Abstract
Background
Consumption of chronic morphine induces neuro-inflammation and addictive seeking behavior. Ginger (Zingiber Officinale Roscoe), a well-known spice plant, has been used traditionally in the treatment of a wide variety of ailments. It has been shown that ginger has anti-inflammatory, anti-oxidative and antinociceptive properties. However, its influences on morphine-induced addictive behaviors have not yet been clarified. The aim of the present study was the inhibition of exploratory behavior of morphine addiction in the conditioned place preference test in male desert rats through ginger.
Methods
For conditioning to the morphine, the male Wistar rats received morphine (12 mg/kg intraperitoneally or i.p.) for 6 consecutive days and treatment groups were given different doses of ginger (25, 50 and 100 mg/kg intragastrically or i.g.) 30 min before morphine injection. For investigating addictive seeking behavior, conditioned place preference test (CPP) was used.
Findings
Our result demonstrated that injection of morphine for 6 days induces dependency to morphine and creates addictive seeking behavior and ginger (100 mg/kg) could decrease time spend in conditioning box (addictive seeking behavior).
Conclusion
The data indicated that ginger extract has a potential anti-addictive property against chronic usage of morphine.
Keywords: Ginger extract, Morphine, Conditioned place preference, Addictive seeking behavior, Rats
Introduction
Opioids are the current standard of care for the management of moderate or severe pain, but treatment with these drugs leads to the induction of side effects such as addiction, tolerance and physical dependence. In addition, conspicuous increases in the abuse of opioids have expanded the need for pharmacotherapeutic interventions. Drug addiction constitutes a major health problem worldwide. Iran is situated along one of the main trafficking routes for cannabis, heroin, opium and morphine. Iran ranks first worldwide in the prevalence of opiate addiction with 2.8% of its population addicted.1 Initiation age for most Iranian addicts is their 20s.2
It has been recently shown that the oxidative/nitrosative stresses may play a critical role in the development of morphine-induced tolerance and dependence and blockade of such stress can attenuate morphine side effects.3,4 In addition, neuroinflammation occurs following chronic usage of opioids, which plays an important role in the induction opioid side effects.5,6
Recently, the anti-tolerance and anti-addictive effects of natural herbal products have drawn intensive interest,7 and need precise scientific experimental testing as well as clinical trials before using as widespread choice in the management of opioid side effects. Wu et al. reported that processed Aconiti tuber (PAT), a traditional Chinese herbal medicine, dose-dependently inhibited morphine-induced conditioned place preference (CPP).8 It has been previously reported that some Iranian traditional herb extract accompanied with their active constituents have inhibitory effects against morphine-induced tolerance and dependence.9-11
Zingiber officinale Roscoe [family: Zingiberaceae], commonly known as ‘ginger’, is one of the frequently used spices in the world. Ginger has been used safely in cooking, and in folk medicine. It is used extensively to treat cold, fever, headache, nausea and digestive problems; and is also used in modern herbal medical practices for the treatment of arthritis, rheumatic disorders and muscular discomfort.12 The main constituents of ginger are the gingerols, shogaols, paradols and zingerone.13 It has been documented that ginger has antioxidant,14,15 anti-inflammatory,16 and antinociceptive properties.17 We have previously reported that this plant could prevent the development of morphine analgesic tolerance and physical dependence through inhibition of morphine-induced calcium channel over-expression.18
Therefore, the present study was designed to test the hypothesis that ginger extract could exert preventive effects against other chronic morphine side effects such as addictive seeking and place preference behavior in rats.
Methods
All experiments were carried out on male Wistar rats, weighing 200-250 g, that were housed four per cage under a 12 h light/dark cycle in a room with controlled temperature (22 ± 1 °C). Food and water were available ad libitum. The animals were handled daily (between 9:00 and 10:00 a.m.) for 3 days, before the experiment days in order to adapt them to manipulation and minimize nonspecific stress responses. Rats were divided randomly into several experimental groups, each comprising 6-8 animals. All experiments followed the guidelines on ethical standards for investigation of experimental pain in animals,19 and approved by the Animal Experimentation Ethic Committee of Kerman Neuroscience Research Center (EC/KNRC/90).
A total of 1 kg of fresh ginger was purchased from the main vegetable market in Khorramabad, Iran. A sample of the rhizome was deposited at the herbarium of the Razi Herbal Medicines Research Center, Lorestan, Iran. Two hundred grams of the air-dried rhizome of the herb was ground into fine powder. The powder was extracted twice, on each occasion with 1 liter of 80% ethyl alcohol. The ethanol extract was filtered, and the filtrate was concentrated until dry under reduced pressure in a rotary evaporator and the resulting ethanol extract was freeze-dried.
Aliquot portions of the crude ginger root extract was weighed and dissolved in warm physiological saline for use on each day of our experiments. Morphine hydrochloride (TEMAD, Iran) was also dissolved in physiological saline. Ginger extract was given intragastrically (i.g.) by gavage and morphine was injected intraperitonealy (i.p.).
We used the CPP test to evaluate drug-induced addictive (drug-seeking) behavior in rats. The rats were intraperitoneally (i.p.) injected with morphine once daily for 6 days and evaluated for drug-induced place preference. The CPP test apparatus was a two-compartment box (60.0 × 29.2 × 29.2 cm) with a transparent plexiglass front separated by a gray cylinder platform (10.3 cm in diameter and 12 cm high). One compartment was white with a textured floor, and the other was black with a smooth floor.
For CPP conditioning, the rats were given saline or morphine (12 mg/kg; i.p.) for 6 days. The two distinctive compartments were paired repeatedly, one with the morphine injections and the other with the saline injections. The rats were kept for 40 min in the corresponding compartment with the guillotine doors closed. The place preference, before conditioning (day 0) and on the days after conditioning (7, 9, and 13), was determined. Each rat was placed on a neutral (gray) platform and allowed to step down from the platform to either the white or black compartment. A sliding wall was then put down on the platform and the rat was free to access either compartment through two openings (9.5 × 12 cm each) on each side of the platform. The amount of time spent in the black or white compartment was automatically measured for 15 min. So that this was drug-seeking behavior which was determined by the increase in the time spent in the compartment previously paired with the morphine injection than in the compartment previously paired with saline. To evaluate the effect of ginger, the extract (25, 50 and 100 mg/kg, i.g.) was given 30 min before each morphine injection (days 1 to 6) once daily for 6 days.
The results are expressed as mean ± SEM. The difference in the mean of time spent in the morphine-paired of the CPP apparatus between groups over the time course of study was determined by one-way analysis of variance (ANOVA) followed by the Tukey test. P < 0.05 was considered statistically significant.
Results
The inhibitory effect of ginger extract on morphine-induced addictive behavior
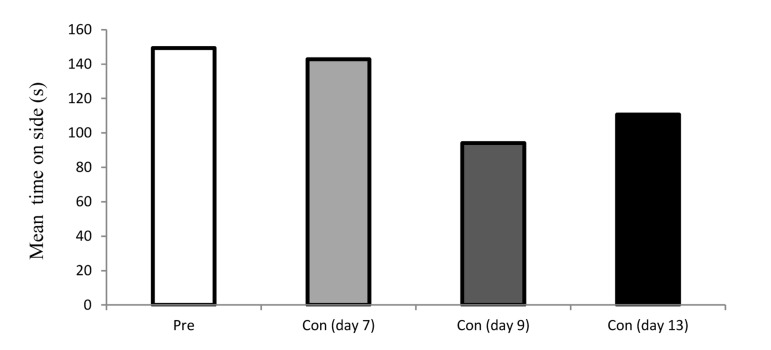
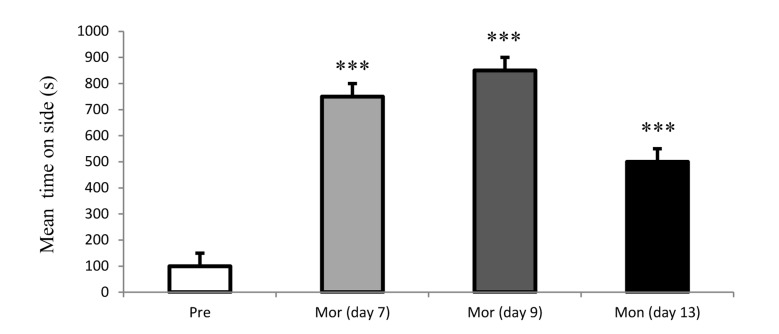
As shown in figure 1, place preference was not occurred following administration of chronic saline in rats. In contrast, a significant dependence was produced in chronic morphine-injected (12 mg/kg for 6 days) rats. The occurrence of CPP (the increased in spending time) revealed rat’s addictive behavior in days 7, 9 and 13 (Figure 2). Due to the amount of time spent by rats in the morphine-paired chamber of the CPP apparatus it seems that the place preference is completely related to morphine administration.
Figure 1.
Time spent in the saline-paired of the CPP (conditioned place preference) test apparatus by rats in preconditioning and post-conditioning days.
Each bar represents the mean ± SEM
Figure 2.
Time spent in the morphine-paired of the CPP (conditioned place preference test) apparatus by rats in preconditioning and post-conditioning phase of test.
Each bar represents the mean ± SEM; ***P < 0.001 compared to preconditioning phase; Mor: Morphine
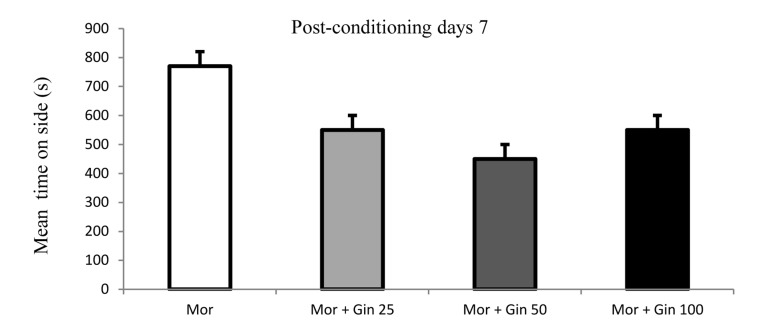
The data indicated that there was a significant preference for the morphine-paired chamber in morphine and ginger administrated rats in day 7 of experiment (Figure 3).
Figure 3.
Time spent in the morphine-paired of the CPP (conditioned place preference test) apparatus in morphine- and morphine plus ginger-treated rats in 7th day of experiment.
Each bar represents the mean ± SEM; Mor: Morphine; Gin: Ginger
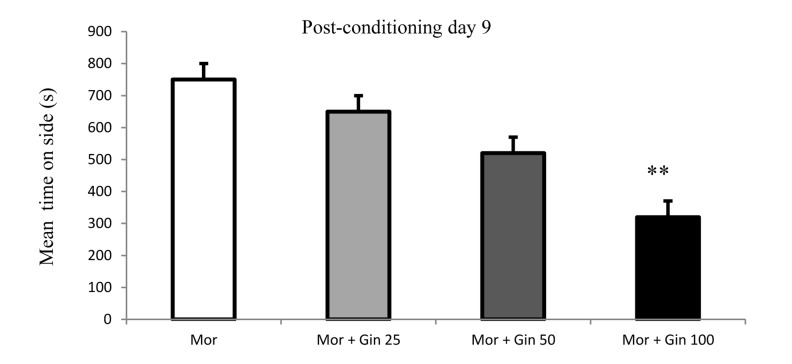
In the 9th day, one way ANOVA test using the preference scores indicated that ginger-treated (100 mg/kg) rats had a significantly lower preference than the morphine-injected rats for the morphine-paired chamber (P < 0.01) (Figure 4). It means concomitant administration of 100 mg/kg ginger extract could attenuate morphine-induced addictive behavior.
Figure 4.
Time spent in the morphine-paired of the CPP apparatus in morphine- and morphine plus ginger-treated rats in 9th day of experiment.
Each bar represents the mean ± SEM; **P < 0.01 compared to morphine-treated rats; Mor: Morphine; Gin: Ginger
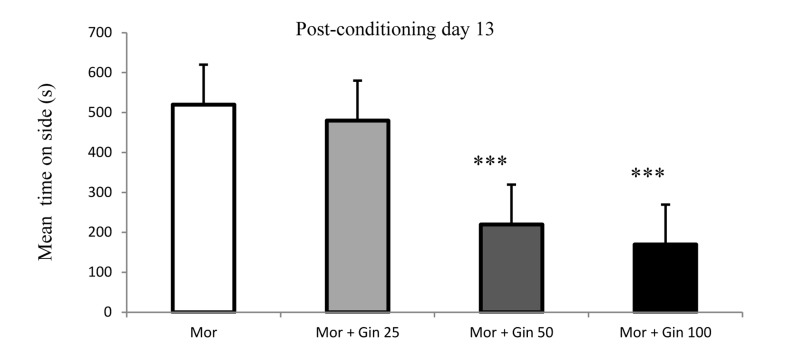
The CPP data in day 13 showed that ginger with doses of 50 and 100 mg/kg significantly reduced preference for the morphine-paired chamber and inhibited addiction landmark (Figure 5).
Figure 5.
Time spent in the morphine-paired of the CPP apparatus in morphine- and morphine plus ginger-treated rats in 13th day of experiment.
Each bar represents the mean ± SEM; ***P < 0.001 compared to morphine-treated rats
Discussion
It has been documented that drug addiction can activate the mesolimbic dopamine rewarding system and increase dopamine content in nucleus accumbens.20 The mesolimbic dopamine structures are involved critically in morphine-induced CPP, since the electrolytic lesion of ventral tegmental area and the nucleus accumbens can block this phenomenon.21
Motivated behaviors are mediated by three important regions in the brain including the amygdala, prefrontal cortex and nucleus accumbens. The contributions of nucleus accumbens to the reinforcement and rewarding behavior have been completely demonstrated.22
Morphine induces a proinflammatory phenotype via μ-opioid receptor in astrocytes and microglial cells. It has been reported that morphine administration causes astrocytes activation in the nucleus accumbens, locus coeruleus, lateral septum, trigeminal nucleus and caudate nucleus, periaqueductal gray and ventral tegmental areas, and prefrontal cortex.23
Commonly abused drugs can induce complex changes in reward neural circuits. However, such changes affect both neuronal and non-neuronal cells. Scientific reports indicated that glial activation is preferentially involved in drug-seeking behavior.24 In addition, neural inflammation contributes to sensitization and neuroplastic processes induced by addictive drugs of abuse.25 It has been documented that chronic morphine activates astrocytes and microglia cells and increases pro-inflammatory cytokine gene expression.26,27 Once activated, microglia can release cytokines, chemokines, ROS, and complement proteins which start progressive cycle of neuroinflammation and cause synaptic plasticity.28,29
Ginger has a long history of human use, especially with regards to its anti-inflammatory properties. This plant elicits anti-inflammatory effects in different models of inflammation.30-32 Ginger extract can reduce TNFα and IL-1β gene expression.33 It seems that the anti-inflammatory property of ginger is responsible, at least in part, for its anti-addictive effect.
Previous reports indicate that oxidative stress and free radicals have important roles in the induction of chronic morphine-induced side effects.4,34-36 Furthermore, the potent antioxidanteffects of ginger and its components have been demonstrated.14,15 Such property can also be involved in the observed effect in this study.
Conclusion
The data indicate that ginger extract has a potential anti-addictive property against chronic usage of morphine and its anti-inflammatory and antioxidant properties as well as its ability to reduce morphine-induced glial activation and neuroinflammation may be involved in its anti-addictive effects.
Footnotes
Conflicts of Interest
The Authors have no conflict of interest.
REFERENCES
- 1.Chawla S. UNODC, World Drug Report 2010. New York, NY: United Nations Publication; 2010. [Google Scholar]
- 2.Tabei SZ, Heydari ST, Mehrabani D, Shamsina SJ, Ahmadi J, Firouzi SM. Current substance use in patients with gastric cancer in Southern Iran. J Cancer Res Ther. 2006;2(4):182–5. doi: 10.4103/0973-1482.29828. [DOI] [PubMed] [Google Scholar]
- 3.Salvemini D. Peroxynitrite and opiate antinociceptive tolerance: A Painful Reality. Arch Biochem Biophys. 2009;484(2):238–44. doi: 10.1016/j.abb.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Zaher AO, Abdel-Rahman MS, ELwasei FM. Blockade of nitric oxide overproduction and oxidative stress by Nigella sativa oil attenuates morphine-induced tolerance and dependence in mice. Neurochem Res. 2010;35(10):1557–65. doi: 10.1007/s11064-010-0215-2. [DOI] [PubMed] [Google Scholar]
- 5.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10(1):40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 6.Shen CH, Tsai RY, Shih MS, Lin SL, Tai YH, Chien CC, et al. Etanercept restores the antinociceptive effect of morphine and suppresses spinal neuroinflammation in morphine-tolerant rats. Anesth Analg. 2011;112(2):454–9. doi: 10.1213/ANE.0b013e3182025b15. [DOI] [PubMed] [Google Scholar]
- 7.Ward J, Rosenbaum C, Hernon C, McCurdy CR, Boyer EW. Herbal medicines for the management of opioid addiction: safe and effective alternatives to conventional pharmacotherapy? CNS Drugs. 2011;25(12):999–1007. doi: 10.2165/11596830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Wu G, Huang W, Zhang H, Li Q, Zhou J, Shu H. Inhibitory effects of processed Aconiti tuber on morphine-induced conditioned place preference in rats. J Ethnopharmacol. 2011;136(1):254–9. doi: 10.1016/j.jep.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 9.Zare L, Esmaeili-Mahani S, Abbasnejad M, Rasoulian B, Sheibani V, Sahraei H, et al. Oleuropein, chief constituent of olive leaf extract, prevents the development of morphine antinociceptive tolerance through inhibition of morphine-induced L-type calcium channel overexpression. Phytother Res. 2012;26(11):1731–7. doi: 10.1002/ptr.4634. [DOI] [PubMed] [Google Scholar]
- 10.Esmaeili Mahani S, Zare L. Olive (Olea europaea L.) leaf extract and its main component (oleuropein) mitigate the development of morphine physical dependence in rats. Physiol Pharmacol. 2013;16(4):360–70. [Google Scholar]
- 11.Esmaeili-Mahani S, Ebrahimi B, Abbasnejad M, Rasoulian B, Sheibani V. Satureja khuzestanica prevents the development of morphine analgesic tolerance through suppression of spinal glial cell activation in rats. Journal of Natural Medicines. 2013;67(1):61–9. doi: 10.1007/s11418-013-0796-6. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen P. Ginger--Zingiber officinale Roscoe, Zingiberaceae. Journal of primary health care. 2011;3(3):235–6. [PubMed] [Google Scholar]
- 13.Jiang H, Xie Z, Koo HJ, McLaughlin SP, Timmermann BN, Gang DR. Metabolic profiling and Phylogenetic analysis of medicinal Zingiber species: Tools for authentication of ginger (Zingiber officinale Rosc). Phytochemistry. 2006;67(15):1673–85. doi: 10.1016/j.phytochem.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Ghasemzadeh A, Jaafar HZE, Rahmat A. Antioxidant activities, total Phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules. 2010;15(6):4324–33. doi: 10.3390/molecules15064324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rehman R, Akram B, Akhtar N, Jabeen Q, Saeed T, Ali Shah SM, et al. Zingiber officinale Roscoe (pharmacological activity). J Med Plants Res. 2011;5(3) [Google Scholar]
- 16.Shimoda H, Shan SJ, Tanaka J, Seki A, Seo JW, Kasajima N, et al. Anti-inflammatory properties of red ginger (Zingiber officinale var. Rubra) extract and suppression of nitric oxide production by its constituents. J Med Food. 2010;13(1):156–62. doi: 10.1089/jmf.2009.1084. [DOI] [PubMed] [Google Scholar]
- 17.Sepahvand R, Esmaeili-Mahani S, Arzi A, Rasoulian B, Abbasnejad M. Ginger (Zingiber officinale Roscoe) elicits antinociceptive properties and potentiates morphine-induced analgesia in the rat radiant heat tail-flick test. J Med Food. 2010;13(6):1397–401. doi: 10.1089/jmf.2010.1043. [DOI] [PubMed] [Google Scholar]
- 18.Darvishzadeh-Mahani F, Esmaeili-Mahani S, Komeili G, Sheibani V, Zare L. Ginger (Zingiber officinale Roscoe) prevents the development of morphine analgesic tolerance and physical dependence in rats. J Ethnopharmacol. 2012;141(3):901–7. doi: 10.1016/j.jep.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 20.Joseph MH, Datla K, Young AM. The interpretation of the measurement of nucleus accumbens dopamine by in vivo dialysis: the kick, the craving or the cognition? Neurosci Biobehav Rev. 2003;27(6):527–41. doi: 10.1016/j.neubiorev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Tzschentke TM, Schmidt WJ. Functional heterogeneity of the rat medial prefrontal cortex: effects of discrete subarea-specific lesions on drug-induced conditioned place preference and behavioural sensitization. Eur J Neurosci. 1999;11(11):4099–109. doi: 10.1046/j.1460-9568.1999.00834.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang B, Luo F, Ge XC, Fu AH, Han JS. Effects of lesions of various brain areas on drug priming or footshock-induced reactivation of extinguished conditioned place preference. Brain Res. 2002;950(1-2):1–9. doi: 10.1016/s0006-8993(02)02980-3. [DOI] [PubMed] [Google Scholar]
- 23.Lazriev IL, Kiknadze GI, Kutateladze II, Nebieridze MI. Effect of morphine on the number and branching of astrocytes in various regions of rat brain. Bull Exp Biol Med. 2001;131(3):248–50. doi: 10.1023/a:1017699315355. [DOI] [PubMed] [Google Scholar]
- 24.Cooper ZD, Jones JD, Comer SD. Glial modulators: a novel pharmacological approach to altering the behavioral effects of abused substances. Expert Opin Investig Drugs. 2012;21(2):169–78. doi: 10.1517/13543784.2012.651123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarz JM, Hutchinson MR, Bilbo SD. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J Neurosci. 2011;31(49):17835–47. doi: 10.1523/JNEUROSCI.3297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shavit Y, Wolf G, Goshen I, Livshits D, Yirmiya R. Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain. 2005;115(1-2):50–9. doi: 10.1016/j.pain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Shen CH, Tsai RY, Wong CS. Role of neuroinflammation in morphine tolerance: effect of tumor necrosis factor-alpha. Acta Anaesthesiol Taiwan. 2012;50(4):178–82. doi: 10.1016/j.aat.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Tai YH, Wang YH, Wang JJ, Tao PL, Tung CS, Wong CS. Amitriptyline suppresses neuroinflammation and up-regulates glutamate transporters in morphine-tolerant rats. Pain. 2006;124(1-2):77–86. doi: 10.1016/j.pain.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Hao S, Liu S, Zheng X, Zheng W, Ouyang H, Mata M, et al. The role of TNFalpha in the periaqueductal gray during naloxone-precipitated morphine withdrawal in rats. Neuropsychopharmacology. 2011;36(3):664. doi: 10.1038/npp.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young HY, Luo YL, Cheng HY, Hsieh WC, Liao JC, Peng WH. Analgesic and anti-inflammatory activities of [6]-gingerol. J Ethnopharmacol. 2005;96(1-2):207–10. doi: 10.1016/j.jep.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46(2):409–20. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 32.Choi YY, Kim MH, Hong J, Kim SH, Yang WM. Dried Ginger (Zingiber officinalis) Inhibits Inflammation in a Lipopolysaccharide-Induced Mouse Model. Evid Based Complement Alternat Med. 2013;2013:914563. doi: 10.1155/2013/914563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li XH, McGrath KC, Nammi S, Heather AK, Roufogalis BD. Attenuation of liver pro-inflammatory responses by Zingiber officinale via inhibition of NF-kappa B activation in high-fat diet-fed rats. Basic Clin Pharmacol Toxicol. 2012;110(3):238–44. doi: 10.1111/j.1742-7843.2011.00791.x. [DOI] [PubMed] [Google Scholar]
- 34.Mori T, Ito S, Matsubayashi K, Sawaguchi T. Comparison of nitric oxide synthase inhibitors, phospholipase A2 inhibitor and free radical scavengers as attenuators of opioid withdrawal syndrome. Behav Pharmacol. 2007;18(8):725–9. doi: 10.1097/FBP.0b013e3282f18da6. [DOI] [PubMed] [Google Scholar]
- 35.Doyle T, Bryant L, Batinic-Haberle I, Little J, Cuzzocrea S, Masini E, et al. Supraspinal inactivation of mitochondrial superoxide dismutase is a source of peroxynitrite in the development of morphine antinociceptive tolerance. Neuroscience. 2009;164(2):702–10. doi: 10.1016/j.neuroscience.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunduz O, Karadag CH, Ulugol A. Modulatory role of the endogenous nitric oxide synthase inhibitor, asymmetric dimethylarginine (ADMA), in morphine tolerance and dependence in mice. J Neural Transm. 2010;117(9):1027–32. doi: 10.1007/s00702-010-0443-2. [DOI] [PubMed] [Google Scholar]