Abstract
Purpose: In this research the effect of vitamin B1 and B6 on cyanocobalamin stability in commercial light protected parenteral formulations and upon adding stabilizing agents will be investigated and best formulation composition and proper storage condition will be introduced.
Methods: In this research some additives such as co solvents and tonicity adjusters, surfactants, antioxidants and chelating agents as well as buffer solutions, were used to improve the stability of the parenteral mixed formulations of B12 in the presence of other B vitamins (B1 and B6). Screening tests and accelerated stability tests were performed according to ICH guidelines Q1A (R2).
Results: Shelf life evaluation revealed the best formulation and the proper storage condition. The results indicated the first kinetic models for all tested formulations and the optimum pH value was determined to be 5.8. There was no evidence of B12 loss when mixed with B1 and B6 in a medical syringe at room temperature for maximum of 8 hours.
Conclusion: It is necessary to formulate vitamin B12 mixed parenteral solutions using proper phosphate buffers (pH=5.8) and to indicate “Store in refrigerator” on the mixed parenteral formulations of vitamin B12 with other B vitamins, which has not been expressed on the label of tested Brand formulations at the time of this study.
Keywords: Parenteral, Stability, Incompatibility, Light protected, Degradation Kinetics
Introduction
It is clearly proved that vitamin B12 or cyanocobalamin is essential for the healthy survival of human cells and because it is not synthesized in mammalian cells, it can only be obtained from external sources.1
In general, many physical and chemical factors can have a negative effect on stability of vitamins. Water-soluble vitamins are prone to degradation in solutions, particularly when exposed to light. B Vitamins are sensitive to factors such as: light, heat, moisture, oxidizing and reducing agents, acids and or bases. There is plenty of literature concerning the poor stability of vitamin B12 and it has been reported that the optimum pH for stability of this vitamin is 4-6.5 pH value.2-13 It is well known that aqueous solutions of cyanocobalamin are photoliable and some B and C vitamins accelerate the photodegradation.14
Recently the effect of vitamin B2 on the photolysis of cyanocobalamin in aqueous solutions has been investigated by Ahmed et al.15 According to kinetic results they concluded that, vitamin B2 acts as a sensitizer in the photolysis of vitamin B12, and thus causes vitamin B12 instability.15 In another study aqueous cyanocobalamin solutions have been photolysed in the presence of individual B (thiamine HCl, riboflavin, nicotinamide and pyridoxine HCl) and C (ascorbic acid) vitamins under high pressure mercury vapor fluorescent lamp. The TLC results indicated that cyanocobalamin changes to hydroxocobalamine, riboflavin degrades to 4-methyl-5-(ßhydroxyethyl) thiazole and 2-methyl-4-amino-5-hydroxymethyl-pyrimidine, riboflavin substituted partially with formylmethylflavin , lumichrome, lumiflavin and carboxymethylflavin, Ascorbic acid oxidized to dehydroascorbic acid and Nicotinamide and pyridoxine HCl did not degrade at all. They concluded that all these reactions are pH dependent and a pH value around 4 is optimum for photostability of all B and C vitamins in pharmaceutical preparations.15
Although poor stability of this vitamin has been previously reported, certain parenteral formulations are commercialized combining this vitamin with other vitamins especially thiamine (B1) and pyridoxine (B6). Authors detected that some manufacturers use high and illegal overages in their light protected formulations to compensate vitamin B12 instability issues. These finding along with the interest in vitamins combination triggered a study of the incompatibility of cyanocobalamin in light protected formulations to prove the overall effect of vitamin B1 and B6 on cyanocobalamin stability in commercialized parenteral concentrations and also to study the effect of some stabilizing agents in order to designate proper formulations and storage conditions.
In this Study, the stability of commercial formulations was first investigated and then parenteral formulations were designed and prepared using some stabilizing additives such as: chelating agents, cosolvents or tonicity-adjusting agents, antioxidants and different buffer solutions. Proper storage conditions according to ICH guidelines were also evaluated and finally aqueous state degradation kinetics was studied and the predominant model was proposed. In addition possible interaction of vitamin B12 in commercial injections with other B complex formulations when mixed in a single syringe for subsequent human injection has also been investigated.
Materials and Methods
Materials
Cyanocobalamin (5, 6-Dimethylbenzimidazolyl cyanocobamide), Thiamine (3-[(4-Amino–2–methyl–5–pyrimidinyl) methyl]-5-(2–hydroxyethyl)-4–methylthiazolium chloride) and pyridoxine (5-Hydroxy–6–methyl–3,4–pyridinedimethanol) were obtained from Sigma-Aldrich, Germany (Figure 1). Hydroxocobalamine (Sigma-Aldrich, Germany), Ethylenediaminetetraacetic acid (EDTA), propylene glycol (PG), polyethylenglycole (PEG 400), sorbitan mono oleate and buffering agents (Citrate, Acetate and Phosphate) were provided by Merck- Schuchardt, Hohenbrunn, Germany. All other chemicals were of HPLC or analytical grade and obtained from Merck- Schuchardt, Hohenbrunn, Germany. Commercial domestic and foreign parenteral formulations containing cyanocobalamin, named Brand-1–3 were acquired from local market.
Figure 1 .
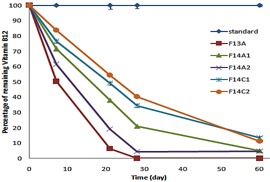
The percentage of remaining vitamin B12 for formulations placed in 40°C oven.
Formulation Methods
Preparation of Parenteral Formulations for Screening and Stability Test
Composition of each prepared formulation has been shown in Table 1. Additives used were solubilizing or tonicity-adjusting agents (lecithin, polyethylene glycol 400, propylene glycol), a surfactant (S.M.O= Sorbitan mono oleate), an antioxidant (S.F.S= Sodium formaldehyde sulfoxylate) and a chelating agent (EDTA). The effect of buffer solutions such as acetate, citrate and phosphate, with different pH values and different concentrations were also studied. It is well known that elevated temperatures are fundamental for screening tests. Based on initial results thermal acceleration of degradation reactions were achieved at 55°C and thus screening tests were performed at this temperature for 5 days. Prepared formulations (n=3) were degassed with bubbling N2 gas and were filled in light protected ampoules and placed in preheated ovens. The amount of remaining vitamin B12 was determined at the end of each day using UV spectrophotometer. Selected formulations were subjected to accelerated stability test according to ICH guidelines.16 Briefly samples (n=3) were stored at 40 and 25°C for 6 months and the remaining vitamin B12 and also vitamin B1 and B6 were measured and recorded using a proper HPLC method. Each stability evaluation has been performed at least 3 times.
Table 1. Prepared formulations for screening test.
| Formulation | vitamins | water | Cosolvents and tonicity adjusting agents | EDTA | S.M.Oc | S.F.Sd | Buffers | |||||||||||||
| B12 | B1 | B6 | P.E.G a | P.Gb | lecithin | acetate | citrate | phosphate | ||||||||||||
| Conc. | 0.33mg/ml | 33mg/ml | 33mg/ml | 50% v/v | 50% v/v | 2% w/v | 0.05% w/v | 0.25% v/v | 1.0% w/v | 0.1 M | 0.2M | 0.05M | 0.1M | 0.025M | 0.05M | |||||
| pH | - | - | - | - | - | - | - | - | - | - | 4.75 | 4.75 | 3.1 | 3.1 | 7 | 8 | 5.8 | 7 | 8 | 5.8 |
| F1 | * | * | ||||||||||||||||||
| F2 | * | * | ||||||||||||||||||
| F3 | * | * | ||||||||||||||||||
| F4 | * | * | * | |||||||||||||||||
| F5 | * | * | * | |||||||||||||||||
| F6 | * | * | * | * | ||||||||||||||||
| F7 | * | * | * | * | * | |||||||||||||||
| F8 | * | * | * | * | * | |||||||||||||||
| F9 | * | * | * | * | * | |||||||||||||||
| F10 | * | * | * | * | * | |||||||||||||||
| F11 | * | * | * | * | * | |||||||||||||||
| F12A | * | * | * | * | * | |||||||||||||||
| F12B | * | * | * | * | * | |||||||||||||||
| F13A | * | * | * | * | * | |||||||||||||||
| F13B | * | * | * | * | * | |||||||||||||||
| F14A1 | * | * | * | * | * | |||||||||||||||
| F14A2 | * | * | * | * | * | |||||||||||||||
| F14B1 | * | * | * | * | * | |||||||||||||||
| F14B2 | * | * | * | * | * | |||||||||||||||
| F14C1 | * | * | * | * | * | |||||||||||||||
| F14C2 | * | * | * | * | * | |||||||||||||||
| F15 | * | * | * | * | * | |||||||||||||||
aP.E.G: Polyethylene glycol
bP.G: Propylene glycol
cS.M.O: Sorbitan mono oleate
dS.F.S: Sodium formaldehyde sulfoxylate
Shelf Life Determination
Instead of the well-known Arrhenius Equation, a recent ICH guideline for shelf life determination introduces a new real time data method with no extrapolation.17 Briefly, the upper and lower accepted USP assay limits for cyanocobalamin injection (115% and 95%) were defined16 and the amount of remaining drug in stability samples (n=3) stored at 25°C, in predetermined time intervals were plotted against time. The results of this test, which was conducted as an accelerated stability test for a product intended for use in refrigerator, were assumed as a 6 month real time data for long term study at room temperature. Data were plotted in excel software and the intersect position of the 95% lower one sided confidence interval of mean with the lower accepted assay limit in the monograph (95%) was detected and shelf life was calculated at room temperature.
Commercial Formulations
Accelerated stability test at 40 and 25°C for 6 months was performed for 3 commercial formulations named Brand-1-3.
Brand 1 and 2 were mixed parenteral formulation containing B12, B1 and B6 and Brand 3 was a mixed B complex formulation containing B1, B2, B6, nicotinamide and dexpanthenol without any cyanocobalamin. The amount of each vitamin (B12, B1 and B6) was analyzed at the end of the first week and then each month during the stability test, using proper HPLC methods.
Stability of Mixed Commercial Formulation
In this section the stability of commercial pure vitamin B12 injection when mixed in a medical grade syringe with vitamin B complex commercial injection (without any vitaminB12 according to the label claim), was studied for 8 hours in 37°C under normal laboratory light.
Liquid-State Kinetics Study
Six month stability data for selected prepared formulations and also brands were fitted to solution kinetics models (zero, First, Second and Third Order) using excel software.18 Mean absolute percentage error19 and R-squared were used to select the best degradation kinetics model.
Analytical Methods
UV Detection
UV analysis was performed on a Shimadzu 1600 UV-visible spectrophotometer (Shimadzu-1600, Japan).
Calibration was performed using standard concentrations of vitamin B12 (20-160 µg/ml) in the presence of constant concentrations of vitamin B1 and B6 (each 0.02 mg/ml). Three samples of each formulation were collected on predetermined time intervals, dilution was performed using 600µl of the ampoule content (except for formulations F2 and F3), into which 2400µl of distilled water was added. Then the UV absorption of prepared solutions against water was detected at 550nm to measure the vitamin B12 quantity. Dilution pattern for formulations F2 and F3 which contain vitamin B1 and vitamin B6, respectively, was as follows. 150µl of each formulation were poured into 3 volumetric flasks and brought to a volume of 250ml using freshly distilled water. Then the UV absorption of diluted solutions was detected at 213nm and 291nm for the determination of vitamin B1 and vitamin B6, respectively. Calibration curve was prepared using different standard concentrations of vitamin B1 and B6 in the presence of constant concentrations of vitamin B12.
HPLC
A liquid chromatographic system (Knauer, Germany) comprising of Knauer K1000 solvent delivery module equipped with a Rheodyne (Cotati, CA) injector and a variable wavelength ultraviolet spectrophotometric detector (Knauer smartline 2500) (PDA) . EZ Chrom Elite version 2.1.7 was used for data acquisition, data reporting and analysis.
Vitamin B1 and B6 quantification was performed isocratically on the reversed phase VP-ODS column (5 µm, 25 cm×4.6 mm; Shimadzu, Japan). The mobile phase consisted of methanol–phosphate buffer (10:90) and 0.018M triethylamine, which was adjusted to pH 3.55 with 85% orthophosphoric acid. The mobile phase was vacuum degassed prior to its use. A volume of 1 ml/min was used as the flow rate, and detection was performed at 283 nm.20 Calibration range used for vitamin B1 mixed with vitamin B6 solutions was 0.0366-0.165 mg/ml.
Vitamin B12 was determined and measured using a newly introduced isocratic reverse phase method at 360 nm. Samples were injected onto a same column, maintained at ambient temperature. Mobile phase was a mixture of acetonitrile:Phosphate buffer (1:6 v/v) and pH was adjusted to 2.6, using orthophosphoric acid. Flow rate was 1.2 ml/min. Calibration was performed using standard solutions of vitamin B12 prepared in mobile phase at concentrations of 0.0103-0.33 mg/ml. Both methods were also applied for Hydroxocobalamine determination.
HPLC method validation
Although there was several methods claiming the simultaneous determination of vitamins B1, B6 and B12, but none of them uses a simple UV detector or common columns.20-22 Method described by Lebiedzinska et al. introduced UV–visible detection only for the determination of thiamine and pyridoxine but not for cyanocobalamin.20-22 The other method introduced by Markopoulou et al., was not sensitive enough to quantitate small concentrations used in commercial parenteral formulations.21 So a new method using C18 column coupled with UV detection as a conventional reversed phase chromatography was introduced for determination of B12 which was sensitive enough to detect small quantities in parenteral formulations. It is a novel and efficient method for determination of vitamin B12 in the presence of other B vitamins using reveres phase HPLC coupled with UV detection. All validation steps including; chromatography method specificity, linearity, accuracy and precision, limit of detection (LOD) and limit of quantification (LOQ), performed for this method and are described in results section.
Results
Formulation Methods
Screening and Stability Test
Based on HPLC results, the cyanocobalamin content of F15 completely decomposed after 2 days. F9 was excluded from the study because of its lecithin content agglomeration after 24 hours. The percentage of remaining vitamin B12 after 5 days of the screening test is shown in Table 2.
Table 2. Remaining actives after 5 days of screening (n=3).
| Formulations | Concentration (n=3)(?) | mean | SD | RSD | Remaining a (%) | ||
| F1 (B12) | 0.070 | 0.071 | 0.068 | 0.070 | 0.001 | 1.612 | 104.78 |
| F2 (B1) | 0.111 | 0.102 | 0.113 | 0.109 | 0.006 | 5.166 | 97.63 |
| F3 (B6) | 0.248 | 0.237 | 0.248 | 0.244 | 0.006 | 2.573 | 91.04 |
| F4 (B12+B1) | 0.053 | 0.055 | 0.057 | 0.055 | 0.002 | 3.688 | 78.94 |
| F5 (B12+B6) | 0.065 | 0.067 | 0.063 | 0.065 | 0.002 | 2.504 | 97.47 |
| F6 (B12+B1+B6) | 0.027 | 0.024 | 0.022 | 0.025 | 0.002 | 8.827 | 40.90 |
| F7 | 0.014 | 0.016 | 0.016 | 0.015 | 0.001 | 6.884 | 25.55 |
| F8 | 0.027 | 0.021 | 0.020 | 0.022 | 0.004 | 15.752 | 31.71 |
| F10 | 0.015 | 0.014 | 0.014 | 0.014 | 0.001 | 5.219 | 24.77 |
| F11 | 0.010 | 0.009 | 0.008 | 0.009 | 0.001 | 12.916 | 15.97 |
| F12A | 0.011 | 0.012 | 0.028 | 0.017 | 0.010 | 56.595 | 29.32 |
| F12B | 0.005 | 0.004 | 0.005 | 0.004 | 0.001 | 16.973 | 7.42 |
| F13A | 0.030 | 0.030 | 0.032 | 0.031 | 0.001 | 3.103 | 50.07 |
| F13B | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 | 1.89 |
| F14 A1 | 0.074 | 0.078 | 0.072 | 0.075 | 0.003 | 3.962 | 67.69 |
| F14 A2 | 0.035 | 0.036 | 0.036 | 0.036 | 0.001 | 2.267 | 52.57 |
| F14 B1 | 0.026 | 0.027 | 0.029 | 0.027 | 0.001 | 4.783 | 39.93 |
| F14 B2 | 0.024 | 0.025 | 0.027 | 0.025 | 0.002 | 6.208 | 32.75 |
| F15 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.00 |
a indicates remaining B12 for all formulations except F2 and F3 for which represents their active ingredients remaining percentage
Based on the screening test results 5 formulations (F13A, F14A1, F14A2, F14C1 and F14C2) were selected for performing a 6-month stability test (Table 1). Formulations were assayed after passing the screening test and selection was performed empirically based on the total decomposition amount of each formulation to less than 40 percent of their initial content. The results of a 6-month stability test for selected formulations in 40°C oven (accelerated stability test for a product intended for storage at room temperature), and room temperature (25°C) (accelerated stability test for a product intended for storage in a refrigerator) are shown in Figure 1 and Figure 2, respectively. These charts demonstrate the percentage of remaining vitamin B12 calculated using a HPLC method. The test ended when more than 90% loss in the amount of vitamin B12 was detectable.
Figure 2 .
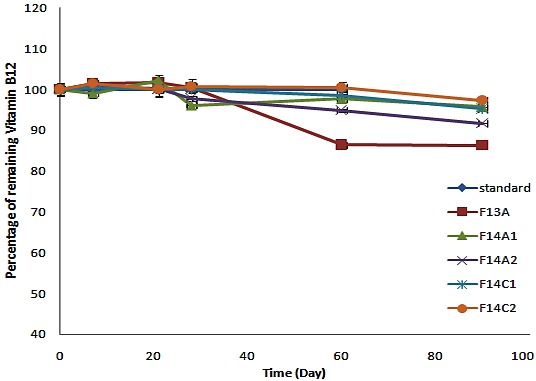
The percentage of remaining vitamin B12 for formulations incubated at room temperature (25°C).
The percentage of remaining vitamin B1 in selected formulations placed in 40°C oven analyzed using HPLC and is shown in Figure 3. No significant change (more than 5% loss) was detected in the amount of vitamin B6 in the formulations kept at 40°C oven. The amount of vitamin B1 and B6 was also constant during incubation at room temperature.
Figure 3 .
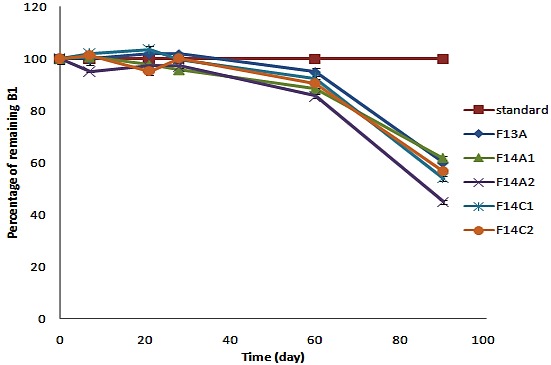
The percentage of remaining vitamin B1 in prepared formulations after incubation at 40°C.
Shelf Life Determination
Calculated shelf lives of five formulations at room temperature are shown in Table 3.
Table 3. Expiration date of selected formulations in room temprature .
| formulation | Expiration date(month) |
| F13A | 1 |
| F14A1 | 2 |
| F14A2 | 1.5 |
| F14C1 | 3 |
| F14C2 | 3.5 |
Commercial Formulations
As it mentioned before the main point in studying the stability of mixed commercial formulations was triggered after analyzing the initial content of vitamin B12 in new commercial products according to their production date. The majority of original innovators had used more than 5 percent of labeled claim as allowable overage excess content in their formulations. This may be due to compensation of the subsequent loss during shelf life period. The initial percentage of vitamin B12, B1 and B6 in commercial formulations according to their label claim in the time of testing (assay date) are listed in Table 4.
Table 4. Remaining vitamins at assay date in commercial formulations .
| Brands | remaining B12 a | remaining B1a | remaining B6a | Remaining shelf life at assay date (months) |
| Brand 1 | 111.8925 | 112.1 | 117.6 | 11 |
| Brand 2 | 68.17738 | 123.2 | 113.3 | 29 |
| Brand 3 | 129.51 | 130.4 | 117.2 | 20 |
a Percentage of label claim
The results of the stability test for commercial formulations incubated in a 40°C oven are shown in Figure 4. Data for vitamin B1 and B6 is not sown as no detectable change (more than 5% loss) was observed in the test samples.
Figure 4 .
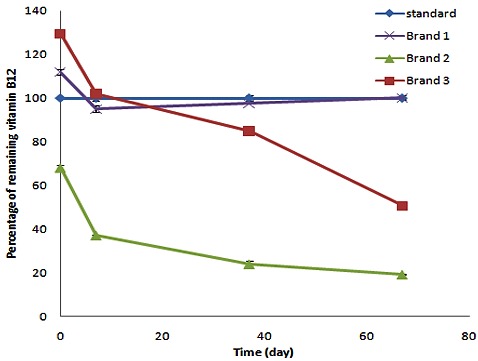
The percentage of remaining vitamin B12 in commercial formulations incubated at 40°C.
Stability of Mixed Commercial Formulation
The percentage of remaining vitamin B12 in light protected mixed commercial formulations after 8 hours at 37°C reduced to 97%.
Liquid-State Kinetics Study
The best model for vitamin B12 degradation kinetics in selected formulations kept at 25°C and commercials at 40°C were determined according to mean absolute percentage error and R-squared, using excel software. The results indicated the first order kinetics models for all tested formulations.
HPLC Methods
Figure 5 depicts the HPLC chromatogram of Hydroxocobalamin standard solution, mixed standard solution of B1 and B6 and stability Sample (F14C2) detected with the HPLC method.
Figure 5 .
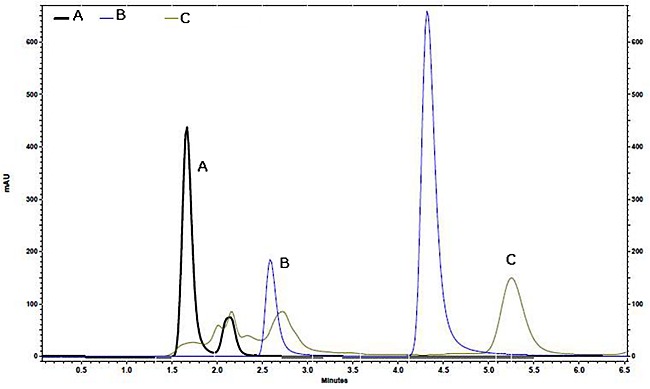
HPLC chromatogram of: A) Hydroxocobalamin standard solution, B) Mixed standard solution of B1 and B6 The first peak (2.6 min) is related to vitamin B1 and the second peak (4.3 min) is related to vitamin B6, C) stability Sample (F14C2) detected with the HPLC method.
HPLC Method Validation for Vitamin B12
The retention time of vitamin B12 was about 6.4 min. Based on HPLC chromatogram the specified B12 peak in stability samples and pure solutions were collected from several HPLC injections and then scanned using UV-spectrophotometer and the purity in stability samples was verified through peak purity analysis using PDA.23
The calibration curve for vitamin B12 was prepared using five points in the range of 0.0103-0.33 mg/ml, and the regression parameter was equal to 1.For determination of the accuracy and precision of the method four samples with concentrations in calibration range were used. Then the mean observed concentrations, standard deviations, C.V. and accuracy were calculated. Results for intra-assay precision (repeatability) and inter-assay precision are shown in Tables 5 and 6.
Table 5. Intra-assay precision achieved from four calibration curves.
| Vitamin B12 added concentration(mg/ml) | Vitamin B12 mean measured concentration(mg/ml) | S.D | C.V.(%) | Accuracy(%) |
| 0.33 | 0.337 | 0.85 | 1.73 | 102.1 |
| 0.0825 | 0.083 | 0.52 | 2.04 | 101.0 |
| 0.0412 | 0.040 | 0.49 | 2.02 | 99.3 |
| 0.0206 | 0.020 | 0.75 | 0.54 | 100.2 |
Table 6. Inter-assay precision achieved from four calibration curves.
| Vitamin B12 added concentration(mg/ml) | Vitamin B12 mean measured concentration(mg/ml) | S.D.(mg/ml) | C.V.(%) | Accuracy(%) |
| 0.33 | 0.332 | 0.51 | 0.82 | 100.6 |
| 0.0825 | 0.0816 | 0.88 | 1.56 | 98.9 |
| 0.0412 | 0.0416 | 0.48 | 1.48 | 100.9 |
| 0.0206 | 0.0208 | 0.62 | 0.32 | 100.9 |
LOD and LOQ are two parameters that are related to the sensitivity of HPLC method. The signal-to-noise ratio of 3:1 for LOD and 10:1 for LOQ were taken. The peaks were clearly identifiable and had acceptable precision. In this method the LOQ value for vitamin B12 was 0.009 mg/ml and the LOD was 0.003 mg/ml.
Discussion
Screening and Stability Test
Vitamin B12 or 5,6-Dimethylbenzimidazolyl cyanocobamide contains several functional groups which makes it prone to different chemical reactions. These groups can be divided into 4 major parts (Figure 6):
Figure 6 .
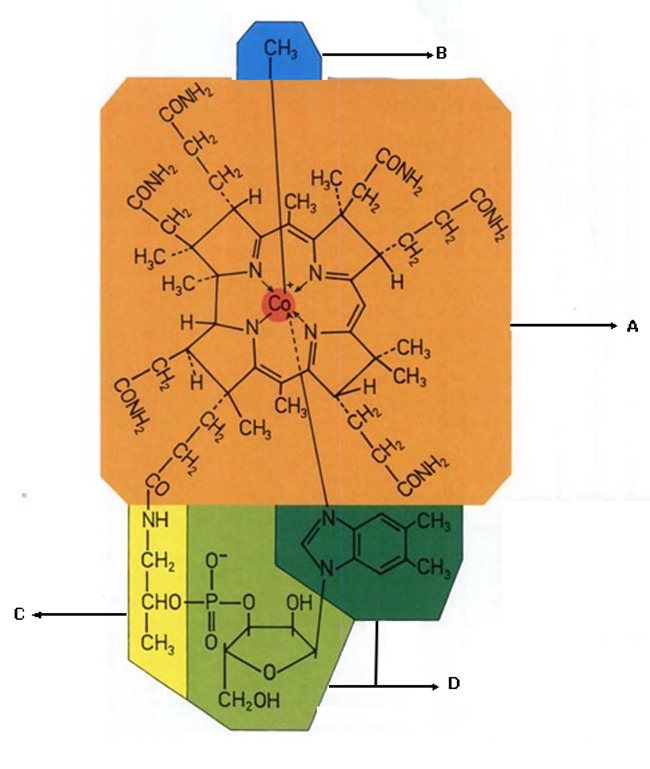
Functional groups of vitamin B12. A) Core ring, B) Beta Legend, C) Amiopropanol and D) Nucleotide moiety adopted from reference (24) with minor modifications.
1-Core Ring which contains side chains
2-Beta legend, Adenosine and methyl cobalamine functional groups
3- Nucleotide moiety which is a Benzimidazole moiety that is an alpha glycosidic bonded benzimidazole and bonds coordinately to cobalt atom in the core ring and is essential for biological activity
4- Aminopropanol residue that links between the side chain of the core ring and the Nucleotide moiety.
The reactions that can be specified for core ring are briefly as; Deamidation of the side chains, Amidation of carboxylic acid side chains, lactam formation by cyclization (producing dehdrovitamin B12), Lactone formation, subistitution (halogenations, nitration) and Isomerization. Reduction of Cobalt inside the ring.The nucleotide moiety may undergo different reactions such as; Hydrolysis of the phosphate bond, chemical attachment of nucleotide moiety and amiopropanol and finally introduction of hetrocycles by microorganisms and the photolytic cleavage of Co-C bond at beta legend part.24
It has previously proved that Pyridoxal phosphate (PLP) is the active form of Vitamin B6 and acts as a cofactor in many reactions of amino acid metabolism inside the living human cells, including transamination, deamination, and decarboxylation. Thiamine pyrophosphate (TPP), the activated form of vitamin B1, is also a coenzyme in the catabolism of sugars and amino acids. Based on vitamin B12 susceptibility to different chemical reactions, vitamin B1 or B6 may trigger its degradation through several pathways. Ahmed et al showed that in aqueous solutions degradation of vitamin B12 mainly results in hydroxylation of cobalt (hyroxocobalamin) and also different oxidation byproducts.3 This information support the utilization of stabilizing agents such as chelating agents, cosolvents or tonicity-adjusting agents, antioxidants and different buffer solutions.
Results revealed that, pure cyanocobalamin solution (F1) is almost stable, but pure thiamin hydrochloride (B1) solution (F2) and pure pyridoxine hydrochloride (B6) solution (F3) have encountered significant degradation (Table 2). It can be concluded that in light protected conditions vitamin B1 aqueous solutions are more stable than vitamin B6 aqueous solutions.
Feller and Macek performed a study and concluded that decomposition of vitamin B12 only occurs in high temperature in the presence of thiamine decomposition products.25 In accordance to previous studies,7-26 combination of cyanocobalamin and vitamin B1 (F4) leads to a remarkable destruction of vitamin B12 compared to combination of cyanocobalamin and vitamin B6 (F5) (Table 2).
The effect of vitamin B1 and B6 on cyanocobalamin stability in parenteral formulations have been investigated previously.3 Ahmed et al. summarized that, "In multi-ingredient (B1 + B6 + B12) preparations cyanocobalamin is unstable and degrades from 28% to 37% with concomitant formation of hydroxocobalamin (1.7% to 25.5%) and oxidation products amounting to 56.4% ± 9.3.3
Crystalline vitamin B12 is stable in solutions of thiamine hydrochloride and niacinamide at pH 3.5 to 4.5 during prolonged storage at room temperature. Thiamine hydrochloride and its decomposition products, which possess reducing properties, are known to destabilize cyanocobalamin solutions at elevated temperatures.25 This finding can explain the difference in the amount of degradation in F1 compared to F4.
Triple-ingredient solution (F6) was degraded much more than previous mentioned formulations (F1-F5), so it can be suggested that the decomposition products of vitamin B1 and vitamin B6 together have a significant influence on the instability of vitamin B12. In a similar study multi-ingredient preparations (vitamin B12 1 mg/ml, B1 and B6 100 mg/ml), that were stored at room temperature (25-28°C) for a period of 12 months under normal laboratory light conditions, showed remarkable degradation percentage in comparison with single vitamin B12 solution.3 In this research the aim was to investigate the effect of allowable additives27 in parenteral solutions in order to make a compatible vitamin B12 triplet parenteral solution with vitamin B1 and B6.
To the best of our knowledge no research is done using PEG, PG, S.M.O, S.F.S, and acetate, citrate and phosphate buffers as stabilizing additives in vitamin B12 mixed parenteral solutions. Based on results, F15, F13B, F8, F12B, F11, F10, F7, F12A, F14B2 and F14B1, decomposed to less than 40 percent of their vitamin B12 content (Table 2).
As the amount of the remaining drug in F6 without any additives was about 40 percent (Table 2), these findings showed that the presence of additives in those has destabilized vitamin B12 and according to Table 1, PEG and PG 50% v/v as solubilizing and tonicity-adjusting agents, EDTA 0.05% w/v as a chelating agent, S.M.O 0.025%v/v as a surfactant and S.F.S 10% w/v as an antioxidant cannot be considered as stabilizing agents in the case of this study. Acetate buffer (pH=4.75), citrate buffer 0.1M (pH=3.1) and phosphate buffer 0.05M (pH=7 or 8) all failed to stabilize vitamin B12 in its parenteral formulations prepared and tested in this study.
In contrast to completely decomposition of F15 in 2 days, some studies have proven the positive effect controversy arises from comes from other investigations revealing vitamin B12 instability in combination with vitamin C as a potent antioxidant agent.8
Others showed the effect of pH in the stability of cyanocobalamin and ascorbic acid, alone or in combination.6 In some mixed vitamins and minerals formulations, EDTA is added as a chelating agent that reduces vitamin degradation28 but our results showed otherwise.29
It is obvious that all selected formulations contained buffer solutions as their stabilizing additives. Selected formulations, incubated at 40°C oven, had decomposed over 80% (Figure 1), and the remaining percentage of vitamin B12 after 2 months in F13A, F14A1, F14A2, F14C1 and F14C2was about 0, 4.2, 4.05, 11.3 and 11.2% respectively. These findings reject the room temperature as optimum storage condition.
According to ICH guidelines accelerated stability test for a product intended for storage in refrigerator needs a study at 25°C. Formulations kept at room temperature (25°C) were evaluated weekly for the first month and monthly for a 6 month period. At the end of the study, the percentage of remaining vitamin B12 for these formulations was as below: F13A (60.5%), F14A2 (73.4%), F14C1 (78.6%), F14A1 (80.5%), F14C2 (85.5%) (Figure 5).
F13A containing citrate buffer 0.05M, pH=3.1 had the most decomposition percentage compared with other selected formulations (about 40%) (Table 1). Among these formulations, F14C2 was the most stable formulation with the remaining B12 content of about 85.5%.
The amount of vitamin B1 and vitamin B6 were also determined weekly for the first month and monthly for a 6 month period. At the end of 3th month, for formulations placed in oven 40°C the amount of vitamin B6 had no significant decline (more than 5% loss), but surprisingly vitamin B1 content of the formulations showed a remarkable reduction.
According to Figure 3 the remaining percentages of vitamin B1 in different formulations were as follow; F14A2 (44.8), F14C1 and F14C2 (56%), F14A1 and F13A (61%). It can be concluded that about half of vitamin B1 content in formulations is destroyed, and it could be an evidence to relate cyanocobalamin decomposition with thiamin hydrochloride.7
Formulations evaluated in room temperature had no significant change (more than 5% loss) in their vitamin B1 and vitamin B6 content, and about 95% of their content was remained intact after a 6-month period.
It is thought that a small percentage of cyanocobalamin degrades to hydroxocobalamin during storage.3 Based on this presumption it was decided to check the presence of hydroxocobalamin that was probably produced during stability test. In this Study, pure hydroxocobalamin solution was prepared and the substance was detected using cyanocobalamin specific HPLC method. A sharp peak appeared in about 2 minutes. Stability sample analysis indicated that hydroxocobalamin may have been present in this sample but unfortunately it was not possible to state this precisely, because the hydroxocobalamin peak in the stability samples containing all three vitamins (B12, B1 and B6) has been overlapped with B1 and B6 peaks.
Shelf Life Determination
USP specifies a pH value of 4.5-7 for the cyanocobalamin injections17 this is a wide range and does not determine the optimum pH for a mixed vitamin B12 parenteral solution with other B vitamins. Based on results, the more and less stable formulations are F14C2 and F13A. It can be concluded that citrate buffer 0.1M (pH=3.1) has less stabilizing effect compared to phosphate buffer (0.05 M, pH= 5.8). The fact of specifying long expiration date for commercial formulations comes from huge overage excess content of their formulations. The initial percentage of vitamin B12 in commercial formulations according to their label claim in the time of testing which was during their shelf lives and far from manufacturing date and also expiration dates were determined as 111.9, 68.2 and 129.5 for Brands 1-3 respectively (Table 4). The B12 content in Brand 3 after 9 weeks storage at 40°C decreased about 80% which is comparable to F14C1 and F14C2.
Commercial Formulations
Stability test was also performed on several commercial formulations. Brand 1 a multi-ingredient domestic formulation and Brand 2 an innovator multi-ingredient parenteral formulation had a drug content of 68% and 130% of their label claim at the first testing date, respectively (Table 4). HPLC data after 9 weeks of storage at 40°C revealed 19 and 50% of the cyanocobalamin label claim for Brands 1 and 2 respectively. Despite the fact that considerable amount of drug had been added to Brand 3 as excess drug, the amount of vitamin B12 decomposition was significantly high. Vitamin B1 and vitamin B6 content of these Brands had a little variation from their primary value, and they remained almost intact during the test period, which is comparable to data acquired at this time point for our prepared formulations. It is interesting that all tested commercial formulations had also excess amounts of vitamin B1 and B6 in their composition (112, 123 and 130% for Brands 2-4 respectively) (Table 4).
Stability of Mixed Commercial Formulation
Based on analyzed data, mixed commercial vitamin B12 injection with commercial vitamin B complex injection (without any vitamin B12 as expressed in label) in a medical grade syringe ready for injection stored at 37°C under normal laboratory light showed no significant loss of B12, B1 and B6 (not more than 5% loss). Thus it may be allowable to mix these vitamins before injection.
Liquid-State Kinetics Study
Analysis of the kinetics data indicated the first order reaction in almost all tested formulations.
HPLC Method Validation for Cyanocobalamin Determination In The Presence Of Vitamin B1 and B6
A new HPLC method was introduced for determination of cyanocobalamin alone and in the presence of vitamins B1 and B6 and possible degradation products. As described before, it is a novel method that has passed all the method validation steps and can be widely used to analysis vitamin B12 in mixed parenteral dosage forms in pharmaceutical industries.
Conclusion
According to the data obtained from 25°C, F14C1 and especially F14C2 are both acceptable parenteral formulations, and can be stabilized at refrigerator condition (4-8°C). The finding of this research proposes a new stable formulation and proper storage conditions rather than expelling the mixed preparations. Although ICH guidelines rejects the formulations with more than 5% loss during the stability test but adding an allowable overage dose of cyanocobalamin to formulations, will increase the shelf life in an acceptable manner. Based on stability studies performed on prepared and commercial formulations, the optimum pH value for the mixed parenteral formulations of B12 was determined to be 5.8. It is necessary to formulate Vitmain B12 mixed parenteral solutions using proper phosphate buffers (pH=5.8) and to indicate “Store in refrigerator” on the mixed parenteral formulations of vitamin B12 with other B vitamins, which has not been expressed on the label of tested Brand formulations at the time of this study.
More detailed investigations are needed to fully describe the mechanism of incompatibilities involved in cyanocobalamin instability in the presence of B1 and B6 in light protected aqueous solutions.
Acknowledgments
This paper was extracted from Pharm.D thesis no. 3546 submitted to the Faculty of Pharmacy of Tabriz University of Medical Sciences and financially supported by grant no. 91/77 from the Drug Applied Research Center of the same university. The authors are thankful to Zahravi Pharmaceutical Co. Tabriz, Iran for supply of vitamins.
Conflict of Interest
The authors declare no financial or other conflict of interests.
References
- 1.Wang X, Wei L, Kotra LP. Cyanocobalamin (vitamin B12) conjugates with enhanced solubility. Bioorg Med Chem . 2007;15(4):1780–7. doi: 10.1016/j.bmc.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 2.Taub A, Lieberman H. Stability of vitamin B12-folic acid parenteral solutions. J Am Pharm Assoc Am Pharm Assoc (Baltim) . 1953;42(4):183–6. doi: 10.1002/jps.3030420402. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad I, Hussain W. Stability of cyanocobalamin in parenteral preparations. Pak J Pharm Sci . 1993;6(1):53–9. [PubMed] [Google Scholar]
- 4.Akers MJ. Excipient-drug interactions in parenteral formulations. J Pharm Sci . 2002;91(11):2283–300. doi: 10.1002/jps.10154. [DOI] [PubMed] [Google Scholar]
- 5.Ansari IA, Vaid FH, Ahmad I. Chromatographic study of photolysis of aqueous cyanocobalamin solution in presence of vitamins B and C. Pak J Pharm Sci . 2004;17(1):19–24. [PubMed] [Google Scholar]
- 6.Bartilucci A, Foss NE. Cyanocobalamin (vitamin B12). I. A study of the stability of cyanocobalamin and ascorbic acid in liquid formulations. J Am Pharm Assoc Am Pharm Assoc (Baltim) 1954;43(3):159–62. [PubMed] [Google Scholar]
- 7.Doerge RF, Ravin LJ, Caldwell HC. Effect of the thiazole moiety of thiamine hydrochloride and selected model compounds on cyanocobalamin stability. J Pharm Sci . 1965;54(7):1038–40. doi: 10.1002/jps.2600540719. [DOI] [PubMed] [Google Scholar]
- 8.Frost DV, Lapidus M, Plaut KA, Scherfling E, Fricke HH. Differential stability of various analogs of cobalamin to vitamin C. Science . 1952;116(3005):119–21. doi: 10.1126/science.116.3005.119. [DOI] [PubMed] [Google Scholar]
- 9.Grissom CB, Chagovetz AM, Wang Z. Use of viscosigens to stabilize vitamin B12 solutions against photolysis. J Pharm Sci . 1993;82(6):641–3. doi: 10.1002/jps.2600820619. [DOI] [PubMed] [Google Scholar]
- 10.Cravioto PJ, Hutchins HH, Macek TJ. A comparison of the stability of cyanocobalamin and its analogs in ascorbate solution. J Am Pharm Assoc Am Pharm Assoc (Baltim) . 1956;45(12):806–8. doi: 10.1002/jps.3030451211. [DOI] [PubMed] [Google Scholar]
- 11.Ichikawa M, Ide N, Shiraishi S, Ono K. Effect of Various Halide Salts on the Incompatibility of Cyanocobalamin and Ascorbic Acid in Aqueous Solution. Chem Pharm Bull . 2005;53(6):688–90. doi: 10.1248/cpb.53.688. [DOI] [PubMed] [Google Scholar]
- 12.Jacob J, Nessel R, Blodinger J. Stability of cyanocobalamin in film-coated multivitamin tablets. J Pharm Sci . 2006;57(11):1854–7. doi: 10.1002/jps.2600571106. [DOI] [PubMed] [Google Scholar]
- 13.Riaz MN, Asif M, Ali R. Stability of vitamins during extrusion. Crit Rev Food Sci Nutr . 2009;49(4):361–8. doi: 10.1080/10408390802067290. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad M. A study of photochemical interaction of cyanocobalamin with thiamine and pyridoxine. Karachi: University of Karachi; 2001. [Google Scholar]
- 15.Ahmad I, Hafeez A, Akhter N, Vaid FHM, Qadeer K. Effect of Riboflavin on the Photolysis of Cyanocobolamin in Aqueous Solution. Open Anal Chem J . 2012;6:22–7. [Google Scholar]
- 16.The European Agency for the Evaluation of Medicinal Products. ICH Q1A (R2) Stability Testing Guidelines: Stability Testing of New Drug Substances and Products (CPMP/ICH/2736/99); 2003.
- 17.United States Pharmacopeia and National Formulary (USP 30/NF 25). Rockville, MD, USA: United States Pharmacopeial Convention; 2007.
- 18.Carstensen JT, Rhodes CT. Drug stability: principles and practice. 3rd ed. New York: Marcel Decker Inc; 2000. [Google Scholar]
- 19.Armstrong JS, Collopy F. Error measures for generalizing about forecasting methods: Empirical comparisons. Int J Forecas . 1992; 8(1):69–80. [Google Scholar]
- 20.Lebiedzinska A, Marszall ML, Kuta J, Szefer P. Reversed-phase high-performance liquid chromatography method with coulometric electrochemical and ultraviolet detection for the quantification of vitamins B(1) (thiamine), B(6) (pyridoxamine, pyridoxal and pyridoxine) and B(12) in animal and plant foods. J Chromatogr A . 2007;1173(1-2):71–80. doi: 10.1016/j.chroma.2007.09.072. [DOI] [PubMed] [Google Scholar]
- 21.Markopoulou CK, Kagkadis KA, Koundourellis JE. An optimized method for the simultaneous determination of vitamins B1, B6, B12 in multivitamin tablets by high performance liquid chromatography. J Pharm Biomed Anal . 2002;30(4):1403–10. doi: 10.1016/s0731-7085(02)00456-9. [DOI] [PubMed] [Google Scholar]
- 22.Marszall ML, Lebiedzinska A, Czarnowski W, Szefer P. High-performance liquid chromatography method for the simultaneous determination of thiamine hydrochloride, pyridoxine hydrochloride and cyanocobalamin in pharmaceutical formulations using coulometric electrochemical and ultraviolet detection. J Chromatogr A . 2005;1094(1-2):91–8. doi: 10.1016/j.chroma.2005.07.091. [DOI] [PubMed] [Google Scholar]
- 23.Stahl M. Peak purity analysis in HPLC and CE using diode-array technology. Waldbronn: Agilent Technologies; 2003. [Google Scholar]
- 24.Schneider Z, Stroinski A. Comprehensive B12: chemistry, biochemistry, nutrition, ecology, medicine. New York: Walter de Gruyter; 1987. [Google Scholar]
- 25.Feller BA, Macek TJ. Effect of thiamine hydrochloride on the stability of solutions of crystalline vitamin B12. J Am Pharm Assoc Am Pharm Assoc (Baltim) 1955;44(11):662–5. doi: 10.1002/jps.3030441106. [DOI] [PubMed] [Google Scholar]
- 26.Dony J, Conter J. Stability of vitamin B12 in presence of thiamine and nicotinamide. J Pharm Belg . 1990;11(3-4):186. [PubMed] [Google Scholar]
- 27.Aulton ME, Taylor K. Aulton's pharmaceutics: the design and manufacture of medicines. 3rd ed. Edinburgh: Churchill Livingstone; 2007. [Google Scholar]
- 28.Hsieh YHP, Hsieh YP. Valence state of iron in the presence of ascorbic acid and ethylenediaminetetraacetic acid. J Agricl Food Chem . 1997;45(4):1126–9. [Google Scholar]
- 29.Heep I, Taterra H. Stabilization of vitamin B12. US Patent 20,110,065,665; 2011.


