Abstract
The shortage of human organs for transplantation has focused research on the possibility of transplanting pig organs into humans. Many factors contribute to the failure of a pig organ graft in a primate. A rapid innate immune response (natural anti-pig antibody, complement activation, and an innate cellular response, e.g., neutrophils, monocytes, macrophages, NK cells) is followed by an adaptive immune response, although T cell infiltration of the graft has rarely been reported. Other factors (e.g., coagulation dysregulation, inflammation) appear to play a significantly greater role than in allotransplantation. The immune responses to a pig xenograft cannot therefore be controlled simply by suppression of T cell activity.
Before xenotransplantation can be introduced successfully into the clinic, the problems of the innate, coagulopathic, and inflammatory responses will have to be overcome, most likely by the transplantation of organs from genetically-engineered pigs. Many of the genetic manipulations aimed at protecting against these responses also reduce the adaptive response. The T cell and elicited antibody responses can be prevented by the biologic and/or pharmacologic agents currently available, in particular, by costimulation blockade-based regimens. The exogenous immunosuppressive regimen may be significantly reduced by the presence of a graft from a pig transgenic for a mutant (human) class II transactivator gene, resulting in downregulation of SLA class II expression, or from a pig with ‘local’ vascular endothelial cell expression of an immunosuppressive gene, e.g., CTLA4-Ig. The immunomodulatory efficacy of regulatory T cells or mesenchymal stromal cells has been demonstrated in vitro, but not yet in vivo.
Keywords: Co-stimulation blockade, immunosuppression, nonhuman primate, pig, xenotransplantation
Introduction
There is a critical and increasing shortage of organs for the purposes of transplantation. In the USA alone approximately 110,000 patients are on the waiting list for an organ of one sort or another, and yet during the current year only approximately 30,000 organs will become available from deceased human donors. Almost 20 patients waiting for a human organ die each day, i.e., almost 7,000 per year. If islet transplantation becomes clinically more successful, as it slowly is, then the situation will be much more serious. There are an estimated 1.5 million type 1 diabetic patients taking insulin each day in the USA.
Xenotransplantation, i.e., the transplantation of organs, tissues, and cells across species, particularly using genetically-engineered pigs as a source for clinical transplantation, offers the potential to resolve this shortage (1-3). In recent years, considerable progress has been made in overcoming the major immunologic and pathophysiologic barriers, and the field is steadily drawing closer to a time when clinical trials would be justified.
Primate response to a pig organ xenograft
Many factors contribute to the failure of a pig organ graft in a primate. These include a rapid innate immune response, characterized by natural anti-pig antibody binding to the vascular endothelium of the graft with activation of the complement cascade (4,5). In addition, an innate cellular response, consisting mainly of neutrophils, monocytes, macrophages, and natural killer [NK] cells, is involved (6). If the initial antibody-mediated complement activation, which results in hyperacute rejection, can be prevented, then the innate cellular response contributes to the development of a delayed form of rejection known variously as acute humoral xenograft rejection, acute vascular rejection, or delayed xenograft rejection (6,7).
The innate response is followed by an adaptive immune response that may be stronger than the adaptive response to an allograft, although this point remains controversial (8-10). Histopathological features of classical acute cellular rejection (i.e., T cell infiltration of the graft), however, have rarely been reported in a pig organ graft, which may be because acute cellular rejection is preceded by acute humoral xenograft rejection or because it has been successfully prevented by the relatively intensive immunosuppressive therapy that nonhuman primate (NHP) recipients of pig grafts have received in studies performed thus far.
In allogeneic systems, the T cell response elicited through the direct pathway is critical in inducing acute rejection, whereas indirect allorecognition may predominate later as a factor in the development of chronic rejection. Primate xenogeneic recognition is through the direct and indirect pathways, and so in pig-to-primate xenotransplantation both pathways are involved in rejection (11-14). It has been suggested that both direct and indirect xenoresponses are stronger than in their counterparts in the alloresponse (11).
Pig stimulator cells directly activate human T cells (8,10), reflecting productive interaction between human T cell receptors and swine leukocyte antigen (SLA) class I and class II peptide complexes (14). Two donor cell types are likely to be the major stimulators of direct T cell recognition - (i) the migratory passenger leukocytes known as antigen-presenting cells, including dendritic cells (15-17), and (ii) SLA class I and class II-positive vascular endothelial cells (ECs) (18,19). Although pig antigen-presenting cells are transient components in the graft, pig vascular ECs are usually permanently present.
In contrast to human aortic ECs, pig aortic ECs constitutively express costimulatory molecules (e.g., CD80/86, CD40) (20-22). This is likely an important factor contributing to rejection of pig xenografts, and thus relatively intensive immunosuppressive therapy has been required to date. This may result in impaired immunity, which is associated with susceptibility to infection (23). Human T cells may proliferate against various peptides (not only SLA class I and II) derived from pig cells through the indirect pathway (11,12,24).
Several studies have investigated the relative contributions of the direct and indirect pathways in xenotransplantation (recently reviewed in 14 and 25). This topic is still debated, but what is known is that the T cell receptor repertoire, accessory molecule interactions, and cytokine production required for both direct and indirect pathways of recognition in the human response to pig antigens are functionally intact (8,25).
The immune response is complicated by factors that appear to play a significantly greater role in xenotransplantation than in allotransplantation. For example, there is major coagulation dysregulation between pig and primate, which contributes towards the development of thrombotic microangiopathy in the graft and/or a consumptive coagulopathy in the recipient (26-29). This dysregulation is believed to be at least partly initiated by the immune response, e.g., activation of the porcine ECs by recipient antibody and/or complement, and/or by direct interaction between recipient platelets and the graft ECs (30), but greatly enhanced by molecular incompatibilities between the coagulation-anticoagulation systems of the two species (31,32).
Factors involved in the coagulation cascade, such as thrombin, may increase the adaptive response (33) (Figure 1), and it may be for this reason that the adaptive response appears stronger than to an allograft, even though the T cell response itself (if unaffected by coagulation factors) may be comparable. Importantly, the mechanism by which this takes place has been demonstrated to be independent of upregulation of expression of SLA class II (Figure 2). Thrombin activation of pig ECs is associated with the upregulation of the costimulatory molecules CD80/86, which can amplify the T cell proliferative response to pig cells independent of the upregulation of SLA class II expression. It is therefore unlikely that complete blockade of SLA class II recognition will prevent T cell proliferation induced by thrombin.
Figure 1. Increased human PBMC and CD4+T cell proliferation in response to thrombin-activated porcine aortic endothelial cells (pAEC).
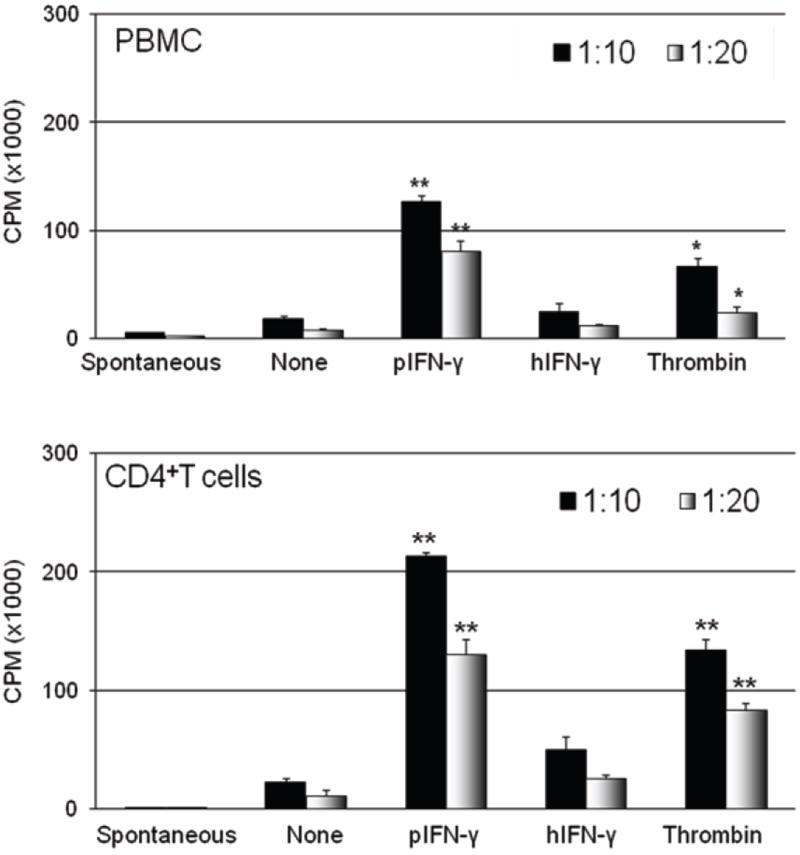
GTKO pAEC were activated using thrombin (40U/mL), pIFN-γ (40U/mL), or hIFN-γ (200U/mL), for 24h. The human PBMC (top) and CD4+T cell (bottom) proliferative responses to pIFN-γ- and thrombin-activated GTKO pAEC were significantly higher than to non-activated pAEC. (Stimulator:responder ratios of 1:10 and 1:20.) Data are representative of three different experiments. (*p<0.01, **p<0.001 in comparison to non-activated pAEC). (Reproduced from Ezzelarab C, et al, Xenotransplantation 2012; 19:311-316 [33] with permission.)
Figure 2. Thrombin does not upregulate SLA class I or II expression on GTKO porcine aortic endothelial cells (pAEC).
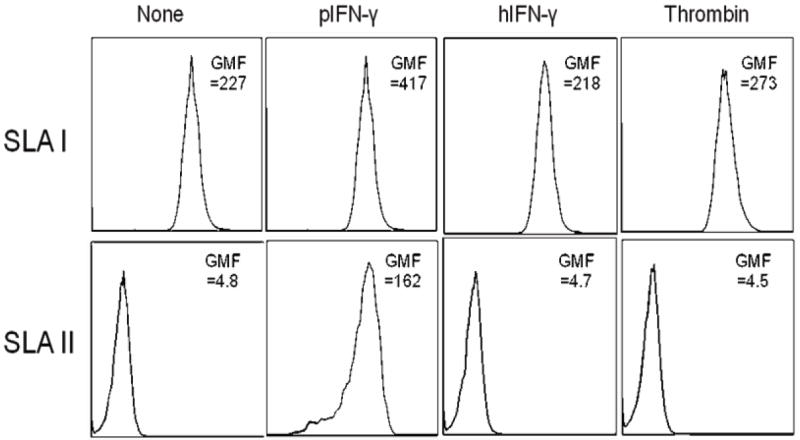
GTKO pAEC were activated using thrombin (40U/mL), pIFN-γ (40U/mL), or hIFN-γ (200U/mL) for 24h. SLA class II expression was upregulated only after pIFN-γ activation, but not after thrombin or hIFN-γ activation. There was no change in SLA class I expression after activation. Data are representative of three different experiments. (Reproduced from Ezzelarab C, et al, Xenotransplantation 2012; 19:311-316 [33] with permission).
There is also an inflammatory response to the graft, which may also contribute towards the xenoreactive immune response appearing stronger. There are data that indicate that there is considerable ‘cross-talk’ between the innate and adaptive responses and between those responses and the factors responsible for coagulation dysfunction and inflammation (34,35). Together, these observations indicate that the immune response to a pig xenograft cannot be considered in isolation, and will not be controlled simply by suppression of T cell activity (as is generally possible in allotransplantation). Equal attention needs to be directed to the innate immune, coagulation, and inflammatory responses. (Of interest, a process by which an innate immune response is induced by the formation of thrombi inside blood vessels – in pathologic conditions unassociated with xenotransplantation - has recently been recognized and termed “immunothrombosis” [36].)
Suppression of the immune responses
The prevention or reduction of the innate immune response has been approached by the genetic engineering of the organ-source pig rather than by the administration of immunosuppressive agents, which are largely ineffective. In this respect, the transgenic expression of one or more human complement-regulatory proteins (hCRPs), e.g., CD46 (hMCP), CD55 (hDAF), CD59, contributes significant protection (37). Similarly, the introduction of pigs in which the gene for α1,3-galactosyltransferase has been deleted (GTKO pigs) (38-40), thus preventing the expression of the important galactose-α1,3-galactose (Gal) antigen, which is the major target for primate anti-pig antibodies (41,42), was a major advance (29,43). GTKO pigs expressing one or more hCRPs provide increased protection than either manipulation alone (44,45). Building on this genetic background, an increasing number of genetically-engineered pigs are becoming available for transplantation studies in NHPs (reviewed by Ekser 2012 [2]).
Although various techniques, e.g., plasmapheresis, extracorporeal immunoadsorption (46), the administration of natural or synthetic oligosaccharides (28,47,48) that prevent anti-Gal antibody binding to the graft, proved valuable in early studies, the value of these has largely been negated by the availability of GTKO pigs. Similarly, although cobra venom factor and other anti-complement agents were administered successfully (49,50), these are no longer necessary when the organ-source pig expresses one or more hCRP. Indeed, there are reasons to believe that cobra venom factor could be detrimental in some respects, as it can result in the release of the anaphylatoxin C5a, which contributes to innate and adaptive immune responses (51). Should an anti-complement agent prove necessary, e.g., if acute humoral xenograft rejection develops, then early evidence from allotransplantation suggests that the monoclonal antibody, eculizumab, might prove successful (52,53).
An unexpected bonus of these genetic manipulations is the observation that the absence of expression of Gal and the expression of a hCRP renders the T cell response to the graft weaker. Studies by Wilhite et al have demonstrated a significant reduction in the primate mixed lymphocyte reaction to pig ECs when Gal is absent or an hCRP is expressed (54,55) (Figure 3). Although there is a definite reduction in the human T cell proliferative response to GTKO/hCRP pig cells in vitro, it is difficult to quantify this effect in vivo in pig-to-NHP transplantation models as so many other factors, e.g., coagulation dysregulation, affect the outcome. In an immunocompetent rodent model, however, GTKO adipose-derived mesenchymal stromal cells (MSCs) induced a weaker anti-pig IgG antibody response than did wild-type pig MSCs, and there was reduced cellular infiltration (lymphocytes, macrophages) in and around the GTKO MSCs (56). Any manipulation of the pig that results in even a small reduction in the human T cell response is clearly welcome.
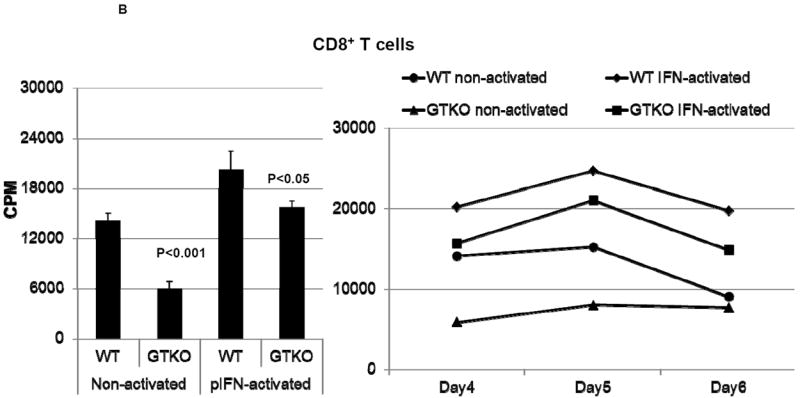
Figure 3. Proliferative response of human T cells to wild-type (WT) and GTKO porcine aortic endothelial cells (pAEC).
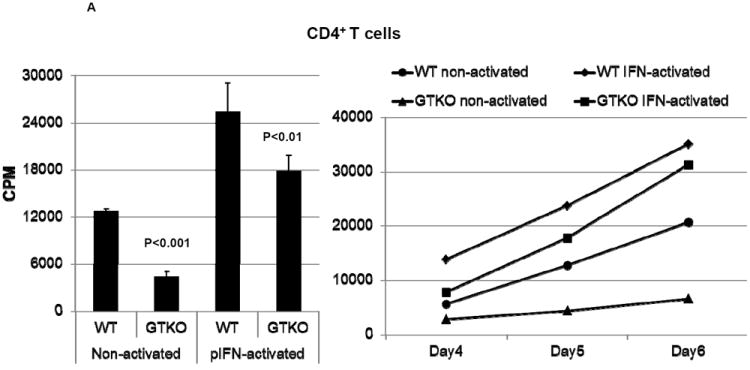
A: The proliferative response of human CD4+T cells (n=3) to WT and GTKO pAEC before and after activation by pIFN-γ (left). The response was significantly less to GTKO pAEC before (p<0.001) and after (p<0.01) activation. Additionally, MLRs were harvested on 3 consecutive days (4, 5 and 6) where CD4+T cell proliferation was consistently lower in response to GTKO pAEC (right).
B: The proliferative response of human CD8+T cells (n=3) to WT and GTKO pAEC before and after activation by pIFN-γ (left). The response was significantly less to GTKO pAEC before (p<0.001) and after (p<0.05) activation. Additionally, MLRs were harvested on 3 consecutive days (4, 5 and 6) where CD8+T cell proliferation was consistently lower in response to GTKO pAEC (right).
In both A and B, 3H incorporation values are presented as CPM. Data represent the mean (+/- SEM) and are representative of three different experiments. (Reproduced from Wilhite T, et al, Xenotransplantation 2012; 19:56-63 [54] with permission).
The adaptive immune response can also be reduced by specific manipulation of the organ-source pig. Initial efforts to express an immunosuppressive agent, such as CTLA4-Ig, in the pig proved unsuccessful in that, although significantly reducing the primate T cell response (22), ubiquitous expression of this agent rendered the pig immune-incompetent and susceptible to infectious complications (57). The efficiency of this approach, however, was illustrated by the fact that, in pigs with ubiquitous expression of CTLA4-Ig, the blood levels of soluble CTLA4-Ig were up to 16-fold higher than the therapeutic levels required by systemic administration of this agent (57).
More recent techniques are allowing this or similar agents to be expressed only in specific target cells of the pig, e.g., the beta cells of the islets (using an insulin promoter) (58,59), vascular ECs (using an endothelium-specific Tie2 enhancer) (60,61), or neuronal cells (using a neuron-specific enolase promoter) (62,63).
There is some concern that, if the pig graft is producing an immunosuppressive agent even in a limited way, this could render the recipient immune-incompetent, particularly as the level of immunosuppression cannot easily be reduced. Nevertheless, the advantage of providing some endogenous ‘local’ immunosuppression to the graft is obvious, and a combination of endogenous and exogenous approaches may prove optimal.
A second approach that has been tested both in vitro and in vivo is rendering the pig transgenic for a mutant human class II transactivator gene, resulting in downregulation of SLA class II expression (CIITA-DN pigs) (64-66) (Figure 4). The primate T cell response to CIITA-DN pig cells/tissues, particularly when these cells have been activated, is significantly reduced (65,66).
Figure 4.
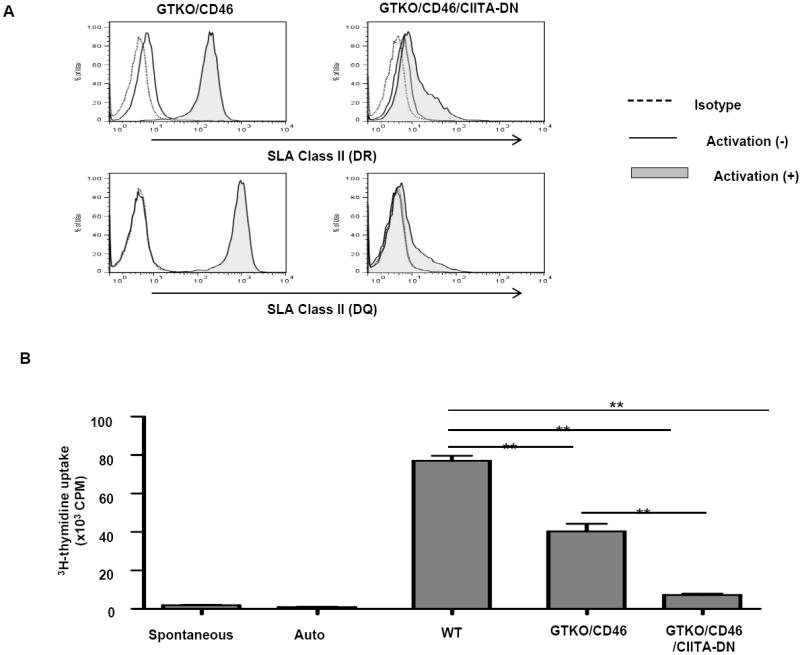
(A) Significant down-regulation of SLA class II expression on aortic endothelial cells from GTKO/CD46/CIITA-DN pigs
The expression of SLA class II DR and DQ on GTKO/CD46/CIITA-DN porcine aortic endothelial cells (pAECs) was compared with those on GTKO/CD46 pAECs. pAECs were activated with pIFN-γ (50ng/mL) for 48h. The pAECs were stained with specific anti-SLA DR or DQ mAbs. Isotype control (dotted line), quiescent (solid line), and activated pAECs (gray filled).
Expression of SLA class II on quiescent pAECs was undetectable or minimal on both GTKO/CD46 and GTKO/CD46/CIITA-DN pAECs. However, expression of SLA class II on GTKO/CD46 pAECs was significantly up-regulated when the pAECs were activated with pIFN-γ for 48h. In contrast, up-regulation of expression of SLA class II on GTKO/CD46/CIITA-DN pAECs was minimal.
(B) Significant reduction of the hCD4+T cell response to CIITA-DN cells
hCD4+T cells were co-cultured with PBMCs from WT, GTKO/CD46, and GTKO/CD46/CIITA-DN pigs for 6 days. The responses of hCD4+T cells were measured by 3H-thymidine incorporation. As a negative control, hCD4+T cells were cultured with medium only (spontaneous) or autologus PBMCs (auto). There was a significantly lower hCD4+T cell response to GTKO/CD46/CIITA-DN pig PBMCs than to either WT or GTKO/CD46 pig PBMCs (**p<0.01).
As human T cells may proliferate against non-SLA pig proteins presented through the indirect pathway, strategies (e.g., costimulation blockade) directed to the prevention of sensitization to pig antigens presented by host antigen-presenting cells will almost certainly be required even when organs from genetically-engineered pigs have been transplanted.
The potency of NK cells in xenograft rejection remains uncertain, but the introduction of transgenes for HLA-E and/or G into the organ-source pig may negate any effect these cells might have (67-72). The mechanism of inhibition by these two HLA class I molecules is different, and therefore expression of both is likely to prove advantageous (71). Depletion or inhibition of other innate immune cells, e.g., neutrophils, monocytes, and macrophages, may be more difficult. Furthermore, these cells may be involved in leukocyte-platelet aggregation, indicating a state of platelet activation which results in the development of thrombotic microangiopathy (6,73).
Genetic modification of the pig may also minimize or prevent coagulation dysregulation. In this respect, pigs are currently available that express human thrombomodulin (TBM), human endothelial protein C receptor (EPCR), as well as CD39 and tissue factor pathway inhibitor. Expression of the human transgenes helps control the known molecular incompatibilities between pig and primate that contribute to this dysfunction (31,32). Transgenic expression of more than one of these human genes will probably be necessary to completely prevent the development of thrombotic microangiopathy in the graft or consumptive coagulopathy in the recipient. It may be necessary, however, to provide additional systemic therapy in the form of an anti-platelet or anti-thrombin agent.
Control or reduction of the inflammatory response is also most likely to be controlled by genetic manipulation of the pig. Expression of TBM, EPCR, and/or CD39 is anticipated to reduce the inflammatory response in addition to coagulation dysfunction (74). Furthermore, pigs are now available that express hemeoxygenase-1 (HO-1) (75), although this gene has only very recently been expressed in pigs that are also protected from the innate response, e.g., on a GTKO/CD46 background, and so its role in controlling inflammation as well as its effect on coagulation has not yet been well-defined. In addition, hemeoxygenase-1 expression may reduce the adaptive response through T cell apoptosis (75). Other anti-inflammatory genes that might prove valuable include A20 (76,77).
Just as there may be a need for exogenous immunosuppression and/or anti-thrombotic therapy, there may also be a need for the administration of anti-inflammatory agents. In this respect, in addition to corticosteroids, there is evidence that high-dose statin therapy not only reduces the inflammatory response and platelet activation (78), but also downregulates the primate cellular response to pig antigens (79). Interleukin-6 receptor (IL-6R) blockade may have a role to play in suppressing the inflammatory response to a xenograft, and intriguing studies in non-xenotransplantation models by Tracey and his colleagues suggest that chronic vagal nerve stimulation or the administration of a parasympathomimetic agent might suppress this response (80).
Specific suppression of the adaptive immune response
‘Rejection’, or more accurately the mechanism of graft failure, of a pig xenograft is therefore complex. However, for the purposes of this review, subsequent attention will be concentrated on the adaptive response and, particularly, on what biologic and pharmacologic agents are likely to play an important role in its control. Pig liver (81) and lung (82) transplantation into NHPs result in rapid graft loss through complex mechanisms that are unrelated to the adaptive response, and in pig islet transplantation the problem of the instant blood-mediated inflammatory reaction has to be overcome (3). These topics deserve in depth consideration in another context, but in this review attention will be directed to immunosuppressive regimens that have been utilized in pig heart and kidney xenotransplantation models.
The early experience in pig-to-NHP heart and kidney transplantation models was comprehensively reviewed by Lambrigts et al in 1998 (83) and will not be repeated here. In summary, almost all studies before 1998 utilized conventional biologic (e.g., anti-thymocyte globulin [ATG]) and/or pharmacologic (e.g., cyclophosphamide, cyclosporine, tacrolimus, corticosteroids) agents. Heterotopic heart graft survival extended to 62 days, with life-supporting kidney graft survival extending to 35 days (83). Acute humoral xenograft rejection was a major cause of graft failure, but, as a result of the necessity for intensive immunosuppressive therapy, many experiments were terminated when irreversible complications of this therapy developed, most commonly infection.
Approaches to exogenous immunomodulatory therapy
More recently, there has been a trend towards costimulation blockade-based regimens, initially introduced in xenotransplantation in 2000 by Buhler et al (84) in a hematopoietic stem cell transplantation model when it was observed that a cyclosporine-based regimen did not prevent an elicited anti-pig antibody response. The suppression of this response is clearly essential if a pig organ xenograft is to survive, and is a good indicator of the efficacy of any immunosuppressive regimen.
Subsequent experience in heterotopic and orthotopic heart transplantation models, and in the life-supporting kidney transplantation model, using either costimulation- or conventional pharmacologic-based regimens, indicates graft survival has increased (2,85). Survival after heterotopic heart transplantation now extends to 8 months (86,87) and after life-supporting kidney transplantation to almost 3 months (88,89). However, this is not yet to the extent that clinical trials can be contemplated. With either immunosuppressive approach, the causes of graft failure are generally no longer rejection, but thrombotic microangiopathy and/or consumptive coagulopathy (6,27-29). It is important to note that there may be significant differences in the primate response to a pig heart than to a pig kidney (90). These may account for the discrepancy in graft survival and for the predominance of thrombotic microangiopathy after heart transplantation and the more rapid onset of consumptive coagulopathy after kidney transplantation.
Despite the efficacy of anti-CD154mAb in xenotransplantation models, its thrombogenicity has necessitated efforts to develop costimulation blockade regimens that do not include this agent. At our own center, we have been able to replace it with a combination of an anti-CD40mAb and LEA29Y (belatacept) (Ekser B and Iwase H, unpublished).
Cell therapy approaches
Although encouraging in vitro data have been reported, the immunomodulatory efficacy of regulatory T cells (Tregs) (10,91,92) or MSCs (55,93,94) has yet to be demonstrated in large animal models of xenotransplantation. Tregs have been demonstrated to suppress the xenoreactive effector T cell response in vitro, as have MSCs.
Importantly, Tregs have been reported to have some effect in suppressing the innate immune response in allotransplantation. Muller et al (14,95) have reviewed the potential role of Tregs in xenotransplantation, and have drawn attention to the increasing evidence for the ability of Tregs to suppress xenogeneic T cell responses, as well as NK and B cell activation. They suggest that it will be important to use immunosuppressive drugs that allow Treg expansion while controlling T effector cell proliferation; targeting the CD154:CD40 pathway is beneficial in this respect (although this will need to be through an anti-CD40mAb unless the thrombogenic effect of anti-CD154mAb can be prevented), as is the administration of rapamycin (96). They also emphasize the tolerogenic environment promoted by IL-10, and speculate that secretion of recombinant IL-10 by genetically-modified pigs could contribute to tolerance induction. The selective recruitment of Tregs to the xenograft mediated by transgenic expression of human cytokines, e.g., CCL17, CCL22, on pig ECs may also help overcome cellular rejection.
The over-expression of programmed cell death ligand 1 (PD-L1) in porcine ECs inhibits the proliferation of T effector cells, augments IL-10 secretion, and allows expansion of CD73+ Tregs in vitro (97). Muller et al have suggested that these studies, together with those reported by Plege (98), indicate that PD-1/PD-ligand interactions may promote the suppressive function of Tregs in xenotransplantation (14).
Survival and function of MSCs across species barriers have been extensively reported (reviewed by Li et al [94]). The immunomodulatory, anti-inflammatory, and/or regenerative potential of pig MSCs is being explored (55,93) as the ready availability of adipose-derived MSCs from large pigs would minimize the logistics of providing unlimited numbers of these cells. Initial in vitro studies indicate that MSCs from genetically-engineered pigs are no more immunogenic than human MSCs, and yet have a comparable immunomodulatory effect on the human T cell response (55,93).
Although Tregs and/or MSCs may augment biologic and/or pharmacologic immunosuppression, we are not optimistic that either will entirely replace more standard therapy in the near future.
Induction of tolerance to a pig xenograft
As with allotransplantation, the ultimate goal is to induce a state of immunological tolerance to the pig graft. In xenotransplantation, this goal has been vigorously explored by Sachs’ group, both through the induction of hematopoietic cell chimerism (99) and thymus transplantation (88,89). It is likely that further genetic manipulations to the pig (to control the innate, coagulopathic, and inflammatory responses) will be required before this goal can be successfully achieved. As outlined above, the in vivo or in vitro expansion and maintenance of Tregs may play a role.
Conclusions
Before xenotransplantation can be introduced successfully into the clinic, the problems of the innate, coagulopathic, and inflammatory responses will have to be overcome, most likely by the transplantation of organs from specifically genetically-engineered pigs. Many of the genetic manipulations aimed at protecting against these responses will also reduce the adaptive response.
The T cell and elicited antibody response can be prevented by the biologic and/or pharmacologic agents currently available. In particular, costimulation blockade-based regimens (that do not include an anti-CD154mAb), augmented by low-dose pharmacologic therapy, have shown encouraging efficacy.
The exogenous immunosuppressive regimen may be significantly reduced by the presence of a graft from a CIITA-DN pig or a pig with ‘local’ expression of an immunosuppressive gene, e.g., CTLA4-Ig or LEA29Y (belatacept).
Acknowledgments
Mohamed Ezzelarab, MD is supported in part by the Joseph A Patrick Research Fellowship in Transplantation of the Thomas E. Starzl Transplantation Institute. Hidetaka Hara MD, PhD is supported in part by NIH grant # RO3A1096296-01. Research on xenotransplantation at the University of Pittsburgh is funded in part by NIH Grants # U19A1090959-01, #U01A1066331, and #P01 HL107152-02, by an Ocular Tissue Engineering and Regenerative Ophthalmology (OTERO) Postdoctoral Fellowship, of the University of Piitsburgh and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor Inc., Blacksburg, VA.
ABBREVIATIONS
- CIITA-DN
MHC class II transactivator dominant-negative mutant
- ECs
endothelial cells
- EPCR
endothelial protein C receptor
- GTKO
α1,3-galactosyltransferase gene-knockout
- hCRP
human complement-regulatory protein
- MSCs
mesenchymal stromal cells
- NHP
nonhuman primate
- TBM
thrombomodulin
- Tregs
regulatory T cells
Footnotes
AUTHORS’ CONTRIBUTIONS
VS and DKCC wrote the original draft, and all authors contributed to revising the draft to produce the final format of the manuscript.
DISCLOSURE OF CONFLICT OF INTEREST
David Ayares and Carol Phelps are employees of Revivicor Inc. No other author has a conflict of interest.
References
- 1.Cooper DKC, Lanza RP. Xeno - The Promise of Transplanting Animal Organs into Humans. Oxford University Press; New York: 2000. pp. 1–274. [Google Scholar]
- 2.Ekser B, Ezzelarab M, Hara H, et al. Clinical xenotransplantation – the next medical revolution? Lancet. 2012;379:672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 3.Van der Windt DJ, Bottino R, Kumar G, et al. Clinical islet xenotransplantation – how close are we? Diabetes. 2012;61:3046–3055. doi: 10.2337/db12-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lexer G, Cooper DKC, Rose AG, et al. Hyperacute rejection in a discordant (pig to baboon) cardiac xenograft model. J Heart Transplant. 1986;5:411–418. [PubMed] [Google Scholar]
- 5.Cooper DKC, Human PA, Lexer G, et al. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J Heart Transplant. 1988;7:238–246. [PubMed] [Google Scholar]
- 6.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in α1, 3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachs DH, Sykes M, Robson SC, Cooper DKC. Xenotransplantation. Adv Immunol. 2001;79:129–223. doi: 10.1016/s0065-2776(01)79004-9. [DOI] [PubMed] [Google Scholar]
- 8.Yamada K, Sachs DH, DerSimonian H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol. 1995;155:5249–5256. [PubMed] [Google Scholar]
- 9.Buhler LH, Cooper DKC. How strong is the T cell response in the pig-to-primate model? Xenotransplantation. 2005;12:85–87. doi: 10.1111/j.1399-3089.2004.00185.x. [DOI] [PubMed] [Google Scholar]
- 10.Lin YJ, Hara H, Tai HC, et al. Suppressive efficacy and proliferative capacity of human regulatory T cells in allogeneic and xenogeneic responses. Transplantation. 2008;86:1452–62. doi: 10.1097/TP.0b013e318188acb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorling A, Lombardi G, Binns R, Lechler RI. Detection of primary direct and indirect human anti-porcine T cell responses using a porcine dendritic cell population. Eur J Immunol. 1996;26:1378–1387. doi: 10.1002/eji.1830260630. [DOI] [PubMed] [Google Scholar]
- 12.Brouard S, Gagne K, Blancho G, Soulillou JP. T cell response in xenorecognition and xenografts: a review. Hum Immunol. 1999;60:455–468. doi: 10.1016/s0198-8859(99)00020-8. [DOI] [PubMed] [Google Scholar]
- 13.Davila E, Byrne GW, LaBreche PT, et al. T-cell responses during pig-to-primate xenotransplantation. Xenotransplantation. 2006;13:31–40. doi: 10.1111/j.1399-3089.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 14.Muller YD, Ehirchiou D, Golshayan D, Buhler LH, Seebach JD. Potential of T-regulatory cells to protect xenografts. Curr Opin Organ Transplant. 2012;17:155–161. doi: 10.1097/MOT.0b013e3283508e17. [DOI] [PubMed] [Google Scholar]
- 15.Bravery CA, Batten P, Yacoub MH, Rose ML. Direct recognition of SLA- and HLA-like class II antigens on porcine endothelium by human T cells results in T cell activation and release of interleukin-2. Transplantation. 1995;60:1024–1033. [PubMed] [Google Scholar]
- 16.Faustman DL, Steinman RM, Gebel HM, Hauptfeld V, Davie JM, Lacy PE. Prevention of rejection of murine islet allografts by pretreatment with anti-dendritic cell antibody. Proc Natl Acad Sci U S A. 1984;81:3864–3868. doi: 10.1073/pnas.81.12.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990;171:307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirzaie M, Meyer T, Berger D, Saalmüller A, Dalichau H. Expression of porcine major histocompatibility antigens in cardiac tissue. APMIS. 1998;106:935–940. doi: 10.1111/j.1699-0463.1998.tb00242.x. [DOI] [PubMed] [Google Scholar]
- 19.Houser SL, Benjamin LC, Wain JC, Madsen JC, Allan JS. Constitutive expression of major histocompatibility complex class II antigens in pulmonary epithelium and endothelium varies among different species. Transplantation. 2004;77:605–607. doi: 10.1097/01.tp.0000114285.63313.e7. [DOI] [PubMed] [Google Scholar]
- 20.Murray AG, Khodadoust MM, Pober JS, Bothwell AL. Porcine aortic endothelial cells activate human T cells: direct presentation of MHC antigens and costimulation by ligands for human CD2 and CD28. Immunity. 1994;1:57–63. doi: 10.1016/1074-7613(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 21.Rogers NJ, Jackson IM, Jordan WJ, Hawardle MA, Dorling A, Lechler RI. Cross-species costimulation: relative contributions of CD80, CD86, and CD40. Transplantation. 2003;75:2068–2076. doi: 10.1097/01.TP.0000069100.67646.08. [DOI] [PubMed] [Google Scholar]
- 22.Koshika T, Phelps C, Fang J, et al. Relative efficiency of porcine and human CTLA4-Ig in inhibiting human CD4+ T cell responses costimulated by porcine and human B7 molecules. Immunology. 2011;134:386–397. doi: 10.1111/j.1365-2567.2011.03496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corcoran PC, Horvath KA, Singh AK, et al. Surgical and nonsurgical complications of a pig to baboon heterotopic heart transplantation model. Transplant Proc. 2010;42:2149–2151. doi: 10.1016/j.transproceed.2010.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorling A, Lechler RI. T cell-mediated xenograft rejection: specific tolerance is probably required for long term xenograft survival. Xenotransplantation. 1998;5:234–245. doi: 10.1111/j.1399-3089.1998.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 25.Scalea J, Hanecamp I, Robson SC, Yamada K. T-cell-mediated immunological barriers to xenotransplantation. Xenotransplantation. 2012;19:23–30. doi: 10.1111/j.1399-3089.2011.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bühler L, Basker M, Alwayn IP, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000;70:1323–1331. doi: 10.1097/00007890-200011150-00010. [DOI] [PubMed] [Google Scholar]
- 27.Houser SL, Kuwaki K, Knosalla C, et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation. 2004;11:416–425. doi: 10.1111/j.1399-3089.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 28.Kuwaki K, Knosalla C, Dor FJ, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-CD154 monoclonal antibody-based regimen. Am J Transplant. 2004;4:363–372. doi: 10.1111/j.1600-6143.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 29.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1, 3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 30.Lin CC, Chen D, McVey JH, Cooper DKC, Dorling A. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008;86:702–709. doi: 10.1097/TP.0b013e31818410a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robson SC, Cooper DKC, d’Apice AJF. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation. 2000;7:166–176. doi: 10.1034/j.1399-3089.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 32.Cowan PJ, Robson SC, d’Apice AJF. Controlling coagulation dysregulation in xenotransplantation. Curr Opin Organ Transplant. 2011;16:214–221. doi: 10.1097/MOT.0b013e3283446c65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ezzelarab C, Ayares D, Cooper DKC, Ezzelarab M. Human T-cell proliferation in response to thrombin-activated GTKO pig endothelial cells. Xenotransplantation. 2012;19:311–316. doi: 10.1111/j.1399-3089.2012.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Semin Thromb Hemost. 2006;32:49–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 35.Esmon CT, Xu J, Lupu F. Innate immunity and coagulation. J Thromb Haemost. 2011;9:182–188. doi: 10.1111/j.1538-7836.2011.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 37.Cozzi E, White DJG. The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1995;1:964–969. doi: 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- 38.Cooper DKC, Koren E, Oriol R. Genetically engineered pigs. Lancet. 1993;342:682–683. doi: 10.1016/0140-6736(93)91791-j. [DOI] [PubMed] [Google Scholar]
- 39.Phelps CJ, Koike C, Vaught TD, et al. Production of α1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolber-Simonds D, Lai L, Watt SR, et al. Production of α1,3-galactosyltransferase null pigs via nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;19:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. [PubMed] [Google Scholar]
- 42.Good AH, Cooper DKC, Malcolm AJ, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in man. Transplant Proc. 1992;24:559–562. [PubMed] [Google Scholar]
- 43.Tseng Y-L, Kuwaki K, Dor FJ, et al. α 1,3-galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching six months. Transplantation. 2005;80:1493–1500. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 44.Azimzadeh AM, Kelishadi S, Ezzelarab M, et al. Early graft failure of GTKO pig organs in baboons is reduced by hCPRP expression. Am J Transplant. 2010;10(Supplement 4):186. Abstract 499. [Google Scholar]
- 45.McGregor CGA, Ricci D, Miyagi N, et al. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation. 2012;93:686–692. doi: 10.1097/TP.0b013e3182472850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kozlowski T, Shimizu A, Lambrigts D, et al. Porcine kidney and heart transplantation in baboons undergoing a tolerance induction regimen and antibody adsorption. Transplantation. 1999;67:18–30. doi: 10.1097/00007890-199901150-00004. [DOI] [PubMed] [Google Scholar]
- 47.Ye Y, Neethling FA, Niekrasz M, et al. Evidence that intravenously administered α-galactosyl carbohydrates reduce baboon serum cytotoxicity to pig kidney cells (PK15) and transplanted pig hearts. Transplantation. 1994;58:330–337. [PubMed] [Google Scholar]
- 48.McGregor CGA, Davies WR, Oi K, et al. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg. 2005;130:844–851. doi: 10.1016/j.jtcvs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 49.Leventhal JR, Dalmasso AP, Cromwell JW, et al. Prolongation of cardiac xenograft survival by depletion of complement. Transplantation. 1993;55:857–865. doi: 10.1097/00007890-199304000-00033. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi T, Taniguchi S, Neethling FA, et al. Delayed xenograft rejection of pig-to-baboon cardiac transplants after cobra venom factor therapy. Transplantation. 1997;64:1255–1261. doi: 10.1097/00007890-199711150-00005. [DOI] [PubMed] [Google Scholar]
- 51.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 52.Locke JE, Magra CM, Singer DL, et al. The use of antibody to complement protein C3 for salvage of severe antibody-mediated rejection. Am J Transplant. 2009;9:231–235. doi: 10.1111/j.1600-6143.2008.02451.x. [DOI] [PubMed] [Google Scholar]
- 53.Dawson KL, Parulekar A, Seethamraju H. Treatment of hyperacute antibody-mediated lung allograft rejection with eculizumab. J Heart Lung Tranplant. 2012;31:1325–1326. doi: 10.1016/j.healun.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 54.Wilhite T, Ezzelarab C, Hara H, et al. The effect of Gal expression on pig cells on the human T-cell xenoresponses. Xenotransplantation. 2012;19:56–63. doi: 10.1111/j.1399-3089.2011.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ezzelarab M, Ezzelarab C, Wilhite T, et al. Genetically-modified pig mesenchymal stromal cells: xenoantigenicity and effect on human T-cell xenoresponses. Xenotransplantation. 2011;18:183–195. doi: 10.1111/j.1399-3089.2011.00635.x. [DOI] [PubMed] [Google Scholar]
- 56.Schubert T, Poilvache H, Galli C, Gianello P, Dufrane D. Galactosyl-knockout engineered pig as a xenogenic donor source of adipose MSCs for bone regeneration. Biomaterials. 2013;34:3279–3289. doi: 10.1016/j.biomaterials.2013.01.057. [DOI] [PubMed] [Google Scholar]
- 57.Phelps C, Ball S, Vaught T, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. 2009;16:477–485. doi: 10.1111/j.1399-3089.2009.00533.x. [DOI] [PubMed] [Google Scholar]
- 58.Phelps C, Vaught T, Ball S, et al. Multi-transgenic pigs designed for xenoislet transplants. Xenotransplantation. 2009;16:374. Abstract # IXA-O-7.3. [Google Scholar]
- 59.Ayares D, Vaught T, Ball S, et al. Islet-specific expression of TFPI, CD39, and CTLA4Ig in transgenic pigs designed for xenoislet transplantation. Xenotransplantation. 2011;18:269. Abstract #119. [Google Scholar]
- 60.Ayares D, Phelps C, Vaught T, et al. Multi-transgenic pigs for vascularized pig organ xenografts. Xenotransplantation. 2011;18:269. Abstract #120. [Google Scholar]
- 61.Ayares D. Multi-transgenic pigs for xenotransplantation. Reprod Fertil Dev. 2012;25:320. doi: 10.1071/RDv25n1Ab344. [DOI] [Google Scholar]
- 62.Martin C, Plat M, Nerriére-Daguin V, et al. Transgenic expression of CTLA4-Ig by fetal pig neurons for xenotransplantation. Transgenic Res. 2005;14:373–384. doi: 10.1007/s11248-004-7268-4. [DOI] [PubMed] [Google Scholar]
- 63.Lévêque X, Cozzi E, Naveilhan P, Neveu I. Intracerebral xenotransplantation: recent findings and perspectives for local immunosuppression. Curr Opin Organ Transplant. 2011;16:190–194. doi: 10.1097/MOT.0b013e32834494b5. [DOI] [PubMed] [Google Scholar]
- 64.Yun S, Gustafsson K, Fabre JW. Suppression of human anti-porcine T-cell immune responses by major histocompatibility complex class II transactivator constructs lacking the amino terminal domain. Transplantation. 1998;66:103–111. doi: 10.1097/00007890-199807150-00016. [DOI] [PubMed] [Google Scholar]
- 65.Hara H, Crossley T, Witt W, et al. Dominant-negative CIITA transgenic pigs – effect on the human anti-pig T cell immune response and immune status. Am J Transplant. 2010;10(Supplement 4):187. Abstract #503. [Google Scholar]
- 66.Hara H, Witt W, Starzl TE, et al. Dominant-negative class II transactivator transgenic pigs – effect on the human anti-pig T cell immune response and immune status. Immunology. 2013 doi: 10.1111/imm.12107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seebach JD, Comrack C, Germana S, LeGuern C, Sachs DH, DerSimonian H. HLA-Cw3 expression on porcine endothelial cells protects against xenogeneic cytotoxicity mediated by a subset of human NK cells. J Immunol. 1997;159:3655–3661. [PubMed] [Google Scholar]
- 68.Dorling A, Monk NJ, Lechler RI. HLA-G inhibits the transendothelial migration of human NK cells. Eur J Immunol. 2000;30:586–593. doi: 10.1002/1521-4141(200002)30:2<586::AID-IMMU586>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 69.Crew MD. Play it in E or G: utilization of HLA-E and –G in xenotransplantation. Xenotransplantation. 2007;14:198–207. doi: 10.1111/j.1399-3089.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- 70.Weiss EH, Lilienfeld BG, Muller S, et al. HLA-E/human beta2-microglobulin transgenic pigs: protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation. 2009;87:35–43. doi: 10.1097/TP.0b013e318191c784. [DOI] [PubMed] [Google Scholar]
- 71.Sasaki H, Xu XC, Mohankumar T. HLA-E and HLA-G expression on porcine endothelial cells inhibit xenoreactive human NK cells through CD94/NKG2-dependent and independent pathways. J Immunol. 1999;163:6301–6305. [PubMed] [Google Scholar]
- 72.Matsunami K, Miyagawa S, Nakai R, Yamada M, et al. Modulation of the leader peptide sequence of the HLA-E gene up regulatesits expression and down regulates natural killer cell mediated swine endothelial cell lysis. Transplantation. 2002;73:1582–1589. doi: 10.1097/00007890-200205270-00010. [DOI] [PubMed] [Google Scholar]
- 73.Lin CC, Ezzelarab M, Shapiro R, et al. Tissue factor expression on recipient platelets is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010;10:1556–1568. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klymiuk N, Aigner B, Brem G, Wolf E. Genetic modification of pigs as organ donors for xenotransplantation. Mol Reprod Dev. 2010;77:209–221. doi: 10.1002/mrd.21127. [DOI] [PubMed] [Google Scholar]
- 75.Petersen B, Ramackers W, Lucas-Hahn A, et al. Transgenic expression of human heme oxygenase-1 in pigs confers resistance against xenograft rejection during ex vivo perfusion of porcine kidneys. Xenotransplantation. 2011;18:355–368. doi: 10.1111/j.1399-3089.2011.00674.x. [DOI] [PubMed] [Google Scholar]
- 76.Oropeza M, Petersen B, Carnwath JW, et al. Transgenic expression of the human A20 gene in cloned pigs provides protection against apoptotic and inflammatory stimuli. Xenotransplantation. 2009;16:522–534. doi: 10.1111/j.1399-3089.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- 77.Schwinzer R, Baars W, Borns K, Petersen B, Kues W, Niemann H. Over-expression of of human HO-1 and A20 in porcine cells: effects on susceptibility to cell-mediated lysis and inflammatory cytokines. Xenotransplantation. 2011;18:269. Abstract #122. [Google Scholar]
- 78.Li Q, Deng SB, Xia S, Du JL, She Q. Impact of intensive statin use on the level of inflammation and platelet activation in stable angina after percutaneous coronary intervention: a clinical study. Med Clin (Barc) 2012 Nov 20; doi: 10.1016/j.medcli.2012.05.042. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 79.Ezzelarab M, Welchons D, Torres C, et al. Atorvastatin downregulates the primate cellular response to porcine aortic endothelial cells in vitro. Transplantation. 2008;86:733–737. doi: 10.1097/TP.0b013e3181821cad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev. 2012;248:188–204. doi: 10.1111/j.1600-065X.2012.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ekser B, Burlak C, Waldman JP, et al. Immunobiology of liver xenotransplantation. Expert Rev Clin Immunol. 2012;8:621–634. doi: 10.1586/eci.12.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pierson RN, 3rd, Dorling A, Ayares D, et al. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009;16:263–280. doi: 10.1111/j.1399-3089.2009.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lambrigts D, Sachs DH, Cooper DKC. Discordant organ xenotransplantation in primates - world experience and current status. Transplantation. 1998;66:547–561. doi: 10.1097/00007890-199809150-00001. [DOI] [PubMed] [Google Scholar]
- 84.Buhler L, Awwad M, Basker M, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced antipig humoral response. Transplantation. 2000;69:2296–2304. doi: 10.1097/00007890-200006150-00013. [DOI] [PubMed] [Google Scholar]
- 85.Ekser B, Kumar G, Cooper DKC. Therapeutic issues in the treatment of vascularized xenotransplants using gal-knockout donors in nonhuman primates. Curr Opin Organ Transplant. 2011;16:222–30. doi: 10.1097/MOT.0b013e3283446c3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu X, Dor FJMF, Cooper DKC. Pig-to-nonhuman primate heart transplantation: immunologic progress over 20 years. J Heart Lung Transplant. 2007;26:210–218. doi: 10.1016/j.healun.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 87.Mohiuddin MM, Corcoran PC, Singh AK, et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012;12:763–771. doi: 10.1111/j.1600-6143.2011.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 89.Griesemer AD, Hirakata A, Shimizu A, et al. Results of gal-knockout porcine thymokidney xenografts. Am J Transplant. 2009;9:2669–2678. doi: 10.1111/j.1600-6143.2009.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Knosalla C, Yazawa K, Behdad A, et al. Renal and cardiac endothelial heterogeneity impact acute vascular rejection in pig-to-baboon xenotransplantation. Am J Transplant. 2009;9:1006–1016. doi: 10.1111/j.1600-6143.2009.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Porter CM, Horvath-Arcidiacono JA, Singh AK, Horvath KA, Bloom ET, Mohiuddin MM. Characterization and expansion of baboon CD4+CD25+ Treg cells for potential use in a non-human primate xenotransplantation model. Xenotransplantation. 2007;14:298–308. doi: 10.1111/j.1399-3089.2007.00416.x. [DOI] [PubMed] [Google Scholar]
- 92.O’Connell PJ, Yi S, Carrington EM, Lew AM. Role of regulatory T cells in xenotransplantation. Curr Opin Organ Transplant. 2010;15:224–229. doi: 10.1097/MOT.0b013e3283373c27. [DOI] [PubMed] [Google Scholar]
- 93.Kumar G, Hara H, Long C, et al. Adipose-derived mesenchymal stem cells from genetically-modified pigs immunogenicity and immune modulatory properties. Cytotherapy. 2012;14:494–504. doi: 10.3109/14653249.2011.651529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li J, Ezzelarab MB, Cooper DKC. Do mesenchymal stem cells function across species barriers? Relevance for xenotransplantation. Xenotransplantation. 2012;19:273–285. doi: 10.1111/xen.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muller YD, Seebach JD, Buhler LH, et al. Transplantation tolerance: clinical potential of T regulatory cells. Self Nonself. 2011;2:26–34. doi: 10.4161/self.2.1.15422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muller YD, Mai G, Morel P, et al. Anti-CD 154 mAb and rapamycin induce T-regulatory cell mediated tolerance in the rat to mouse islet-transplantation. PLoS One. 2010;5:e 10352. doi: 10.1371/journal.pone.0010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ding Q, Lu L, Zhou X, et al. Human PD-L1 overexpressing porcine vascular endothelial cells induce functionally suppressive human CD4+CD25hiFoxp3+ Treg cells. J Leukoc Biol. 2011;90:77–86. doi: 10.1189/jlb.1210691. [DOI] [PubMed] [Google Scholar]
- 98.Plege A, Borns K, Baars W, Schwinzer R. Supression of human T-cell activation and expansion of regulatory T-cells by pig cells overexpressing PD ligands. Transplantation. 2009;87:975–982. doi: 10.1097/TP.0b013e31819c85e8. [DOI] [PubMed] [Google Scholar]
- 99.Tseng Y-L, Sachs DH, Cooper DKC. Porcine hematopoietic progenitor cell transplantation in nonhuman primates: a review of progress. Transplantation. 2005;79:1–9. doi: 10.1097/01.tp.0000146504.73727.13. [DOI] [PubMed] [Google Scholar]


