Abstract
Purpose: Nonsteroidal anti-inflammatory drugs (NSAIDs) are used for the treatment of many joint disorders, inflammation and to control pain. Numerous reports have indicated that NSAIDs are capable of producing nephrotoxicity in human. Therefore, the objective of this study was to evaluate mefenamic acid, a NSAID nephrotoxicity in an animal model.
Methods: Mice were dosed intraperitoneally with mefenamic acid either as a single dose (100 or 200 mg/kg in 10% Dimethyl sulfoxide/Palm oil) or as single daily doses for 14 days (50 or 100 mg/kg in 10% Dimethyl sulfoxide/Palm oil per day). Venous blood samples from mice during the dosing period were taken prior to and 14 days post-dosing from cardiac puncture into heparinized vials. Plasma blood urea nitrogen (BUN) and creatinine activities were measured.
Results: Single dose of mefenamic acid induced mild alteration of kidney histology mainly mild glomerular necrosis and tubular atrophy. Interestingly, chronic doses induced a dose dependent glomerular necrosis, massive degeneration, inflammation and tubular atrophy. Plasma blood urea nitrogen was statistically elevated in mice treated with mefenamic acid for 14 days similar to plasma creatinine.
Conclusion: Results from this study suggest that mefenamic acid as with other NSAIDs capable of producing nephrotoxicity. Therefore, the study of the exact mechanism of mefenamic acid induced severe nephrotoxicity can be done in this animal model.
Keywords: NSAIDS, Mefenamic acid, Nephrotoxicity, Histopathology
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are the most widely used therapeutic agents.1 According to Mintzes (2012), more than 100 million prescriptions of NSAIDs were made throughout the world, which media was the main route for advertising.2 Mefenamic acid is used for inflammatory pain such as dental pain or after traumas. Despite the wide usage, they cause a large variety of serious toxicity which include severe gastrointestinal tract disorders, hepatotoxicity and nephrotoxicity.3
Like other NSAIDs, mefenamic acid or Ponstan® (2',3'-dimethyl-N-phenyl-anthranilic acid) (Figure 1), inhibits prostaglandin biosynthesis. This effect may be responsible for the analgesic, antipyretic and anti-inflammatory properties of all drugs of this group.4 In spite of the potential for nephrotoxicity, particularly in the elderly, the drug continues to be widely used at high dosage in patients.5 NSAIDs have produced a variety of distinct renal syndromes which include acute ischemic renal insufficiency and acute interstitial nephritis caused by haemodynamic effect, which is a direct result of COX inhibition.6,7 Analgesic-associated nephropathy is a severe adverse reaction, where the cause remains unknown.8,9 We have reported previously that mefenamic acid induced hepatotoxicity in an animal model.10 However, in this current investigation, we evaluated the nephrotoxicity caused by mefenamic acid in mice and determine the usefulness of this animal model in studying the mechanism of nephrotoxicity.
Figure 1 .
Chemical Structure of mefenamic acid
Materials and Methods
Mefenamic acid (Ponstan®) was purchased from United Pharma Ltd. Baar, Switzerland. All other chemicals were of analytical grade were purchased from Sigma Chemicals or BDH, UK. Male Balb/c mice from Institute of Medical Research (IMR), Malaysia 30-40g in bodyweight were kept in plastic cages (8 mice/cage) with wood shavings as bedding. They were made to acclimatize to the vivarium environment for 1 week. They were fed standard laboratory pellets with tap water ad libitum. All procedures are approved by the Institutional Animal Care and Use Committee (ACUC).
The mice (n=8/group) received single intraperitoneal injections of mefenamic acid (at 100 or 200 mg/kg in 10% Dimethyl sulfoxide/Palm oil) or daily intraperitoneal dosing (at 50 or 100 mg/kg in 10% Dimethyl sulfoxide/Palm oil) for 14 days. Control animals received equivalent amount of vehicle.
Venous blood samples from mice during the dosing period (100 µl) were taken prior to and 14 days post-dosing from cardiac puncture into heparinized vials (0.3 mL vials containing 15 UmL-1 lithium heparin: Sarstedt, UK). Blood samples were centrifuged at 4000 rpm for 15 min and the plasma transferred to 0.5 mL plastic eppendorf tubes, stored at -20°C until analysis. Plasma blood urea nitrogen (BUN) activity was measured using a commercial kit (Sigma Chemicals, UK). BUN levels were calculated from the decrease in NADH absorbance at λ 340 nm using a BUN Sigma standard. Plasma creatinine level was performed with an automatic chemical analyzer (Hitachi Roche 902, Japan). Six hours after the final dose, mice were sacrificed by cervical dislocation and their kidneys removed. A section was fixed in formalin for 24 hr before processing for histological examination.
Data are expressed as mean ± s.d and were analyzed for statistical significance (p<0.05) using analysis of variance (ANOVA) with Duncan multiple post test or Student’s t test.
Results
Figure 2 demonstrates mefenamic acid-induced nephrotoxicity. BUN levels were determined in this study, since changes in serum BUN level are considered as the marker for kidney functions. BUN is regulated by the metabolism of proteins and the rate of renal excretion of urea nitrogen. All doses of mefenamic acid were able to induce nephrotoxicity as measured by sharp increases in BUN that exceeded control (20 mg/dl) by greater than 3-fold (50 mg/kg/day: 62 mg/dl) and 6.5-fold (100 mg/kg: 130 mg/dl). Similar trends were also observed for creatinine parameter (Table 1). The single doses of mefenamic acid revealed normal plasma BUN and creatinine activities.
Figure 2 .
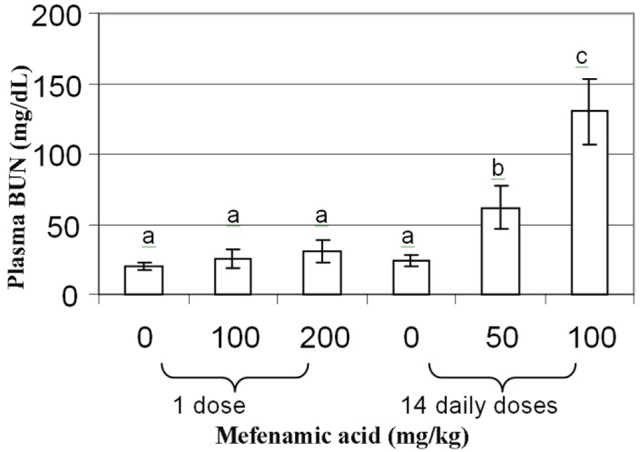
Blood Urea Nitrogen activity in mice treated with mefenamic acid
Mean with different superscript differ significantly (p<0.05). n=8/group
Table 1. Plasma creatinine level of mice .
| Group (n=8) mg/kg mefenamic acid | Pre treatment | Post Treatment | |
| Single doses | 0 | 0.37 ± 0.003ax | 0.47 ± 0.016ax |
| 100 | 0.43 ± 0.012ax | 0.33 ± 0.042ax | |
| 200 | 0.39 ± 0.025ax | 0.31 ± 0.075ax | |
| 14 days doses | 0 | 0.27 ± 0.034ax | 0.47 ± 0.022ax |
| 50 | 0.53 ± 0.078ax | 0.79 ± 0.011by | |
| 100 | 0.34 ± 0.065ax | 0.99 ± 0.075by | |
a-cMean with different superscript differ significantly in the same column(p<0.05). n=8/group
x-y Mean with different superscript differ significantly in the same row(p<0.05). n=8/group
Histopathological examination of kidney sections vividly mirrored the biochemical findings. Some necrotic glomeruli were observed at renal cortex of mice treated with single doses of 100mg/kg mefenamic acid. At renal medulla, there was presence of tubular atrophy. 200mg/kg of mefenamic acid in single doses produced glomerular necrosis and medullary tubular atrophy (Figure 3A). There were also some cortical and papillary tubular congestion with eosinophilic secretory material in the tubular lumen.
Figure 3 .
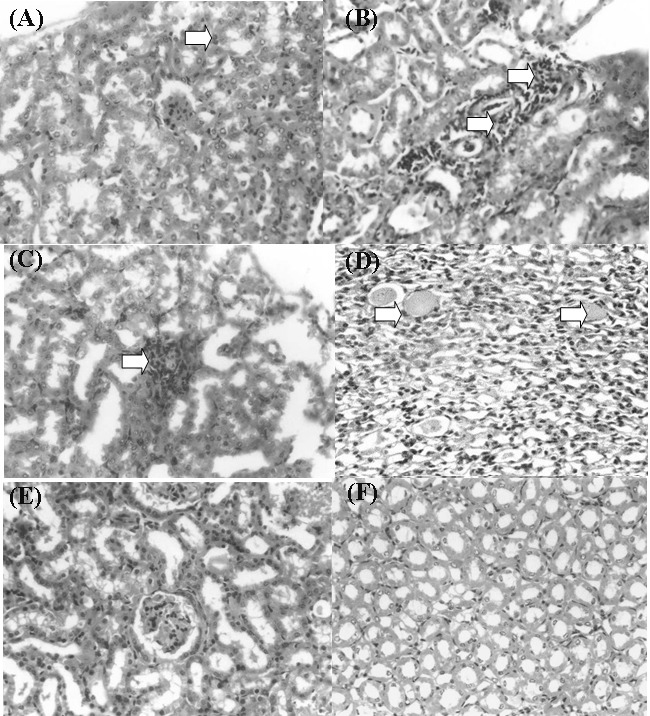
Histopathological changes in the kidneys of mice treated with mefenamic acid.
A: Mild interstitial nephritis in the renal cortex of mouse (Arrow) received 200 mg/kg mefenamic acid for 1 day (Mag. 400x)
B: Interstitial nephritis and glomerular necrosis (Arrows) in the renal cortex of mouse received 100 mg/kg mefanamic acid for 14 days (Mag. 400x)
C: Interstitial nephritis in the renal cortex of mouse (Arrow) received 100 mg/kg mefanamic acid for 14 days (Mag. 400x)
D: Medullary tubular atrophy (Arrows) of mouse received 100 mg/kg mefanamic acid for 14 days (Mag. 400x)
E: Section from control mouse showing normal renal cortex histology (Mag. 400x)
F: Section from control mouse showing normal renal medulla histology (Mag. 400x)
Repeated doses (50mg/kg and 100mg/kg) of mefenamic acid produces the same lesions with glomerular necrosis and medullary tubular atrophy. However, the lesions were more severe. The focal infiltration of inflammatory cells seen at renal cortex of mice treated repeatedly with 100mg/kg mefenamic acid (Figure 3B and 3C) is also seen in the rare interstitial nephritis associated with chronic use of NSAIDs.9 Papillary necrosis, which was suggested as the potential mechanism of nephropathy induced by mefenamic acid was not detected in this study.1 However, papillary tubular atrophy was observed in all (single and repeated doses) mefenamic acid treated mice (Figure 3D). The controls showed normal histology at both renal cortex and medulla (Figure 3E and 3F). Proximal convoluted tubules with smaller amount of distal convoluted tubules making up the bulk of the parenchyma.
Discussion
An astounding number of prescriptions (over 100 million) are written every year for NSAIDs.2 This number is excluding those which are available as nonprescription agents. The 2009 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS) showed that NSAIDs were the most common in acute overdose in adult patients.11 They are popular analgesics of choice due to the potency, often used clinically for the short-term alleviation of postoperative pain, dysmenrrhoea, and gastrointestinal complications.1 However, severe renal dysfunctions are occasional consequences of its toxicity.5,6 Although mefenamic acid is extensively used, mechanisms for causing multiorgan toxicity remain unknown.
Mefenamic acid induced kidney damage but results from this current investigation revealed that the kidney lesions were mild and not life threatening for single doses of mefenamic acid. However, repeated doses of mefenamic acid caused severe lesions in the kidney. Further studies are needed to elucidate the mechanism of which mefenamic acid induced severe nephopathy. Approximately 1–5% of patients taking NSAIDs develop diverse nephrotoxic syndromes warranting potential physician intervention.12 Fortunately, NSAID-induced renal complications are typically fully reversible if the clinician suspects NSAID complications when presented with laboratory and histologic findings, and swiftly discontinues the offending NSAID.13
The mechanism suggested is the involvement of prostaglandin I2 and PGE2 which are synthesized by both the glomerular and medullary interstitial cells. Prostaglandins will diminish vascular resistance, dilate renal vascular beds and enhance organ perfusion. This will lead to redistribution of blood flow from the renal cortex to nephrons in the juxtamedullary region.14 NSAIDs inhibit prostaglandin synthesis, decreasing the blood supply to the nephrons causing acute ischemic renal insufficiency.
Due to numerous reports for adverse drug reactions (ADRs) induced by NSAIDs, researchers have come up with new strategies for lowering ADRs by improving their efficacy through drug delivery.15,16 Work on piroxicam, another NSAID has revealed exciting findings of significantly improve its efficacy by nano-encapsulation with much reduced ADRs.17 Synthetic drugs are usually induce more ADRs than naturally occurring compounds.18 Therefore, others had opted to find new anti-inflammatory drug candidates from herbal plants or animals.19,20
A large number of the previous publications primarily describe the involvement of variants of hepatic cytochrome P450 that metabolize diclofenac and sulindac (two other NSAIDs) and their toxic metabolites as prime suspects in generating oxidative stress.10,15 Interestingly however, the majority of such observations were derived either from intact livers, or isolated hepatocyte models. Similar mechanism may be involved for mefenamic acid in the kidneys.
Although mefenamic acid is an older NSAIDs, but it is still being prescribed for various causes in developing countries for pain syndrome and gynecological disorders.1 Indeed, the studies into the mechanism of nephrotoxicity are vital for the progress of this drug. These data suggest that mefenamic acid causes nephrotoxicity and thus warrants circumspection and appropriate clinical monitoring. Indeed, this animal model is useful for the study of the mechanism of nephrotoxicity induced by mefenamic acid.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Rang HP, Dale MM, Ritter JM. Pharmacology. International edition of Seventh edition. USA: Churchill Livingstone; 2007. [Google Scholar]
- 2.Mintzes B. Advertising of prescription-only medicines to the public: does evidence of benefit counterbalance harm? Annu Rev Public Health. 2012;33:259–77. doi: 10.1146/annurev-publhealth-031811-124540. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien WM. Rare adverse reaction to non-steroidal anti-inflammatory drugs. In: Rainsford KD, Velo GP, editors. Side effects of anti-inflammatory drugs. Part 1: Clinical and epidemiological aspects. First edition. USA: MTP press limited;1987. P. 123-37.
- 4.Barr M, Buckley M, O'morain C. Review article: non-steroidal anti-inflammatory drugs and Helicobacter pylori. Aliment Pharmacol Ther. 2000;14 Suppl 3:43–7. doi: 10.1046/j.1365-2036.2000.00399.x. [DOI] [PubMed] [Google Scholar]
- 5.Robertson CE, Ford MJ, Van Someren V, Dlugolecka M, Prescott LF. Mefenamic acid nephropathy. Lancet . 1980;2(8188):232–3. doi: 10.1016/s0140-6736(80)90122-1. [DOI] [PubMed] [Google Scholar]
- 6.Kenny GN. Potential renal, haematological and allergic adverse effects associated with nonsteroidal anti-inflammatory drugs. Drugs . 1992;44 Suppl 5:31–6; discussion 7. doi: 10.2165/00003495-199200445-00005. [DOI] [PubMed] [Google Scholar]
- 7.Brater DC. Clinical aspects of renal prostaglandins and NSAID therapy. Semin Arthritis Rheum . 1988;17(3 Suppl 2):17–22. doi: 10.1016/0049-0172(88)90040-6. [DOI] [PubMed] [Google Scholar]
- 8.Bennett WM, Debroe ME. Analgesic nephropathy--a preventable renal disease. N Engl J Med . 1989;320(19):1269–71. doi: 10.1056/NEJM198905113201908. [DOI] [PubMed] [Google Scholar]
- 9.Murray MD, Brater DC. Renal toxicity of the nonsteroidal anti-inflammatory drugs. Annu Rev Pharmacol Toxicol . 1993;33:435–65. doi: 10.1146/annurev.pa.33.040193.002251. [DOI] [PubMed] [Google Scholar]
- 10.Somchit N, Sanat F, Gan EH, Shahrin IAW, Zuraini A. Liver Injury induced by the nonsteroidal anti-inflammatory drug Mefenamic Acid (Ponstan): An animal model. Singapore Med J . 2004; 45(11):530–2. [PubMed] [Google Scholar]
- 11.Hunter LJ, Wood DM, Dargan PI. The patterns of toxicity and management of acute nonsteroidal anti-inflammatory drug (NSAID) overdose. Open Access Emerg Med . 2011;3:39–48. doi: 10.2147/OAEM.S22795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whelton A, Watson J. Nonsteroidal anti-inflammatory drugs: effects on kidney function. In: De Broe ME, Porter GA, Bennett WM, Verpooten GA, editors. Clinical Nephrotoxins: Renal Injury From Drugs and Chemicals. Dordrecht, Netherlands: Kluwer Academic Publishers;1997. P. 209-22.
- 13.Whelton A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med. 1999;106(5B):13S–24S. doi: 10.1016/s0002-9343(99)00113-8. [DOI] [PubMed] [Google Scholar]
- 14.Oates JA, Fitzgerald GA, Branch RA, Jackson EK, Knapp HR, Roberts LJ, 2nd. Clinical implications of prostaglandin and thromboxane A2 formation (1) N Engl J Med . 1988;319(11):689–98. doi: 10.1056/NEJM198809153191106. [DOI] [PubMed] [Google Scholar]
- 15.Somchit MN, Faizah S, Zuraini A, Khairi HM, Hasiah AH, Zakaria ZA. Selective in vitro cytotoxic effects of piroxicam and mefenamic acid on several cancer cells lines. Res J Pharmacol . 2009;3(1):15–8. [Google Scholar]
- 16.Hussein MZ, Al Ali SH, Zainal Z, Hakim MN. Development of antiproliferative nanohybrid compound with controlled release property using ellagic acid as the active agent. Int J Nanomedicine . 2011;6:1373–83. doi: 10.2147/IJN.S21567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiong HS, Yong YK, Ahmad Z, Sulaiman MR, Zakaria ZA, Yuen KH. et al. Cytoprotective and enhanced anti-inflammatory activities of liposomal piroxicam formulation in lipopolysaccharide-stimulated RAW 264.7 macrophages. Int J Nanomedicine. 2013;8:1245–55. doi: 10.2147/IJN.S42801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somchit N, Hassim SM, Samsudin SH. Itraconazole- and fluconazole-induced toxicity in rat hepatocytes: a comparative in vitro study. Hum Exp Toxicol . 2002;21(1):43–8. doi: 10.1191/0960327102ht208oa. [DOI] [PubMed] [Google Scholar]
- 19.Sulaiman MR, Zakaria ZA, Bujarimin AS, Somchit MN, Israf DA, Moin S. Evaluation of moringa oleifera aqueous extract for antinociceptive and anti-inflammatory activities in animal models. Pharmaceut Biol . 2008;46(12):838–45. [Google Scholar]
- 20.Zakaria ZA, Mat Jais AM, Goh YM, Sulaiman MR, Somchit MN. Amino acid and fatty acid composition of an aqueous extract of Channa striatus (Haruan) that exhibits antinociceptive activity. Clin Exp Pharmacol Physiol . 2007;34(3):198–204. doi: 10.1111/j.1440-1681.2007.04572.x. [DOI] [PubMed] [Google Scholar]