Abstract
Although an altered vaginal microbiota has been demonstrated to affect parturition, its role in assisted reproductive technologies is uncertain. Nevertheless, the effect of known pathogens such as Mycoplasma tuberculosis, Chlamydia trachomatis, and Neisseria gonorrhoeae is clear, causing subclinical changes thought to be risk factors in subfertility. The Human Microbiome Project (HMP) has allowed for metagenomic studies to aid in characterizing normal vaginal flora. Recent findings from the HMP demonstrate that many different species of Lactobacillus are present in the vaginal tract, with a few that predominate. Studies that characterize the vaginal microbiome in assisted reproductive technology support the hypothesis that colonizing the transfer-catheter tip with Lactobacillus crispatus at the time of embryo transfer may increase the rates of implantation and live birth rate while decreasing the rate of infection. In addition, there is some evidence that a progesterone-resistant endometrium might increase the risk of an abnormal vaginal microbiome.
Keywords: microbiome, in vitro fertilization, Lactobacillus, progesterone
The publication of the human genome sequence was heralded in 2001 as a notable achievement in the field of biology.1 However, many noted that without the knowledge of the “second genome,” namely, the genome of the microorganisms that inhabit the human body, our understanding would be woefully incomplete.2,3 Thus, by 2007, there was a global effort to sequence the microbiome, which is the ecological community of commensal, symbiotic, and pathogenic microorganisms. Worldwide efforts by China, Canada, Singapore, France, Australia, Japan, the European Union, and the United States sought to understand the microbial community in states of good health and disease.4
In the United States, the project was named the “Human Microbiome Project” (HMP) and was launched in 2007 by the National Institutes of Health (NIH) as an initiative of the NIH Roadmap for Biomedical Research.5 Although some countries like the Sino-French project sought to understand the microbiome in states of disease like obesity, the HMP desired to characterize the human microbiome from at least four body sites from normal volunteers, including the vagina as a major site of study in healthy women. In reproductive age women, the vaginal microbiota is affected by factors including age, sexual activity, pregnancy, and exogenous hormones.5,6
Many studies have implicated the vaginal microbiota in parturition. Neonates generally acquire intestinal microbial communities within the first week of life with relative equilibrium reached within the first year. Infants delivered via cesarean birth have higher rates of colonization by environmentally acquired microbes such as Clostridium difficile and Klebsiella and Enterobacter species, and have enhanced colonization of the neonatal oral cavity and intestine by components of the skin microbiome. In addition, the Ureaplasma species as well as Mycoplasma hominis are associated with preterm birth (PTB).7–13
The role of the vaginal microbiome in fertility and assisted reproductive technology (ART) is not as clear. Although known pathogens such as Mycoplasma tuberculosis, Chlamydia trachomatis, and Neisseria gonorrhoeae can cause infertility, subclinical changes in the microbiota in states like bacterial vaginosis (BV) are also thought to be risk factors in subfertility. Infertility affects a substantial proportion of reproductive-aged women and men in the United States. Data from CDC’s 2002 National Survey of Family Growth show that among surveyed married U.S. women 15 to 44 years of age, an estimated 7.4% were affected by infertility (defined as failing to become pregnant after 1 year of trying with the same partner), while 12% of all women 15 to 44 years of age had impaired fecundity (defined as difficulty in getting pregnant or carrying a child to term).14–19
In this article, we review the potential role of the reproductive-tract microbiome in ART outcomes. We discuss the current status of knowledge, the direction of research now under way, and a research agenda to answer the questions most likely to allow optimization of outcomes.
The Vaginal Microbiome Environment
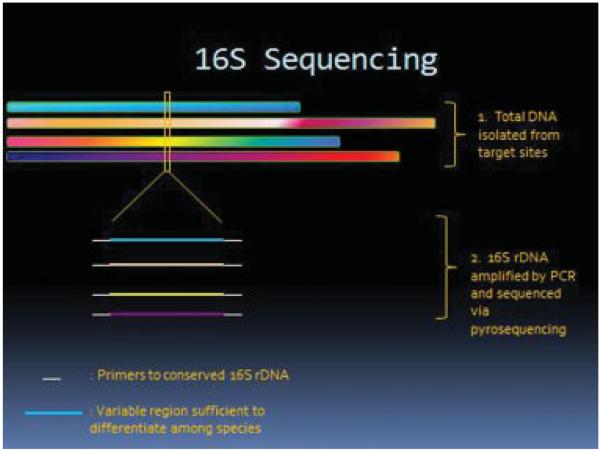
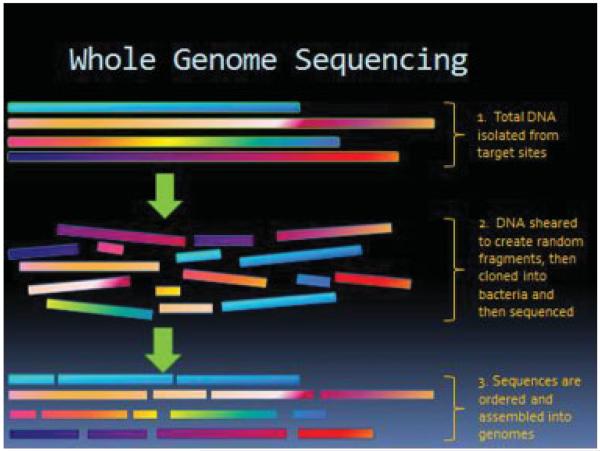
The urogenital site comprises 9% of the total human microbiome compared with the gastrointestinal tract which makes up 29% of the total. The methodology to characterize vaginal microbiome profiles is thoroughly reviewed by our esteemed contributors. Briefly, microbiome profiles are created using via either 16S rDNA amplification by polymerase chain reaction and pyrosequencing as shown in Fig. 1, or whole genome sequencing, as depicted in Fig. 2. Metagenomics reveals that a majority of microbial diversity has not been identified by traditional cultivation-based methods, and less than 1% of bacteria grows and forms colonies on agar plates. Structure and function of the normal human microbiome demonstrates that the vaginal site has low α diversity which measures diversity within samples, demonstrating that the vagina has the least diverse micropopulations with a predominance of Lactobacillus species.5,7,20
Figure 1.
16S rDNA for microbiota identification.
Figure 2.
Whole genome sequencing for microbiota identification.
Lactobacilli and Their Effect on Implantation
The “normal” flora of the reproductive tract includes a variety of Lactobacillus species, which promote a healthy, supportive environment for the embryo in the pre- and periconceptual period. Not only by their presence but also by production of lactic acid,21 hydrogen peroxide (H2O2)22, bacteriocins, antibiotic toxic hydroxyl radicals, and probiotics,23 do lactobacilli promote a supportive environment for implantation.
Since 1928, when Stanley Thomas identified Lactobacillus acidophilus, cultivation-based techniques continued to improve dramatically; between those and the recently developed cultivation-independent molecular-based techniques, over 20 lactobacilli species have now been identified in the genital tract environment.24–30 Lactobacillus comprises 90 to 95% of the total bacterial count in the reproductive tract, with four species showing numerical dominance: Lactobacillus crispatus, Lactobacillus iners, Lactobacillus jensenii, and Lactobacillus gasseri.24,25,29,31–37 Jakobsson and Forsum obtained similar results in 2008 while checking for changes in the cultivatable genital tract flora during the in vitro fertilization (IVF) cycle.38 The Lactobacillus species and their proportions differ among human races and geographical locations,24 and nutrition may have some effect on the diversity as well, as it affects the gastrointestinal Lactobacillus species.39
Two main attributes of lactobacilli have been shown to play a pivotal role in shifting the balance of the reproductive tract environment in favor of successful implantation and pregnancy. Lactobacilli produce lactic acid, which lowers the vaginal pH and makes it an unfavorable habitat for many pathogens, including BV.40 In addition, live birth rate (LBR) has been directly correlated with recovery of H2O2-producing lactobacilli from the embryo transfer-catheter tip31 and inversely correlated with BV.21,41
Pathogens and the Vaginal Microbiome: Bacterial Vaginosis and Infertility
BV describes a perturbation in the Lactobacillus-dominated environment of the vagina and historically refers to an overgrowth of Gardnerella vaginalis. BV is the most common vaginal disorder in reproductive age women and results in millions of health care visits per year in the United States. It is associated with infertility, PTB, endometritis, pelvic inflammatory disease, and increased risk of acquiring human immunodeficiency virus. Thus, the term BV is deceptive since it is a disease of both the lower and upper genital tracts.42
In 1932, Curtis discovered that in women with “white discharge syndrome,” there was a paucity of Lactobacillus and overgrowth of black-pigmented anaerobes, curved anaerobic motile rods, anaerobic cocci, and gram-variable diphtheroidal rods. Drs. Gardner and Duke reported a strong association of nonspecific vaginitis with Haemophilus vaginalis.43 They believed that this organism was the cause of the syndrome despite the fact that direct inoculation of cultivated G. vaginalis did not reproduce the disease. BV is most likely a polymicrobial disease dominated by anaerobes that produce a biofilm. Since anaerobic bacteria are very difficult to culture, sequencing techniques employed by microbiome profiling provide an innovative method of identifying other key culprits other than G. vaginalis, this furthering our understanding of BV and creating treatments to prevent its well-known sequelae. In the clinical setting, the Amsel criteria are used for diagnosis.44,45 In the laboratory setting, the gold standard is the Nugent score which is calculated by assessing for the presence of large gram-positive rods, small gram-variable rods (morphotypes), and curved gram-variable rods. A score of 7 to 10 is consistent with BV.46
Although BV is strongly associated with late fetal loss and PTB, several studies demonstrate a higher prevalence of BV in the infertile population but do not confirm negative effects on pregnancy outcomes.47–50 Previous studies report that up to 40% of patients undergoing IVF cycles have abnormal reproductive tract microbiota.51,52 One study conducted on 91 women undergoing IVF with embryo transfer (IVF-ET), a reduction of LBR was associated with the recovery of Streptococcus viridans from the embryo transfer-catheter tip, but not with other virulent pathogens, including H2O2-nonproducing Lactobacillus, Enterococcus, Staphylococcus epidermidis, Escherichia coli, anaerobic gram-positive cocci, Ureaplasma urealyticum, and M. hominis.53 However, in other studies, a 50% reduction in pregnancy rate was reported when these bacteria were recovered from the embryo transfer-catheter tip,54–57 with dominant microorganisms differing by patient’s geographic location.53,54,56
A recent ART study attempted to correlate patterns of vaginal microbiota during in vitro cycles using modern 16S sequencing method. Thirty-one patients undergoing IVF cycles were enrolled, none of whom had signs of active infection. Swabs of the posterior fornix were obtained on day of baseline ultrasound, day of oocyte retrieval, day of embryo transfer, and at 6 to 8 weeks gestation. They found a diverse vaginal microflora at the time of embryo transfer appeared to be an important factor in the success of the IVFET procedure with a vaginal microbiome composed solely of Lactobacillus yielding the most successful outcome.37
A recent meta-analysis attempted to elucidate if there is a negative effect of BV on conception rates. Twelve studies that used the Nugent criteria were evaluated. Meta-analysis demonstrated that BV is significantly more prevalent in women with infertility (odds ratio [OR], 3.32; 95% confidence interval [CI], 1.53–7.20). Women with tubal factor had significantly higher prevalence BV (OR, 2.77; 95% CI, 1.62–4.75) compared with women with other causes of infertility. BV was not associated with decreased conception rates (OR, 1.03; 95% CI, 0.79–1.33) but was associated with significantly elevated risk of preclinical pregnancy loss (OR, 2.36; 95% CI, 1.24–4.51). BV was not associated with an increased risk of first trimester miscarriage (OR, 1.20; 95%CI, 0.53–2.75).6
The Cytokine Network and Its Importance in the ART Environment
The cytokine network is an important factor in the periconceptual period; the cytokine network allows the embryo to navigate itself successfully toward mature life by overcoming the obstacles of environmental conditions. By sending and receiving signals, this microenvironment system affects gene expression and the epigenome in early embryonic life.58
In recent years, much of the focus regarding intrauterine inflammation has been on the cytokine network. This signaling system is thought to play an important role from conception until implantation.57 Expression of cytokine receptors by the embryo allows cytokines and growth factors secreted from the maternal oviduct and uterine epithelial cells to influence proper development of the embryo and adaptation to its microenvironment.57 The effects of those growth factors and cytokines on the preimplantation embryo have been studied.59–63 Mouse models have shown that Th2 cytokines, particularly interleukin (IL)-10 and IL-4, are essential for implantation and formation of trophoblastic tissue.64 Th1 proinflammatory cytokines are responsible for the down-regulation of Th2 cytokines, and the Th1-Th2 cytokine balance affects the degree of inflammation.65 Growth factors such as granulocyte-macrophage colony-stimulating factor (GM-CSF), colony-stimulating factor (CSF-1), leukemia inhibitory factor (LIF), heparin-binding EGF-like growth factor (HB-EGF), insulin-like growth factor (IGF-1, IGF-2) and cytokines IL-4, IL-10, IL-11 are crucial for normal blastocyst development, whereas other growth factors such as tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), and cytokine IL-2 inhibit implantation.58
The male factor also contributes to the implantation success. Robertson et al found that in mice, transforming growth factor beta (TGF)-β and IL-6 had an embryotrophic effect on the preimplantation period.66 Removal of the seminal vesicles compromised the ability to conceive,67 as well as the ability to maintain adequate growth in utero.68
The delicate cytokine signaling system is self-regulatory and finely tuned, balancing the positive and negative feedback from its surrounding environment. Current data show that the Toll-like receptor (TLR) pathway plays a major role in this periconceptual regulation system, as do the Nod-like receptors.58 The TLR family is dominant in the epithelial cell lining of the female reproductive tract. It binds a wide range of ligands called pathogen-associated molecular patterns (PAMPs) and the result is a wide expression of cytokines and growth factors.69,70 A recent study by Robertson et al found that a preimplantation administration of the systemic low-dose gram-negative bacteria mimetic lipopolysaccharide, a TLR-4 ligand, resulted in increased expression of TNF-α and IFN-γ, which are known to have negative effects on implantation and later adverse effects on fetal and placental development.58
In addition to binding PAMPs, the TLR family also binds danger-associated molecular patterns (DAMPs), also known as alarmins, which are released from cells during tissue damage caused by systemic inflammation, stress, trauma, ischemia, or chemical toxins.71 Some examples of DAMPS are high mobility group box protein 1, expressed in the endometrium; heat shock proteins; S100 calcium-binding proteins; and others.72,73 Their ability to bind to the TLR increases the inflammatory reaction and was found to have a stress-induced effect on miscarriage in mice.74
As discussed previously, the reproductive tract microbiome is not an isolated, closed system. Environmental factors such as stress, nutrition, obesity, chemical toxins, and excessive amounts of alcohol consumption have been found to mimic pathogens in their adverse effects on embryo development and take part in the regulation of cytokines.71
The Role of Prophylactic Antibiotics
Prophylactic antibiotics are commonly used as part of IVF-ET protocols to prevent infections after invasive pelvic procedures. Moore et al53 found no evidence of reduced virulent bacteria or H2O2-producing lactobacilli with the use of doxycycline in IVF-ET setting. Other studies conducted with prophylactic administration of ceftriaxone or intravenous metronidazole demonstrated reduction in virulent bacteria on the transfer catheter and improved pregnancy rate, but were lacking a control group and did not check for the presence of H2O2-producing lactobacilli.54,55 Thus, the role of prophylactic antibiotics and its effect on implantation in the setting of IVF-ET still remains to be determined.
Association of the Reproductive-Tract Microbiome Composition with Circulating Estrogen (E2) and Progesterone (P4) Levels
Measurement of hormone concentrations in the stages of the IVF cycle is a routine matter, but little is known about the effect of these hormones on the uterine microbiome. In 2008, Bezirtzoglou et al75 revealed that in the reproductive-tract microbiota of rats that underwent ovariectomy, lactobacilli concentrations depend on estrogen (E2) levels. In a recent study using the metagenomic approach, Hyman et al37 examined the association of the reproductive-tract microbiome composition with circulating E2 and progesterone (P4) levels. A substantial decrease in serum-E2 concentration between the time of human chorionic gonadotropin (hCG) administration and that of embryo transfer was found in all women who had a live birth.37 These findings are consistent with previous studies that proved this decrease in E2 to be a necessary, but not sufficient, condition for successful outcome of IVF-ET cycles.76,77 Correlation between changes in serum-E2 concentration and changes in the reproductive-tract microbiome showed that 54% of the women had a change in their flora while all serum-E2 concentrations rose significantly between the baseline swab and the late-follicular-stage swab. During the time elapsed from the administration of hCG to embryo transfer, 76% of the patients’ reproductive-tract microbiota changed, while their serum E2 decreased.37 In addition, measurements of serum P4 at time of embryo transfer were not statistically significant between women who had a live birth and those who did not.
Progesterone Function in Implantation
Progesterone is essential for successful implantation and pregnancy maintenance. It promotes endometrial receptivity to blastocyst implantation; in addition, it regulates a large number of cytokines, growth factors, and molecular changes of the endometrial surface epithelium.78–80
Furthermore, P4 is essential for regulation of the decidualization of endometrial stromal fibroblasts, as is evident by morphologic changes and up-regulation of insulin-like growth factor-binding protein 1 (IGFBP-1), glycodelin, prolactin (PRL), HB-EGF, and other biomarkers.81–91 Regulation of trophoblast invasion and leukocyte migration by P4 at the time of decidualization is critical for successful implantation.
Progesterone Resistance
Recent evidence from a study of normal fertile women in an artificial hormonally controlled cycle demonstrated that the minimum P4 concentration needed for endometrial maturation was as low as 4 ng/mL and might be even lower than that seen in ovulatory women.92 Despite that, supplementation of P4 during IVF cycles has proven to increase the clinical pregnancy rate of infertility patients.92–94 Because P4 concentrations approaching the lowest of those observed in ovulatory women support normal endometrial structural and functional maturation in normal individuals, while infertility patients appear to benefit from higher P4 concentrations, it is possible that it is the resistance of the endometrium to P4, rather than a reduced concentration of P4, that may contribute to infertility.
It has been hypothesized that resistance to P4 is caused by altered P4-receptor expression or activity.95 Supporting data come from endometriosis studies, which demonstrate local changes in the responsiveness of the tissue to P4 and lower expression of “P4-regulated” genes such as IGFBP-1 and PRL.83 Resistance to cyclic adenosine monophosphate, an intracellular signaling molecule that enhances P4-receptor activity and supports decidualization, has been found to contribute to P4 resistance in women with endometriosis as well.96 Savaris et al demonstrated P4-resistant endometria in women with polycystic ovary syndrome (PCOS) and hyperandrogenemia.97 On the basis of the role of inflammation in the induction of a progesterone-resistant endometrium, we hypothesize that failure of implantation might be explained, perhaps in part, by alteration in the uterine microbiome in response to inflammation.
This hypothesis finds support in the ability of environmental factors to alter progesterone sensitivity. As noted before, environmental factors play an important role in the success of implantation. Bruner-Tran and Osteen showed that exposure to in utero environmental toxins, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), in mice led to a progesterone-resistant phenotype in adult animals that can persist for several generations.98 Maternal exposure to TCDD promoted a progesterone-resistant endometrium, similar to the endometrium of women with endometriosis, which manifested as infertility or PTB,99–101 likely due in part to loss of P4-receptor expression.
A very important finding derived from this study demonstrated that TCDD-mediated P4 resistance might increase sensitivity to inflammation, even in subsequent generations not exposed to the toxicants, resulting in PTB.98 By means of extrapolation, these data support the hypothesis that a combination of environmental factors, taken together, are a risk for recurrent pregnancy loss and PTB in humans; the mechanism being creation of a P4-resistant endometrium and the presence of inflammation.
Therefore, we conclude that the association between the microbiome of the reproductive tract and circulating serum E2 concentrations may reflect the environment and availability of glycogen. However, progesterone resistance, albeit an unproven relationship to the microbiome, might contribute to implantation failure and infertility. This putative role of undetected endometrial colonization and progesterone resistance requires further investigation.
Conclusions
The majority of data pertaining to the reproductive-tract microbiome in ART have been gleaned from studies on the cervicovaginal flora. The upper genital tract is generally considered to be sterile, but previous studies using endometrial cultures obtained via surgical hysterotomy have demonstrated growth of one or more microorganisms in the uterus, with Lactobacillus species, M. hominis, G. vaginalis, and Enterobacter species the most frequently recovered.102,103 Svenstrup et al104 further supported the concept of a non-sterile uterine environment; they demonstrated the ability of certain bacteria to attach to spermatozoa and be transported into the uterine cavity.
In the era of metagenomic studies, the molecular-based techniques have revolutionized our knowledge of the vaginal microbiome. With the combination of cultivation-dependent and independent molecular-based techniques, we are now able to explore microorganisms that were not detected previously, adding missing pieces to the puzzle called “the reproductive-tract microbiome.”
Our ability to discover different species of lactobacilli, previously underappreciated, has shed light on the composition of the reproductive-tract flora during IVF-ET cycles. It is now clear that only specific Lactobacillus species are present with numerical supremacy in the healthy flora; these species are likely to play a pivotal role in maintaining a supportive environment for implantation and future pregnancy outcome.
Several studies have supported the hypothesis that the reproductive-tract microbiome on the day of embryo transfer affects pregnancy outcome, but no study has used the metagenomic approach for analyzing samples from the transfer-catheter tip. Therefore, further studies are needed to evaluate the association of H2O2-producing Lactobacillus recovered from the transfer-catheter tip with implantation and LBR by using the cultivation-independent molecular-based techniques, and compare results to those measured with the cultivation-dependent techniques.
The idea of colonizing the reproductive tract flora with different species of Lactobacillus to achieve healthy, “normal” flora has been a topic of interest. Probiotic species not numerically dominant in the vagina were used to colonize the already-infected vaginal environment, but unfavorable results were observed. When a more common species, H2O2-producing L. crispatus, was used to colonize vaginas of sexually active healthy women, the success rate was 69 to 90%. This result may support the hypothesis that colonizing the transfer-catheter tip with L. crispatus at the time of IVF-ET would increase the rates of implantation and LBR while decreasing the rate of infection.
It is unclear if the use of prophylactic antibiotics in IVF-ET cycles has an effect on implantation and LBR. Previous studies have recommended using broad-spectrum antibiotics for prophylaxis, but by doing so, there is always a risk of diminishing the dominant H2O2-producing Lactobacillus species in the reproductive tract. Another potential direction for future research would be to identify prophylactic antibiotics that are specific for the virulent microorganisms in the flora of the reproductive tract, while not affecting the H2O2-producing lactobacilli, and compare rates of implantation, infection, and LBR with and without the use of these antibiotics.
On the basis of the previous studies on the progesterone-resistant endometrium, it is possible that failure of implantation might be explained, in part, by alteration in the uterine microbiome in response to inflammation, leading to the development of a progesterone-resistant endometrium. Many questions about the interaction between genes and environment are still to be answered. When these answers are in hand, we envision that appropriately controlling for these factors and the common environmental factors described earlier would create a supportive habitat for the embryo in cases of IVF-ET, resulting in a successful implantation, healthy pregnancy, and healthy neonate.
Acknowledgments
This research was supported, in part, by Intramural research program of the Program in Reproductive and Adult Endocrinology, NICHD, NIH.
Footnotes
The views expressed in this article are those of the authors and do not reflect the official policy or position of the U.S. Government.
References
- 1.Venter JC, Adams MD, Myers EW, et al. The sequence of the human genome. Science. 2001;291(5507):1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 2.Relman DA. New technologies, human-microbe interactions, and the search for previously unrecognized pathogens. J Infect Dis. 2002;186(Suppl 2):S254–S258. doi: 10.1086/344935. [DOI] [PubMed] [Google Scholar]
- 3.Relman DA, Falkow S. The meaning and impact of the human genome sequence for microbiology. Trends Microbiol. 2001;9(5):206–208. doi: 10.1016/s0966-842x(01)02041-8. [DOI] [PubMed] [Google Scholar]
- 4.Mullard A. Microbiology: the inside story. Nature. 2008;453(7195):578–580. doi: 10.1038/453578a. [DOI] [PubMed] [Google Scholar]
- 5.Peterson J, Garges S, Giovanni M, et al. NIH HMP Working Group The NIH Human Microbiome Project. Genome Res. 2009;19(12):2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Oostrum N, De Sutter P, Meys J, Verstraelen H. Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta-analysis. Hum Reprod. 2013;28(7):1809–1815. doi: 10.1093/humrep/det096. [DOI] [PubMed] [Google Scholar]
- 7.Aagaard K, Petrosino J, Keitel W, et al. The Human Microbiome Project strategy for comprehensive sampling of the human microbiome and why it matters. FASEB J. 2013;27(3):1012–1022. doi: 10.1096/fj.12-220806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69(5):1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 9.Favier CF, Vaughan EE, De Vos WM, Akkermans AD. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol. 2002;68(1):219–226. doi: 10.1128/AEM.68.1.219-226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 11.Kurokawa K, Itoh T, Kuwahara T, et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007;14(4):169–181. doi: 10.1093/dnares/dsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grönlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28(1):19–25. doi: 10.1097/00005176-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunderam S, Kissin DM, Flowers L, et al. Assisted reproductive technology surveillance–United States. MMWR Surveill Summ. 2009;61:1–23. [PubMed] [Google Scholar]
- 15.Petrou S, Abangma G, Johnson S, Wolke D, Marlow N. Costs and health utilities associated with extremely preterm birth: evidence from the EPICure study. Value Health. 2009;12(8):1124–1134. doi: 10.1111/j.1524-4733.2009.00580.x. [DOI] [PubMed] [Google Scholar]
- 16.Keirse MJ. New perspectives for the effective treatment of preterm labor. Am J Obstet Gynecol. 1995;173(2):618–628. doi: 10.1016/0002-9378(95)90292-9. [DOI] [PubMed] [Google Scholar]
- 17.Young LE. Imprinting of genes and the Barker hypothesis. Twin Res. 2001;4(5):307–317. doi: 10.1375/1369052012632. [DOI] [PubMed] [Google Scholar]
- 18.Kaminsky ZA, Tang T, Wang SC, et al. DNA methylation profiles in monozygotic and dizygotic twins. Nat Genet. 2009;41(2):240–245. doi: 10.1038/ng.286. [DOI] [PubMed] [Google Scholar]
- 19.Watkins AJ, Papenbrock T, Fleming TP. The preimplantation embryo: handle with care. Semin Reprod Med. 2008;26(2):175–185. doi: 10.1055/s-2008-1042956. [DOI] [PubMed] [Google Scholar]
- 20.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawes SE, Hillier SL, Benedetti J, et al. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis. 1996;174(5):1058–1063. doi: 10.1093/infdis/174.5.1058. [DOI] [PubMed] [Google Scholar]
- 22.Aroutcheva AA, Simoes JA, Behbakht K, Faro S. Gardnerella vaginalis isolated from patients with bacterial vaginosis and from patients with healthy vaginal ecosystems. Clin Infect Dis. 2001;33(7):1022–1027. doi: 10.1086/323030. [DOI] [PubMed] [Google Scholar]
- 23.Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis. 2009;15(2):300–310. doi: 10.1002/ibd.20602. [DOI] [PubMed] [Google Scholar]
- 24.Pavlova SI, Kilic AO, Kilic SS, et al. Genetic diversity of vaginal lactobacilli from women in different countries based on 16S rRNA gene sequences. J Appl Microbiol. 2002;92(3):451–459. doi: 10.1046/j.1365-2672.2002.01547.x. [DOI] [PubMed] [Google Scholar]
- 25.Burton JP, Reid G. Evaluation of the bacterial vaginal flora of 20 postmenopausal women by direct (Nugent score) and molecular (polymerase chain reaction and denaturing gradient gel electrophoresis) techniques. J Infect Dis. 2002;186(12):1770–1780. doi: 10.1086/345761. [DOI] [PubMed] [Google Scholar]
- 26.Klein G, Pack A, Bonaparte C, Reuter G. Taxonomy and physiology of probiotic lactic acid bacteria. Int J Food Microbiol. 1998;41(2):103–125. doi: 10.1016/s0168-1605(98)00049-x. [DOI] [PubMed] [Google Scholar]
- 27.Antonio MA, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis. 1999;180(6):1950–1956. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- 28.Reid G, McGroarty JA, Tomeczek L, Bruce AW. Identification and plasmid profiles of Lactobacillus species from the vagina of 100 healthy women. FEMS Immunol Med Microbiol. 1996;15(1):23–26. doi: 10.1111/j.1574-695X.1996.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 29.Vásquez A, Jakobsson T, Ahrné S, Forsum U, Molin G. Vaginal lactobacillus flora of healthy Swedish women. J Clin Microbiol. 2002;40(8):2746–2749. doi: 10.1128/JCM.40.8.2746-2749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tärnberg M, Jakobsson T, Jonasson J, Forsum U. Identification of randomly selected colonies of lactobacilli from normal vaginal fluid by pyrosequencing of the 16S rDNA variable V1 and V3 regions. APMIS. 2002;110(11):802–810. doi: 10.1034/j.1600-0463.2002.1101106.x. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto T, Zhou X, Williams CJ, Hochwalt A, Forney LJ. Bacterial populations in the vaginas of healthy adolescent women. J Pediatr Adolesc Gynecol. 2009;22(1):11–18. doi: 10.1016/j.jpag.2008.01.073. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, Forney LJ. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology. 2004;150(Pt 8):2565–2573. doi: 10.1099/mic.0.26905-0. [DOI] [PubMed] [Google Scholar]
- 33.Zhou X, Brown CJ, Abdo Z, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 2007;1(2):121–133. doi: 10.1038/ismej.2007.12. [DOI] [PubMed] [Google Scholar]
- 34.Thies FL, König W, König B. Rapid characterization of the normal and disturbed vaginal microbiota by application of 16S rRNA gene terminal RFLP fingerprinting. J Med Microbiol. 2007;56(Pt 6):755–761. doi: 10.1099/jmm.0.46562-0. [DOI] [PubMed] [Google Scholar]
- 35.Shi Y, Chen L, Tong J, Xu C. Preliminary characterization of vaginal microbiota in healthy Chinese women using cultivation-independent methods. J Obstet Gynaecol Res. 2009;35(3):525–532. doi: 10.1111/j.1447-0756.2008.00971.x. [DOI] [PubMed] [Google Scholar]
- 36.Vitali B, Pugliese C, Biagi E, et al. Dynamics of vaginal bacterial communities in women developing bacterial vaginosis, candidiasis, or no infection, analyzed by PCR-denaturing gradient gel electrophoresis and real-time PCR. Appl Environ Microbiol. 2007;73(18):5731–5741. doi: 10.1128/AEM.01251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hyman RW, Herndon CN, Jiang H, et al. The dynamics of the vaginal microbiome during infertility therapy with in vitro fertilization-embryo transfer. J Assist Reprod Genet. 2012;29(2):105–115. doi: 10.1007/s10815-011-9694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakobsson T, Forsum U. Changes in the predominant human Lactobacillus flora during in vitro fertilisation. Ann Clin Microbiol Antimicrob. 2008;7:14. doi: 10.1186/1476-0711-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahrné S, Nobaek S, Jeppsson B, Adlerberth I, Wold AE, Molin G. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J Appl Microbiol. 1998;85(1):88–94. doi: 10.1046/j.1365-2672.1998.00480.x. [DOI] [PubMed] [Google Scholar]
- 40.Skarin A, Sylwan J. Vaginal lactobacilli inhibiting growth of Gardnerella vaginalis, Mobiluncus and other bacterial species cultured from vaginal content of women with bacterial vaginosis. Acta Pathol Microbiol Immunol Scand [B] 1986;94(6):399–403. doi: 10.1111/j.1699-0463.1986.tb03074.x. [DOI] [PubMed] [Google Scholar]
- 41.Eschenbach DA, Davick PR, Williams BL, et al. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 1989;27(2):251–256. doi: 10.1128/jcm.27.2.251-256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiegel CA, Amsel R, Eschenbach D, Schoenknecht F, Holmes KK. Anaerobic bacteria in nonspecific vaginitis. N Engl J Med. 1980;303(11):601–607. doi: 10.1056/NEJM198009113031102. [DOI] [PubMed] [Google Scholar]
- 43.Gardner HL, Dukes CD. Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified non-specific vaginitis. Am J Obstet Gynecol. 1955;69(5):962–976. [PubMed] [Google Scholar]
- 44.Spiegel CA, Amsel R, Holmes KK. Diagnosis of bacterial vaginosis by direct gram stain of vaginal fluid. J Clin Microbiol. 1983;18(1):170–177. doi: 10.1128/jcm.18.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74(1):14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 46.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ralph SG, Rutherford AJ, Wilson JD. Influence of bacterial vaginosis on conception and miscarriage in the first trimester: cohort study. BMJ. 1999;319(7204):220–223. doi: 10.1136/bmj.319.7204.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C, Pearson J. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. BMJ. 1994;308(6924):295–298. doi: 10.1136/bmj.308.6924.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaudoin M, Rekha P, Morris A, Lynch J, Acharya U. Bacterial vaginosis and past chlamydial infection are strongly and independently associated with tubal infertility but do not affect in vitro fertilization success rates. Fertil Steril. 1999;72(4):730–732. doi: 10.1016/s0015-0282(99)00310-6. [DOI] [PubMed] [Google Scholar]
- 50.Witkin SS, Kligman I, Grifo JA, Rosenwaks Z. Chlamydia trachomatis detected by polymerase chain reaction in cervices of culture-negative women correlates with adverse in vitro fertilization outcome. J Infect Dis. 1995;171(6):1657–1659. doi: 10.1093/infdis/171.6.1657. [DOI] [PubMed] [Google Scholar]
- 51.Leitich H, Kiss H. Asymptomatic bacterial vaginosis and intermediate flora as risk factors for adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. 2007;21(3):375–390. doi: 10.1016/j.bpobgyn.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 2003;189(1):139–147. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 53.Moore DE, Soules MR, Klein NA, Fujimoto VY, Agnew KJ, Eschenbach DA. Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertil Steril. 2000;74(6):1118–1124. doi: 10.1016/s0015-0282(00)01624-1. [DOI] [PubMed] [Google Scholar]
- 54.Egbase PE, al-Sharhan M, al-Othman S, al-Mutawa M, Udo EE, Grudzinskas JG. Incidence of microbial growth from the tip of the embryo transfer catheter after embryo transfer in relation to clinical pregnancy rate following in-vitro fertilization and embryo transfer. Hum Reprod. 1996;11(8):1687–1689. doi: 10.1093/oxfordjournals.humrep.a019470. [DOI] [PubMed] [Google Scholar]
- 55.Egbase PE, Udo EE, Al-Sharhan M, Grudzinskas JG. Prophylactic antibiotics and endocervical microbial inoculation of the endometrium at embryo transfer. Lancet. 1999;354(9179):651–652. doi: 10.1016/s0140-6736(99)02415-0. [DOI] [PubMed] [Google Scholar]
- 56.Fanchin R, Harmas A, Benaoudia F, Lundkvist U, Olivennes F, Frydman R. Microbial flora of the cervix assessed at the time of embryo transfer adversely affects in vitro fertilization outcome. Fertil Steril. 1998;70(5):866–870. doi: 10.1016/s0015-0282(98)00277-5. [DOI] [PubMed] [Google Scholar]
- 57.Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis. 1993;16(Suppl 4):S273–S281. doi: 10.1093/clinids/16.supplement_4.s273. [DOI] [PubMed] [Google Scholar]
- 58.Robertson SA, Chin PY, Glynn DJ, Thompson JG. Peri-conceptual cytokines—setting the trajectory for embryo implantation, pregnancy and beyond. Am J Reprod Immunol. 2011;66(Suppl 1):2–10. doi: 10.1111/j.1600-0897.2011.01039.x. [DOI] [PubMed] [Google Scholar]
- 59.Hardy K, Spanos S. Growth factor expression and function in the human and mouse preimplantation embryo. J Endocrinol. 2002;172(2):221–236. doi: 10.1677/joe.0.1720221. [DOI] [PubMed] [Google Scholar]
- 60.Díaz-Cueto L, Gerton GL. The influence of growth factors on the development of preimplantation mammalian embryos. Arch Med Res. 2001;32(6):619–626. doi: 10.1016/s0188-4409(01)00326-5. [DOI] [PubMed] [Google Scholar]
- 61.Kane MT, Morgan PM, Coonan C. Peptide growth factors and preimplantation development. Hum Reprod Update. 1997;3(2):137–157. doi: 10.1093/humupd/3.2.137. [DOI] [PubMed] [Google Scholar]
- 62.Kaye PL, Harvey MB. The role of growth factors in preimplantation development. Prog Growth Factor Res. 1995;6(1):1–24. doi: 10.1016/0955-2235(95)00001-1. [DOI] [PubMed] [Google Scholar]
- 63.Pampfer S, Arceci RJ, Pollard JW. Role of colony stimulating factor-1 (CSF-1) and other lympho-hematopoietic growth factors in mouse pre-implantation development. Bioessays. 1991;13(10):535–540. doi: 10.1002/bies.950131007. [DOI] [PubMed] [Google Scholar]
- 64.Robertson SA. Control of the immunological environment of the uterus. Rev Reprod. 2000;5(3):164–174. doi: 10.1530/ror.0.0050164. [DOI] [PubMed] [Google Scholar]
- 65.Eckert LO, Moore DE, Patton DL, Agnew KJ, Eschenbach DA. Relationship of vaginal bacteria and inflammation with conception and early pregnancy loss following in-vitro fertilization. Infect Dis Obstet Gynecol. 2003;11(1):11–17. doi: 10.1155/S1064744903000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robertson SA, Mayrhofer G, Seamark RF. Uterine epithelial cells synthesize granulocyte-macrophage colony-stimulating factor and interleukin-6 in pregnant and nonpregnant mice. Biol Reprod. 1992;46(6):1069–1079. doi: 10.1095/biolreprod46.6.1069. [DOI] [PubMed] [Google Scholar]
- 67.Tremellen KP, Seamark RF, Robertson SA. Seminal transforming growth factor beta1 stimulates granulocyte-macrophage colony-stimulating factor production and inflammatory cell recruitment in the murine uterus. Biol Reprod. 1998;58(5):1217–1225. doi: 10.1095/biolreprod58.5.1217. [DOI] [PubMed] [Google Scholar]
- 68.Robertson SA, Mayrhofer G, Seamark RF. Ovarian steroid hormones regulate granulocyte-macrophage colony-stimulating factor synthesis by uterine epithelial cells in the mouse. Biol Reprod. 1996;54(1):183–196. doi: 10.1095/biolreprod54.1.183. [DOI] [PubMed] [Google Scholar]
- 69.Herbst-Kralovetz MM, Quayle AJ, Ficarra M, et al. Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. Am J Reprod Immunol. 2008;59(3):212–224. doi: 10.1111/j.1600-0897.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 70.Soboll G, Shen L, Wira CR. Expression of Toll-like receptors (TLR) and responsiveness to TLR agonists by polarized mouse uterine epithelial cells in culture. Biol Reprod. 2006;75(1):131–139. doi: 10.1095/biolreprod.106.050690. [DOI] [PubMed] [Google Scholar]
- 71.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zicari A, Centonze C, Realacci M, Buchetti B, Pietropolli A, Ticconi C. Estradiol 17-beta and progesterone modulate inducible nitric oxide synthase and high mobility group box 1 expression in human endometrium. Reprod Sci. 2008;15(6):559–566. doi: 10.1177/1933719107312560. [DOI] [PubMed] [Google Scholar]
- 73.Tabibzadeh S, Broome J. Heat shock proteins in human endometrium throughout the menstrual cycle. Infect Dis Obstet Gynecol. 1999;7(1-2):5–9. doi: 10.1155/S1064744999000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Friebe A, Douglas AJ, Solano E, et al. Neutralization of LPS or blockage of TLR4 signaling prevents stress-triggered fetal loss in murine pregnancy. J Mol Med (Berl) 2011;89(7):689–699. doi: 10.1007/s00109-011-0743-5. [DOI] [PubMed] [Google Scholar]
- 75.Bezirtzoglou E, Voidarou Ch, Papadaki A, Tsiotsias A, Kotsovolou O, Konstandi M. Hormone therapy alters the composition of the vaginal microflora in ovariectomized rats. Microb Ecol. 2008;55(4):751–759. doi: 10.1007/s00248-007-9317-z. [DOI] [PubMed] [Google Scholar]
- 76.Kim YJ, Ku SY, Jee BC, et al. Dynamics of early estradiol production may be associated with outcomes of in vitro fertilization. Fertil Steril. 2010;94(7):2868–2870. doi: 10.1016/j.fertnstert.2010.06.070. [DOI] [PubMed] [Google Scholar]
- 77.Var T, Tonguc E, Dogan M, Mollamahmutoglu L. Relationship between the oestradiol/oocyte ratio and the outcome of assisted reproductive technology cycles with gonadotropin releasing hormone agonist. Gynecol Endocrinol. 2011;27(8):558–561. doi: 10.3109/09513590.2010.501887. [DOI] [PubMed] [Google Scholar]
- 78.Giudice LC, Telles TL, Lobo S, Kao L. The molecular basis for implantation failure in endometriosis: on the road to discovery. Ann N Y Acad Sci. 2002;955:252–264. doi: 10.1111/j.1749-6632.2002.tb02786.x. discussion 293–295, 396–406. [DOI] [PubMed] [Google Scholar]
- 79.Lessey BA. Two pathways of progesterone action in the human endometrium: implications for implantation and contraception. Steroids. 2003;68(10-13):809–815. doi: 10.1016/j.steroids.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 80.Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Semin Reprod Med. 2010;28(1):5–16. doi: 10.1055/s-0029-1242988. [DOI] [PubMed] [Google Scholar]
- 81.Lessey BA, Gui Y, Apparao KB, Young SL, Mulholland J. Regulated expression of heparin-binding EGF-like growth factor (HB-EGF) in the human endometrium: a potential paracrine role during implantation. Mol Reprod Dev. 2002;62(4):446–455. doi: 10.1002/mrd.10129. [DOI] [PubMed] [Google Scholar]
- 82.Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol Reprod. 2009;80(1):105–114. doi: 10.1095/biolreprod.108.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25(6):445–453. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- 84.Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178(3):357–372. doi: 10.1677/joe.0.1780357. [DOI] [PubMed] [Google Scholar]
- 85.Bischof P. Three pregnancy proteins (PP12, PP14, and PAPP-A): their biological and clinical relevance. Am J Perinatol. 1989;6(2):110–116. doi: 10.1055/s-2007-999559. [DOI] [PubMed] [Google Scholar]
- 86.Rutanen EM, Koistinen R, Seppälä M, Julkunen M, Suikkari AM, Huhtala ML. Progesterone-associated proteins PP12 and PP14 in the human endometrium. J Steroid Biochem. 1987;27(1-3):25–31. doi: 10.1016/0022-4731(87)90290-1. [DOI] [PubMed] [Google Scholar]
- 87.Julkunen M, Raikar RS, Joshi SG, Bohn H, Seppälä M. Placental protein 14 and progestagen-dependent endometrial protein are immunologically indistinguishable. Hum Reprod. 1986;1(1):7–8. doi: 10.1093/oxfordjournals.humrep.a136349. [DOI] [PubMed] [Google Scholar]
- 88.Julkunen M, Koistinen R, Sjöberg J, Rutanen EM, Wahlström T, Seppälä M. Secretory endometrium synthesizes placental protein 14. Endocrinology. 1986;118(5):1782–1786. doi: 10.1210/endo-118-5-1782. [DOI] [PubMed] [Google Scholar]
- 89.Rutanen EM, Koistinen R, Sjöberg J, et al. Synthesis of placental protein 12 by human endometrium. Endocrinology. 1986;118(3):1067–1071. doi: 10.1210/endo-118-3-1067. [DOI] [PubMed] [Google Scholar]
- 90.Bohn H, Kraus W. [Isolation and characterization of a new placenta specific protein (PP12) (author’s transl)] Arch Gynecol. 1980;229(4):279–291. doi: 10.1007/BF02108579. [DOI] [PubMed] [Google Scholar]
- 91.Martin KL, Barlow DH, Sargent IL. Heparin-binding epidermal growth factor significantly improves human blastocyst development and hatching in serum-free medium. Hum Reprod. 1998;13(6):1645–1652. doi: 10.1093/humrep/13.6.1645. [DOI] [PubMed] [Google Scholar]
- 92.Usadi RS, Groll JM, Lessey BA, et al. Endometrial development and function in experimentally induced luteal phase deficiency. J Clin Endocrinol Metab. 2008;93(10):4058–4064. doi: 10.1210/jc.2008-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fritz M, Adamson GD, Barnhart K, et al. Practice Committee of the American Society for Reproductive Medicine. Progesterone supplementation during the luteal phase and in early pregnancy in the treatment of infertility: an educational bulletin. Fertil Steril. 2008;89(4):789–792. doi: 10.1016/j.fertnstert.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 94.Daya S, Gunby JL. WITHDRAWN: Luteal phase support in assisted reproduction cycles. Cochrane Database Syst Rev. 2008;(3):CD004830. doi: 10.1002/14651858.CD004830.pub2. [DOI] [PubMed] [Google Scholar]
- 95.Aghajanova L, Velarde MC, Giudice LC. Altered gene expression profiling in endometrium: evidence for progesterone resistance. Semin Reprod Med. 2010;28(1):51–58. doi: 10.1055/s-0029-1242994. [DOI] [PubMed] [Google Scholar]
- 96.Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;85(3):564–572. doi: 10.1016/j.fertnstert.2005.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Savaris RF, Groll JM, Young SL, et al. Progesterone resistance in PCOS endometrium: a microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. J Clin Endocrinol Metab. 2011;96(6):1737–1746. doi: 10.1210/jc.2010-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bruner-Tran KL, Osteen KG. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol. 2011;31(3):344–350. doi: 10.1016/j.reprotox.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bruner-Tran KL, Ding T, Osteen KG. Dioxin and endometrial progesterone resistance. Semin Reprod Med. 2010;28(1):59–68. doi: 10.1055/s-0029-1242995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bruner-Tran KL, Yeaman GR, Crispens MA, Igarashi TM, Osteen KG. Dioxin may promote inflammation-related development of endometriosis. Fertil Steril. 2008;89(5, Suppl):1287–1298. doi: 10.1016/j.fertnstert.2008.02.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nayyar T, Bruner-Tran KL, Piestrzeniewicz-Ulanska D, Osteen KG. Developmental exposure of mice to TCDD elicits a similar uterine phenotype in adult animals as observed in women with endometriosis. Reprod Toxicol. 2007;23(3):326–336. doi: 10.1016/j.reprotox.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Møller BR, Kristiansen FV, Thorsen P, Frost L, Mogensen SC. Sterility of the uterine cavity. Acta Obstet Gynecol Scand. 1995;74(3):216–219. doi: 10.3109/00016349509008942. [DOI] [PubMed] [Google Scholar]
- 103.Cowling P, McCoy DR, Marshall RJ, Padfield CJ, Reeves DS. Bacterial colonization of the non-pregnant uterus: a study of pre-menopausal abdominal hysterectomy specimens. Eur J Clin Microbiol Infect Dis. 1992;11(2):204–205. doi: 10.1007/BF01967084. [DOI] [PubMed] [Google Scholar]
- 104.Svenstrup HF, Fedder J, Abraham-Peskir J, Birkelund S, Christiansen G. Mycoplasma genitalium attaches to human spermatozoa. Hum Reprod. 2003;18(10):2103–2109. doi: 10.1093/humrep/deg392. [DOI] [PubMed] [Google Scholar]