Abstract
Background
Forty percent of in-hospital deaths among injured patients involve massive truncal hemorrhage. These deaths may be prevented with rapid hemorrhage control and improved resuscitation techniques. The Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) Trial was designed to determine if there is a difference in mortality between subjects who received different ratios of FDA approved blood products. This report describes the design and implementation of PROPPR.
Study Design
PROPPR was designed as a randomized, two-group, Phase III trial conducted in subjects with the highest level of trauma activation and predicted to have a massive transfusion. Subjects at 12 North American level 1 trauma centers were randomized into one of two standard transfusion ratio interventions: 1:1:1 or 1:1:2, (plasma, platelets, and red blood cells). Clinical data and serial blood samples were collected under Exception from Informed Consent (EFIC) regulations. Co-primary mortality endpoints of 24 hours and 30 days were evaluated.
Results
Between August 2012 and December 2013, 680 patients were randomized. The overall median time from admission to randomization was 26 minutes. PROPPR enrolled at higher than expected rates with fewer than expected protocol deviations.
Conclusion
PROPPR is the largest randomized study to enroll severely bleeding patients. This study showed that rapidly enrolling and successfully providing randomized blood products to severely injured patients in an EFIC study is feasible. PROPPR was able to achieve these goals by utilizing a collaborative structure and developing successful procedures and design elements that can be part of future trauma studies.
Keywords: trauma, transfusion, clinical trial, plasma, resuscitation, platelets
1. Introduction
Injury is the leading cause of death between the ages of 1 and 44 years. Nearly 50% of injury-related deaths occur before the individual reaches the hospital, and much of this mortality is currently difficult to prevent.[1–4] However, approximately 40% of the in-hospital deaths among injured patients involve massive truncal hemorrhage that is considered potentially salvageable with rapid hemorrhage control and improved resuscitation techniques.[5–11]
One method to improve the outcome of rapidly bleeding patients is to deliver predetermined ratios of platelets, plasma, and red blood cells (RBCs). However, the optimal ratio of these products is unclear. The current United States (US) Department of Defense (DoD) guideline specifies the use of 1:1:1.[12] In civilian observational studies, investigators have reported good outcomes across a range of different blood product ratios;[13–19] the largest observational transfusion study of bleeding trauma patients, the PRospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study, was conducted in 10 Level I trauma centers in the US. In PROMMTT, clinicians generally delivered transfusion ratios that cumulated in the range of 1:1 or 1:2.[20]
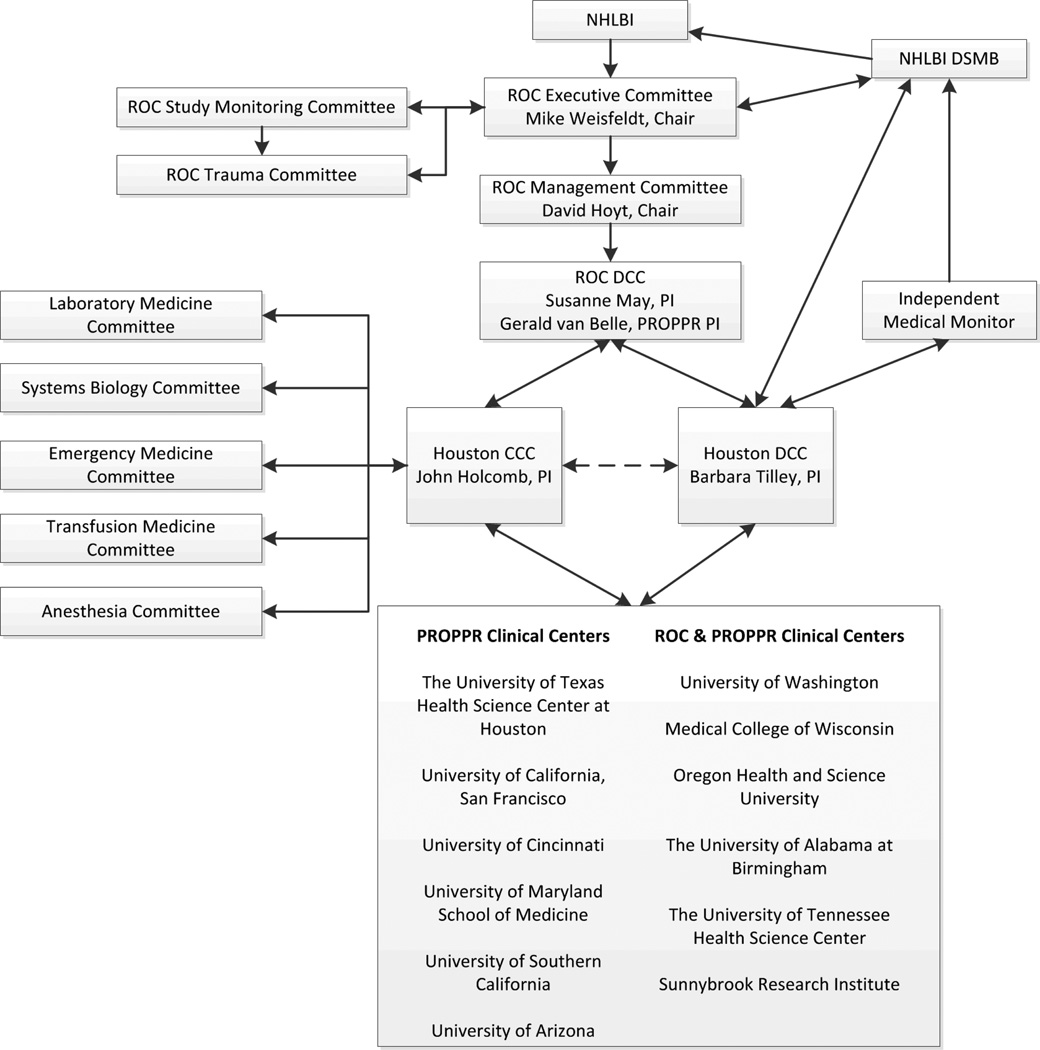
The DoD and the National Heart, Lung and Blood Institute (NHLBI) recognized the need to address optimal blood product ratios and to characterize the natural history of coagulopathy and inflammation in bleeding patients.[21, 22] They recommended a large trial comparing blood product ratios and collecting serial blood samples and subsequently funded the Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial through the Resuscitation Outcomes Consortium (ROC) in 2010, with additional funds from Defense Research and Development Canada and the Canadian Institutes of Health Research. PROPPR worked within a complex structure of review and oversight for the design and conduct of the trial (Figure 1).
Figure 1.
PROPPR Administrative Structure and Participating Sites
2. Methods
2.1 Study design and hypotheses
PROPPR was a randomized, two-group, Phase III trial conducted in subjects requiring the highest level of trauma activation and predicted to receive a massive transfusion as defined by ABC score or physician gestalt.[23] Subjects were randomized into transfusion ratio interventions: 1:1:1 or 1:1:2 (Table 1). Based upon the timing of hemorrhagic death the Food and Drug Administration (FDA) approved two co-primary endpoints.[24] The clinical hypotheses are below:
Ha1: A greater proportion of subjects predicted to have a massive transfusion and randomized to the 1:1:1 ratio group will survive to 24 hours after Emergency Department (ED) admission compared with subjects randomized to the 1:1:2 ratio, and
Ha2: A greater proportion of subjects predicted to have a massive transfusion and randomized to the 1:1:1 ratio group will survive to 30 days after ED admission compared with subjects randomized to the 1:1:2 ratio group.
Table 1.
Contents of container cycles for each ratio group.
| Container 1 | Container 2 | ||
|---|---|---|---|
| Group 1a | Platelets | 1 | 1 |
| 1:1:1 | Plasma | 6 | 6 |
| RBCs | 6 | 6 | |
| Group 2b | Platelets | 0 | 1 |
| 1:1:2 | Plasma | 3 | 3 |
| RBCs | 6 | 6 |
Group 1: Platelets first, then alternate RBCs and Plasma, as clinically required
Group 2: Platelets first (if available), then alternate 2 RBCs and 1 Plasma, as clinically required
The container cycles were repeated until hemostasis was achieved and resuscitation completed.
Ancillary clinical aims include comparisons between treatment groups on the number of ventilator-free, ICU-free days, and hospital-free days. Other ancillary analyses include comparisons of time to completion of resuscitation, incidence of major surgical procedures during initial hospitalization, incidence of transfusion-related serious adverse events during initial hospitalization and other complications, amount of study blood products given until hemostasis, re-bleeding after resuscitation was complete, amount of blood products given from hemostasis to 24 hours, and functional status (Glasgow Outcome Scale) at hospital discharge.
The laboratory hypotheses were defined as:
Ha1: Severely injured trauma patients enrolled into PROPPR will differ in their coagulation and inflammatory phenotypes at admission by subject demographic and injury characteristics;
Ha2: Coagulation and inflammatory phenotypes identified at admission will display dynamic changes. These phenotype changes will be driven by injury demographics and resuscitation.
Ha3: Coagulation and inflammatory profiles will be associated with primary, secondary, and ancillary clinical outcomes.
The PROPPR trial employed a pragmatic design. While the assignment of transfusion ratios was randomized, no other clinical procedures or aspects of patient care were altered by the study.
2.2 Site Selection
Site selection was critical to the success of PROPPR. Surveys were sent to 25 North American Level I trauma centers. Sites were rated based on geographic distribution, volume of admissions, estimated number of massive transfusion patients, previous research experience, ability to collect data and samples 24/7, and the ability to deliver blood components in a timely manner. Twelve sites were selected and are listed as part of the organizational structure (Figure 1).
2.3 Exception from Informed Consent (EFIC)
Rapid randomization required Exception From Informed Consent (EFIC). EFIC allows subjects to be randomized before they or their legally authorized representative are consented.[25, 26]The use of EFIC mandated extensive review and approval prior to the start of the trial, despite the use of approved blood products in commonly employed ratios.[20] ROC, National Institutes of Health, DoD, Health Canada, FDA and all local Institutional Review Boards/Research Ethics Boards reviewed and approved the PROPPR protocol and use of EFIC.
2.4 Eligibility Criteria
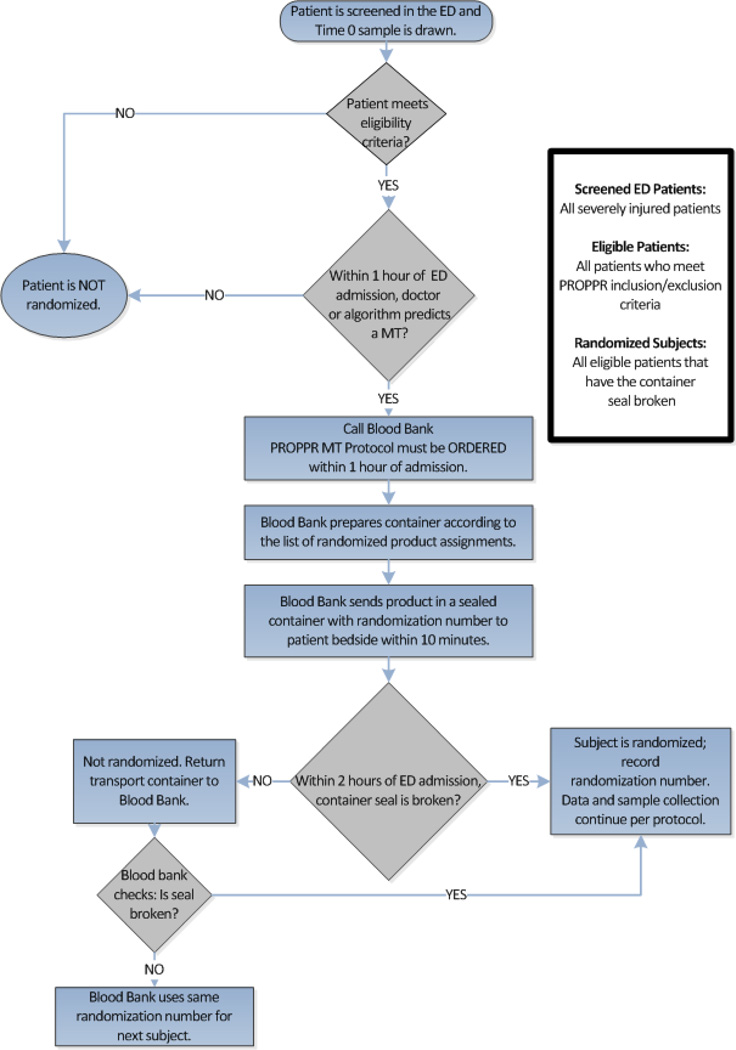
PROPPR incorporated standard inclusion and exclusion criteria common to trauma trials to facilitate enrollment of severely injured patients who had a high risk of mortality from bleeding (Table 2). An ABC score ≥2 and 1 unit of transfused RBCs within one hour of ED admission were the criteria used to automatically call the blood bank for randomized products. If the ABC score was <2, the attending physician could use clinical judgment to call for study products (Figure 2).[23] Lastly, patients who received substantial pre-randomization transfusions (three units of RBCs) were excluded. There was no set limit on other blood products. The amount of pre-randomized RBCs was based on data from PROMMTT and a qualitative assessment of all site principal investigators (PIs).[20] To be enrolled, the seal on the randomized blood product container had to be broken within two hours of ED admission. Limiting the time of enrollment to two hours from arrival and using administration of at least one unit of RBCs and the ABC score or the attending physician judgment excluded many patients who were mortally wounded or slowly or minimally bleeding.
Table 2.
Criteria for Eligibility in the PROPPR Trial
|
Eligible subjects must meet all of the following: |
Ineligible subjects meet at least one of the following criteria: |
|---|---|
| 1) Highest trauma level activation; | 1) Received care from an outside hospital or healthcare facility (defined as receiving a lifesaving intervention); |
| 2) Estimated age 15 years or older or weight of 50 kg or greater if age unknown; | 2) Moribund patient with devastating injuries and expected to die within one hour of ED admission; (e.g. lethal traumatic brain injury) |
| 3) Received directly from the injury scene; | 3) Prisoners directly admitted from a correctional facility; |
| 4) Initiated transfusion of at least one unit of blood component within the first hour of arrival or during pre-hospital transport; | 4) Patients requiring an ED thoracotomy prior to receiving randomized blood products; |
| 5) Children under the age of 15 years or under 50 kg body weight if age unknown; | |
| 5) Predicted to receive a MT by exceeding the threshold score of either the ABC score of 2 or greater23 or based on the attending trauma physician’s judgment. | 6) Known pregnancy in the ED; |
| 7) Greater than 20% total body surface area (TBSA) burns; | |
| 8) Suspected inhalation injury; | |
| 9) Received greater than five consecutive minutes of cardiopulmonary resuscitation (CPR with chest compressions) in the pre-arrival or ED setting; | |
| 10) Known DNR prior to randomization; | |
| 11) Enrolled in a concurrent ongoing interventional, randomized clinical trial; | |
| 12) Activated the “opt-out” process for the PROPPR trial (usually by wearing a bracelet given out at community consent presentations); | |
| 13) No more than 3 RBCs given before randomization. |
Figure 2.
Protocol Flowchart
2.5 Randomization, Blinding, and Protocol Completion
Subjects were randomized using a stratified (by site) permuted- block design. Treatment assignment labels, generated by the Houston Data Coordinating Center (HDCC), were kept in secure files at each clinical site’s blood bank. Complete masking (blinding) of the intervention assignment was not logistically possible without interfering with the delivery and utilization of approved life-saving blood products. However, masking of the clinicians for as long as possible was considered extremely important so that potential bias could be minimized. For this reason, randomized blood products were placed into sealed containers in the blood bank and the clinicians at the bedside remained blinded to group assignment until the container seal was broken.
A subject was considered randomized when a study blood product container was opened. If a patient was ineligible, blood products could be returned in the unopened containers to the blood bank inventory. If an ineligible patient needed an immediate emergency transfusion, the container could be opened after the patient was declared ineligible by a member of the study team. The ineligible patient was not considered randomized and blood products were never withheld from any bleeding patient. To maintain the randomization sequence and masking, an HDCC representative was available 24/7 to provide a new treatment assignment and randomization sequence to the blood bank as soon as they were notified of emergency use of products. A subject declared ineligible after either container was opened or consent was withdrawn was considered randomized, and was followed only for safety and mortality.
Derived from the experience of Nascimento, et al., a novel two-step method determined when hemostasis and resuscitation were considered achieved and the study protocol was discontinued.[27] First, the time of anatomic hemostasis noted by the attending surgeon was recorded. Secondly, a separate physiologic assessment of adequate resuscitation was made by the attending surgeon and anesthesiologist. Adequate resuscitation was based on qualitative improvement in blood pressure, urine output, and heart rate as well as decreased vasopressor requirements. When both anatomic hemostasis and adequate resuscitation criteria were met, randomized products were stopped.
2.6 Sample Size
Initally, the trial planned to enroll 580 subjects with 290 subjects per group. A 10% difference in mortality at 24 hours was considered to be clinically meaningful. The design provided 90% power to detect a difference of 10% or larger in 24-hour mortality and 88% power to detect a difference of 12% or greater in 30-day mortality, assuming a two-sided alpha=0.044 (adjusted from 0.05 for two interim efficacy analyses). The 24-hour and 30-day outcomes were considered separate co-primary outcomes requiring no adjustment of alpha for multiple comparisions.[28] The 24-hour and 30-day mortality in the 1:1:1 group were assumed to be 11% and 23%, respectively, based on extensive retrospective data[19] and the prospective observational study, PROMMTT.[20]
2.7 Vanguard Phase
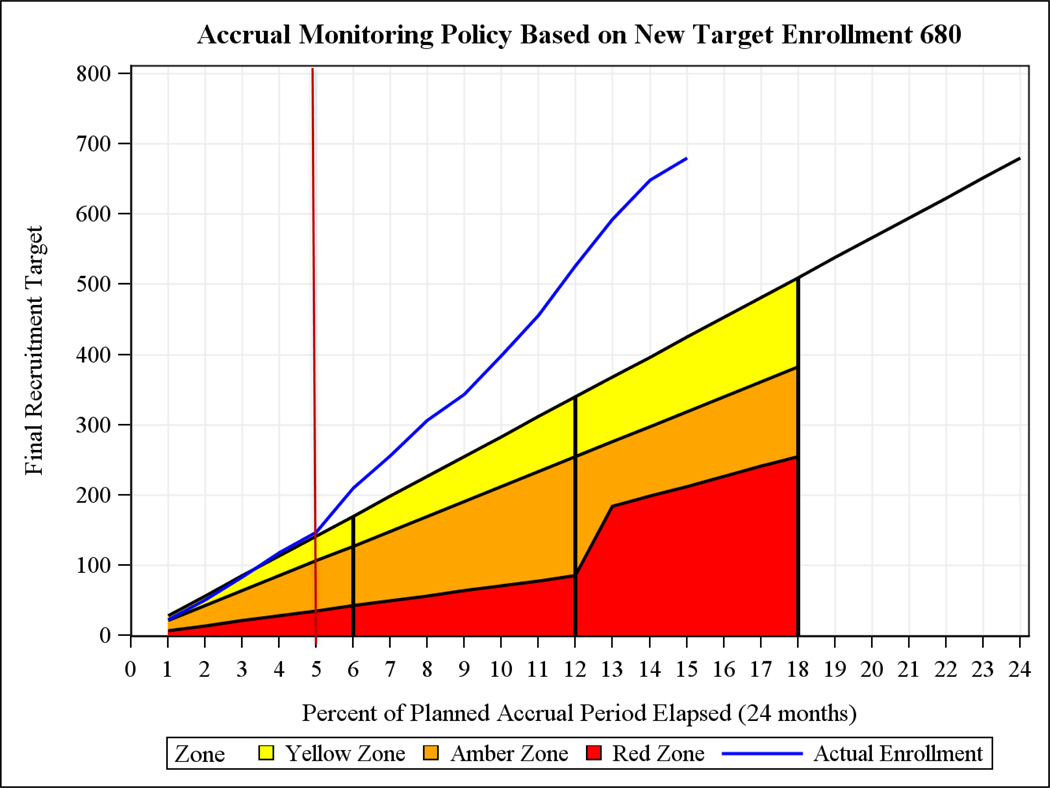
A Vanguard Phase was built into the trial to assess process feasibility because the protocol was challenging to implement and the sites’ ability to recruit and deliver randomized blood products rapidly was unknown.[29, 30] The Vanguard Phase was not considered a pilot study because data from this phase were expected to be part of the Phase III trial. Six months after at least four sites had begun enrollment, the Data Safety Monitoring Board (DSMB) reviewed process measures related to recruitment and data quality. The DSMB determined if the protocol could be followed and whether modifications to the protocol, data collection procedures or trial manual of operations were needed. If substantive changes were required, the DSMB would determine if the Vanguard stage data could be used in the Phase III trial. In April 2013, based on recruitment (Figure 3) and other administrative information, the DSMB concluded that the trial was feasible, and that data collected during the Vanguard Phase would be included in the Phase III trial.
Figure 3.
Recruitment graph.
The vertical lines in the above figure indicate 25%, 50%, and 75% of the planned accrual period elapsed, respectively. The red vertical line indicates the end of the Vanguard stage. The red, orange and yellow shaded areas represent NHLBIs level of concern with low enrolling studies.
2.8 Adaptive Design
Prior to the first interim efficacy analysis and after successful completion of the Vanguard Phase, a pre-planned re-analysis of power was presented to members of the DSMB by a biostatistician from the ROC to determine whether the sample size should be increased. All participants in the discussion were masked to the overall mortality and treatment group differences per FDA guidelines for adaptive designs.[31] Power was recalculated based on observed 24-hour mortality and 30-day mortality in the 1:1:1 group (the comparator arm) using the clinically meaningful difference assumed in the original sample size calculations. Based on the power calculations, the DSMB recommended increasing the sample size to 680 to maintain an overall power of 85% across a range of alternatives.
2.9 Analysis
Analyses for the Phase III trial co-primary outcomes (24-hour and 30-day mortality) and secondary analyses are intention-to-treat, i.e., all subjects are analyzed as randomized. For the primary analyses, 24-hour and 30-day mortality are considered fixed points in time and will be compared using separate Mantel-Haenszel tests taking site, the stratifying variable, into account. Two interim analyses for the DSMB were planned after 1/3 and 2/3 of the projected 24-hour or 30-day mortality events were observed (whichever reached its projected 1/3 and 2/3 first). The two co-primary outcomes were separately monitored using a two-sided O’Brien-Fleming boundary[32] with Lan-DeMets alpha spending function based on numbers of events for each of the two comparisons.[33]
No test for futility (stochastic curtailment[34]) was planned, since the null hypotheses were also of clinical interest for both co-primary outcomes. If differences are not detected, the full sample size will be required to produce as narrow and informative confidence limits as possible.
2.10 Endpoint Ascertainment
The FDA indicated the trial would be considered scientifically invalid if 30-day outcome information was missing on more than 10% of enrolled subjects at the end of the trial. Under EFIC, searches of all available public data sources to determine vital status at 30 days, even for those who withdrew consent, are allowed.
Deaths were classified (Table 3) by a blinded, independent medical monitor (Houston Clinical Coordinating Center (HCCC, PI) for deaths outside of Houston and by the HDCC’s Independent Medical Monitor for deaths at the Houston site. If disagreement between the local PI and the HCCC PI or HDCC Medical Monitor occurred, then final death classification was decided by the independent HDCC Medical Monitor.
Table 3.
Adjudicated Cause of Death Categories*
| Cause of Death Definitions |
|---|
| Exsanguination / Hemorrhagic Shock: Exsanguination: death caused by uncontrolled bleeding. Hemorrhagic Shock: shock associated with the sudden and rapid loss of significant amounts of blood. |
| Traumatic Brain Injury (TBI): An injury to the brain caused by penetration of the skull or movement of the brain within the skull. TBI as a cause of death usually occurs with several days of admission. |
| Respiratory/Pulmonary Contusion/Tension Pneumothorax: Respiratory: any loss of ventilatory capability, usually from a mechanical issue somewhere between the ventilator and the pulmonary parenchyma. Pulmonary contusion: injury to lung parenchyma, leading to edema and blood collecting in alveolar spaces and loss of normal lung structure & function. |
| Sepsis: An overwhelming systemic response to documented infection. Patients dying of sepsis usually do so > 72 hours after admission. |
| MOF: Altered organ function in at least 2 organ systems. Progressive and profound organ dysfunction that is incompatible with life. Patients dying of MOF usually do so > 48 hours after admission. |
| Stroke: New neurological deficit not present prior to injury which is sudden or rapid in onset, lasts > 24 hours and is confirmed as an infarction by CT or MRI, acutely causing death. |
| Myocardial Infarction: Acute, irreversible myocardial injury documented by both: (1) Abnormal increase in CK-MB or troponin and (2) New, serial T-wave, S-T segment or Q wave ECG abnormalities acutely causing death. |
| Pulmonary Embolism: A blood clot lodged in the lumen of a pulmonary artery acutely causing death, diagnosed by CT angiogram, pulmonary angiogram or ventilation perfusion scan. |
| Transfusion Related Fatality: fatality as a direct result of a complication of blood component transfusion. |
Patients could experience more than one cause of death.
2.11 Data Collection, Management, and Quality
Data were collected via direct observation by study staff on standardized case report forms PIas instructed in the manual of operations. Research laboratory samples were collected on admission and an additional 5 times over 72 hours. One assay on fresh samples was performed locally (thromboelastography [TEG] and Multiplate). A second fresh sample was sent to the Houston Core Lab for flow analysis. The remaining samples were processed, frozen and shipped to the Houston core lab for later analysis. A series of webinars, posters, individual site visits, and site certification by the HDCC were used to familiarize and train the sites on PROPPR procedures including data and sample collection. These training methods were supplemented with monthly site coordinator calls, joint PI and coordinator calls, committee calls (lab committee meetings were bimonthly), in person meetings every six months, and ad hoc calls as needed.
All data were maintained in a web-based data entry and management system (OpenClinica, LLC, Waltham, MA) and extensively queried for appropriate ranges and consistency across forms. Site inspections and monitoring were carried out by an independent company. All sites had a first monitoring visit after the site’s initial enrollment occurred, a follow up visit every 6 months thereafter, and more frequent visits if necessary. At the end of the trial, each site has a closeout visit.
A major concern was the number of protocol deviations that might occur when transfusing lifesaving products in the correct order (Table 1) while simultaneously caring for seriously injured patients. Each deviation was reviewed at weekly HDCC and HCCC meetings and, if found to be serious, the local PI and involved parties were called to discuss the deviation. Site PIs also reviewed any deviations at their site during regular meetings and calls and suggested a mitigation plan. This process is similar in concept to the weekly morbidity and mortality (M&M) conferences that the trauma community holds to improve patient care. By utilizing the familiar M&M concept, all coordinators and PIs, as well as the HCCC and HDCC, could quickly and openly understand issues, devise solutions and implement changes across all 12 sites, thus minimizing repetition of serious protocol deviations.
2.12 Continuous Quality Improvement (CQI)
The success of protocol implementation hinged on the ability of the entire team at each site to work together smoothly and provide the correct blood products to the bedside within 10 minutes of blood bank notification. Each site had dramatically different blood bank, ED and operating room (OR) arrangements. A team from the PROPPR HDCC and HCCC walked through the study protocol at each site prior to trial initiation. Working with each site team, protocol conduct and blood delivery processes were refined to not only take into account individual site requirements, but also ensure maintenance of protocol rigor.
The Research laboratory committee oversaw collection, storage and prioritization of trial specimens. The systems biology committee established procedures for novel analytic methods. Additional committees were established representing three clinical groups (anesthesia, emergency medicine, and transfusion medicine) to facilitate hospital-wide buy-in and to help solve problems unique to their respective specialties. This CQI approach, actively involving key stakeholders in developing and implementing the protocol has previously proven to anticipate problems and provide a ready pathway for solutions.[35]
3. Results
3.1 Enrollment
Figure 3 shows enrollment over the course of the trial. Throughout the trial, recruitment was higher than projected. Four sites began enrolling within 19 days of the first subject (3 Aug 2012), and all 12 sites were enrolling within a 6 month span.
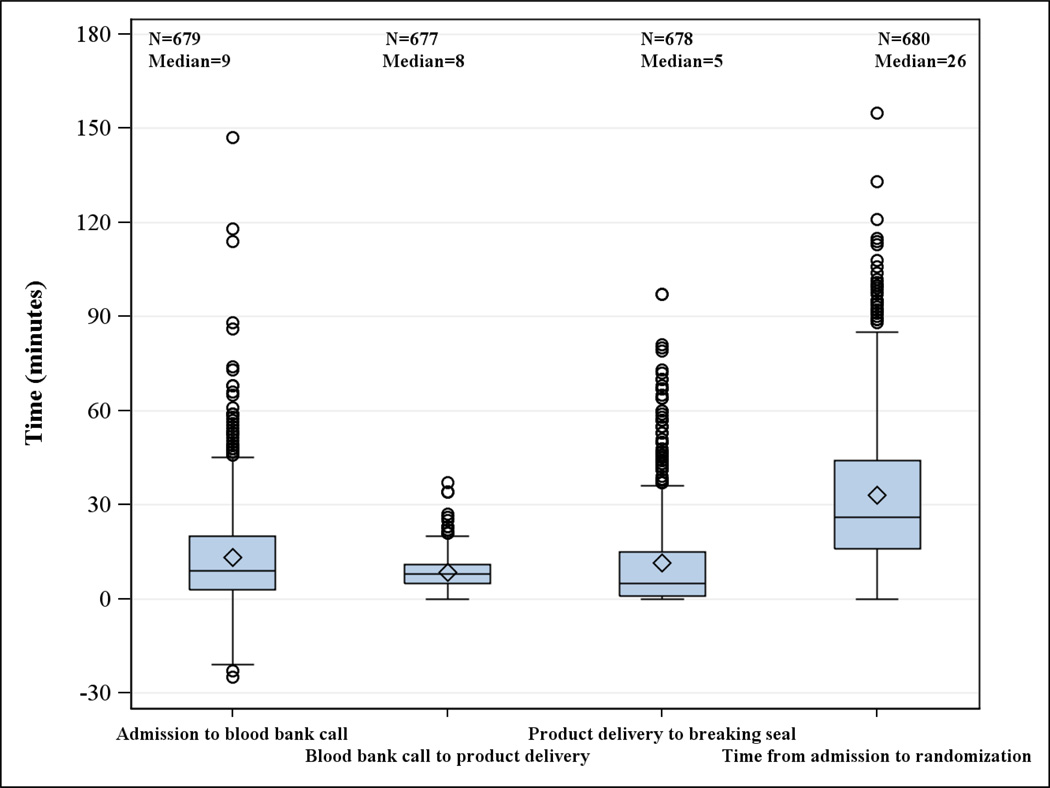
3.2 Process Time Measures
Figure 4 graphs time from blood bank notification to delivery of study products to the bedside and randomization. The median time from admission to blood bank call was 9 minutes. The median time from blood bank call to product delivery was 8 minutes, which is under the stated protocol goal of 10 minutes. The time from product delivery to breaking the seal was 5 minutes. The overall median time from admission to randomization was 26 minutes.
Figure 4.
Process time measures
3.3 Protocol Deviations
As a result of implementation of the M&M procedures and CQI approach, deviations decreased from an early high of 22% to a final rate of 15% over the course of the trial (Table 4).
Table 4.
Types of Deviations
| # of patients (% with deviations) |
Blood products given out of order |
Early stop by treating physician |
Enrollment in another randomized study |
ITT subject not followed correctly |
Misrandomization by blood bank |
Randomization of ineligible subject |
|
|---|---|---|---|---|---|---|---|
| All Sites | 680* (15.0) | 40 | 11 | 4 | 1 | 14 | 36 |
4 patients had two protocol deviations.
4. Discussion
Previous Phase III studies of hemostasis or resuscitation in acutely injured trauma patients have largely failed to show mortality differences between groups.[36–40] Reasons for the negative findings have included enrolling either moribund or minimally injured patients, delays in enrollment due to not utilizing EFIC, lack of blinding, high protocol deviation rates after unblinding, lack of blood product availability and limited biological effect of the study agent.[36–40] Several observational trauma transfusion studies have also identified survival bias as a significant issue.[41]
The PROPPR investigators spent considerable time designing a protocol attempting to address the lessons learned from previous studies.[20, 27, 36–40] Because clinicians were treating critically injured patients, a guiding principle was that PROPPR would not create processes that slowed the delivery of clinical care, including blood products. By combining the ABC score and clinical judgment and working with each blood bank, we created a system that has improved blood product delivery. The timeliness of these actions is demonstrated in Figure 4, with a median time from admission to randomization of 26 minutes. To reduce bias from lack of blinding, PROPPR clinicians were required to declare a patient eligible prior to knowing the treatment assignment and were required to make every effort to ascertain mortality outcomes using methods allowed under EFIC. Thus we maintained rigor and reduced bias while caring for critically ill patients.
The PROPPR investigators designed a trial that rapidly enrolled a group of patients with substantial transfusion requirements. The methodology and dedication of the study personnel allowed timely delivery of blood products to the bedside of bleeding trauma patients. While the outcomes have yet to be analyzed, the effort of all involved is a testament to their dedication to improving the outcome of injured patients everywhere.
Acknowledgements
We would like to thank the members of the DSMB, including Lance Becker, Charles Cairns, Ralph D’Agostino, Karl Jern, Nigel Key, Laurence McCullough, Jeremy Perkins, Herbert Wiedemann, Janet Wittes, and Jay Mason. The members of the PROPPR External Advisory Committee, including Kenneth G. Mann, Kathleen Brummel, Beth Hartwell, Charles Esmon, Morris Blajchman, Andrew P. Cap, Andrei Kindzelski, and Anthony E. Pusateri, also contributed to the design of PROPPR and we thank them for their time and effort. We also thank the ROC Protocol Review Committee for their important contributions.
This work was sponsored by the U.S. National Heart, Lung, and Blood Institute (U01HL077863) and the U.S. Department of Defense, as well as Defence Research and Development Canada in partnership with the Canadian Institutes of Health Research (CIHR)- Institute of Circulatory and Respiratory Health (CRR-120612). NHLBI and the DoD were consulted regarding study design only. No sponsors were involved in the collection, analysis, and interpretation of data; the writing of the manuscript; or the decision to submit the manuscript for publication. The content is the sole responsibility of the authors and is not to be construed as official or as reflecting the views of any sponsor.
*Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) Study Group
Clinical Coordinating Center: John B. Holcomb, MD; Charles E. Wade, PhD; Deborah J. del Junco, PhD; Erin E. Fox, PhD; Nena Matijevic, PhD (Laboratory Committee co-Chair); Jeanette Podbielski, RN; Angela M. Beeler, BS.
Data Coordinating Center: Barbara C. Tilley, PhD; Sarah Baraniuk, PhD; Joshua Nixon, MS; Hui Yang, MS; Michael O. Gonzalez, MS.
Core Laboratory: Lisa Baer, MS; Yao-Wei Willa Wang, MD; Brittany S. Hula, MS; Elena Espino, BS; An Nguyen, BS; Nicholas Pawelczyk, BS; Kisha D. Arora-Nutall, BS; Rishika Sharma, MD; Jessica C. Cardenas, PhD; Elaheh Rahbar, PhD; Tyrone Burnett, Jr., BS; David Clark, BS.
Resuscitation Outcomes Consortium: Gerald van Belle, PhD; Susanne May, PhD; Brian Leroux, PhD; David Hoyt, MD; Judy Powell, BSN, RN; Kellie Sheehan, BSN.
Systems Biology Committee: Alan Hubbard, PhD (co-Chair); Adam P. Arkin, PhD.
Transfusion Committee: John R. Hess, MD (co-Chair); Jeannie Callum, MD (co-Chair)
PROPPR Clinical Sites (listed in order of number of patients enrolled):
University of Texas Health Science Center at Houston: Bryan A. Cotton, MD, MPH; Laura Vincent, BSN, RN, CCRP; Timothy Welch; Tiffany Poole, DC; Evan G. Pivalizza, MD; Sam D. Gumbert, MD; Yu Bai, MD, PhD; James J. McCarthy, MD; Amy Noland, MD; Rhonda Hobbs, MT(ASCP)SBB.
University of Washington: Eileen M. Bulger, MD; Patricia Klotz, RN; Lindsay Cattin, BA; Keir J. Warner, BS; Angela Wilson, BA; David Boman, BA; Nathan White, MD, MS; Andreas Grabinsky, MD; Jennifer A. Daniel-Johnson, MBBS.
University of California, San Francisco: Mitchell Jay Cohen, MD (Systems Biology and Laboratory Committees co-Chair); Rachael A. Callcut, MD, MSPH; Mary Nelson, RN, MPA; Brittney Redick, BA; Amanda Conroy, BA; Marc P. Steurer, MD, DESA; Preston C. Maxim, MD; Eberhard Fiebig, MD; Joanne Moore; Eireen Mallari, MT.
University of Cincinnati: Peter Muskat, MD; Jay A. Johannigman, MD; Bryce R. H. Robinson, MD; Richard D. Branson, MSc, RRT; Dina Gomaa, BS, RRT; Christopher Barczak, BS, MT(ASCP); Suzanne Bennett, MD; Patricia M. Carey, MD; Christopher N. Miller, MD; Helen Hancock, BS, MT(ASCP); Carolina Rodriguez, BA.
University of Southern California: Kenji Inaba, MD; Jay G. Zhu, MD; Monica D. Wong, MS; Michael Menchine, MD, MPH; Kelly Katzberg, MD, FACEP; Sean O. Henderson, MD; Rodney McKeever, MD; Ira A. Shulman, MD; Janice M. Nelson, MD; Christopher W. Tuma, BA, MT(ASCP), SBB; Cheryl Y. Matsushita, BS, MT(ASCP).
Shock, Trauma and Anesthesiology Research - Organized Research Center (STAR-ORC), R Adams Cowley Shock Trauma Center, University of Maryland Medical Center: Thomas M. Scalea, MD; Deborah M. Stein, MD, MPH; Cynthia K. Shaffer, MS, MBA; Christine Wade, BA; Anthony V. Herrera, MS; Seeta Kallam, MBBS; Sarah E. Wade, BS; Cynthia K. Shaffer, MS, MBA; Samuel M. Galvagno, Jr, DO, PhD; Magali J. Fontaine, MD, PhD; Janice M. Hunt, BS, MT(ASCP) SBB; Rhonda K. Cooke, MD.
University of Tennessee Health Science Center, Memphis: Timothy C. Fabian, MD; Jordan A. Weinberg, MD; Martin A. Croce, MD; Suzanne Wilson, RN; Stephanie Panzer-Baggett, RN; Lynda Waddle-Smith, BSN; Sherri Flax, MD.
Medical College of Wisconsin: Karen J. Brasel, MD, MPH; Pamela Walsh, AS, CCRC; David Milia, MD; Allia Nelson, BS, BA; Olga Kaslow, MD, PhD; Tom P. Aufderheide, MD, MS; Jerome L. Gottschall, MD; Erica Carpenter, MLS(ASCP).
University of Arizona: Terence O’Keeffe, MBChB, MSPH; Laurel L. Rokowski, RN, BSN, MKT; Kurt R. Denninghoff, MD; Daniel T. Redford, MD; Deborah J. Novak, MD; Susan Knoll, MS, MT(ASCP) SBB.
University of Alabama at Birmingham: Jeffrey D. Kerby, MD, PhD; Jean-Francois Pittet, MD (Anesthesia Chair); Patrick L. Bosarge, MD; Carolyn R. Williams, RN, BSN, BSME; Shannon W. Stephens, EMTP; Henry E. Wang, MD, MS ; Marisa B. Marques, MD .
Oregon Health and Science University: Martin A. Schreiber, MD ; Jennifer M. Watters, MD; Samantha J. Underwood, MS; Tahnee Groat, MPH; Craig Newgard, MD, MPH; Matthias Merkel, MD, PhD ; Richard M. Scanlan, MD; Beth Miller, MT(ASCP)SBB.
Sunnybrook Health Science Center: Sandro Rizoli, MD, PhD; Homer Tien, MD; Barto Nascimento, MD, MSc, CTBS; Sandy Trpcic; Skeeta Sobrian-Couroux, RN, CCRP, BHA; Marciano Reis; Adic Pérez, MD; Susan E. Belo, MD, PhD; Connie Colavecchia, BSc, MLT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This effort is dedicated to the soldiers, sailors, marines, and airmen who put their lives on the line every day. We hope this effort will help improve the care of seriously injured patients, both military and civilian.
Conflicts of Interest Statement:
No conflicts of interest have been declared by any author in regards to this manuscript.
References
- 1.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 2.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 3.Kauvar DS, Wade CE. The epidemiology and modern management of traumatic hemorrhage: US and international perspectives. Crit Care. 2005;9(Suppl 5):S1–S9. doi: 10.1186/cc3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perkins JG, Cap AP, Spinella PC, Blackbourne LH, Grathwohl KW, Repine TB, et al. An evaluation of the impact of apheresis platelets used in the setting of massively transfused trauma patients. J Trauma. 2009;66:S77–S84. doi: 10.1097/TA.0b013e31819d8936. discussion S-5. [DOI] [PubMed] [Google Scholar]
- 5.Demetriades D, Murray J, Charalambides K, Alo K, Velmahos G, Rhee P, et al. Trauma fatalities: time and location of hospital deaths. J Am Coll Surg. 2004;198:20–26. doi: 10.1016/j.jamcollsurg.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Gruen RL, Jurkovich GJ, McIntyre LK, Foy HM, Maier RV. Patterns of errors contributing to trauma mortality: lessons learned from 2,594 deaths. Ann Surg. 2006;244:371–380. doi: 10.1097/01.sla.0000234655.83517.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, et al. Causes of death in U.S Special Operations Forces in the global war on terrorism: 2001–2004. Ann Surg. 2007;245:986–991. doi: 10.1097/01.sla.0000259433.03754.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoyt DB, Bulger EM, Knudson MM, Morris J, Ierardi R, Sugerman HJ, et al. Death in the operating room: an analysis of a multi-center experience. J Trauma. 1994;37:426–432. [PubMed] [Google Scholar]
- 9.Kelly JF, Ritenour AE, McLaughlin DF, Bagg KA, Apodaca AN, Mallak CT, et al. Injury severity and causes of death from Operation Iraqi Freedom and Operation Enduring Freedom: 2003–2004 versus 2006. J Trauma. 2008;64:S21–S26. doi: 10.1097/TA.0b013e318160b9fb. discussion S6–7. [DOI] [PubMed] [Google Scholar]
- 10.Teixeira PG, Inaba K, Hadjizacharia P, Brown C, Salim A, Rhee P, et al. Preventable or potentially preventable mortality at a mature trauma center. J Trauma. 2007;63:1338–1346. doi: 10.1097/TA.0b013e31815078ae. discussion 46-7. [DOI] [PubMed] [Google Scholar]
- 11.Tien HC, Spencer F, Tremblay LN, Rizoli SB, Brenneman FD. Preventable deaths from hemorrhage at a level I Canadian trauma center. J Trauma. 2007;62:142–146. doi: 10.1097/01.ta.0000251558.38388.47. [DOI] [PubMed] [Google Scholar]
- 12.Simmons JW, White CE, Eastridge BJ, Mace JE, Wade CE, Blackbourne LH. Impact of policy change on US Army combat transfusion practices. J Trauma. 2010;69(Suppl 1):S75–S80. doi: 10.1097/TA.0b013e3181e44952. [DOI] [PubMed] [Google Scholar]
- 13.Cotton BA, Gunter OL, Isbell J, Au BK, Robertson AM, Morris JA, Jr, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177–1182. doi: 10.1097/TA.0b013e31816c5c80. discussion 82-3. [DOI] [PubMed] [Google Scholar]
- 14.Dente CJ, Shaz BH, Nicholas JM, Harris RS, Wyrzykowski AD, Patel S, et al. Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. J Trauma. 2009;66:1616–1624. doi: 10.1097/TA.0b013e3181a59ad5. [DOI] [PubMed] [Google Scholar]
- 15.Duchesne JC, Hunt JP, Wahl G, Marr AB, Wang YZ, Weintraub SE, et al. Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma. 2008;;65:272–276. doi: 10.1097/TA.0b013e31817e5166. discussion 6–8. [DOI] [PubMed] [Google Scholar]
- 16.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–270. doi: 10.1097/TA.0b013e31817de3e1. discussion 70-1. [DOI] [PubMed] [Google Scholar]
- 17.Moore FA, Nelson T, McKinley BA, Moore EE, Nathens AB, Rhee P, et al. Is there a role for aggressive use of fresh frozen plasma in massive transfusion of civilian trauma patients? Am J Surg. 2008;196:948–958. doi: 10.1016/j.amjsurg.2008.07.043. discussion 58–60. [DOI] [PubMed] [Google Scholar]
- 18.Sperry JL, Ochoa JB, Gunn SR, Alarcon LH, Minei JP, Cuschieri J, et al. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65:986–993. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 19.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 20.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA surgery. 2013;148:127–136. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josephson CD, Glynn SA, Kleinman SH, Blajchman MA. A multidisciplinary “think tank”: the top 10 clinical trial opportunities in transfusion medicine from the National Heart, Lung, and Blood Institute-sponsored 2009 state-of-the-science symposium. Transfusion. 2011;51:828–841. doi: 10.1111/j.1537-2995.2010.02898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NHLBI. Trans-Agency Coagulopathy in Trauma Workshop. https://www.nhlbi.nih.gov/meetings/workshops/tactrauma.htm)
- 23.Cotton BA, Dossett LA, Haut ER, Shafi S, Nunez TC, Au BK, et al. Multicenter validation of a simplified score to predict massive transfusion in trauma. J Trauma. 2010;;69(Suppl 1):S33–S39. doi: 10.1097/TA.0b013e3181e42411. [DOI] [PubMed] [Google Scholar]
- 24.Holcomb JB, Weiskopf R, Champion H, Gould SA, Sauer RM, Brasel K, et al. Challenges to effective research in acute trauma resuscitation: consent and endpoints. Shock. 2011;35:107–113. doi: 10.1097/SHK.0b013e3181f7fd01. [DOI] [PubMed] [Google Scholar]
- 25.Bulger EM, Schmidt TA, Cook AJ, Brasel KJ, Griffiths DE, Kudenchuk PJ, et al. The random dialing survey as a tool for community consultation for research involving the emergency medicine exception from informed consent. Ann Emerg Med. 2009;53:341–350. doi: 10.1016/j.annemergmed.2008.07.021. 50 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.FDA. Exception from Informed Consent Requirments for Emergency Reseach. http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM249673.pdf.
- 27.Nascimento B, Rizoli S, Rubenfeld G, Lin Y, Callum J, Tien HC. Design and preliminary results of a pilot randomized controlled trial on a 1:1:1 transfusion strategy: the trauma formula-driven versus laboratory-guided study. J Trauma. 2011;71:S418–S426. doi: 10.1097/TA.0b013e318232e591. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien PC. The appropriateness of analysis of variance and multiple-comparison procedures. Biometrics. 1983;39:787–794. [PubMed] [Google Scholar]
- 29.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 30.Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT, Jr, Cushman WC, et al. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) ALLHAT Research Group. American journal of hypertension. 1996;9:342–360. doi: 10.1016/0895-7061(96)00037-4. [DOI] [PubMed] [Google Scholar]
- 31.FDA. Adaptive Design Clinical Trials for Drugs and Biologics. http://www.fda.gov/downloads/Drugs/.../Guidances/ucm201790.pdf.
- 32.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 33.Lan KK, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 34.Proschan MA, Lan KK, Wittes JT. Statistical Monitoring of Clinical Trials: A Unified Approach. New York, New York: Springer; 2006. [Google Scholar]
- 35.Tilley BC, Lyden PD, Brott TG, Lu M, Levine SR, Welch KM. Total quality improvement method for reduction of delays between emergency department admission and treatment of acute ischemic stroke The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Arch Neurol. 1997;54:1466–1474. doi: 10.1001/archneur.1997.00550240020008. [DOI] [PubMed] [Google Scholar]
- 36.Bulger EM, May S, Kerby JD, Emerson S, Stiell IG, Schreiber MA, et al. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Ann Surg. 2011;253:431–441. doi: 10.1097/SLA.0b013e3181fcdb22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hauser CJ, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, et al. Results of the CONTROL trial: efficacy and safety of recombinant activated Factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69:489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- 38.Moore EE, Moore FA, Fabian TC, Bernard AC, Fulda GJ, Hoyt DB, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 2009;208:1–13. doi: 10.1016/j.jamcollsurg.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 39.Chen JY, Scerbo M, Kramer G. A review of blood substitutes: examining the history, clinical trial results, and ethics of hemoglobin-based oxygen carriers. Clinics (Sao Paulo) 2009;64:803–813. doi: 10.1590/S1807-59322009000800016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nascimento B, Callum J, Tien H, Rubenfeld G, Pinto R, Lin Y, et al. Effect of a fixed-ratio (1:1:1) transfusion protocol versus laboratory-results-guided transfusion in patients with severe trauma: a randomized feasibility trial. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2013;185:E583–E589. doi: 10.1503/cmaj.121986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho AM, Dion PW, Yeung JH, Holcomb JB, Critchley LA, Ng CS, et al. Prevalence of survivor bias in observational studies on fresh frozen plasma:erythrocyte ratios in trauma requiring massive transfusion. Anesthesiology. 2012;116:716–728. doi: 10.1097/ALN.0b013e318245c47b. [DOI] [PubMed] [Google Scholar]