Abstract
Imidazopyridine 1 was identified from a phenotypic screen against P. falciparum (Pf) blood stages and subsequently optimized for activity on liver-stage schizonts of the rodent parasite P. yoelii (Py) as well as hypnozoites of the simian parasite P. cynomolgi (Pc). We applied these various assays to the cell-based lead optimization of the imidazopyrazines, exemplified by 3 (KAI407), and show that optimized compounds within the series with improved pharmacokinetic properties achieve causal prophylactic activity in vivo and may have the potential to target the dormant stages of P. vivax malaria.
Keywords: Liver-stage antimalarial, imidazopyrazines, structure−activity relationship, lead optimization
Malaria continues to be a significant public health problem, threatening up to 40% of the global population.1 Among the five species of malaria parasites that infect humans,2P. vivax malaria alone causes approximately 80–300 million clinical cases annually.3−5 Primaquine, the only licensed drug for the radical cure of P. vivax, is contraindicated in glucose-6-phosphate dehydrogenase (G6PD)-deficient populations,6 and the recommended long-term treatment (30 mg/kg for 14 days) precludes its widespread use. Thus, there remains an urgent need to develop non-8-aminoquinolines as safe and effective therapeutics for radical cure of P. vivax. From a drug discovery perspective, finding potential radical curative agents requires identifying compounds that also target the liver stages of the parasite.
We recently described two in vitro assays, which can identify compounds targeting developing schizonts and quiescent hypnozoites in hepatocytes.7−9 These assays utilize the rodent P. yoelii (Py) and the simian P. cynomolgi (Pc) parasites to target the liver schizonts and hypnozoites, respectively.10 We sought to develop a screening strategy involving both blood- and liver-stage assays to identify compounds with biological activity on multiple parasite life stages with the ultimate goal of developing radical curative agent for P. vivax malaria. A subsequent candidate with optimal drug-like properties could then be evaluated in the P. cynomolgi infected monkey model as a test for radical cure activity.11
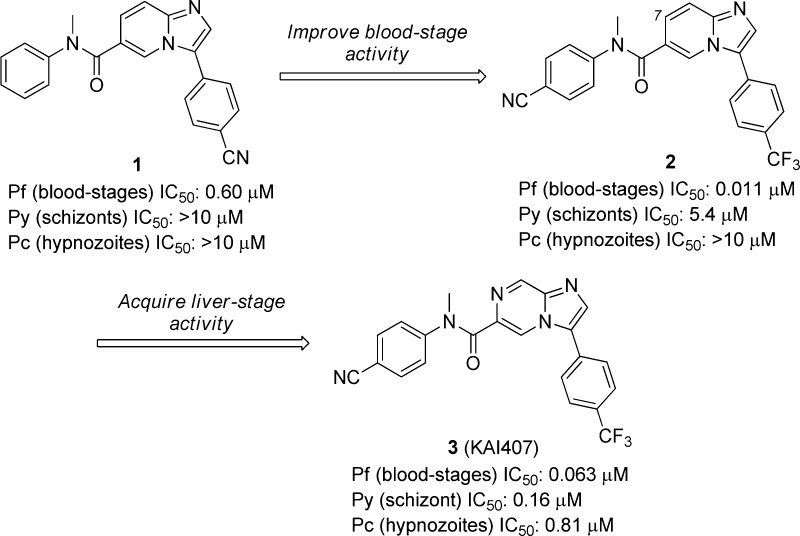
From a previous phenotypic screening effort on Pf blood stages, we selected imidazopyridine 1 as a starting point despite its relatively weak activity (Figure 1).12 Early hit-to-lead chemistry on the two distal aromatic rings resulted in the identification of 2, a compound that showed a greater than 50-fold increase in potency. Although compound 2 was active on blood stages, it was still relatively inactive on Py schizonts and Pc and suffered from poor physicochemical properties, presumably due to the high lipophilicity (cLogP = 4.3). Modification of the bicyclic core by installation of a nitrogen atom at the 7 position reduced the cLogP by one log unit and resulted in 3 (KAI407).8 Although the potency on Pf blood stages was diminished, incorporating the imidazolopyrazine core resulted in a compound with improved activity on Py and Pc liver stages.
Figure 1.
In vitro activity of compounds 1–3 on multiple parasite life stages.
The added activity of compound 3 on Py schizonts and Pc hypnozoites suggested that changes to the bicyclic core could impart the desired liver-stage activity to the molecule. We further hypothesized that activity in the Py assay might be predictive of potency on Pc hypnozoites for this chemical class and could therefore be used as a first-pass screen due to the low throughput of the Pc assay.
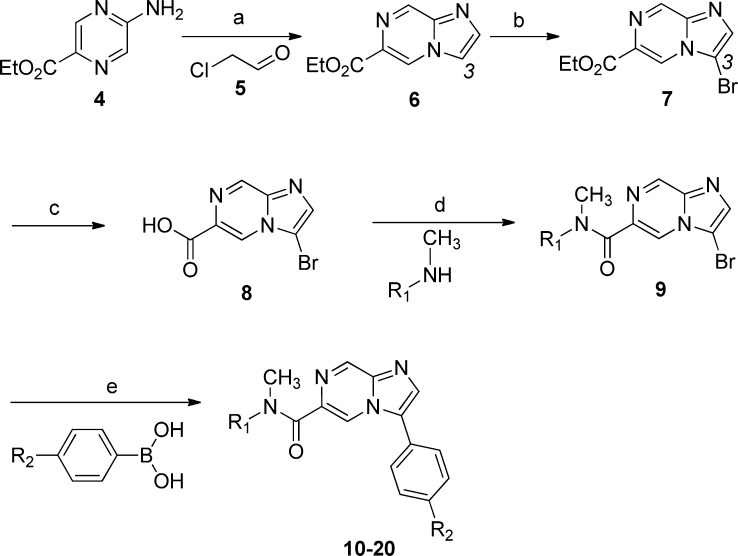
Following the discovery of compound 3, we initiated a lead optimization effort in an attempt to identify a compound with an optimal potency and PK profile. The overall strategy focused on making peripheral changes around the bicyclic imidazopyrazine core. This feature, along with the C3 phenyl and amide linker was found to be the main pharmacophore responsible for activity on Pc hypnozoites in vitro. Thus, analogues could be prepared from a primary building block consisting of the imidazopyrazine core (8) and chemically elaborated to 10–20 (Scheme 1).
Scheme 1. Synthetic Route to the Imidazopyrazines.
Reagents and conditions: (a) 2-chloroacetaldehyde 5 (50 wt % solution in water), NaHCO3, ethanol, reflux, 70%; (b) N-bromosuccinimide (NBS), CH2Cl2, room temperature (rt), 94%; (c) NaOH, THF/H2O, 60 °C, 56%; (d) oxalyl dichloride, CH2Cl2, DMF (a drop), 30 min, followed by amine, rt; (e) boronic acid, Pd(PPh3)4 (5–10 mol %), aqueous KF solution (2 M), microwave, 110 °C, 30 min to 1 h.
Briefly, 2-chloroacetaldehyde 5 was added to a solution of aminopyrazine 4 in ethanol and heated to reflux to afford the imidazopyrazine core (6).13 A regioselective bromination at C3 with NBS provided 3-bromo-6-carboxylate 7 as the sole product isolated.14 Hydrolysis of 7 to the corresponding acid (8) followed by amide bond formation with an appropriate amine provided compound 9. Finally, Suzuki coupling with a series of boronic acids yielded the corresponding imidazopyrazine analogues.15
As we explored the SAR around the R1 position of the imidazopyrazine core, we found that para-substituted N-phenyl or N-pyridyl were favored, while other heterocycles such as pyridazine (12) or benzothiazole (13) resulted in a loss of activity on both blood and liver stages (Table 1). In addition, the benzyl derivative (14), or replacement with a piperidine ring (15), resulted in a loss of potency suggesting a narrow range of tolerated substitutions at the R1-position. The C3 phenyl was an essential part of the pharmacophore, and para-substitutions were preferred. Optimization of the R2 groups revealed the carboxamide as the optimal substituent, providing, a good balance between potency and physicochemical properties (18–20). Other R2 substituents such as dimethylamine, methylsulfones, and carboxylic acids were inactive (data not shown). Interestingly adding an additional heterocycle such as the pyrazole (16) or aminothiadiazole (17) resulted in some of the most potent compounds. However, the poor solubility for these analogues led to low Cmax and poor oral exposure in mice.
Table 1. Blood- and Liver-Stage (Schizont) Activity of the Imidazopyrazinesa.
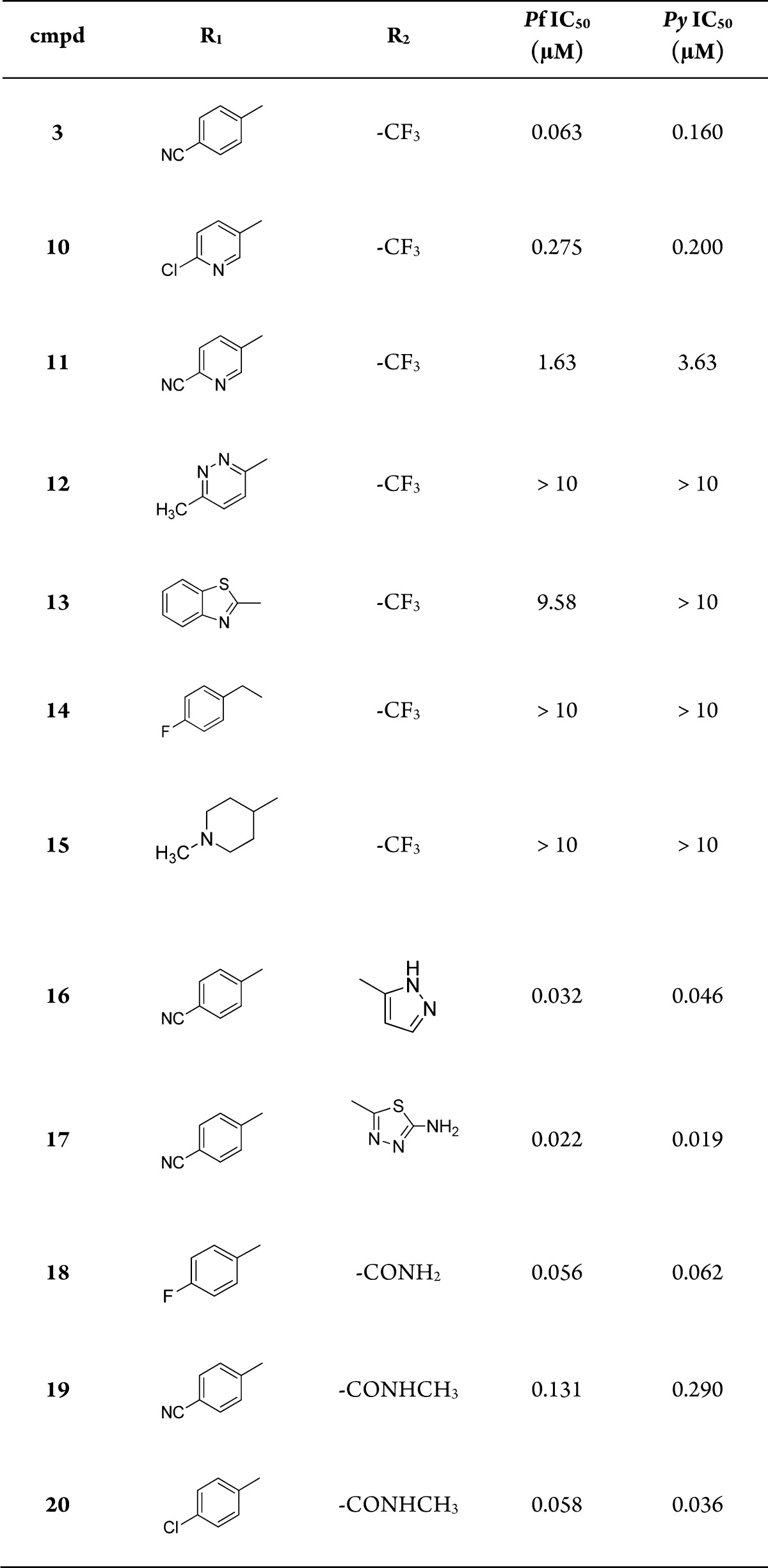
IC50 values are the average of at least two independent experiments.
Only those compounds displaying blood- and liver-schizont activity (<300 nM) were selected for testing on Pc hypnozoites. This assay can also differentiate between the developing schizonts and dormant hypnozoites, which are not present in the Py assay. For this chemical series, we found that activity in the Py assay was predictive of activity on both Pc liver stages (Table 2).
Table 2. Activity of the Imidazopyrazines on P. cynomolgi Liver Stagesa.
|
P. cynomolgi IC50 (μM) |
||
|---|---|---|
| cmpd | schizonts | hypnozoites |
| 3 | 0.12 | 0.81 |
| 16 | 0.30 | 0.30 |
| 17 | 0.040 | 0.060 |
| 18 | 0.19 | 0.26 |
| 19 | 0.081 | 0.094 |
| 20 | 0.105 | 0.196 |
IC50 values are the average of at least two independent experiments.
The specific physicochemical properties of the imidazopyrazines were largely dictated by the peripheral aromatic substituents (R1 and R2) (Table 3). In order to determine if the improved solubility would translate to a better in vivo PK profile, compounds 17–20 were evaluated in mice by oral (p.o.) and intravenous (i.v.) routes at 20 and 5 mg/kg, respectively (Table 4). Of the four compounds evaluated, 18 and 19 were more soluble and displayed favorable PK properties with moderate clearance, low volume of distribution, and moderate bioavailability (∼50%). Increasing the clogP slightly by alkylating the primary carboxamide (20) further boosted Cmax and exposure levels while lowering clearance and increasing the bioavailability (60%).
Table 3. Summary of Key Physicochemical Properties of the Imidazopyrazines.
| mouse
microsome stability |
||||
|---|---|---|---|---|
| cmpd | solubility (μM, pH 6.8) | cLogP | ERa (%) | T1/2 (min) |
| 3 | 9 | 3.3 | 40 | 82 |
| 17 | <5 | 1.9 | nab | na |
| 18 | 170 | 1.7 | 52 | 57 |
| 19 | 430 | 1.3 | 33 | 125 |
| 20 | 820 | 2.5 | 49 | 63 |
Hepatic extraction ratio.
Not available.
Table 4. Pharmacokinetic Parameters Following 20 mg/kg oral and 5 mg/kg Intravenous Dosing in Micea.
| oral PKb parameters |
intravenous PK parameters |
|||||||
|---|---|---|---|---|---|---|---|---|
| cmpd | Cmax (μM) | Tmax (h) | AUC (μM·h) | T1/2 (h) | F (%) | Vss (L/kg) | CL (mL/min/kg) | T1/2 (h) |
| 17 | 0.02 | 1.7 | 0.12 | 3.39 | 0.24 | 0.99 | 16.2 | 2.12 |
| 18 | 8.3 | 0.5 | 11.14 | 1.35 | 51.6 | 0.90 | 39.7 | 0.62 |
| 19 | 5.2 | 0.5 | 12.4 | 1.63 | 51.9 | 1.02 | 34.0 | 0.42 |
| 20 | 20.5 | 0.5 | 31.2 | 3.63 | 60 | 0.96 | 19.3 | 0.83 |
Cmax, maximum concentration of drug in plasma; Tmax, time to maximum concentration of drug in plasma; AUC, area under the curve extrapolated to infinity; Vss, volume of distribution at steady state; CL, clearance; T1/2, half-life; F, oral bioavailability;
Formulation used for oral and intravenous dosing is PEG300/D5W (3:1, V/V).
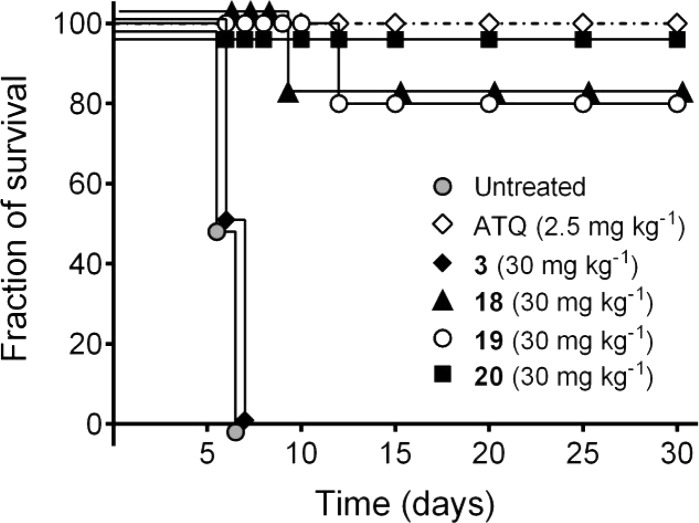
If Py liver schizonts are being inhibited in vitro, then the assay should predict that active compounds with an acceptable pharmacokinetic profile display causal prophylactic activity in vivo by preventing the parasite from establishing itself in the liver. In order to evaluate the in vivo efficacy of this class of compounds, analogues 3 and 18–20 were tested in a causal prophylaxis P. berghei mouse model (Figure 2). When administrated orally at a single dose of 30 mg/kg at the time of infection, compounds 18 and 19 were able to completely protect 80% of mice as compared to untreated control or compound 3-treated. More attractively, compound 20 (KDU691)16 displayed comparable activity to atovaquone (ATQ) and was able to completely prevent a developing liver-stage infection; an indication that developing schizonts were being inhibited and precluding blood-stage patency.
Figure 2.
Single dose mouse causal prophylactic activity of the imidazopyrazines.
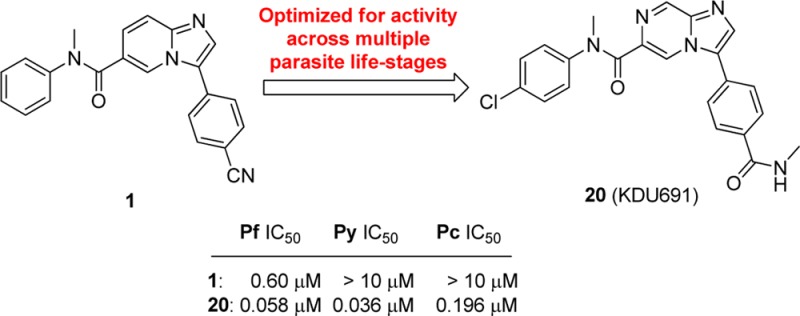
In conclusion, we described the SAR around the imidazopyrazine class of antimalarials by exploring substitutions at the R1- and R2-substitutions of the bicyclic core. Optimization of pharmacokinetic properties by improving the solubility led to the identification of a series of compounds for in vivo efficacy evaluation. Compound 20 (KDU691) combined the optimal potency and PK properties to provide 100% protection from a developing P. berghei infection in a causal prophylaxis model at a single oral dose.
The target of the imidazopyrazines was recently identified and reported as Plasmodium PI4K, which we believe is the source of the broad antimalarial activity across the various parasite life stages.16 At the time of the lead optimization, this target was suspected, but recombinant protein was unavailable, and hence, the optimization was primarily driven by cellular activity in three different parasite assays. We have shown that the imadazopyrazines with dual activity on blood stages and liver schizonts also translated to potency on hypnozoites in vitro. Although the activity of 20 on liver-stage schizonts was confirmed in a casual prophylaxis mouse model, the test for in vivo antihypnozoite activity will require the P. cynomolgi infected monkey model for validation.
Acknowledgments
We gratefully acknowledge translational research grants (WT078285 and WT096157) from the Wellcome Trust and funding from the Medicines for Malaria Venture (MMV) to the Genomics Institute of the Novartis Research Foundation, the Swiss Tropical and Public Health Institute, the Biomedical Primate Research Centre, and the Novartis Institute for Tropical Diseases.
Supporting Information Available
Full experimental details for compounds synthesized and description of assays. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- World Health Organization. World Malarial Report 2011. http://www.who.int/malaria/world_malaria_report_2011/en/index.html.
- White N. J. Plasmodium knowlesi: the fifth human malaria parasite. Clin. Infect. Dis. 2008, 46, 172–173. [DOI] [PubMed] [Google Scholar]
- Mendis K.; Sina B. J.; Marchesini P.; Carter R. The neglected burden of plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 2001, 64, 97–106. [DOI] [PubMed] [Google Scholar]
- Hay S. I.; Guerra C. A.; Tatem A. J.; Noor A. M.; Snow R. W. The global distribution and population at risk of malaria: past, present, and future. Lancet Infet. Dis. 2004, 4, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller I.; Galinski M. R.; Baird J. K.; Carlton J. M.; Kochar D. K.; Alonso P. L.; del Portillo H. A. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect. Dis. 2009, 9, 555–566. [DOI] [PubMed] [Google Scholar]
- Alving A. S.; Carson P. E.; Flanagan C. L.; Ickes C. E. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science 1956, 124, 484–485. [DOI] [PubMed] [Google Scholar]
- Meister S.; Plouffe D. M.; Kuhen K. L.; Bonamy G. M.; Wu T.; Barnes S. W.; Bopp S. E.; Borboa R.; Bright A. T.; Che J.; Cohen S.; Dharia N. V.; Gagaring K.; Gettayacamin M.; Gordon P.; Groessl T.; Kato N.; Lee M. C.; McNamara C. W.; Fidock D. A.; Nagle A.; Nam T. G.; Richmond W.; Roland J.; Rottmann M.; Zhou B.; Froissard P.; Glynne R. J.; Mazier D.; Sattabongkot J.; Schultz P. G.; Tuntland T.; Walker J. R.; Zhou Y.; Chatterjee A.; Diagana T. T.; Winzeler E. A. Imaging of plasmodium liver stages to drive next-generation antimalarial drug discovery. Science 2011, 334, 1372–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman A. M.; van Amsterdam S. M.; McNamara C. W.; Voorberg-van der Wel A.; Klooster E. J.; van den Berg A.; Remarque E. J.; Plouffe D. M.; van Gemert G. J.; Luty A.; Sauerwein R.; Gagaring K.; Borboa R.; Chen Z.; Kuhen K.; Glynne R. J.; Chatterjee A. K.; Nagle A.; Roland J.; Winzeler E. A.; Leroy D.; Campo B.; Diagana T. T.; Yeung B. K. S.; Thomas A. W.; Kocken C. H. KAI407, a potent non 8-aminoquinoline compound that kills Plasmodium cynomolgi early dormant liver stage parasites in vitro. Antimicrob. Agents Chemother. 2013, 58, 1586–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembele L.; Franetich J. F.; Lorthiois A.; Gego A.; Zeeman A. M.; Kocken C. H.; Le Grand R.; Dereuddre-Bosquet N.; van Gemert G. J.; Sauerwein R.; Vaillant J. C.; Hannoun L.; Fuchter M. J.; Diagana T. T.; Malmquist N. A.; Scherf A.; Snounou G.; Mazier D. Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nat. Med. 2014, 20, 307–312. [DOI] [PubMed] [Google Scholar]
- P. cynomolgi has historically been used as a surrogate for P. vivax in a disease monkey model as both simian and human species form dormant liver-stages in vivo.
- Schmidt L. H. Appraisals of compounds of diverse chemical classes for capacities to cure infections with sporozoites of Plasmodium cynomolgi. Am. J. Trop. Med. Hyg. 1983, 32, 231–257. [DOI] [PubMed] [Google Scholar]
- Plouffe D.; Brinker A.; McNamara C.; Henson K.; Kato N.; Kuhen K.; Nagle A.; Adrián F.; Matzen J. T.; Anderson P.; Nam T. G.; Gray N. S.; Chatterjee A.; Janes J.; Yan S. F.; Trager R.; Caldwell J. S.; Schultz P. G.; Zhou Y.; Winzeler E. A. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 9059–9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chezal J. M.; Moreau E.; Delmas G.; Gueiffier A.; Blache Y.; Grassy G.; Lartigue C.; Chavignon O.; Teulade J. C. Heterocyclization of functionalized vinylic derivatives of imidazo[1,2-a]pyridines. J. Org. Chem. 2001, 66, 6576–6584. [DOI] [PubMed] [Google Scholar]
- Chuaqui C.; Cossrow J.; Dowling J.; Guan B.; Hoemann M.; Ishchenko A.; Jones J. H.; Kabigting L.; Kumaravel G.; Peng H.; Powell N.; Raimundo B.; Tanaka H.; Van Vloten K.; Vessels J.; Xin Z. Preparation of heteroaryl compunds useful as Raf kinase inhibitors. WO2010/078408 A1.
- Note that a weakly basic solution of aqueous potassium fluoride (KF) was required to avoid cleavage of the amide bond under the microwave conditions of the Suzuki reactions. Alternatively the order of the Suzuki and amide bond formation can be reversed.
- McNamara C. W.; Lee M. C. S.; Lim C. S.; Lim S. H.; Roland J.; Nagle A.; Simon O.; Yeung B. K. S.; Chatterjee A. K.; McCormack S. L.; Manary M. J.; Zeeman A. M.; Dechering K. J.; Kumar T. R. S.; Henrich P. P.; Gagaring K.; Ibanez M.; Kato N.; Kuhen K. L.; Rischli C.; Rottmann M.; Plouffe D. M.; Bursulaya B.; Meister S.; Rameh L.; Trappe J.; Haasen D.; Timmerman M.; Sauerwein R. W.; Suwanarusk R.; Russel B.; Renia L.; Nosten F.; Tully D. C.; Kocken C. H. M.; Glynne R. J.; Bodenreider C.; Fidock D. A.; Diagana T. T.; Winzeler E. A. Targeting Plasmodium PI(4)K to eliminate malaria. Nature 2013, 504, 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.