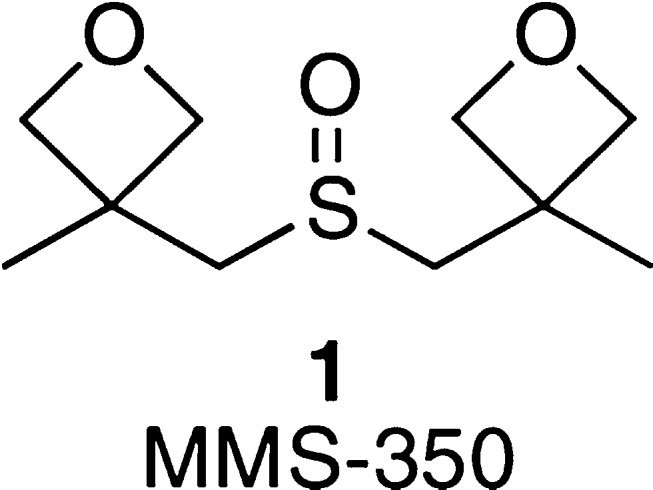
Structure of MMS-350 (1),18 an oxetanyl DMSO derivative that is an effective cosolvent for increasing the aqueous solubility of lipophilic organic molecules.
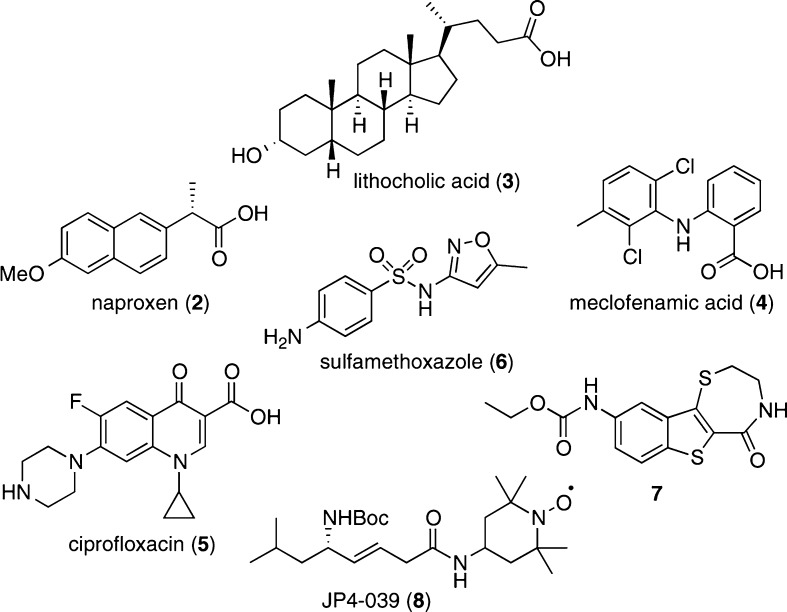
We envisioned that a covalently attachable variant of 1 would further expand upon the use of the oxetanyl sulfoxide moiety as a novel solubility enhancing function in medicinal chemistry. Bioactive compounds with low solubility that require an ionizable group were chosen for covalent modification (Figure 2). Specifically, bioactive agents containing carboxylic acid groups, such as naproxen (2), lithocholic acid (3), and meclofenamic acid (4), were modified to form oxetanyl sulfoxide ester derivatives. Amine-containing bioactive molecules, which are either used as the hydrochloride salt (as in ciprofloxacin (5) and sulfamethoxazole (6)) or acylated to prevent salt formation (as in some kinase inhibitors (e.g., 7) and the radiation mitigator JP4-039 (8)), were derivatized to form the corresponding oxetanyl sulfoxide carbamates.
Figure 2.
Structures of poorly water-soluble drugs and drug candidates used in this study.
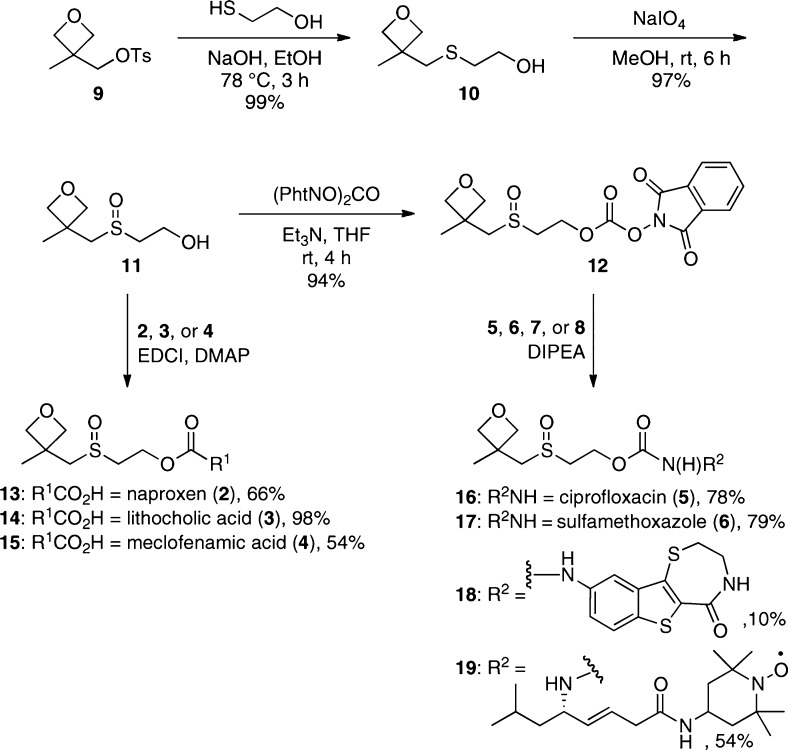
The synthesis of the covalent solubility modifier moiety 11 began with the known oxetanyl tosylate 9 (Scheme 1).20 Reaction with mercaptoethanol under basic conditions led to thioether 10, which was then oxidized to sulfoxide 11 using sodium periodate. The alcohol was converted to the activated carbonate 12 using diphthalimidyl carbonate.21 This alkoxycarbonyl transfer agent was chosen in order to provide a balance between stability and reactivity along with ease of purification.
Scheme 1. Synthesis of Oxetanyl Sulfoxide Derivatives of Carboxylic Acid (13, 14, 15) and Amine (16, 17, 18, 19)-Containing Bioactive Compounds.
Since we had success in solubilizing naproxen (2) in water with MMS-350 as an additive,18 it was chosen for validation studies of our covalent modification. Using the conditions outlined in Scheme 1, naproxen (2) was esterified with alcohol 11 to provide compound 13. Analogously, esters 14 and 15 were accessed via standard coupling conditions using alcohol 11. Activated carbonate 12 was converted to carbamates 16, 17, 18, and 19 by reaction with the free amine functional groups of the selected compounds under basic conditions. Since sulfoxides 11 and 12 are racemic, it should be noted that the derivatization of a chiral parent compound results in a diastereomeric product mixture (e.g., 13, 14, and 19), which can complicate NMR analyses.
Measuring solubility by UV–vis spectroscopy is a common, straightforward method of analysis. Unfortunately, because several of our test compounds did not contain a suitable chromophore, UV–vis could not be used as a standard method of detection. Instead, we chose to measure the solubility by mass recovery. To ascertain that this method was accurate, we chose UV-active naproxen derivative 13 and compared the solubility of 13 by both methods. The results obtained for these measurements were in good agreement (for details see Supporting Information) and supported our use of mass recovery determinations for other non-UV active compounds.
The thermodynamic solubility of 13 was measured in water after an equilibration at 30 °C for 24 h in an end-over-end rotator. Gratifyingly, the oxetanyl sulfoxide derivative showed a >10-fold increase in solubility compared to naproxen (Table 1, 13 and 2, respectively). To determine whether the sulfoxide was necessary and represented the optimal oxidation state of the sulfur atom, sulfide 20 and sulfone 21 were also synthesized. Naproxen was esterified with thioether alcohol 10 to furnish ester 20, which was also oxidized to the corresponding sulfone 21 by treatment with oxone (see Supporting Information). The thioether displayed similar solubility to naproxen itself (Table 1, 20), while the sulfone provided a 3-fold increase in solubility over the free acid (Table 1, 21). Following indentification of the sulfoxide as the optimal oxidation state with regard to solubility, the influence of the oxetane on the solubility was tested next. Analog 22, which contains a sulfoxide but lacks the oxetanyl ring, was synthesized by coupling of naproxen to 2-(methylthio)ethanol followed by oxidation. Compound 22 was more soluble than either the sulfone or the thioether derivatives but less soluble than the oxetanyl sulfoxide with solubility of 830 μM. Overall, the oxetanyl sulfoxide led to the most significant improvement in the aqueous solubility of naproxen. In this instance, and in the case of most of the oxetanyl sulfoxide derivatives, the calculated log D values did not closely track the solubility trends (Table 1).
Table 1. Aqueous Solubility of Naproxen Derivativesa.
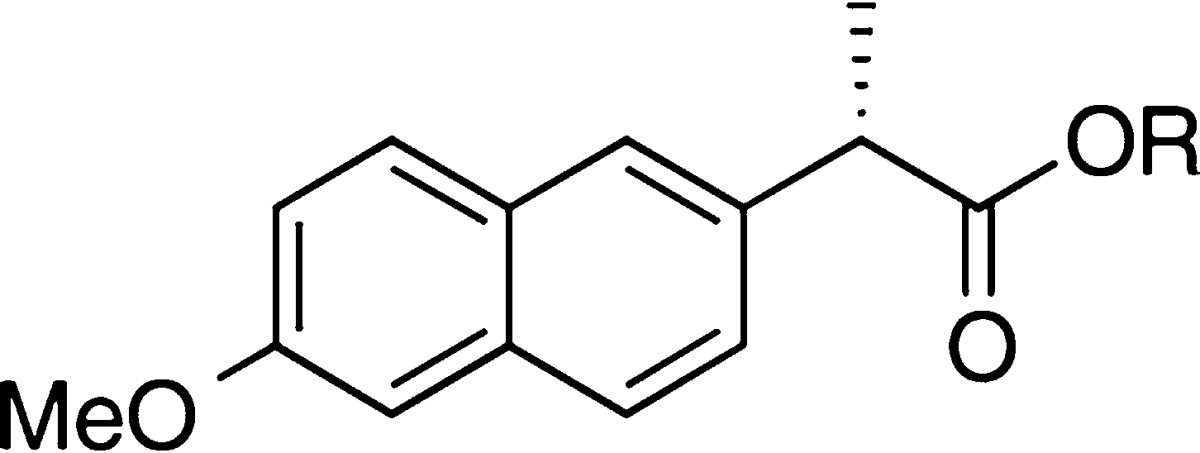
Solubility was measured in H2O after an incubation period of 24 h at 30 °C and rounded to 2 significant digits. See SI for full experimental details including standard deviations.
Calculated with Instant JChem 6.3, 2014, ChemAxon (http://www.chemaxon.com).
n = 1, consistent with literature results.26
n = 3.
n = 2.
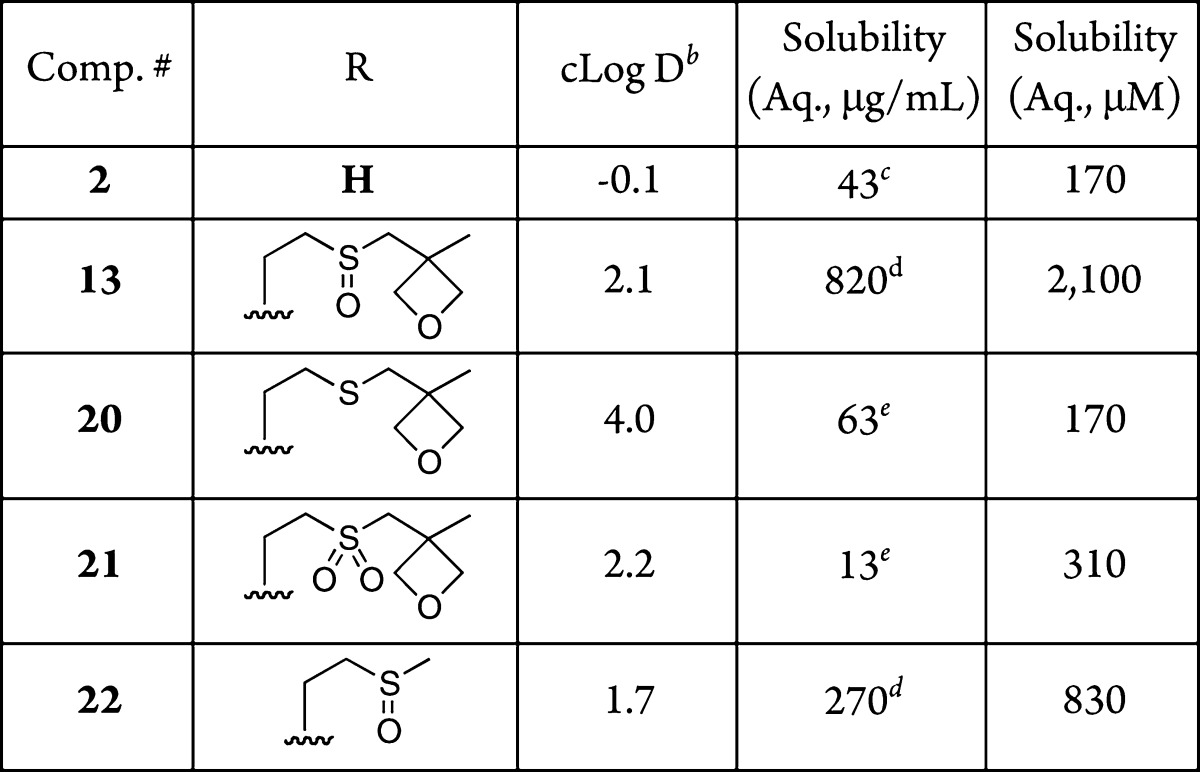
After this successful validation of the oxetanyl sulfoxide as a covalent solubility enhancer, we sought to test additional derivatives to determine the scope of this approach. The bile constituent and selective antineuroblastoma agent lithocholic acid (3), which has detergent-like properties due to a polar carboxylic acid–lipophilic steroid backbone combination,22 has a reported aqueous solubility of 1 μM at pH 2,23 consistent with our experimental results in unbuffered water (Table 2, 3). Esterification with the oxetanyl sulfoxide alcohol 11 resulted in a solubility of 480 μM for 14, thus providing a very substantial improvement over lithocholate 3 (Table 2, 14). In contrast, meclofenamic acid (4), an NSAID that inhibits prostaglandin synthesis24 and has an aqueous solubility of 1,900 μM exhibited a decrease in solubility to 540 μM when esterified with 11 (Table 2, 4 and 5, respectively). We attribute this negative effect of the oxetanyl sulfoxide attachment in 15 to the potential for increasing solubility by anionic dimer and trimer formation of the ionizable parent, anthranilic acid derivative 4.25
Table 2. Aqueous Solubility of Carboxylic Acid-Containing Bioactive Compoundsa.
Solubility in H2O was measured after an incubation period of 24 h at 30 °C and rounded to 2 significant digits. See SI for full experimental details including standard deviations.
Calculated with Instant JChem 6.3, 2014, ChemAxon (http://www.chemaxon.com).
The mass recovery of this compound was undetectable, which is consistent with the reported trace solubility of 1 μM.23
n = 4.
n = 3.
The relative decrease in solubility for ester 15 vs meclofenamate 4 highlights an apparent downside in generating nonionizable derivatives for solubility purposes. However, in general, neutral compounds are also often much more membrane permeable than their ionized counterparts. If the solubility of the oxetanyl sulfoxide derivatives is at an acceptable level, then the expected increase in permeability may well compensate for a slight decrease in solubility. The naproxen derivative 13 was compared to the parent naproxen (2) to test how the permeability was affected by the incorporation of the solubilizing moiety. Using a PAMPA permeability protocol, it was found that 13 had improved permeability over 2 with a log Pe (log of the effective permeability) of −4.9 vs −6.2, respectively.27
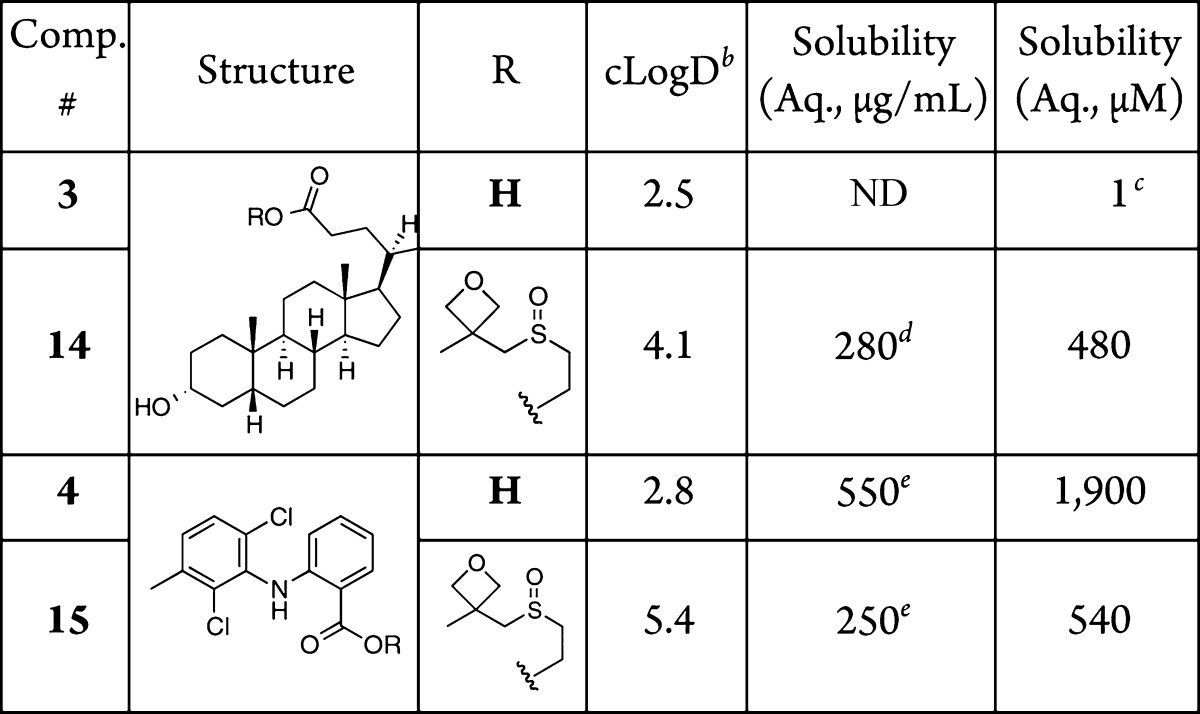
Subsequent to the esterification of carboxylic acid-containing bioactive compounds, a series of biologically active amines were derivatized as carbamates by reaction with carbonate 12 to study the effects of the oxetanyl sulfoxide on their solubility. Ciprofloxacin, an antibacterial agent, is a BCS class III drug with a solubility of 90 μM for the neutral amine (Table 3, 5). While the solubility of its HCl salt is more than sufficient for oral dosing, the compound is not very permeable, likely due to the basic piperazine.28 The oxetanyl sulfoxide derivative of 5 increased the solubility to 350 μM; that is, it was more than three times as soluble as the parent compound (Table 3, 16). Sulfamethoxazole, a BCS class IV antibacterial agent, has a solubility of 1,000 μM. The oxetanyl sulfoxide derivative of this compound at 2,200 μM was more than two times as soluble (Table 3, 6 and 17, respectively).
Table 3. Aqueous Solubility of Amine-Containing Bioactive Compoundsa.
Solubility was measured in H2O after an incubation period of 24 h at 30 °C and rounded to 2 significant digits. See SI for full experimental details including standard deviations.
Calculated with Instant JChem 6.3, 2014, ChemAxon (http://www.chemaxon.com).
n = 1 (literature value for HCl salt was 1,000 μg/mL).28
n = 3.
n = 1 (literature value for HCl salt is 100 μg/mL).28
n = 2.
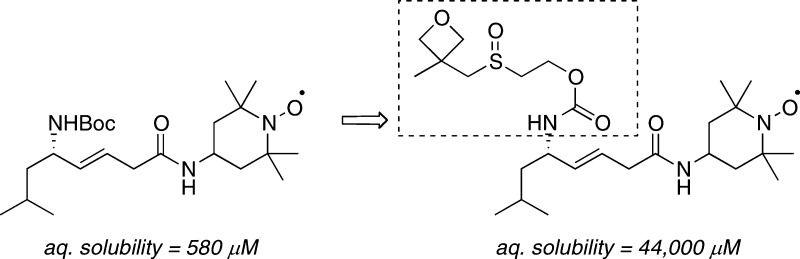
Experimental drug candidates from our own laboratory were also derivatized and analyzed for solubility enhancements. First, benzothienothiazepine 7, a selective PKD inhibitor29,30 that suffers from low aqueous solubility, was derivatized with oxetanyl sulfoxide to yield 18. This new carbamate analog showed a marked increase in aqueous solubility to 960 μM, an almost 5-fold increase over the parent ethyl carbamate (Table 3, 18). Finally, our mitochondrial-targeted nitroxide, JP4-039 (8),31−33 was converted to the oxetanyl sulfoxide derivative 19. The parent compound has an aqueous solubility of 580 μM (Table 3, 8), while 19 displayed an aqueous solubility of 44 mM (Table 3), a 76-fold increase in solubility.
A comparison of the microsomal stability of these analogs further demonstrates a subtle balance between solubility and other physical and biological properties. For example, when JP4-039 (8) was incubated with mouse liver microsomes, 15% of the parent compound remained after 1 h. The oxetanyl sulfoxide derivative 19 displayed equivalent results to those of the microsome assay, with 16% remaining after 1 h.34 Conversely, when naproxen and its oxetanyl sulfoxide derivative were exposed to mouse liver microsomes, a decrease in stability from 81% to 2%, respectively, was observed. These results could be due, in part, to the presence of a negative charge in the parent naproxen, vs the neutral character of the oxetanyl sulfoxide ester and/or ester hydrolysis.
In conclusion, we have developed a new oxetanyl sulfoxide solubility modifier and demonstrated its utility for several bioactive carboxylic acids and amines. In most cases, the oxetanyl sulfoxide group increased the solubility of the parent compound by 5–10-fold. In two instances, a more significant improvement was observed. The solubility of JP4-039 (8) was increased 76-fold by addition of the oxetanyl sulfoxide (19) while the solubility of lithocholic acid (3) was even increased from 0.05 μM to 480 μM in analog 14. However, the conversion of an ionizable acid group to a neutral ester derivative can also lead to a decrease in solubility, as shown for meclofenamic acid analog 15. Not surprisingly, metabolic stability is variable between parent compounds and solubilized analogs.
For this preliminary study, the bioactivity of the resulting derivatives was not evaluated along with the physical properties, and it is feasible that some solubilized analogs might not be compatible with the mechanism of action of the parent compounds. However, as a proof of principle, we demonstrated that the oxetanyl sulfoxide functional group can be incorporated in high yield into low solubility bioactive molecules to improve in most cases the aqueous solubility of the parent structures and that the resulting derivatives can be more membrane permeable. Accordingly, this work introduces a novel covalent modification tool that does not increase solubility at the expense of adding charge or decreasing permeability for poorly water-soluble pharmaceutical drug candidates. Further studies to assess the potential of oxetanyl sulfoxides to serve as prodrug functions35,36 will be reported in due course.
Acknowledgments
We thank Mr. Peter Chambers for QC analysis and stability testing of MMS-350 and Dr. Melissa M. Sprachman for the synthesis of the MMS-350 sample used in the stability testing.
Glossary
Abbreviations
- BCS
biopharmaceutics classification system
- DIPEA
N,N-diisopropylethylamine
- DMAP
4-dimethylaminopyridine
- EDCI
1-(3-(dimethylamino)propyl)-3-ethylcarbodiimide hydrochloride
- MMS-350
3,3′-sulfinylbis(methylene)bis(3-methyloxetane) (1)
- NSAID
nonsteroidal anti-inflammatory drug
- PAMPA
parallel artificial membrane permeability assay
- PKD
protein kinase D
- SAR
structure activity relationship
Supporting Information Available
Full experimental procedures with NMR spectra for all new compounds as well as detailed results from the solubility testing. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
‡ The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. M.Z.K. and J.S. contributed equally to this work.
This research was supported by the NIH/NIGMS CMLD program (P50 GM067082).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Amidon G.; Lennernäs H.; Shah V. P.; Crison J. R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [DOI] [PubMed] [Google Scholar]
- Baurin N.; Baker R.; Richardson C.; Chen I.; Foloppe N.; Potter A.; Jordan A.; Roughley S.; Parratt M.; Greaney P.; Morley D.; Hubbard R. E. Drug-like annotation and duplicate analysis of a 23-supplier chemical database totaling 2.7 million compounds. J. Chem. Inf. Comput. Sci. 2004, 44, 643–651. [DOI] [PubMed] [Google Scholar]
- Di L.; Fish P. V.; Mano T. Bridging solubility between drug discovery and development. Drug Disc. Today 2012, 17, 486–495. [DOI] [PubMed] [Google Scholar]
- Ishikawa M.; Hashimoto Y. Improvement in aqueous solubility in small molecule drug discovery programs by disruption of molecular planarity and symmetry. J. Med. Chem. 2011, 54, 1539–1554. [DOI] [PubMed] [Google Scholar]
- Gleeson M. P. Generation of a set of simple, interpretable ADMET rules of thumb. J. Med. Chem. 2008, 51, 817–834. [DOI] [PubMed] [Google Scholar]
- Wermuth C. G.Preparation of water-soluble compounds by covalent attachment of solubilizing moieties. In The Practice of Medicinal Chemistry, 3rd ed.; Wermuth C. G., Ed.; Elsevier: 2008; pp 767–785. [Google Scholar]
- Mouzin G.; Cousse H.; Autin J. M. A new, convenient synthesis of glafenine and floctafenine. Synthesis 1980, 54–55. [Google Scholar]
- Greenwald R. B. PEG drugs: An overview. J. Controlled Release 2001, 74, 159–171. [DOI] [PubMed] [Google Scholar]
- Miescher K.; Fischer W. H.; Meystre C. Über Steroide. (33. Mitteilung). Über Glucoside des Desoxycorticosterons. Helv. Chim. Acta 1942, 25, 40–42. [Google Scholar]
- Christiansen E.; Due-Hansen M. E.; Urban C.; Grundmann M.; Schröder R.; Hudson B. D.; Milligan G.; Cawthorne M. A.; Kostenis E.; Kassack M. U.; Ulven T. Free fatty acid receptor 1 (FFA1/GPR40) agonists: Mesylpropoxy appendage lowers lipophilicity and improves ADME properties. J. Med. Chem. 2012, 55, 6624–6628. [DOI] [PubMed] [Google Scholar]
- Wuitschik G.; Carreira E. M.; Wagner B.; Fischer H.; Parrilla I.; Schuler F.; Rogers-Evans M.; Müller K. Oxetanes in drug discovery: Structural and synthetic insights. J. Med. Chem. 2010, 53, 3227–3246. [DOI] [PubMed] [Google Scholar]
- Burkhard J. A.; Wuitschik G.; Rogers-Evans M.; Müller K.; Carreira E. M. Oxetanes as versatile elements in drug discovery and synthesis. Angew. Chem., Int. Ed. 2010, 49, 9052–9067. [DOI] [PubMed] [Google Scholar]
- Lucht B. L.; Collum D. B. Etheral solvation of lithium hexamethyldisilazide—unexpected relationships of solvation number, solvation energy, and aggregation state. J. Am. Chem. Soc. 1995, 117, 9863–9874. [Google Scholar]
- Burkhard J. A.; Wuitschik G.; Plancher J.-M.; Rogers-Evans M.; Carreira E. M. Synthesis and stability of oxetane analogs of thalidomide and lenalidomide. Org. Lett. 2013, 15, 4312–4315. [DOI] [PubMed] [Google Scholar]
- Stepan A. F.; Karki K.; McDonald W. S.; Dorff P. H.; Dutra J. K.; DiRico K. J.; Won A.; Subramanyam C.; Efremov I. V.; O’Donnell C. J.; Nolan C. E.; Becker S. L.; Pustilnik L. R.; Sneed B.; Sun H.; Lu Y.; Robshaw A. E.; Riddell D.; O’Sullivan T. J.; Sibley E.; Capetta S.; Atchison K.; Hallgren A. J.; Miller E.; Wood A.; Obach R. S. Metabolism-directed design of oxetane-containing arylsulfonamide derivatives as γ-secretase inhibitors. J. Med. Chem. 2011, 54, 7772–7783. [DOI] [PubMed] [Google Scholar]
- Stepan A. F.; Kauffman G. W.; Keefer C. E.; Verhoest P. R.; Edwards M. Evaluating the differences in cycloalkyl ether metabolism using the design parameter ″Lipophilic Metabolism Efficiency″ (LipMetE) and a matched molecular pairs analysis. J. Med. Chem. 2013, 56, 6985–6990. [DOI] [PubMed] [Google Scholar]
- Sprachman M. M. Ph.D. Dissertation, University of Pittsburgh, 2012. [Google Scholar]
- Sprachman M. M.; Wipf P. A bifunctional dimethylsulfoxide substitute enhances the aqueous solubility of small organic molecules. Assay Drug Dev. Technol. 2012, 10, 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The stability of MMS-350 was studied in water over a period of 6 months. The compound was 99% pure after 6 months. See Supporting Information for experimental details.
- Rose N. G. W.; Blaskovich M. A.; Evindar G.; Wilkinson S.; Luo Y.; Fishlock D.; Reid C.; Lajoie G. A. Preparation of 1-[N-benzyloxycarbonyl-(1S)-1-amino-2-oxoethyl]-4-methyl-2,6,7-trioxabicyclo[2.2.2]octane. Org. Synth. 2002, 79, 216–227. [Google Scholar]
- Kurita K.; Imajo H. Diphthalimido carbonate: A new reagent for active ester synthesis. J. Org. Chem. 1982, 47, 4584–4586. [Google Scholar]
- Small D. M.; Admirand W. Solubility of bile salts. Nature 1969, 221, 265–267. [DOI] [PubMed] [Google Scholar]
- Hofmann A. F.; Mysels K. J. Bile acid solubility and precipitation in vitro and in vivo: The role of conjugation, pH, and Ca2+ ions. J. Lipid Res. 1992, 33, 617–626. [PubMed] [Google Scholar]
- Syed M. M.; Parekh A. B.; Tomita T. Receptors involved in mechanical responses to catecholamines in the circular muscle of guinea-pig stomach treated with meclofenamate. Br. J. Pharmacol. 1990, 101, 809–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdeef A.; Bendels S.; Tsinman O.; Tsinman K.; Kansy M. Solubility-excipient classification gradient map. Pharm. Res. 2007, 24, 530–545. [DOI] [PubMed] [Google Scholar]
- McNamara D. P.; Amidon G. L. Dissolution of acidic and basic compounds from the rotating disk: Influence of convective diffusion and reaction. J. Pharm. Sci. 1986, 75, 858–868. [DOI] [PubMed] [Google Scholar]
- These experiments were performed by Pharmaron, Inc. (Irvine, CA). The detailed results are included in the Supporting Information.
- Kasim N. A.; Whitehouse M.; Ramachandran C.; Bermejo M.; Lennernäs H.; Hussain A. S.; Junginger H. E.; Stavchansky S. A.; Midha K. K.; Shah V. P.; Amidon G. L. Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Mol. Pharm. 2003, 1, 85–96. [DOI] [PubMed] [Google Scholar]
- Bravo-Altamirano K.; George K. M.; Frantz M.-C. l.; LaValle C. R.; Tandon M.; Leimgruber S.; Sharlow E. R.; Lazo J. S.; Wang Q. J.; Wipf P. Synthesis and structure-activity relationships of benzothienothiazepinone inhibitors of protein kinase D. ACS Med. Chem. Lett. 2010, 2, 154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George K. M.; Frantz M.-C.; Bravo-Altamirano K.; LaValle C. R.; Tandon M.; Leimgruber S.; Sharlow E. R.; Lazo J. S.; Wang Q. J.; Wipf P. Design, synthesis, and biological evaluation of PKD inhibitors. Pharmaceutics 2011, 3, 186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan M. S.; Gupta K.; Epperly M. W.; Franicola D.; Zhang X.; Wang H.; Zhao H.; Tyurin V. A.; Pierce J. G.; Kagan V. E.; Wipf P.; Kanai A. J.; Greenberger J. S. The mitochondria-targeted nitroxide JP4-039 augments potentially lethal irradiation damage repair. In Vivo 2009, 23, 717–726. [PMC free article] [PubMed] [Google Scholar]
- Frantz M.-C.; Pierce J. G.; Pierce J. M.; Kangying L.; Qingwei W.; Johnson M.; Wipf P. Large-scale asymmetric synthesis of the bioprotective agent JP4-039 and analogs. Org. Lett. 2011, 13, 2318–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz M.-C.; Skoda E. M.; Sacher J. R.; Epperly M. W.; Goff J. P.; Greenberger J. S.; Wipf P. Synthesis of analogs of the radiation mitigator JP4-039 and visualization of BODIPY derivatives in mitochondria. Org. Biomol. Chem. 2013, 11, 4147–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- These experiments were performed by Pharmaron, Inc. (Irvine, CA).
- Huttunen K. M.; Raunio H.; Rautio J. Prodrugs—from serendipity to rational design. Pharmacol. Rev. 2011, 63, 750–771. [DOI] [PubMed] [Google Scholar]
- Wipf P.; Lynch S. M.; Powis G.; Birmingham A.; Englund E. E. Synthesis and biological activity of prodrug inhibitors of the thioredoxin-thioredoxin reductase system. Org. Biomol. Chem. 2005, 3, 3880–3882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.