Summary
Background
The glycoprotein (GP)Ib-IX complex is critical to hemostasis and thrombosis. Its proper assembly is closely correlated with its surface expression level and requires cooperative interactions among extracellular and transmembrane domains of Ibα, Ibβ and IX subunits. Two interfaces have been previously identified between the extracellular domains of Ibβ and IX.
Objective
To understand how extracellular domains interact in GPIb-IX.
Methods
The Ibβ extracellular domain (IbβE) or the IX counterpart (IXE) in GPIb-IX was replaced with a well-folded IbβE/IXE chimera called IbβEabc, and the effect of domain replacement on assembly and expression of the receptor complex in transiently transfected Chinese hamster ovary cells was analyzed.
Results
Replacing IXE with IbβEabc in GPIb-IX retained interface 1 but not interface 2 between the extracellular domains. While this domain replacement preserved complex integrity, the expression levels of Ibβ and Ibα were significantly reduced. Additional domain replacement with IbβEabc or IbβE in GPIb-IX produced the complex at disparate expression levels that cannot be simply explained by two separate interfaces. In particular, when IbβE in GPIb-IX was replaced by IbβEabc, Ibα and IX were expressed at approximately 70% of the wild-type level. Their levels were not reduced when IXE was changed further to IbβE.
Conclusions
Our results demonstrate the importance of the association between Ibβ and IX extracellular domains for complex assembly and efficient expression, and provide evidence for the structural malleability of these domains that may accommodate and propagate conformational changes therein.
Keywords: Bernard-Soulier syndrome, leucine-rich repeat proteins, platelet glycoprotein GPIb-IX complex, protein-protein interaction domains, von Willebrand factor receptors
Introduction
The platelet glycoprotein (GP)Ib-IX complex consists of GPIbα, GPIbβ and GPIX subunits (herein Ibα, Ibβ and IX) at a 1:2:1 stoichiometry [1–3]. Ibα is the major subunit in the complex, with its N-terminal domain harboring the binding sites for many hemostatically important ligands [4–6]. In comparison, the functions of Ibβ and IX, especially the extracellular domains thereof, remain sketchy. The only well-documented function of Ibβ and IX is that both are required for efficient expression of Ibα in the plasma membrane [7–9]. A number of mutations in the Ibβ or IX gene have been identified in patients with Bernard-Soulier syndrome (BSS) [10]. Consistently, genetic deletion of Ibβ produced BSS-like phenotypes in mice [11,12]. However, it is not clear what additional roles Ibβ and IX may play in mediating the platelet function. Although there have been several reports of the involvement of Ibβ in GPIb-IX-mediated signaling and the procoagulant activity of the platelet [13–15], the molecular basis supporting these functional claims is not elucidated. This is partly due to the lack of understanding of the assembly of GPIb-IX extracellular domains and, correspondingly, the lack of tools to dissect interactions of Ibβ and IX.
In GPIb-IX, Ibα is linked through membrane-proximal disulfide bonds to 2 Ibβ subunits to form the GPIb complex [2]. Formation of these disulfide bonds depends on the interaction between the transmembrane domains in GPIb-IX [16]. Disruption of these interactions not only abolishes native Ibα-Ibβ disulfide bonds, and thus GPIb formation, but also increases formation of Ibα-containing complexes of higher molecular weights that we now call hmwGPIb [8]. In addition to the interaction between transmembrane domains, the assembly of GPIb-IX also involves the relatively weak interaction between homologous Ibβ and IX extracellular domains (IbβE and IXE, respectively) [17–19]. The structures of IbβE and IXE both can be divided into three parts: the N-capping region, the central leucine-rich repeat (LRR) region and the C-capping region [19]. The central LRR region takes on a parallel β-coil structure, with each coil consisting of a convex loop and a concave β-strand on the opposite side. Unlike IbβE, IXE cannot be expressed as a stand-alone protein, presumably due to its instability [18]. This precludes not only in vitro characterization of the interaction between recombinant IbβE and IXE but also dissection of any interfaces in GPIb-IX that involve IXE because it is not possible to distinguish whether a mutation in IXE disrupts its own folding or its interaction with other domains. Instead, a stable IbβE/IXE chimeric protein called IbβEabc, in which three convex loops of IXE are grafted onto the IbβE scaffold, contains a IX-derived Ibβ-binding site [18]. The crystal structure of IbβEabc provides the structural detail of an interface between the convex loops of IXE and the C-capping region of IbβE (named interface 1), which has been confirmed by site-directed mutagenesis of full-length GPIb-IX [19]. Furthermore, recombinant IbβEabc can form a homo-tetramer, suggesting the existence of a second interface (interface 2) between the C-capping region of IXE and the convex loops of the other IbβE in the complex. Figure 1 shows a current structural model of the interacting domains in GPIb-IX, which can be simplified into a sketch with all the inter-subunit contacts.
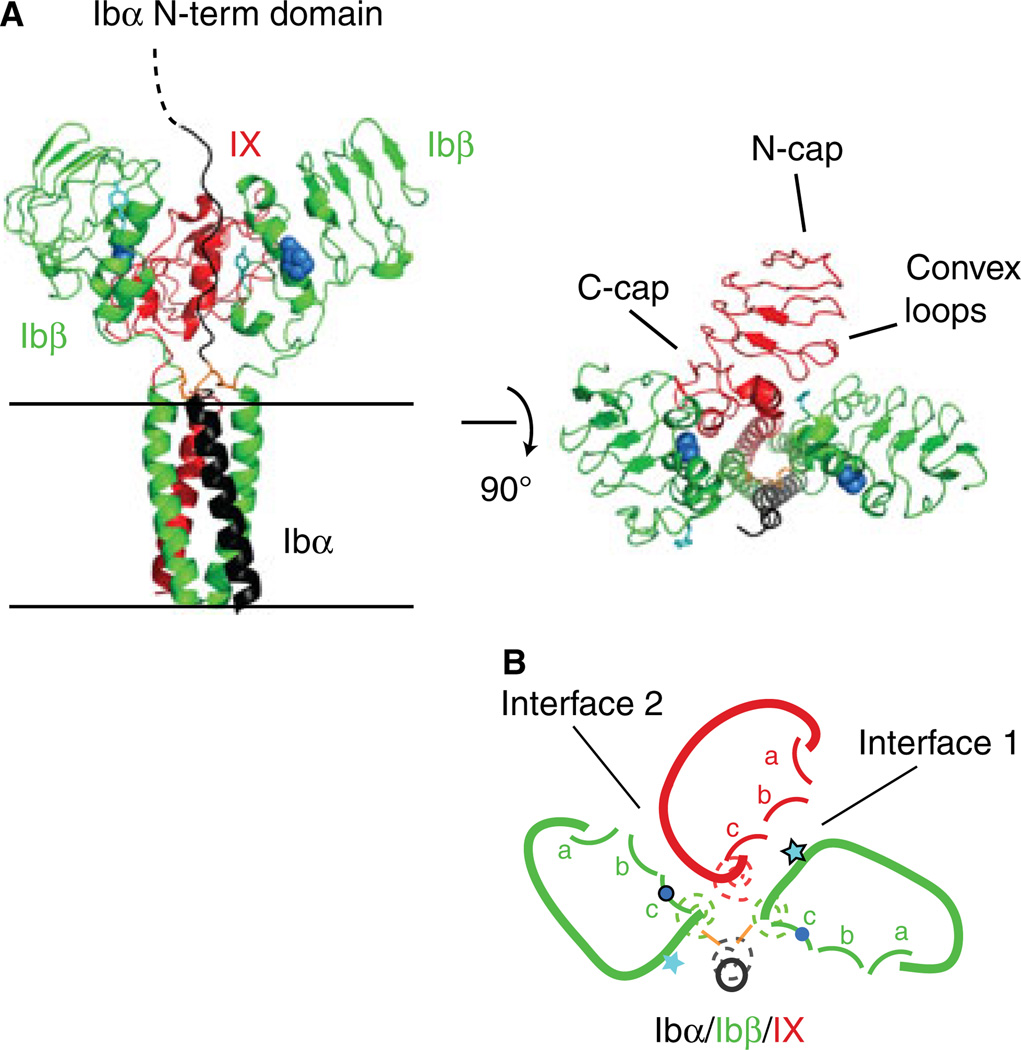
Fig. 1.
The organization of the GPIb-IX complex, viewed from the extracellular space towards the membrane, is summarized in a sketch for easy visualization and comprehension. (A) The membrane-proximal extracellular and transmembrane domains of GPIb-IX have been modeled in ribbon diagrams, with its side view shown on the left and top view in the middle (adapted from [3]). Ibα, Ibβ and IX subunits are shown in black, green and red color, respectively. The general locations of N- and C-capping regions and convex loops in IXE are marked. The side chains of Tyr106 in Ibβ are shown as ball-and-sticks in teal, and those of Pro74 in Ibβ as spheres in marine. The juxtamembrane Ibα-Ibβ disulfide bonds are shown in orange. These domains are shown in (B) as sketches of the same color. The transmembrane domains are represented by dashed coils. The extracellular domains are drawn in solid curves, with convex loops (a, b, c) marked in each domain. Positions of Tyr106 and Pro74 are marked with a star and dot, respectively.
In this paper we have characterized the assembly of various chimeric GPIb-IX complexes in transiently transfected Chinese hamster ovary (CHO) cells. Our results demonstrate the importance of interface 2 for complex assembly and particularly expression of Ibβ. Moreover, we report two chimeric complexes that contain significantly altered extracellular domains yet largely preserve their structural integrity. These complexes demonstrate the structural malleability of extracellular domains in the GPIb-IX complex, which may accommodate and propagate conformational changes therein.
Materials and methods
Materials
The CHO K1 cell line was obtained from ATCC (Manassas, VA, USA). Monoclonal antibody WM23 was kindly provided by Dr Michael Berndt. Anti-HA and anti-actin antibodies were purchased from Sigma (St Louis, MO, USA). Expression vector pDX, used to express GPIb-IX in CHO cells, including HA-tagged Ibβ or IX, has been described [7,18,20,21]. The pDX vector containing the IbβEabc-IbβTC gene, HA-tagged or otherwise, was constructed in a similar manner to those containing HA-tagged IbβEabc-IXTC and IbβE-IXTC genes [18]. All constructs were confirmed by DNA sequencing (Genewiz, South Plainfield, NJ, USA).
Expression of GPIb-IX in transiently transfected CHO K1 cells
Vectors containing Ibα, Ibβ and IX-derived genes were transiently transfected into CHO cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) as described before [9]. Transfected cells were grown in Dulbecco’s modified Eagle’s medium (Invitrogen) with 10% fetal bovine serum for an additional 48 h before being analyzed for protein expression. Surface expression of Ibα was measured by flow cytometry after staining with WM23 followed by FITC-conjugated anti-mouse antibody. Surface expression of Ibβ, IX and derivatives was measured using anti-HA antibody. The quantified mean fluorescence intensity (MFI) of each cell population (10 000 cells) was normalized, with the value of CHO cells expressing wild-type GPIb-IX being 100% and that of CHO cells transfected with only empty pDX vector 0% [9]. Groups were compared using the non-paired t-test.
SDS-polyacrylamide gel electrophoresis and western blot
Two days after transfection, transfected CHO cells were harvested and lysed in the 1% triton X-100 lysis buffer containing 1:50-diluted protease inhibitor cocktail for mammalian tissues (Sigma). SDS gel electrophoresis and western blot to detect individual GPIb-IX subunits, and formation of Ibα-Ibβ disulfide bonds, were performed as described before [9,18].
Results
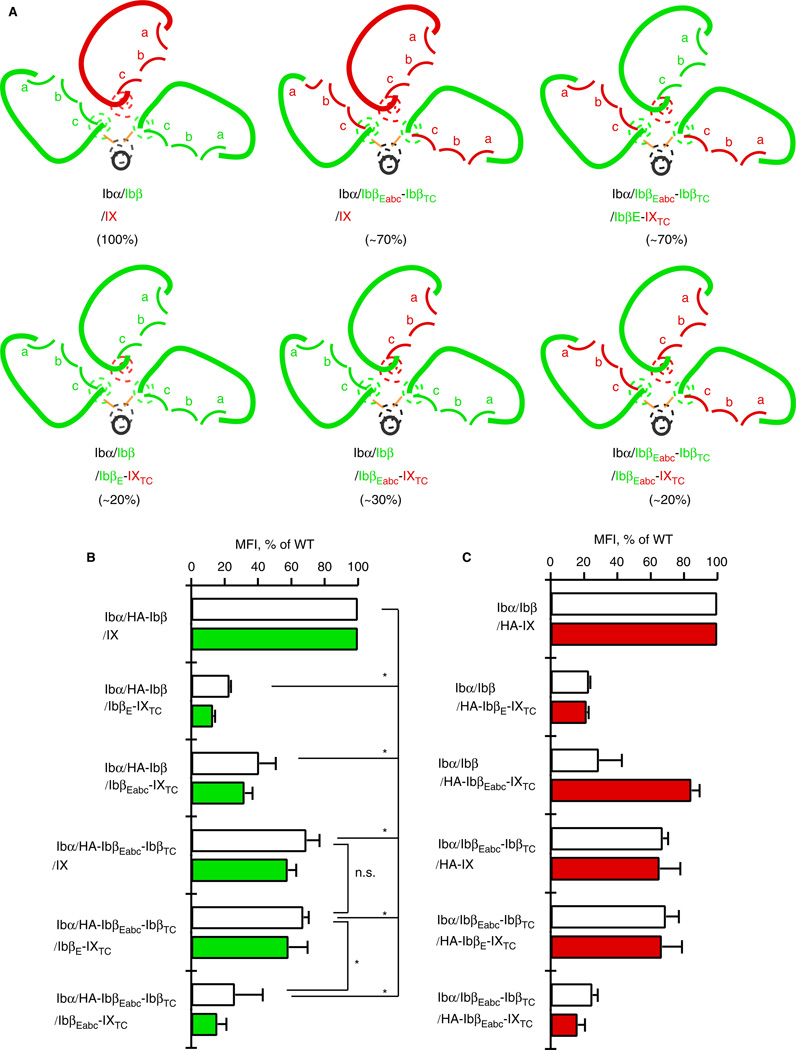
To clearly describe the GPIb-IX complexes containing various chimeric constructs, we have adopted a nomenclature that largely follows the traditional names but has small differences. In this paper, only a complex keeps ‘GP’ in its name (e.g. GPIb). A full-length subunit is named without ‘GP’ and without a subscript (e.g. Ibβ). A domain in the subunit is identified by the subunit name with a subscript (e.g. IbβE). The subscripts E, T and C denote the extracellular, transmembrane and cytoplasmic domains, respectively. A chimeric subunit is identified by the individual domains therein, listed sequentially from the N- to C-terminus. For instance, HA-IbβEabc-IXTC is N-terminally HA-tagged IbβEabc chimeric extracellular domain fused to the transmembrane and cytoplasmic domains of IX. Finally, various GPIb-IX complexes as well as CHO cells expressing them are identified by the subunits therein, separated by ‘/’. For instance, Ibα/Ibβ/IbβEabc-IXTC cells are the cells expressing a mutant complex that contains wild-type Ibα, wild-type Ibβ and the chimeric IbβEabc-IXTC subunit. Also, to appreciate the effect of a mutation, it will be helpful to view it in the three-dimensional context, for instance as sketched in Figure 2(A.)
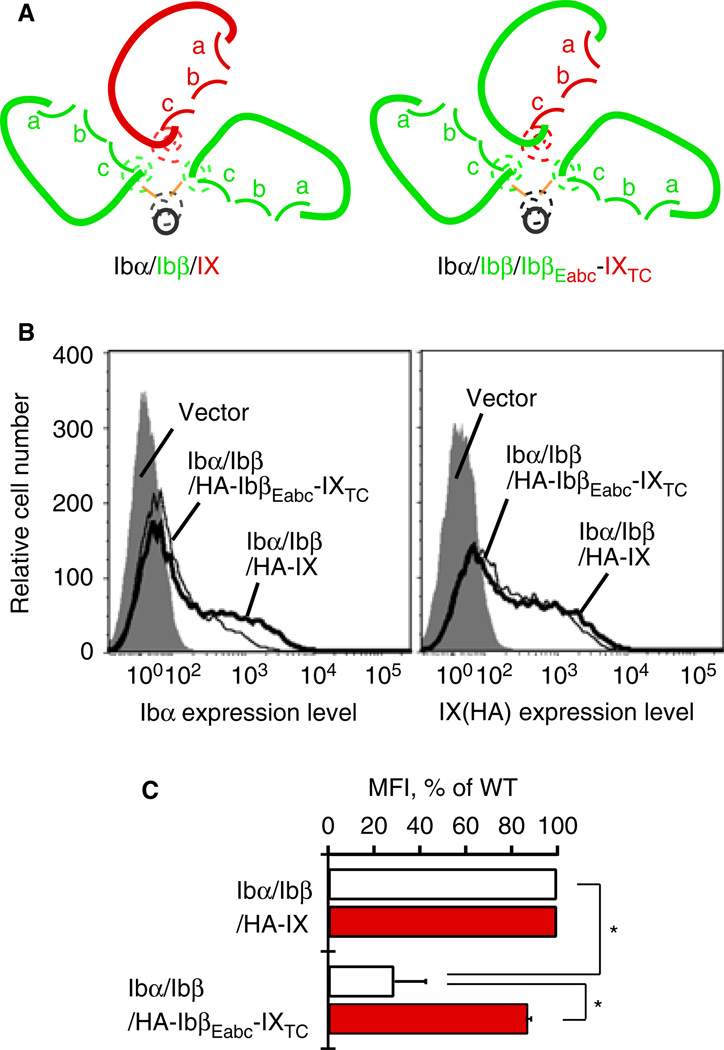
Fig. 2.
Replacing IXE in GPIb-IX with IbβEabc significantly decreases surface expression of Ibα in transiently transfected CHO cells. (A) Sketches of the mutant GPIb-IX complex containing IbβEabc-IXTC in comparison with the wild type. In GPIbβEabc, three convex loops of IXE are grafted onto the IbβE scaffold [18]. (B) Overlaid histograms showing surface expression of Ibα and IX derivatives in transfected CHO cells measured by flow cytometry using WM23 and anti-HA antibodies, respectively. Each trace is identified by the subunits transfected. Gray peak: cells transfected with empty vector. Thick line: Ibα/Ibβ/HA-IX cells. Thin line: Ibα/Ibβ/HA-IbβEabc-IXTC cells. Each plot is representative of at least four independent experiments. (C) Quantitative representation of relative surface expression levels of Ibα (white bar) and HA-tagged IX derivatives (red) in aforementioned transfected cells. The measured mean fluorescence intensity (MFI), obtained for the entire cell population (10 000 cells per sample), was normalized with the expression level in Ibα/Ibβ/HA-IX (WT) cells being 100% and cells transfected with empty vectors 0% [9,18]. All data are presented as mean ± SD (n = 4 or 6). *P < 0.01.
Ibβ enhances IbβEabc-IXTC surface expression but not vice versa
As the surface expression levels of GPIb-IX subunits in transiently transfected CHO cells closely correlate with the extent of GPIb-IX assembly [17,22], they have been used to analyze the complex assembly [8,9,18,19,23]. We have previously shown that appending the HA tag to the N-terminus of Ibβ or IX does not affect GPIb-IX assembly or its expression, and that surface expression of HA-IbβEabc-IXTC is significantly enhanced by co-expression of Ibβ because the former associates with the latter through IX-derived convex loops [18,19]. Consistently, when HA-IbβEabc-IXTC was transiently transfected with wild-type Ibα and Ibβ into CHO cells (i.e. Ibα/Ibβ/HA-IbβEabc-IXTC cells), its surface expression level, measured by flow cytometry using anti-HA antibody, was similar to that of HA-IX in Ibα/Ibβ/HA-IX cells (Fig. 2B,C). However, the measured mean fluorescence intensity of Ibα in Ibα/Ibβ/HA-IbβEabc-IXTC cells was only about 30% of that in ‘wild-type’ Ibα/Ibβ/HA-IX cells, indicating that Ibα in the mutant complex was not as stably assembled as in the wild type. The significant difference between the relative expression levels of Ibα and HA-IbβEabc-IXTC subunits in the same complex is very unusual and has never been reported.
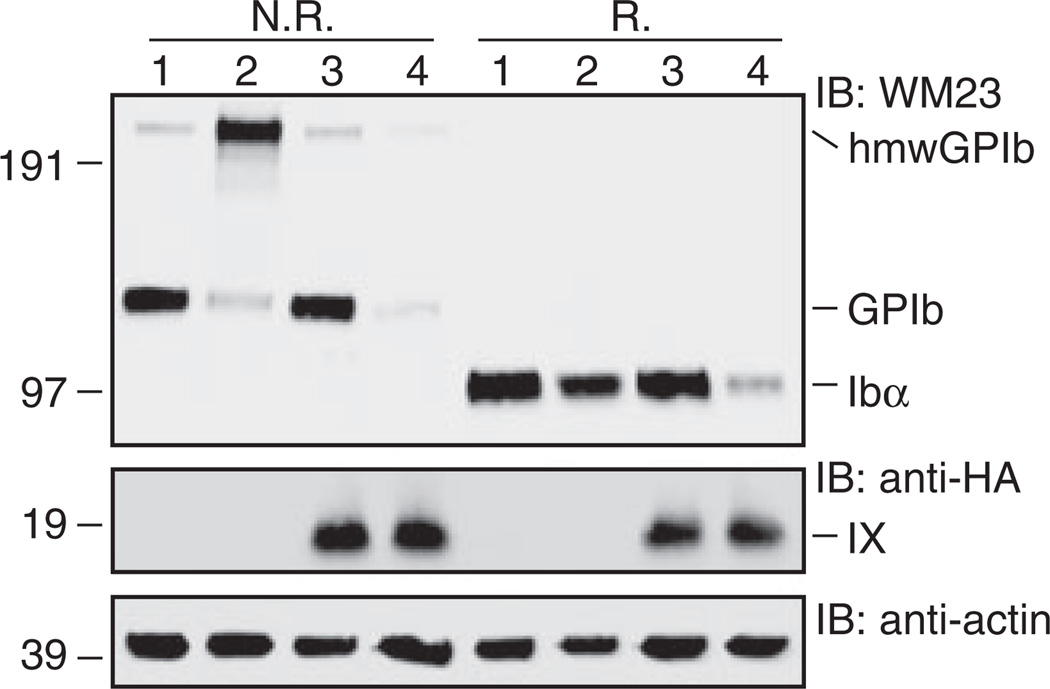
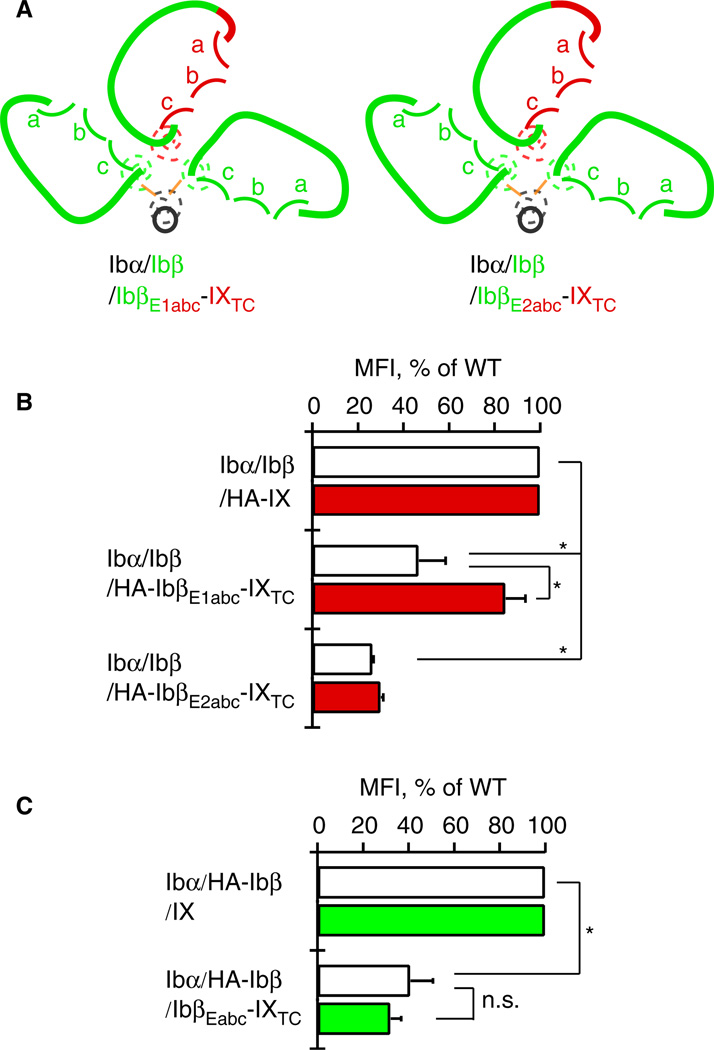
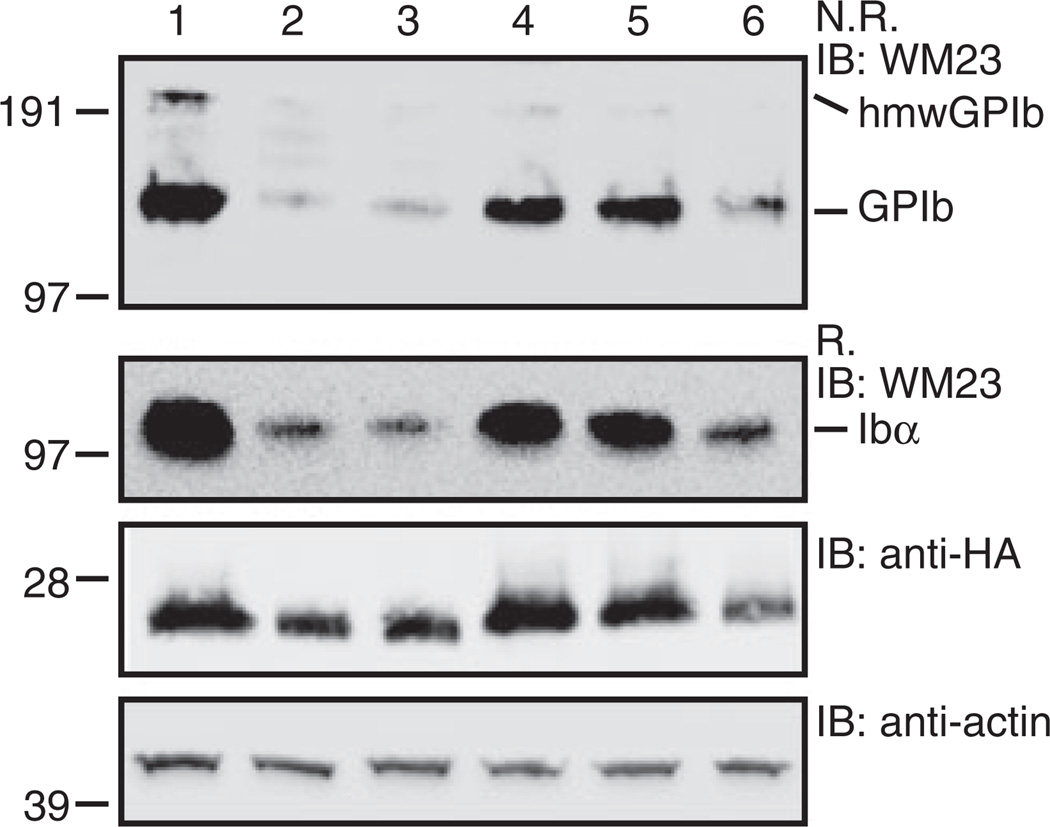
We next sought to explain the low expression of Ibα in Ibα/Ibβ/HA-IbβEabc-IXTC cells. First, western blot of cell lysates under non-reducing conditions showed that native Ibα-Ibβ disulfide bonds were formed in the Ibα/Ibβ/HA-IbβEabc-IXTC complex (Fig. 3). Similarly, no increase in the formation of hmwGPIb was observed, indicating that the association of transmembrane domains was not affected by the domain replacement. Second, to explore the possibility that IXE may contain a separate binding interface for Ibα that was abolished by the domain replacement, we systematically changed residues in IbβEabc back to those in IXE, especially those located on the surface and distal from both interfaces with Ibβ. Only two types of results were observed for all the tested mutations (Fig. 4). The first type, exemplified by IbβE1abc-IXTC, in which the N-capping region is replaced with that in IXE (Fig. 4A), had little effect on the disparate expression levels of Ibα and mutant IX in transfected cells. The other type, exemplified by IbβE2abc-IXTC, in which all residues preceding the convex loops were changed to IX-derived ones, exhibited low expression levels of both Ibα and mutant IX. This was likely because the mutation disrupted proper folding of IbβEabc. No mutations in IbβEabc-IXTC that we had tested could restore the wild-type-like Ibα expression level (for a list of tested mutations, see supporting information Table S1). Finally, in order to assess the Ibβ surface expression level in transfected cells, the HA tag was appended to the N-terminus of Ibβ and concurrently removed from IX. As shown in Figure 4(C), the measured fluorescence intensity of Ibβ in Ibα/HA-Ibβ/IbβEabc-IXTC cells was around 30% of that in Ibα/HA-Ibβ/IX cells, matching the relative ratio of Ibα. This result indicates that the low expression level of Ibβ in Ibα/HA-Ibβ/IbβEabc-IXTC cells is the limiting factor for complex expression. Therefore, although surface expression of IbβEabc-IXTC is enhanced by the presence of Ibβ [19], surface expression of Ibβ is not reciprocally enhanced by IbβEabc-IXTC. Of all the inter-subunit interfaces in the Ibα/HA-Ibβ/IbβEabc-IXTC complex, only interface 2 is altered from the wild type (Fig. 2A). Thus, our results suggest that interface 2 is important in stabilizing the second Ibβ subunit and, by extension, the entire complex.
Fig. 3.
SDS gels showing correct formation of native Ibα-Ibβ disulfide bonds in the Ibα/Ibβ/HA-IbβEabc-IXTC complex. Lysates from various transfected CHO cells were separated in Bis-Tris SDS gels under non-reducing (N.R.) or reducing (R.) conditions, transferred to a PVDF membrane and immunoblotted by noted antibodies. Lane 1, Ibα/Ibβ/IX; 2, Ibα/Ibβ; 3, Ibα/Ibβ/HA-IX; 4, Ibα/Ibβ/HA-IbβEabc-IXTC. This figure is representative of four independent experiments.
Fig. 4.
Reduced surface expression of Ibα is due to the reduced expression of Ibβ in the Ibα/Ibβ/HA-IbβEabc-IXTC complex. (A) Sketches of mutant GPIb-IX complexes used. The style follows that described in Figure 1. (B) Relative surface expression levels of Ibα (white bar) and HA-tagged IX derivatives (red) in transfected CHO cells measured by flow cytometry. Each cell is identified by the subunits transfected. (C) Relative surface expression levels of Ibα (white bar) and HA-tagged Ibβ (green) in transfected CHO cells. The HA tag was appended to Ibβ and removed from IX derivatives to allow detection of Ibβ by flow cytometry. The measurement and quantitation follow the procedure described in Figure 2(C). All data are presented as mean ± SD (n = 3). *P < 0.01.
Replacing IbβE in GPIb-IX with IbβEabc largely preserves complex assembly and stability
We have constructed two additional Ibβ/IX chimeric subunits, both of which could be expressed alone at a modest level in transfected CHO cells. In IbβEabc-IbβTC, IbβEabc is linked to the transmembrane and cytoplasmic domains of Ibβ. In IbβE-IXTC, IbβE is linked to the transmembrane and cytoplasmic domains of IX (Fig. 5A). Genes encoding these proteins, along with those of Ibβ, IX and IbβEabc-IXTC, were co-transfected transiently, in various combinations, with the wild-type Ibα gene into CHO cells. An N-terminal HA tag was attached to Ibβ, IX or the chimeric subunit to allow measurement and comparison of their surface expression levels in the cell (Fig. 5B,C).
Fig. 5.
Extracellular domains of Ibβ and IX adopt different conformations to stabilize certain domain-swapped GPIb-IX complexes and enhance their surface expression. (A) Sketches of wild-type GPIb-IX and five chimeric complexes, each of which is identified by the subunits therein in colors as described in Figure 1. The measured mean fluorescence intensity of surface Ibα in transfected CHO cells, expressed as the percentage of that in wild-type cells and indicative of GPIb-IX assembly and stability, is listed in parenthesis for each complex. Note that although the extracellular domains are similarly sketched to denote their high structural homology to one another, they adopt neither the same conformation nor interaction in different complexes. (B) Relative surface expression levels of Ibα (white bar) and HA-tagged Ibβ derivatives (green) in transfected CHO cells. Each CHO cell is identified by the subunits transfected. (C) Relative surface expression levels of Ibα (white bar) and HA-tagged IX derivatives (red) in transfected CHO cells. The measurement and quantitation follow that described in Figure 2(C). All data are presented as mean ± SD (n = 3–5). Only examples of statistical analysis results are shown. *P < 0.01.
A combination of two Ibβ-equivalent subunits (Ibβ, IbβEabc-IbβTC) and three IX-equivalent ones (IX, IbβE-IXTC, IbβEabc-IXTC) produced a total of five chimeric complexes in addition to the wild-type Ibα/Ibβ/IX complex (Fig. 5A). All of them formed native Ibα-Ibβ disulfide bonds (Fig. 6). Although the Ibα expression level in cells expressing these complexes varied greatly, no increased formation of hmwGPIb was observed. The preservation of wild-type Ibα-Ibβ disulfide bonds and transmembrane domain interactions in these chimeric complexes indicates that the organization of individual subunits in each complex is the same as the wild type (Fig. 5A). As correct assembly of GPIb-IX involves association between both extracellular and transmembrane domains [3], the preservation of transmembrane domain association also indicates that these five chimeric complexes differ only in the association of the Ibβ and IX extracellular domains.
Fig. 6.
SDS gels showing the effect of domain replacement on the expression and Ibα-Ibβ disulfide formation in various transfected CHO cells. Lysates from various CHO cells transfected with GPIb-IX subunits were separated in Bis-Tris SDS gels under non-reducing (N.R.) or reducing (R.) conditions, transferred to PVDF membrane and immunoblotted by noted antibodies. Lane 1, Ibα/HA-Ibβ/IX; 2, Ibα/HA-Ibβ/IbβE-IXTC; 3, Ibα/HA-Ibβ/IbβEabc-IXTC; 4, Ibα/HA-IbβEabc-IbβTC/IX; 5, Ibα/HA-IbβEabc-IbβTC/IbβE-IXTC; 6, Ibα/HAIbβEabc-IbβTC/IbβEabc-IXTC. This figure is representative of three independent experiments.
Comparison of surface expression levels of Ibα, Ibβ- and IX-equivalent subunits in wild-type and chimeric GPIb-IX complexes produced results that could not be explained simply by two separate interfaces in the extracellular domains (Fig. 5). For instance, Ibα surface expression was low in Ibα/Ibβ/IbβE-IXTC cells, with the measured mean fluorescence intensity at about 20% of that in wild-type Ibα/Ibβ/IX cells. Western blot of the cell lysates also produced similar results (Fig. 6). The reduced Ibα expression level was not surprising because IbβE does not self-associate [19] and replacing IXE with IbβE was expected to abolish both interfaces 1 and 2 (Fig. 5A). However, it was surprising that expression levels of Ibα and associated subunits in Ibα/IbβEabc-IbβTC/IbβEabc-IXTC cells were as low as those in Ibα/Ibβ/IbβE-IXTC cells, despite IbβEabc domains in this complex being expected to form two interfaces [19]. Moreover, both Ibα/IbβEabc-IbβTC/IbβE-IXTC and Ibα/Ibβ/IbβEabc-IXTC complexes contain the same interfaces, except that these interfaces are in different orders or locations. Yet these two complexes produced significantly different levels of Ibα. Finally, despite the obvious difference in the extracellular domain of their IX-equivalent subunits, Ibα/IbβEabc-IbβTC/IX and Ibα/IbβEabc-IbβTC/IbβE-IXTC complexes were expressed at the same level, about 70% of those in Ibα/Ibβ/IX cells (Fig. 5), suggesting that both complexes are reasonably assembled like the wild type and have the same level of stability. Therefore, it appears that the effect of changing interfaces is not additive, because simply counting the number of preserved interfaces in these chimeric complexes would not explain all of the reported results. A more plausible explanation is that interfaces 1 and 2 are connected. Changing one interface is likely to affect the other, probably through a conformational change or allostery. Similarly, for the IbβEabc domain in IbβEabc-IbβTC to associate with IXE and IbβE domains in the Ibα/IbβEabc-IbβTC/IX and Ibα/IbβEabc-IbβTC/IbβE-IXTC complexes, respectively, to achieve similar complex stability, it should adopt different conformations in these complexes.
In summary, by analyzing and comparing the organization and expression levels of various GPIb-IX complexes in which IbβE and/or IXE were replaced by homologous domains, we have provided evidence for the importance of interface 2 in the assembly and stability of GPIb-IX. The results further indicate that Ibβ and IX extracellular domains are capable of conformational changes.
Discussion
Proper assembly and efficient expression of the GPIb-IX complex requires all of its subunits [7]. Systematic assessment of the mutational effect on surface expression of GPIb-IX subunits in transiently transfected CHO cells, corroborated in many instances by direct characterization of GPIb-IX from BSS patients bearing the same mutation, has greatly advanced our understanding of GPIb-IX structure and organization [3]. Inter-subunit interfaces in the extracellular and transmembrane domains have been identified [8,9,18,19,23]. Two IbβE and one IXE interact with one another through two interfacial regions (Fig. 1). Analysis of site-specific mutations at interface 1 demonstrates its importance for efficient expression of IX [19]. By comparison, only one mutation at interface 2 that reduces expression (P74R of Ibβ) has been characterized [19]. In this study, by analyzing the effects of co-expressing IbβEabc-IXTC with Ibα and Ibβ, we have provided additional evidence for the importance of interface 2 in assembly and expression of GPIb-IX, particularly stability of Ibβ.
Unlike IX, Ibβ can express alone on the cell surface, albeit at a modest level [7,20,24]. This is likely to be due to the juxtamembrane sequence in its cytoplasmic domain that is involved in protein topology and trafficking [21], its transmembrane domain that is capable of self-oligomerization to prevent exposure of polar residues therein [16,25], and its extracellular domain that can be expressed as a well-folded protein [19,26], all of which are features that IX lacks. Yet like IX, expression of Ibβ is significantly enhanced when it is co-expressed with the other subunits of GPIb-IX [7,20]. The mechanism for this enhancement has not been elucidated. In this study, we report that replacing IXE in GPIb-IX with IbβEabc specifically disrupted interface 2 while preserving interface 1 and other inter-subunit interactions, which led to significantly reduced expression of Ibβ in the Ibα/Ibβ/IbβEabc-IXTC complex. Thus, we have identified interface 2, or the convex loops of Ibβ, as a site of interaction for improved stability and expression of Ibβ. This is similar to the enhancement of IX expression through interaction of its convex loops [18]. Elucidation of the molecular basis for interaction-mediated enhancement of complex expression will contribute to our understanding of the assembly and organization of the GPIb-IX complex.
This study has also produced the first evidence suggesting that IbβE and IXE domains in GPIb-IX can undergo conformational changes. We found, unexpectedly, that both Ibα/IbβEabc-IbβTC/IX and Ibα/IbβEabc-IbβTC/IbβE-IXTC complexes are equally stable and can be expressed at a reasonable level (Figs 4 and 5). Both complexes share the same domain organization as the wild type, and differ from the wild type by only changes in extracellular domains of Ibβ and IX (Fig. 5). In these complexes, it is likely that IbβE, IbβEabc and IXE domains adopt different conformations in response to different domain contacts. This is consistent with the reported crystal structures of IbβE and IbβEabc, both of which share the same sequence for the C-capping region. However, the C-capping region of IbβE in the crystal structure does not have contact with another protein, while that of IbβEabc, taking on a slightly different conformation, forms extensive contact with the convex loops of another IbβEabc [19]. It is also consistent with the fact that the GPIb-IX complex contains two IbβE domains that are not symmetrically located. The two IbβE domains form different contacts with IXE and possibly also Ibα (Fig. 1), and therefore should adopt different conformations. Although we have presented evidence suggesting different conformations of IbβE, IXE and IbβEabc domains in various complexes, their structural details remain to be defined.
That IbβE and IXE can undergo conformational change in GPIb-IX may help to address a fundamental question about the complex. Ibα possesses the binding sites for all the known ligands of GPIb-IX, but why does it need to co-express with Ibβ and IX? One likely possibility is that Ibβ and IX participate in Ibα-initiated signaling. Consistently, the cytoplasmic domain of Ibβ plays a role in modulating Ibα function [13,27,28]. RAM.1, a monoclonal antibody targeting IbβE in GPIb-IX, reduces platelet adhesion and GPIb signaling via intracellular signaling events [15]. Thus, it is possible that RAM.1 binding, or ligand binding of Ibα, induces a conformational change in IbβE and IXE, which transmits the signal across the platelet membrane. Whether antibody or ligand binding induces a change of IbβE and IXE conformation in the GPIb-IX complex, and the underlying structural basis for such change if it occurs, will require future investigation.
Supplementary Material
Acknowledgements
We thank F. Lanza for suggesting the term of hmwGPIb and Emory Children’s Pediatric Research Center Flow Cytometry Core for technical support. This work was supported by NIH (HL082808). WY acknowledges the support of the Natural Science Foundation of China (81200355).
Footnotes
Addendum
L. Zhou and W. Yang designed and performed research, analyzed data and wrote the manuscript. R. Li designed research, analyzed data and wrote the manuscript.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interests.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. A list of not-so-productive mutations generated in this study.
References
- 1.Berndt MC, Gregory C, Kabral A, Zola H, Fournier D, Castaldi PA. Purification and preliminary characterization of the glycoprotein Ib complex in the human platelet membrane. Eur J Biochem. 1985;151:637–649. doi: 10.1111/j.1432-1033.1985.tb09152.x. [DOI] [PubMed] [Google Scholar]
- 2.Luo S-Z, Mo X, Afshar-Kharghan V, Srinivasan S, Lopez JA, Li R. Glycoprotein Ibα forms disulfide bonds with 2 glycoprotein Ibβ subunits in the resting platelet. Blood. 2007;109:603–609. doi: 10.1182/blood-2006-05-024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li R, Emsley J. The organizing principle of the platelet glycoprotein Ib-IX-V complex. J Thromb Haemost. 2013;11:605–614. doi: 10.1111/jth.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huizinga EG, Tsuji S, Romijn RA, Schiphorst ME, de Groot PG, Sixma JJ, Gros P. Structures of glycoprotein Ibα and its complex with von Willebrand factor A1 domain. Science. 2002;297:1176–1179. doi: 10.1126/science.107355. [DOI] [PubMed] [Google Scholar]
- 5.Dumas JJ, Kumar R, Seehra J, Somers WS, Mosyak L. Crystal structure of the GpIbα-thrombin complex essential for platelet aggregation. Science. 2003;301:222–226. doi: 10.1126/science.1083917. [DOI] [PubMed] [Google Scholar]
- 6.Celikel R, McClintock RA, Roberts JR, Mendolicchio GL, Ware J, Varughese KI, Ruggeri ZM. Modulation of α-thrombin function by distinct interactions with platelet glycoprotein Ibα. Science. 2003;301:218–221. doi: 10.1126/science.1084183. [DOI] [PubMed] [Google Scholar]
- 7.Lopez JA, Leung B, Reynolds CC, Li CQ, Fox JEB. Efficient plasma membrane expression of a functional platelet glycoprotein Ib-IX complex requires the presence of its three subunits. J Biol Chem. 1992;267:12851–12859. [PubMed] [Google Scholar]
- 8.Luo S-Z, Mo X, López JA, Li R. Role of the transmembrane domain of glycoprotein IX in assembly of the glycoprotein Ib-IX complex. J Thromb Haemost. 2007;5:2494–2502. doi: 10.1111/j.1538-7836.2007.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mo X, Lu N, Padilla A, López JA, Li R. The transmembrane domain of glycoprotein Ibβ is critical to efficient expression of glycoprotein Ib-IX complex in the plasma membrane. J Biol Chem. 2006;281:23050–23059. doi: 10.1074/jbc.M600924200. [DOI] [PubMed] [Google Scholar]
- 10.Berndt MC, Andrews RK. Bernard-Soulier syndrome. Haematologica. 2011;96:355–359. doi: 10.3324/haematol.2010.039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato K, Martinez C, Russell S, Nurden P, Nurden A, Fiering S, Ware J. Genetic deletion of mouse platelet glycoprotein Ibβ produces a Bernard-Soulier phenotype with increased α-granule size. Blood. 2004;104:2339–2344. doi: 10.1182/blood-2004-03-1127. [DOI] [PubMed] [Google Scholar]
- 12.Strassel C, Nonne C, Eckly A, David T, Leon C, Freund M, Cazenave JP, Gachet C, Lanza F. Decreased thrombotic tendency in mouse models of the Bernard-Soulier syndrome. Arterioscler Thromb Vasc Biol. 2007;27:241–247. doi: 10.1161/01.ATV.0000251992.47053.75. [DOI] [PubMed] [Google Scholar]
- 13.Bodnar RJ, Xi X, Li Z, Berndt MC, Du X. Regulation of glycoprotein Ib-IX-von Willebrand factor interaction by cAMP-dependent protein kinase-mediated phosphorylation at Ser 166 of glycoprotein Ibβ. J Biol Chem. 2002;277:47080–47087. doi: 10.1074/jbc.M208329200. [DOI] [PubMed] [Google Scholar]
- 14.Ravanat C, Strassel C, Hechler B, Schuhler S, Chicanne G, Payrastre B, Gachet C, Lanza F. A central role of GPIb-IX in the procoagulant function of platelets that is independent of the 45-kDa GPIbα N-terminal extracellular domain. Blood. 2010;116:1157–1164. doi: 10.1182/blood-2010-01-266080. [DOI] [PubMed] [Google Scholar]
- 15.Maurer E, Tang C, Schaff M, Bourdon C, Receveur N, Ravanat C, Eckly A, Hechler B, Gachet C, Lanza F, Mangin PH. Targeting platelet GPIbβ reduces platelet adhesion, GPIb signaling and thrombin generation and prevents arterial thrombosis. Arterioscler Thromb Vasc Biol. 2013;33:1221–1229. doi: 10.1161/ATVBAHA.112.301013. [DOI] [PubMed] [Google Scholar]
- 16.Luo SZ, Li R. Specific heteromeric association of four transmembrane peptides derived from platelet glycoprotein Ib-IX complex. J Mol Biol. 2008;382:448–457. doi: 10.1016/j.jmb.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenny D, Morateck PA, Gill JC, Montgomery RR. The critical interaction of glycoprotein (GP) Ibβ with GPIX – a genetic cause of Bernard-Soulier syndrome. Blood. 1999;93:2968–2975. [PubMed] [Google Scholar]
- 18.Mo X, Nguyen NX, McEwan PA, Zheng X, Lopez JA, Emsley J, Li R. Binding of platelet glycoprotein Ibβ through the convex surface of leucine-rich repeats domain of glycoprotein IX. J Thromb Haemost. 2009;7:1533–1540. doi: 10.1111/j.1538-7836.2009.03536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McEwan PA, Yang W, Carr KH, Mo X, Zheng X, Li R, Emsley J. Quaternary organization of GPIb-IX complex and insights into Bernard-Soulier syndrome revealed by the structures of GPIbβ and a GPIbβ/GPIX chimera. Blood. 2011;118:5292–5301. doi: 10.1182/blood-2011-05-356253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez JA, Weisman S, Sanan DA, Sih T, Chambers M, Li CQ. Glycoprotein (GP) Ibβ is the critical subunit linking GP Ibα and GP IX in the GP Ib-IX complex. Analysis of partial complexes. J Biol Chem. 1994;269:23716–23721. [PubMed] [Google Scholar]
- 21.Mo X, Luo S-Z, Lóopez JA, Li R. Juxtamembrane basic residues in glycoprotein Ibβ cytoplasmic domain are required for assembly and surface expression of glycoprotein Ib-IX complex. FEBS Lett. 2008;582:3270–3274. doi: 10.1016/j.febslet.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong JF, Gao S, Lopez JA. Synthesis, assembly, and intracellular transport of the platelet glycoprotein Ib-IX-V complex. J Biol Chem. 1998;273:31449–31454. doi: 10.1074/jbc.273.47.31449. [DOI] [PubMed] [Google Scholar]
- 23.Mo X, Liu L, Lopez JA, Li R. Transmembrane domains are critical to the interaction between platelet glycoprotein V and glycoprotein Ib-IX complex. J Thromb Haemost. 2012;10:1875–1886. doi: 10.1111/j.1538-7836.2012.04841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staron M, Wu S, Hong F, Stojanovic A, Du X, Bona R, Liu B, Li Z. Heat-shock protein gp96/grp94 is an essential chaperone for the platelet glycoprotein Ib-IX-V complex. Blood. 2011;117:7136–7144. doi: 10.1182/blood-2011-01-330464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei P, Liu X, Hu MH, Zuo LM, Kai M, Wang R, Luo SZ. The dimerization interface of the glycoprotein Ibbeta transmembrane domain corresponds to polar residues within a leucine zipper motif. Protein Sci. 2011;20:1814–1823. doi: 10.1002/pro.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finch CN, Lyle VA, Cunningham D, Miller JL. Expression of human platelet glycoprotein Ibβ in insect cells. Thromb Res. 1996;81:679–686. doi: 10.1016/0049-3848(96)00045-x. [DOI] [PubMed] [Google Scholar]
- 27.Perrault C, Mangin P, Santer M, Baas MJ, Moog S, Cranmer SL, Pikovski I, Williamson D, Jackson SP, Cazenave JP, Lanza F. Role of the intracellular domains of GPIb in controlling the adhesive properties of the platelet GPIb/V/IX complex. Blood. 2003;101:3477–3484. doi: 10.1182/blood-2002-06-1847. [DOI] [PubMed] [Google Scholar]
- 28.Dai K, Bodnar R, Berndt MC, Du X. A critical role for 14-3-3ζ protein in regulating the VWF binding function of platelet glycoprotein Ib-IX and its therapeutic implications. Blood. 2005;106:1975–1981. doi: 10.1182/blood-2005-01-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.