Abstract
Purpose of the review
There is a need for improved diagnosis and for optimal classification of patients with infectious diseases. An alternative approach to the pathogen-detection strategy is based on a comprehensive analysis of the host response to the infection. This review focuses on the value of transcriptome analyses of blood leukocytes for the diagnosis and management of patients with infectious diseases.
Recent findings
Initial studies showed that RNA from blood leukocytes of children with acute viral and bacterial infections carried pathogen-specific transcriptional signatures. Subsequently, transcriptional signatures for several other infections have been described and validated in humans with malaria, dengue, salmonella, meloidosis, RSV, influenza, tuberculosis and HIV. In addition, transcriptome analyses represent an invaluable tool to understand disease pathogenesis, and to objectively classify patients according to clinical severity.
Summary
Microarray studies have shown to be highly reproducible using different platforms, and in different patient populations, confirming the value of blood transcriptome analyses to study pathogen-specific host immune responses in the clinical setting. Combining the detection of the pathogen with a comprehensive assessment of the host immune response will provide a new understanding of the correlations between specific etiologic agents, the host response and the clinical manifestations of the disease.
Keywords: gene expression profiles, microarrays, infection, disease severity, transcriptome
Introduction
Despite major advances in the development and implementation of vaccines and antimicrobial agents, infectious diseases continue to represent a major cause of morbidity and mortality worldwide [1]. Recent examples such as the MERS coronavirus and measles outbreaks [2,3], the 2009 H1N1 influenza pandemic [4,5], the ongoing epidemic of methicillin-resistant Staphylococcus aureus infections [6,7], as well as the increased frequency of hospital acquired infections caused by multiple-resistant gram-negative bacilli [8] and highly virulent strains of Clostridium difficile [9] highlight the challenges we continue to experience in managing patients with infectious diseases. In this context of outbreaks of emergent and re-emergent pathogens linked to increased antimicrobial resistance, there is a need for improved diagnostic tools and for optimal patient classification and management.
Host responses for improving the diagnosis of Infectious Diseases
One of the most frequent challenges that physicians face in the clinical setting is the difficulty to establish an appropriate etiologic diagnosis, or even distinguish between bacterial or viral infections in patients presenting with an acute febrile illness. These obstacles can delay initiation of appropriate therapy, which can result in unnecessary morbidity and even mortality. On the other hand, the need to promptly start appropriate antimicrobial therapy to control the infection has to be balanced with a rationale use of antibiotics. Within this context, there is an obvious need for improved diagnostics tools to help with patient classification, which in turn should allow the use of targeted therapies.
Microbial pathogens are detected in clinically relevant specimens using a variety of assays including cultures, rapid antigen detection tests, and PCR assays. To date, to be able to establish causality, growth of the specific pathogen (bacteria, virus and fungus) remains the gold standard. However, this is a flawed approach particularly if the organism is not present in the blood or other easily accessible sites. In addition, many pathogens grow slowly or require complex media, and a significant number of clinically important microbes remain unrecognized as they are resistant to cultivation in the laboratory, limiting the physician’s clinical decision-making [10,11]. The introduction of more sensitive molecular diagnostic assays has significantly improved the diagnosis of viral infections [12]. Unfortunately, this is not the case for bacterial pathogens. Moreover, in the clinical setting is not uncommon to encounter situations in which the sole identification of a pathogen is not sufficient to establish causality, e.g. the detection of respiratory viruses in patients with no respiratory symptoms or in patients with pneumonia, which oftentimes have a bacterial pathogen co-detected. An alternative approach to the pathogen-detection strategy is based on a comprehensive analysis of the host response to the infection caused by different pathogens (Fig. 1) [13–17].
Figure 1. Different classes of pathogens induce specific gene expression profiles that can be identified by analyses of blood leukocytes.
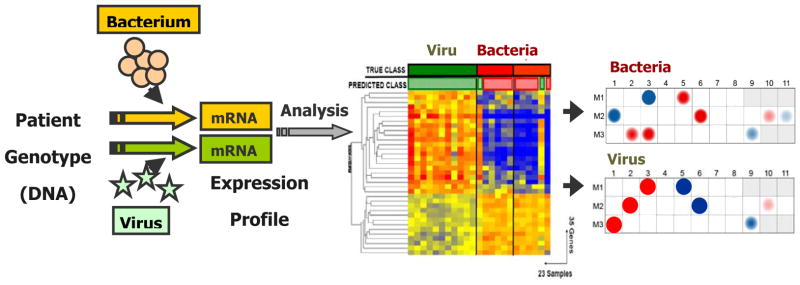
Using microarray technology we can measure in a quantitative and qualitative manner the differences in gene expression patterns present in blood immune cells as induced by various types of infectious agents.
Reproduced with permission from Ramilo O, Mejias A: Shifting the paradigm: host gene signatures for diagnosis of infectious diseases. Cell Host Microbe 2009, 6:199–200. [17]
Different classes of pathogens trigger specific pattern-recognition receptors (PRRs) differentially expressed on peripheral blood leukocytes [18–20]. Blood represents both a reservoir and a migration compartment for these immune cells that become educated and implement their function by circulating between central and peripheral lymphoid organs and migrating to and from the site of infection via blood. Therefore, blood leukocytes constitute an accessible source of clinically relevant information, and a comprehensive molecular phenotype of these cells can be obtained using gene expression microarrays [21]. Because they provide a comprehensive assessment of all immune related cells and pathways, genomic studies are well suited to study the host-pathogen interaction. In fact, studies in children and adults with acute infections have shown that different classes of pathogens induce distinct gene expression profiles that can be identified by analyses of blood leukocytes [13–16,22–25].
In vitro Studies
The initial studies supporting the hypothesis that pathogen-specific gene expression profiles can be measured in immune cells were derived from in vitro studies. The comparative analysis of a compendium of host-pathogen microarray datasets identified both a common host transcriptional response to infection and a pathogen-specific signature [26–29]. Upon activation, Toll-like receptors (TLRs) trigger signaling pathways that share common components while retaining unique characteristics accounting in part for the specificity of transcriptional responses [30]. In fact, in vitro microarray studies have shown the ability of HSV, West Nile virus, pseudorabies virus, HCV, VZV or rhinovirus to limit the ability of the host to develop effective anti-viral responses by a variety of mechanisms [31–33]. Altogether the vast body of in vitro experimental data accumulated over the years suggests that hosts can mount pathogen-specific transcriptional responses to infections.
Human Studies: Proof of Concept
Initial studies tested the hypothesis that leukocytes isolated from peripheral blood of patients with acute infections carry unique transcriptional signatures, which would in turn permit pathogen discrimination and classification. In those initial studies gene expression patterns derived from PBMCs of pediatric patients hospitalized with acute infections showed that there are pathogen-specific signatures that can be measured in the blood and distinguished children with influenza A from Staphylococcus aureus, Streptococcus pneumoniae and Escherichia coli acute infections with > 95% accuracy [13]. Analysis of PBMC samples requires processing in real time, which has limitations from a practical application in the clinical setting with large numbers of patients. In addition, PBMC samples do not include neutrophils, which is a relevant cell population for the pathogenesis of bacterial and viral infections. For these reasons in recent years there has been a shift towards whole blood samples to study transcriptional profiles in the clinical setting. Indeed, whole blood signatures for several other infections have also been described from infected subjects including: malaria, dengue, salmonella, melioidosis, TB, RSV, influenza virus and the novel H1N1/09, adenovirus, HTLV-1 and HIV (Table 1) [15,16,22,24,34–42].
Table 1.
| Country/Study year | Pathogens | Population | Sample type | Validation |
|---|---|---|---|---|
| USA, 2007 [13] | * Virus vs. bacterial | Children < 18 yr; n=131 Ctrl age matched; n=7 |
PBMCs | 3 patient cohorts and RT-PCR |
| Vietnam, 2009 [34] | Salmonella typhi | Adults; n=29 Ctrl; (OD n=10; HC n=16) |
Whole blood | ----- |
| Thailand, 2009 [24] | Burkholderiapseudomallei | Adults; n=32 Ctrl; (OD n=31; HC n=29) |
Whole blood | 3 patient cohorts |
| Cambodia, 2010 [35] | Dengue | Children <15 yr; n=48 (DSS n=19; DF n=16; DHF n=13) | Whole blood | RT-PCR validation |
| UK, South Africa, 2010 [22] | Mycobacterium tuberculosis (TB) | Adults TB; n=123 Ctrl; (OD =96; HC n=24) |
Whole blood | 3 patient cohorts |
| Switzerland, 2010 [36] | HIV | Adults; n=137 Ctrl; n=19 |
CD4+ T-cells | Genome wide SNP |
| West Africa, 2012 [37] | Plasmodium falciparum | Children <10 yr; n=94 Ctrl age-matched; n=61 |
Whole blood | Mouse model |
| UK, 2012 [38] | HTLV-1 associated myelopathy | Adults; n=57 Ctrl; (OD n=144; HC n=17) |
Whole blood | 2 patient cohorts |
| USA, 2012 [39] | S. aureus (invasive) | Children; n=99 Ctrl; n=44 |
Whole blood | 2 patient cohorts |
| UK, 2012 [40] | H1N1 Influenza A | Adults; n=11 Ctrl; (OD n=28; HC n= 18) |
Whole blood | 2 patient cohorts Public available data sets [13, 25] |
| USA 2012 [28] | RSV, influenza | Children < 2yr; n=79 Ctrl age-matched; n= 22 |
PBMCs | Mouse model Primary human epithelial cells |
| USA, Finland, 2013 [15] | RSV, influenza, HRV | Children < 2 yr; n=181 Ctrl age-matched; n=39 |
Whole blood | 4 patient cohorts |
| UK, 2013 [41] | H1N1/09 influenza, RSV, bacteria | Children < 8 yr; n=77 Ctrl children; n=33 |
Whole blood | Public available data set [34] |
| USA, 2013 [31] | ** Virus vs bacterial | Febrile children <3yr; n=30 Afebrile children; n=22 |
Whole blood | Public available data set [13] |
| South Africa Malawi, 2013 [16] |
TB ± HIV | Adults TB ± HIV; n=361 OD; n=175 |
Whole blood | 4 patient cohorts and public available data set [22] |
| USA, 2013 [42] Australia |
*** Virus vs bacterial | Experimental infection; n=41[25] Febrile adults; n=102 Ctrl; n=35 |
Whole blood | 3 patient cohorts |
Virus: Influenza A; bacteria: S. pneumoniae, S. aureus, E. coli.
Virus: HHV-6, adenovirus, enterovirus.
Virus: influenza A, HRV; bacterial: S. aureus, S. pneumoniae, E. coli. Ctrl: controls; DSS: dengue shock syndrome; DF: dengue fever; DHF: dengue hemorrhagic fever. RT-PCR: real time PCR. OD: other diseases. SNP: single nucleotide polymorphism.
A study performed in adult volunteers experimentally infected with RSV, HRV or influenza A found an “acute respiratory viral signature” which was independently validated in a previously published dataset of pediatric patients with pneumonia [13]. Despite the technical challenges involved in such analysis and the differences in the patient populations analyzed (children naturally infected vs adults with experimental infection), their identified “viral signature” classified pediatric patients with influenza from age-matched healthy controls with 100% accuracy [25]. This is a critical observation that confirms the reproducibility and potential value of blood transcriptome analysis to study host immune responses to respiratory viruses in the clinical setting. Additional studies will be necessary to evaluate this approach in other relevant clinical situations where the application of this methodology has the potential to transform the standard of care. In this regard, two studies have already shown the utility of host gene expression profiles as a diagnostic tool when effective treatment depends of rapid identification of the infectious agent or even the need for treatment. In the first study using also adult volunteers experimentally infected with influenza A H1N1 or H3N2 [25], the authors found a blood RNA signature that was detectable more than 24 hours before the peak of clinical symptoms. [43] Subsequently, the same group of investigators used the transcriptome profiles derived from the experimental influenza signature [25] to develop a targeted host based RT-PCR low-density microarray assay. This assay was applied to adult patients presenting with fever to the ED and differentiated viral vs. bacterial infections with 89% sensitivity and 94% specificity [42], demonstrating that gene expression profiles identify by microarray analyses can be successfully applied to custom made platforms with potential for a fast, point of care patient diagnosis and classification. To a certain point it is remarkable that although in the majority of studies patient samples were collected at different time points after pathogen exposure and disease onset, a robust and pathogen-specific biosignature have been derived and validated in independent cohorts of patients in complete different settings.
Areas for Improving Diagnosis
There are two areas that represent a challenge for improving diagnosis in infectious diseases, especially in pediatrics: lower respiratory tract infections/pneumonia and febrile infants without a clear source. We will review these sections separately:
(A) Lower Respiratory Tract Infections (LRTI)/Pneumonia
Acute LRTI/pneumonia represent the leading cause of hospitalization in the US and the main cause of death worldwide in children less than 5 years of age [1, 44–46]. In the current clinical practice establishing the precise etiologic diagnosis of pneumonia, or even simply discriminating viral from bacterial respiratory infections, remains challenging. Unfortunately, the pressure to achieve a rapid resolution of symptoms has commonly lead medical practitioners to take an overcautious approach and to treat many patients unnecessarily with antibiotics. Recent studies have provided an initial proof of concept of how application of blood gene expression profiles could represent an alternative approach for the diagnosis of viral and bacterial LRTI [15,16,22,25, 41, 42]. Two landmark studies published in 2013 have shown the potential of transcriptome analysis for diagnosis and patient classification in two completely different clinical situations in which traditional tools have demonstrated to be insufficient: TB and RSV [15,16].
Tuberculosis remains a major diagnostic challenge, especially in the developing world where the prevalence of HIV is high. A study conducted in Africa showed the value of whole blood transcriptome analysis for the diagnosis of TB in a large cohort of HIV infected and uninfected patients. From the expression data authors developed a disease risk score that discriminated with high sensitivity and specificity (>90%) between patients with active TB from those with an alternative diagnosis but who presented initially with suspected TB and even from patients with latent TB infection [16]. In young children, respiratory viral infections and specifically RSV represent the most common cause of LRTI leading to hospitalization worldwide. In the clinical setting it is impossible to predict, based on the physical examination and available diagnostic tools, which patients with RSV infection will progress to worse disease requiring hospitalization and which patients can be discharged home safely. Hence, there is a clear need to better understand the immune response to RSV and how it relates to disease pathogenesis, progression and severity. A recent study conducted in the USA analyzed a cohort of 220 children < 2 years of age who were hospitalized with acute RSV, HRV and influenza A LRTI and showed that blood RNA profiles differentiated these three viral infections with 95% accuracy [15]. In addition, and as previously suggested [28, 47], RSV infection induced overexpression of interferon and neutrophil genes and suppression of B and T-cell genes, which persisted beyond the acute disease and was greatly impaired in infants < 6 months of age. These results may explain in part the lack of protective antibody responses observed after acute RSV infection. Moreover, the authors identified a genomic score that significantly correlated with outcomes of care. Altogether, these studies demonstrate that large amounts of microarray data can be translated into a biologically meaningful context that can be correlated with disease severity and applied in the relevant clinical setting to accurately classify patients. It is remarkable that blood signatures can achieve such accuracy for the diagnosis of respiratory pathogens that are thought to be mostly confined to the respiratory tract. From the practical perspective, in the majority of the clinical situations obtaining a blood sample is more feasible than obtaining infected tissue.
Febrile Infant without a source
The evaluation of febrile infants and specially those less than 2 months of age who present to the Emergency Department (ED) continues to represent a major challenge for clinicians. Although viral infections are a common cause of fever with no apparent source in young infants, many children receive antibiotics unnecessarily for the risk of serious bacterial infections. This is mainly due to the deceiving clinical appearance of the infant that pairs up with the poor sensitivity of our current diagnostic tools. A study conducted in children 2–36 months of age presenting with fever to the ED showed that transcriptional profiles clearly distinguished between children with bacterial and viral infections and classified these patients with better accuracy than traditional white blood cells counts (WBC). Moreover, gene expression profiles clearly differentiated symptomatic febrile from non-symptomatic control children infected with the same virus (HHV-6 or adenovirus)[31]. Thus, whole blood RNA expression profiles represent much more than an expensive traditional WBC and have significantly superior capacity at discriminating bacterial from viral infections [13,31, 48]. The readout is a combined representation of the number and cell types and the activation or suppression of all these cell types in peripheral blood. These findings also demonstrate the applicability of these tools in the clinical setting where gene expression profiles can help to determine the clinical significance of detecting viral nucleic acid in individual patients.
Currently, an ongoing large multicenter study is being conducted by the Pediatric Emergency Care And Research Network (PECARN) in the United States evaluating the application of transcriptional profiles for diagnosis of young febrile infants < 2 months of age in the ED [32].
New Analytical Tools
The practical application of gene profiling assays in the clinical setting requires appropriate tools that facilitate the analysis of large datasets generated from these studies. Chaussabel and colleagues [14] [23,24, 49] developed different analytical tools to decrease the background noise and to simplify these analyses:
A) Modular Analyses
Transcriptional modules are sets of genes that follow similar expression patterns across a large number of samples (identified through co-expression meta-analysis) and share a similar biological function. This tool was applied to analyze gene expression profiles of patients presenting with a variety of bacterial and viral infections [14,15,22, 39, 50]. When comparing the modular maps in different infectious diseases processes, we found that certain specific modules displayed similar expression patterns in different diseases; however, each disease displayed a unique combination of modules that generates a characteristic “disease fingerprint” (Fig. 2). In fact, the expression of some modules alone (i.e interferon) is sufficient to distinguish different type of conditions.
Figure 2. Mapping transcriptional changes at the module-level identifies disease specific biosignatures in patients with infectious diseases.
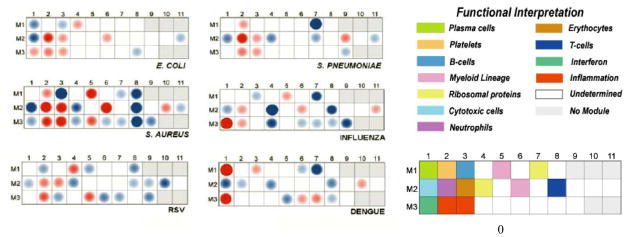
Expression levels were compared between patients and appropriately matched healthy controls on a module-by-module basis. The spots represent the percentage of significantly over-expressed (red) or under-expressed (blue) transcripts within a module (i.e. set of coordinately expressed gene). Blank spots indicate that there are no differences in the genes included in that module between patients and healthy controls. This information is displayed on a grid, with the coordinates corresponding to one of 28 module IDs (e.g. Module M3.1 is at the intersection of the 3rd row & 1st column.). Each pathogen induces a disease-specific biosignature that is easily identifiable. Reproduced with permission from Genomic and Personalized Medicine, 2nd edition, Chapter 93. Diagnosis and classifications of Pathogens. Editors: Geoffrey Ginsburg & Huntington Willard (Elsevier; 2013 ISBN: 978-0-12-415937-2)
B) Molecular distance to health (MDTH) [24]
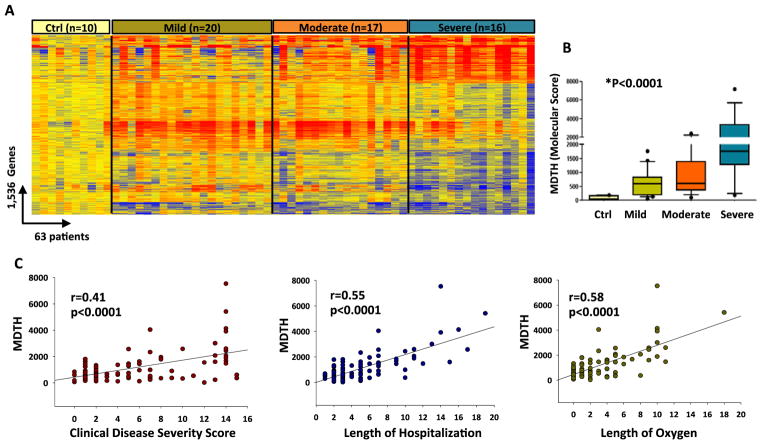
Developing an objective genomic disease severity score in patients with infectious diseases promises to be extremely helpful to monitor disease status, response to therapy and eventually predict clinical outcomes. A recent study conducted by a multinational group of investigators applied the MDTH score to determine the value of transcriptome analyses to assess clinical disease severity in adults with TB. MDTH scores significantly correlated with the radiologic extent of TB disease, as patients with more severe pulmonary involvement had higher MDTH scores. More importantly, as patients were treated, MDTH scores progressively decreased and normalized at around 12 months [22]. Another study conducted in young children with RSV bronchiolitis showed that MDTH scores derived at 24h of hospitalization classified patients based on clinical disease severity, as infants with less severe RSV LRTI had lower MDTH scores compared with those requiring PICU care. More importantly MDTH scores predicted length of stay and duration of supplemental oxygen requirement (Fig. 3) [15].
Figure 3. Molecular Distance to Health (MDTH) scores correlate with clinical disease severity.
(A). Hierarchical clustering of genes differentially expressed (Kruskal-Wallis p<0.01, Benjamini-Hochberg multiple test correction) between infants RSV LRTI and 10 healthy matched controls, demonstrated a higher proportion of underexpressed genes in children with severe RSV (n=16) that gradually declined in patients with moderate (n=17) and mild RSV disease (n=20). Each column represents a patient’s sample and each row a transcript. (B). Using those 1,536 transcripts, MDTH scores were calculated and were higher in children with severe disease than in children with mild or moderate disease (p<0.001). (C). The genomic MDTH score significantly correlated with the clinical disease severity score, the total length of hospitalization and duration of supplemental O2 in the overall RSV cohort (n=91).
Reproduced with permission from Mejias A, Dimo B, Suarez NM, Garcia C, Suarez-Arrabal MC, Jartti T, Blankenship D, Jordan-Villegas A, Ardura MI, Xu Z, et al.: Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med 2013, 10:e1001549. [15]
Conclusion
In summary, the studies conducted to date highlight the strength and value of applying gene expression profiling to improve the diagnosis and classification of patients with infectious diseases, to better understand the pathogenesis of the infections and to better assess disease severity. The challenge now is designing relevant clinical studies to identify and validate diagnostic and prognostic immune profiles induced by relevant pathogens that correlate with outcomes of infection. Combining the detection of the pathogen using state of the art molecular assays with a comprehensive assessment of the host immune response will provide a broad new understanding of the correlations between specific etiologic agents, the host response and the clinical manifestations of the disease. It will also improve our understanding of the disease pathogenesis, and will help to determine the role of a pathogen (viral, bacterial, or viral-bacterial) detected in a clinical sample, whether it implies that the microbe is causing the disease or that simply reflects colonization and/or asymptomatic shedding. This combined approach will help developing a valuable databank of diagnostic signatures and biomarkers of disease severity that will assist in treatment decisions on an individual basis.
Key Points (3–5).
Pathogen-specific gene expression profiles can be measured in blood immune cells from infected subjects.
Gene expression profiles identify by microarray analyses have shown to have superior diagnostic accuracy than traditional methods.
Transcriptome analyses can help to determine the clinical significance of detecting a pathogen in a clinical specimen.
Specific blood RNA profiles allows for a better assessment of disease severity
Acknowledgments
AM and OR are supported in part by NIH grants AI089987, AI057234, HD062477 and Nationwide Children’s Hospital intramural funds.
Abbreviations
- MERS
Middle East respiratory syndrome
- PCR
polymerase chain reaction
- PBMC
peripheral blood mononuclear cells
- HSV
herpes simplex virus
- HCV
hepatitis C virus
- VZV
varicella zoster virus
- TB
Tuberculosis
- LTBI
latent tuberculosis infection
- RSV
respiratory syncytial virus
- HRV
human rhinovirus
- HTLV-1
human T-lymphotropic virus type-1
- ED
emergency department
- LRTI
lower respiratory tract infection
- CAP
community acquired pneumonia
- O2
oxygen
- HHV-6
human herpes virus 6
- WBC
white blood cell counts
- PECARN
Pediatric Emergency Care Applied Research Network
- SNP
single nucleotide polymorphism
- PICU
pediatric intensive care unit
Footnotes
The authors declare that they have no competing interests.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 3.Kuehn BM. Public health officials mark 50th year of measles vaccine: concern remains about outbreaks in pockets of unvaccinated. JAMA. 2014;311:345–346. doi: 10.1001/jama.2013.285502. [DOI] [PubMed] [Google Scholar]
- 4.Libster R, Bugna J, Coviello S, Hijano DR, Dunaiewsky M, Reynoso N, Cavalieri ML, Guglielmo MC, Areso MS, Gilligan T, et al. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N Engl J Med. 2010;362:45–55. doi: 10.1056/NEJMoa0907673. [DOI] [PubMed] [Google Scholar]
- 5.Chang ML, Jordan-Villegas A, Evans A, Bhore R, Brock E, Mejias A, Siegel JD. Respiratory viruses identified in an urban children’s hospital emergency department during the 2009 influenza A(H1N1) pandemic. Pediatr Emerg Care. 2012;28:990–997. doi: 10.1097/PEC.0b013e31826ca980. [DOI] [PubMed] [Google Scholar]
- 6.Iwamoto M, Mu Y, Lynfield R, Bulens SN, Nadle J, Aragon D, Petit S, Ray SM, Harrison LH, Dumyati G, et al. Trends in invasive methicillin-resistant Staphylococcus aureus infections. Pediatrics. 2013;132:e817–824. doi: 10.1542/peds.2013-1112. [DOI] [PubMed] [Google Scholar]
- 7.Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, Lessa FC, Lynfield R, Nadle J, Petit S, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970–1978. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guervil DJ, Chau T. Trends in multidrug-resistant gram-negative bacilli and the role of prolonged beta-lactam infusion in the intensive care unit. Crit Care Nurs Q. 2013;36:345–355. doi: 10.1097/CNQ.0b013e3182a10d2f. [DOI] [PubMed] [Google Scholar]
- 9.Sammons JS, Toltzis P, Zaoutis TE. Clostridium difficile Infection in children. JAMA Pediatr. 2013;167:567–573. doi: 10.1001/jamapediatrics.2013.441. [DOI] [PubMed] [Google Scholar]
- 10.Cummings CA, Relman DA. Genomics and microbiology. Microbial forensics--”cross-examining pathogens”. Science. 2002;296:1976–1979. doi: 10.1126/science.1073125. [DOI] [PubMed] [Google Scholar]
- 11.Relman DA. The human body as microbial observatory. Nat Genet. 2002;30:131–133. doi: 10.1038/ng0202-131. [DOI] [PubMed] [Google Scholar]
- 12.Jartti T, Soderlund-Venermo M, Hedman K, Ruuskanen O, Makela MJ. New molecular virus detection methods and their clinical value in lower respiratory tract infections in children. Paediatr Respir Rev. 2013;14:38–45. doi: 10.1016/j.prrv.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramilo O, Allman W, Chung W, Mejias A, Ardura M, Glaser C, Wittkowski KM, Piqueras B, Banchereau J, Palucka AK, et al. Gene expression patterns in blood leukocytes discriminate patients with acute infections. Blood. 2007;109:2066–2077. doi: 10.1182/blood-2006-02-002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaussabel D, Quinn C, Shen J, Patel P, Glaser C, Baldwin N, Stichweh D, Blankenship D, Li L, Munagala I, et al. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity. 2008;29:150–164. doi: 10.1016/j.immuni.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **15.Mejias A, Dimo B, Suarez NM, Garcia C, Suarez-Arrabal MC, Jartti T, Blankenship D, Jordan-Villegas A, Ardura MI, Xu Z, et al. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med. 2013;10:e1001549. doi: 10.1371/journal.pmed.1001549. Authors found that specific blood RNA profiles in infants with RSV bronchiolitis allowed for specific diagnosis compared to influenza and human rhinovirus, better understanding of disease pathogenesis and better assessment of disease severity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **16.Kaforou M, Wright VJ, Oni T, French N, Anderson ST, Bangani N, Banwell CM, Brent AJ, Crampin AC, Dockrell HM, et al. Detection of tuberculosis in HIV-infected and -uninfected African adults using whole blood RNA expression signatures: a case-control study. PLoS Med. 2013;10:e1001538. doi: 10.1371/journal.pmed.1001538. Using transcriptome analyses authors developed a disease risk score that allowed discrimination between active vs. latent tuberculosis as well as tuberculosis from other diseases with high sensitivity and specificity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramilo O, Mejias A. Shifting the paradigm: host gene signatures for diagnosis of infectious diseases. Cell Host Microbe. 2009;6:199–200. doi: 10.1016/j.chom.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Ingle H, Prasad DV, Kumar H. Recognition of bacterial infection by innate immune sensors. Crit Rev Microbiol. 2013;39:229–246. doi: 10.3109/1040841X.2012.706249. [DOI] [PubMed] [Google Scholar]
- 20.Broz P, Monack DM. Newly described pattern recognition receptors team up against intracellular pathogens. Nat Rev Immunol. 2013;13:551–565. doi: 10.1038/nri3479. [DOI] [PubMed] [Google Scholar]
- 21.Chaussabel D, Pascual V, Banchereau J. Assessing the human immune system through blood transcriptomics. BMC Biol. 2010;8:84. doi: 10.1186/1741-7007-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaussabel D, Allman W, Mejias A, Chung W, Bennett L, Ramilo O, Pascual V, Palucka AK, Banchereau J. Analysis of significance patterns identifies ubiquitous and disease-specific gene-expression signatures in patient peripheral blood leukocytes. Ann N Y Acad Sci. 2005;1062:146–154. doi: 10.1196/annals.1358.017. [DOI] [PubMed] [Google Scholar]
- 24.Pankla R, Buddhisa S, Berry M, Blankenship DM, Bancroft GJ, Banchereau J, Lertmemongkolchai G, Chaussabel D. Genomic transcriptional profiling identifies a candidate blood biomarker signature for the diagnosis of septicemic melioidosis. Genome Biol. 2009;10:R127. doi: 10.1186/gb-2009-10-11-r127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaas AK, Chen M, Varkey J, Veldman T, Hero AO, 3rd, Lucas J, Huang Y, Turner R, Gilbert A, Lambkin-Williams R, et al. Gene expression signatures diagnose influenza and other symptomatic respiratory viral infections in humans. Cell Host Microbe. 2009;6:207–217. doi: 10.1016/j.chom.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaussabel D, Semnani RT, McDowell MA, Sacks D, Sher A, Nutman TB. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood. 2003;102:672–681. doi: 10.1182/blood-2002-10-3232. [DOI] [PubMed] [Google Scholar]
- 27.Boldrick JC, Alizadeh AA, Diehn M, Dudoit S, Liu CL, Belcher CE, Botstein D, Staudt LM, Brown PO, Relman DA. Stereotyped and specific gene expression programs in human innate immune responses to bacteria. Proc Natl Acad Sci U S A. 2002;99:972–977. doi: 10.1073/pnas.231625398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ioannidis I, McNally B, Willette M, Peeples ME, Chaussabel D, Durbin JE, Ramilo O, Mejias A, Flano E. Plasticity and virus specificity of the airway epithelial cell immune response during respiratory virus infection. J Virol. 2012;86:5422–5436. doi: 10.1128/JVI.06757-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenner RG, Young RA. Insights into host responses against pathogens from transcriptional profiling. Nat Rev Microbiol. 2005;3:281–294. doi: 10.1038/nrmicro1126. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- **31.Hu X, Yu J, Crosby SD, Storch GA. Gene expression profiles in febrile children with defined viral and bacterial infection. Proc Natl Acad Sci U S A. 2013;110:12792–12797. doi: 10.1073/pnas.1302968110. Transcriptional profiles classified febrile children with better accuracy than traditional white blood cell counts and clearly differentiated symptomatic febrile from non-symptomatic control children infected with the same virus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahajan P, Kuppermann N, Suarez N, Mejias A, Casper C, Dean M, Ramilo O. Transcriptional Biosignature Analysis for Identifying Febrile Infants with Serious Bacterial Infections in the Emergency Department: A Feasibility Study. Ped Emergency Care. 2014 doi: 10.1097/PEC.0000000000000324. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian F, Chung L, Zheng W, Bruno V, Alexander RP, Wang Z, Wang X, Kurscheid S, Zhao H, Fikrig E, et al. Identification of genes critical for resistance to infection by West Nile virus using RNA-Seq analysis. Viruses. 2013;5:1664–1681. doi: 10.3390/v5071664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson LJ, Dunstan SJ, Dolecek C, Perkins T, House D, Dougan G, Nguyen TH, Tran TP, Doan CD, Le TP, et al. Transcriptional response in the peripheral blood of patients infected with Salmonella enterica serovar Typhi. Proc Natl Acad Sci U S A. 2009;106:22433–22438. doi: 10.1073/pnas.0912386106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devignot S, Sapet C, Duong V, Bergon A, Rihet P, Ong S, Lorn PT, Chroeung N, Ngeav S, Tolou HJ, et al. Genome-wide expression profiling deciphers host responses altered during dengue shock syndrome and reveals the role of innate immunity in severe dengue. PLoS One. 2010;5:e11671. doi: 10.1371/journal.pone.0011671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotger M, Dang KK, Fellay J, Heinzen EL, Feng S, Descombes P, Shianna KV, Ge D, Gunthard HF, Goldstein DB, et al. Genome-wide mRNA expression correlates of viral control in CD4+ T-cells from HIV-1-infected individuals. PLoS Pathog. 2010;6:e1000781. doi: 10.1371/journal.ppat.1000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Idaghdour Y, Quinlan J, Goulet JP, Berghout J, Gbeha E, Bruat V, de Malliard T, Grenier JC, Gomez S, Gros P, et al. Evidence for additive and interaction effects of host genotype and infection in malaria. Proc Natl Acad Sci U S A. 2012;109:16786–16793. doi: 10.1073/pnas.1204945109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tattermusch S, Skinner JA, Chaussabel D, Banchereau J, Berry MP, McNab FW, O’Garra A, Taylor GP, Bangham CR. Systems biology approaches reveal a specific interferon-inducible signature in HTLV-1 associated myelopathy. PLoS Pathog. 2012;8:e1002480. doi: 10.1371/journal.ppat.1002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banchereau R, Jordan-Villegas A, Ardura M, Mejias A, Baldwin N, Xu H, Saye E, Rossello-Urgell J, Nguyen P, Blankenship D, et al. Host immune transcriptional profiles reflect the variability in clinical disease manifestations in patients with Staphylococcus aureus infections. PLoS One. 2012;7:e34390. doi: 10.1371/journal.pone.0034390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *40.Parnell GP, McLean AS, Booth DR, Armstrong NJ, Nalos M, Huang SJ, Manak J, Tang W, Tam OY, Chan S, et al. A distinct influenza infection signature in the blood transcriptome of patients with severe community-acquired pneumonia. Crit Care. 2012;16:R157. doi: 10.1186/cc11477. Whole blood transcriptional profiles highly distinguished influenza infection from other causes of respiratory failure, including bacterial pneumonia and non-infectious systemic inflammatory response syndrome in critically ill adults with CAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Herberg JA, Kaforou M, Gormley S, Sumner ER, Patel S, Jones KD, Paulus S, Fink C, Martinon-Torres F, Montana G, et al. Transcriptomic profiling in childhood H1N1/09 influenza reveals reduced expression of protein synthesis genes. J Infect Dis. 2013;208:1664–1668. doi: 10.1093/infdis/jit348. Reduced expression of protein synthesis is the most significant pathway distinguishing children hospitalized with H1N1/2009 influenza A from controls, and children with RSV or bacterial pneumonia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **42.Zaas AK, Burke T, Chen M, McClain M, Nicholson B, Veldman T, Tsalik EL, Fowler V, Rivers EP, Otero R, et al. A host-based RT-PCR gene expression signature to identify acute respiratory viral infection. Sci Transl Med. 2013;5:203ra126. doi: 10.1126/scitranslmed.3006280. Gene expression profiles identified by microarray analyses can be successfully applied to a custom made platform with potential for a fast, point-of-care patient diagnosis and classification. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *43.Woods CW, McClain MT, Chen M, Zaas AK, Nicholson BP, Varkey J, Veldman T, Kingsmore SF, Huang Y, Lambkin-Williams R, et al. A host transcriptional signature for presymptomatic detection of infection in humans exposed to influenza H1N1 or H3N2. PLoS One. 2013;8:e52198. doi: 10.1371/journal.pone.0052198. Authors showed the utility of gene expression for presymptomatic identification of influenza infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 45.Lee GE, Lorch SA, Sheffler-Collins S, Kronman MP, Shah SS. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics. 2010;126:204–213. doi: 10.1542/peds.2009-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bucasas KL, Mian AI, Demmler-Harrison GJ, Caviness AC, Piedra PA, Franco LM, Shaw CA, Zhai Y, Wang X, Bray MS, et al. Global gene expression profiling in infants with acute respiratory syncytial virus broncholitis demonstrates systemic activation of interferon signaling networks. Pediatr Infect Dis J. 2013;32:e68–76. doi: 10.1097/INF.0b013e318278b4b3. [DOI] [PubMed] [Google Scholar]
- 48.Openshaw PJ. A gene expression signature for RSV: clinical implications and limitations. PLoS Med. 2013;10:e1001550. doi: 10.1371/journal.pmed.1001550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang E, Marincola FM. Bottom up: a modular view of immunology. Immunity. 2008;29:9–11. doi: 10.1016/j.immuni.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ardura MI, Banchereau R, Mejias A, Di Pucchio T, Glaser C, Allantaz F, Pascual V, Banchereau J, Chaussabel D, Ramilo O. Enhanced monocyte response and decreased central memory T cells in children with invasive Staphylococcus aureus infections. PLoS One. 2009;4:e5446. doi: 10.1371/journal.pone.0005446. [DOI] [PMC free article] [PubMed] [Google Scholar]