SUMMARY
The incidence of extrapulmonary tuberculosis is increasing, possibly due to the high frequency of co-infection with HIV. Extrapulmonary infections complicate diagnosis, have higher mortality rates and are more difficult to treat. Insight into the mechanisms involved in extrapulmonary spread of tuberculosis is critical to improving management. We set out to better understand extrapulmonary spread kinetics in mice and guinea pigs as well as the effects of infectious dose. We found that extrapulmonary spread occurs at a discrete time point when infected by low-dose aerosol, but at high-dose aerosol it occurs within the first 24 h. The ability to follow tuberculosis in real-time during infection would allow us to better address the mechanisms involved. We found that mycobacteria can be optically imaged after pulmonary infection in the mouse lung, suggesting that this technology could be applied to study of extrapulmonary spread of tuberculosis.
Keywords: Mice, Guinea pigs, Mycobacterium, Infection, Respiratory, Pathogenesis
Extrapulmonary tuberculosis occurs in 5–20% of all tuberculosis cases, making it a relatively frequent complication of disease.1-3 In children from birth to 4 years old tuberculosis nearly always becomes extrapulmonary and has high mortality rates.4-7 The immunological status of the host can impact the ability of Mycobacterium tuberculosis (Mtb) to cause extrapulmonary disease.3,8-10 Children, in particular, are more susceptible to tuberculosis and often develop the extrapulmonary form.11-13 This includes miliary tuberculosis that has a very high mortality rate in children (15–20%) and adults (25–30%).14-24 Since miliary tuberculosis will arise in 8% of all extrapulmonary cases of tuberculosis,24 the rising frequency of extrapulmonary tuberculosis is of great concern. Extended therapy is recommended to prevent relapse, suggesting that extrapulmonary tuberculosis is more difficult to treat than pulmonary tuberculosis.3
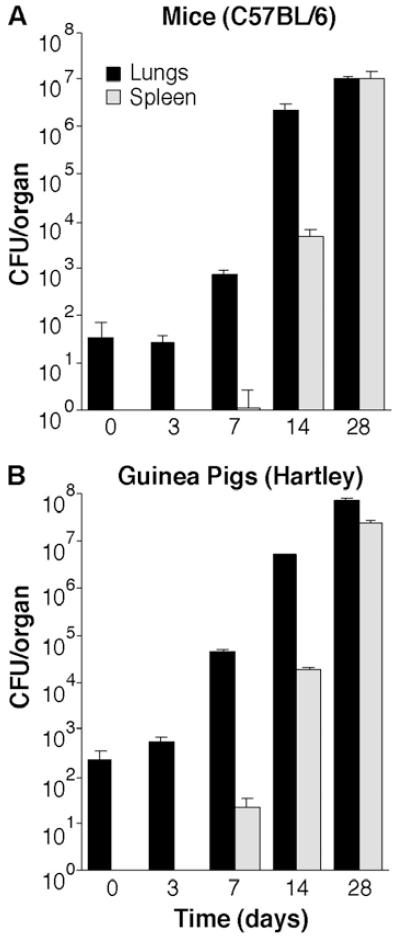
It has been suggested that there is no distinct time point for extrapulmonary spread of tuberculosis in mice, since Mtb is found in multiple organs from the first few days after infection by aerosol.25,26 However, this observation is not consistent with data suggesting that extrapulmonary spread can occur at discrete time points in certain strains of mice.27 Since extrapulmonary spread is thought to occur at around two weeks post-infection in human tuberculosis,28-31 we were curious whether there were differences in the kinetics in mice as opposed to other animal models. To address this question, we carried out a direct comparison of the kinetics of dissemination in mice and guinea pigs (GP). We found that in both mice and GP dissemination occurs between 3 and 14 days post-infection (Fig. 1). Although it appears that extrapulmonary spread occurs somewhat earlier in GP, they were infected with approximately four-fold higher numbers of bacteria than mice, accounting for this difference in CFU in the spleen at seven days. Overall, the kinetics of extrapulmonary spread are comparable in both animal models, suggesting that extrapulmonary spread occurs through a similar mechanism in both mice and guinea pigs.
Fig. 1.
Kinetics of extrapulmonary spread of tuberculosis over the first 28 days post-infection in (A) mice and (B) guinea pigs infected with approximately 100 colony-forming units (CFU) by aerosol. In both mice and guinea pigs, bacteria first spread from the lungs to the spleen at between three and fourteen days post-infection. The kinetics of extrapulmonary spread of tuberculosis in mice and guinea pigs are very similar. Data shown are the means and standard deviations of five animals per group.
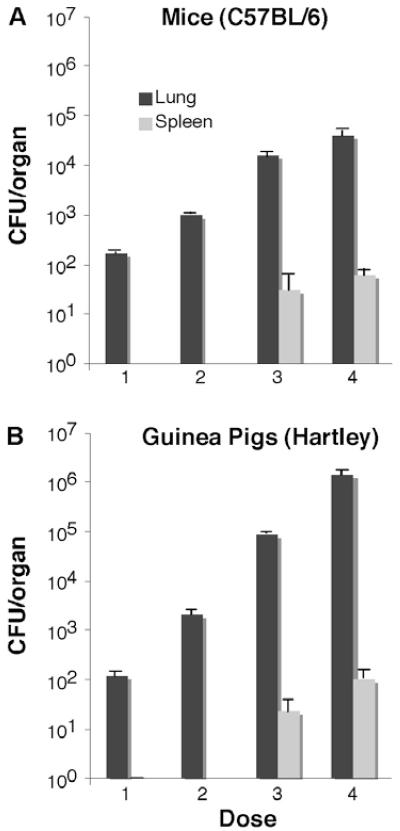
It is possible that the controversy regarding whether extrapulmonary spread occurs at a discrete time point in mice arises from differences in the experimental conditions used. One parameter that might impact the kinetics of extrapulmonary spread is the initial infectious dose. We infected mice and guinea pigs with between 100 and 106 CFU of tuberculosis by aerosol and examined whether extrapulmonary spread had occurred at day one post-infection. Since dissemination after low-dose aerosol occurs at around the same point in mice, guinea pigs and humans, between 8 and 14 days post-infection, we were surprised to find that at bacterial doses above 104 CFU in the lung, bacteria can be found in the spleen at 24 h post-infection (Fig. 2). These observations suggest that infecting with high numbers of bacilli by aerosol circumvents the normal route of extrapulmonary spread through the draining lymph nodes, entry into the thoracic duct and then the blood stream, but might allow direct entry into the blood stream through the alveolar epithelial barrier or via inappropriate sites other than the lung, such as the upper airways or gastrointestinal system. Regardless of the mechanism, these data argue that infecting mice and guinea pigs by aerosol with numbers of bacilli greater than 103 CFU leads to more rapid extrapulmonary spread. More rapid extrapulmonary spread could be due to several potential mechanisms, including greater cytopathology in the respiratory epithelium leading to increased permeability, increased access to normally uncommon portals of entry in the gastrointestinal system or upper airways, more efficient access to relatively rare cell types involved in spread, or an increased likelihood of bacteria being primed for extrapulmonary spread.
Fig. 2.
Effects of M. tuberculosis aerosol infectious dose from approximately 100 to 105 colony-forming units (CFU) on the number of bacteria that spread to the spleen by day one post-infection in (A) mice and (B) guinea pigs. Aerosol infection with more than 10,000 CFU results in early extrapulmonary spread of tuberculosis to the spleen in both mice and guinea pigs. Data shown are the means and standard deviations of five animals per group.
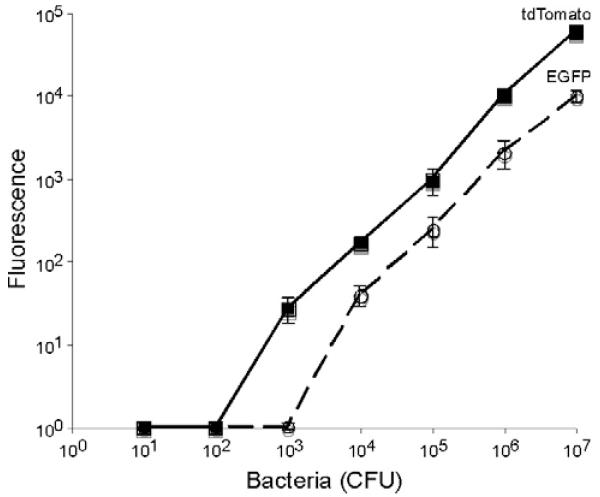
Our data suggest that it would be extremely difficult under normal circumstances to follow Mtb throughout the process of extrapulmonary spread, since it is necessary to infect with less than 104 CFU to ensure that normal kinetics are observed. In order to better track tuberculosis during infections, we constructed green and red fluorescent protein expressing tuberculosis strains using EGFP and tdTomato.32,33 Although EGFP and tdTomato allowed detection of less than 104 bacteria in vitro (Fig. 3), tdTomato displays higher levels of fluorescence and has a lower threshold of detection (P < 0.01 by two-way ANOVA), approximately ten-fold. These observations suggest that tdTomato will serve as a superior marker for tuberculosis during infection in mice due to its greater fluorescence intensity. In addition, we assumed that the longer wavelength of tdTomato (Ex: 554, Em: 581) as compared to EGFP (Ex: 484, Em: 510) would have significant advantages for in vivo imaging because the long emission tail, going out to nearly 700 nm, of tdTomato would avoid the light-absorbing activity of hemoglobin seen at wavelengths below 600 nm.34
Fig. 3.
Threshold of detection for tuberculosis expressing EGFP and tdTomato fluorescence proteins and correlation with colony-forming units (CFU). Data shown are the means and standard deviations of a representative experiment done in triplicate. Data points below background were corrected to one so that they could be plotted on a log scale.
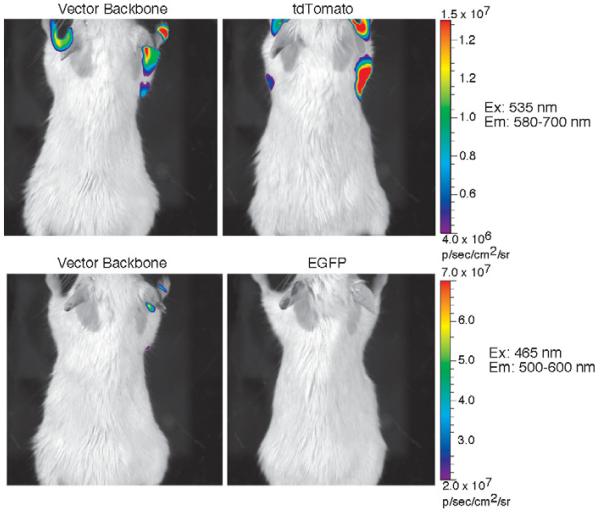
In order to test this hypothesis, we evaluated tuberculosis strains expressing EGFP and tdTomato for fluorescence by in vivo imaging using an IVIS Spectrum (Caliper/Xenogen) in live mice (Balb/c). We inoculated various numbers of tuberculosis bacilli from 107 to 104 CFU subcutaneously that express EGFP and tdTomato along the dorsal surface of mice. We found that EGFP could be detected after subcutaneous inoculation at 107 CFU, but lower numbers were not detectable. In contrast, tdTomato allowed detection of bacteria at 105 CFU (data not shown). These observations suggest that we could detect mycobacteria after pulmonary infection. We infected mice intranasally with approximately 106 M. bovis BCG expressing EGFP and tdTomato and imaged the living animals using the IVIS Spectrum. We found that mice infected with mycobacteria expressing tdTomato display approximately three- to four-fold higher fluorescence under tdTomato imaging conditions than mice infected with bacteria that carry vector alone (Fig. 4). However, no fluorescent signal was detected in mice infected with mycobacteria expressing EGFP above that of mice infected with bacteria that carry vector alone under EGFP imaging conditions.
Fig. 4.
Fluorescence emission in photons/second/square cm/surface area (p/s/cm2/sr) of mice infected intranasally to deliver approximately 105 mycobacteria into the lungs. Bacteria carry either the vector backbone, vector expressing EGFP (EGFP) or vector expressing tdTomato (tdTomato). The color bar to the right of images applies to both images in a row and displays the colorized intensity of fluorescence emission from each animal under the excitation (Ex) conditions shown to the right of each row of images by transillumination with spectral unmixing over the wavelength range shown for emission (Em). Imaging was carried out using the IVIS Spectrum (Caliper/Xenogen) and LivingImage Software provided by the manufacturer. All images were captured under the same conditions and analyzed in an identical fashion, except that the final fluorescence scales for tdTomato and EGFP were set differently due to their different optical properties.
These observations are exciting because they suggest, for the first time, that tuberculosis can be imaged after pulmonary infection directly in living mice. One caveat of the current imaging method is that it requires higher numbers of bacteria to be present (106) in the lung than would allow observation of physiologically normal extrapulmonary spread (<104). However, we are in the process of optimizing codon usage, ribosomal binding sites and promoter used for expression of fluorescent proteins with the expectation that we can significantly improve upon our current threshold of detection. Regardless of whether we can greatly improve the sensitivity of fluorescent proteins for imaging tuberculosis, they serve an important purpose as a secondary marker for optical imaging due to their great stability, allowing bacteria to be readily observed in fixed tissues. We envision that, ultimately, fluorescent proteins will be an important component of emerging dual- and triple-optical imaging approaches that will use other luminescent and/or fluorescent strategies to first identify and quantify infection of tissues followed by cellular and ultrastructural localization of the bacilli within the tissues with fluorescent proteins, allowing a level of detailed analysis of disease processes not previously possible.
Acknowledgments
This work was supported by the Bill & Melinda Gates Foundation and grant AI47866 from the National Institutes of Health.
Footnotes
Competing interests: The authors declare no competing interests with regard to the content of or research that went into this manuscript.
References
- 1.Smith I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev. 2003;16:463–96. doi: 10.1128/CMR.16.3.463-496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362:887–99. doi: 10.1016/S0140-6736(03)14333-4. [DOI] [PubMed] [Google Scholar]
- 3.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120:316–53. [PubMed] [Google Scholar]
- 4.Rieder HL, Snider DE, Cauthen GM. Extrapulmonary tuberculosis in the United States. Am Rev Respir Dis. 1990;141:347–51. doi: 10.1164/ajrccm/141.2.347. [DOI] [PubMed] [Google Scholar]
- 5.Starke JR, Taylor-Watts KT. Tuberculosis in the pediatric population of Houston, TX. Pediatrics. 1989;84:28–35. [PubMed] [Google Scholar]
- 6.Marais BJ. Childhood tuberculosis: reflections from the front line. Pediatr Ann. 2004;33:695–8. doi: 10.3928/0090-4481-20041001-13. [DOI] [PubMed] [Google Scholar]
- 7.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8:392–402. [PubMed] [Google Scholar]
- 8.Small PM, Schecter GF, Goodman PC, Sande MA, Chaisson RE, Hopewell PC. Treatment of tuberculosis in patients with advanced human immunodeficiency virus infection. N Engl J Med. 1991;324:289–94. doi: 10.1056/NEJM199101313240503. [DOI] [PubMed] [Google Scholar]
- 9.Aaron L, Saadoun D, Calatroni I, Launay O, Memain N, Vincent V, et al. Tuberculosis in HIV-infected patients: a comprehensive review. Clin Microbiol Infect. 2004;10:388–98. doi: 10.1111/j.1469-0691.2004.00758.x. [DOI] [PubMed] [Google Scholar]
- 10.Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148:1292–7. doi: 10.1164/ajrccm/148.5.1292. [DOI] [PubMed] [Google Scholar]
- 11.Lewinsohn DA, Gennaro ML, Scholvinck L, Lewinsohn DM. Tuberculosis immunology in children: diagnostic and therapeutic challenges and opportunities. Int J Tuberc Lung Dis. 2004;8:658–74. [PubMed] [Google Scholar]
- 12.Starke JR, Jacobs RF, Jereb J. Resurgence of tuberculosis in children. J Pediatr. 1992;120:839–55. doi: 10.1016/s0022-3476(05)81949-3. [DOI] [PubMed] [Google Scholar]
- 13.Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. 1974;99:131–8. doi: 10.1093/oxfordjournals.aje.a121593. [DOI] [PubMed] [Google Scholar]
- 14.Hussey G, Chisholm T, Kibel M. Miliary tuberculosis in children: a review of 94 cases. Pediatr Infect Dis J. 1991;10:832–6. doi: 10.1097/00006454-199111000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Kim PK, Lee JS, Yun DJ. Clinical review of miliary tuberculosis in Korean children. 84 cases and review of the literature. Yonsei Med J. 1969;10:146–52. doi: 10.3349/ymj.1969.10.2.146. [DOI] [PubMed] [Google Scholar]
- 16.Aderele WI. Miliary tuberculosis in Nigerian children. East Afr Med J. 1978;55:166–71. [PubMed] [Google Scholar]
- 17.Gurkan F, Bosnak M, Dikici B, Bosnak V, Yaramis A, Tas MA, et al. Miliary tuberculosis in children: a clinical review. Scand J Infect Dis. 1998;30:359–62. doi: 10.1080/00365549850160648. [DOI] [PubMed] [Google Scholar]
- 18.Rahajoe NN. Miliary tuberculosis in children. A clinical review. Paediatr Indones. 1990;30:233–40. [PubMed] [Google Scholar]
- 19.Slavin RE, Walsh TJ, Pollack AD. Late generalized tuberculosis: a clinical pathologic analysis and comparison of 100 cases in the preantibiotic and antibiotic eras. Medicine (Baltimore) 1980;59:352–66. [PubMed] [Google Scholar]
- 20.Long R, O’Connor R, Palayew M, Hershfield E, Manfreda J. Disseminated tuberculosis with and without a miliary pattern on chest radiograph: a clinicalpathologic-radiologic correlation. Int J Tuberc Lung Dis. 1997;1:52–8. [PubMed] [Google Scholar]
- 21.Munt PW. Miliary tuberculosis in the chemotherapy era: with a clinical review in 69 American adults. Medicine (Baltimore) 1972;51:139–55. doi: 10.1097/00005792-197203000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Campbell IG. Miliary tuberculosis in British Columbia. Can Med Assoc J. 1973;108:1517–9. passim. [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JH, Langston AA, Gallis HA. Miliary tuberculosis: epidemiology, clinical manifestations, diagnosis, and outcome. Rev Infect Dis. 1990;12:583–90. doi: 10.1093/clinids/12.4.583. [DOI] [PubMed] [Google Scholar]
- 24.Sharma SK, Mohan A, Sharma A, Mitra DK. Miliary tuberculosis: new insights into an old disease. Lancet Infect Dis. 2005;5:415–30. doi: 10.1016/S1473-3099(05)70163-8. [DOI] [PubMed] [Google Scholar]
- 25.Garcia de Viedma D, Lorenzo G, Cardona PJ, Rodriguez NA, Gordillo S, Serrano MJ, et al. Association between the infectivity of Mycobacterium tuberculosis strains and their efficiency for extrarespiratory infection. J Infect Dis. 2005;192:2059–65. doi: 10.1086/498245. [DOI] [PubMed] [Google Scholar]
- 26.Cardona PJ, Llatjos R, Gordillo S, Diaz J, Ojanguren I, Ariza A, et al. Evolution of granulomas in lungs of mice infected aerogenically with Mycobacterium tuberculosis. Scand J Immunol. 2000;52:156–63. doi: 10.1046/j.1365-3083.2000.00763.x. [DOI] [PubMed] [Google Scholar]
- 27.Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun. 2002;70:4501–9. doi: 10.1128/IAI.70.8.4501-4509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medlar EM. The pathogenesis of minimal pulmonary tuberculosis: a study of 1225 necropsies in cases of sudden and unexpected death. Am Rev Tuberc. 1948;58:583–611. doi: 10.1164/art.1948.58.6.583. [DOI] [PubMed] [Google Scholar]
- 29.Sweany HC, Cook CE, Kegerreis R. A study of the position of primary cavities in pulmonary tuberculosis. Am Rev Tuberc. 1931;24:558–82. [Google Scholar]
- 30.Balasubramanian V, Wiegeshaus EH, Smith D. Pathogenesis of tuberculosis: pathways to apical localization. Tuberc Lung Dis. 1994;75:168–78. doi: 10.1016/0962-8479(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 31.Stead WW. Pathogenesis of tuberculosis: Clinical and epidemiological perspectives. Rev Infect Dis. 1989;11:366–8. doi: 10.1093/clinids/11.supplement_2.s366. [DOI] [PubMed] [Google Scholar]
- 32.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–72. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 33.Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat Methods. 2005;2:905–9. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- 34.Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19:316–7. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]