Abstract
During an infection, T cells can differentiate into multiple types of effector and memory T cells, which help to mediate pathogen clearance and provide long-term protective immunity. These cells can vary in their phenotype, function and location, and in their long-term fate in terms of their ability to populate the memory T cell pool. Over the past decade, the signalling pathways and transcriptional programmes that regulate the formation of heterogeneous populations of effector and memory CD8+ T cells have started to be characterized, and this Review discusses the major advances in these areas.
A T cell response to an acute infection can be characterized by three distinguishable phases: clonal expansion, contraction of the T cell population and memory formation (FIG. 1). As the antigen-specific T cells clonally expand, they differentiate into effector cells, many of which enter the blood and migrate to sites of infection. Infections with viruses or intracellular bacteria induce type 1 responses, which are the focus of this Review. These responses promote the differentiation of CD8+ T cells into cytotoxic T lymphocytes (CTLs) that kill infected cells (through granzymes and perforin) and secrete cytokines such as interferon-γ (IFNγ) and tumour necrosis factor (TNF). CD4+ T cells concurrently differentiate into T helper 1 (TH1) cells, which also produce IFNγ, TNF and interleukin-2 (IL-2), to coordinate the antiviral immune response and mediate direct killing of virus-infected immune cells. In addition, CD4+ T follicular helper cells (TFH cells) form and initiate B cell germinal centre responses to generate high-affinity neutralizing antibodies. Although these pools of effector CD4+ and CD8+ T cells formed during type 1 responses are dominated by particular effector traits (such as IFNγ production and cytotoxicity), closer inspection shows that these cells are not uniform and can be separated into subsets based on differences in gene and protein expression, additional effector functions, migratory patterns, proliferative capacity and long-term fate1–3.
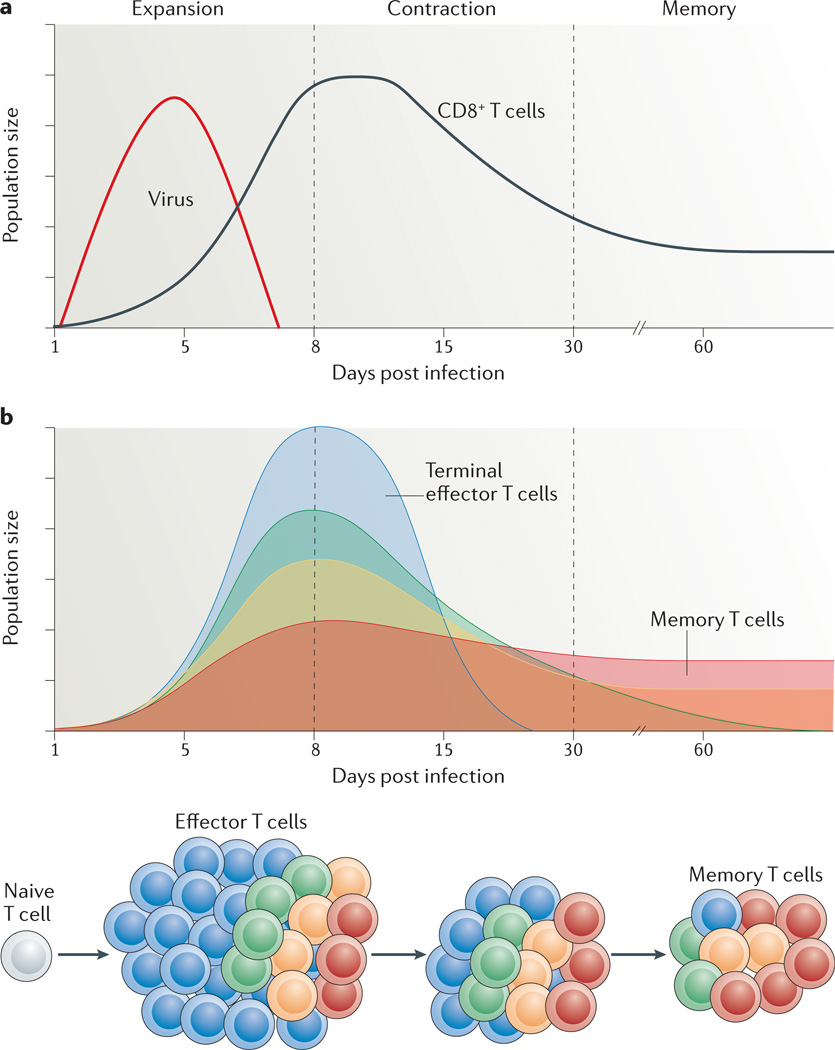
Figure 1. Kinetics of a T cell response and distribution of memory cell potential.
a | During an acute viral infection, antigen-specific T cells rapidly proliferate (during the expansion phase) and differentiate into cytotoxic T lymphocytes (CTLs) that mediate viral clearance. Most of these cells die over the next several weeks during the contraction phase of the response. Only a small percentage of effector T cells (5–10%) survive and further develop into functional mature memory CD8+ T cells. b | The pool of effector T cells can be separated into multiple diverse subsets based on differences in gene and protein expression, effector functions, migratory patterns, proliferative capacity and long-term fate. Ultimately, not all effector T cells have equal potential to form memory T cells. Some cell-surface markers correlate with distinct effector and memory T cell fates: terminal effector T cells (shown in blue) are KLRG1hiIL-7RαlowCD27lowBCL-2low, and long-lived memory (and memory precursor) cells (shown in red) are KLRG1lowIL-7RαhiCD27hiBCL-2hi. However, other T cell subsets with intermediate differentiation states also exist that have mixed phenotypes, longevities and abilities to self-renew, as depicted by the yellow and green populations. Over time, there may also be some interconversion between these subsets.
Following the elimination of the infecting pathogen, effector CD8+ T cells undergo a precipitous contraction phase wherein the majority of pathogen-specific effector CD8+ T cells die by apoptosis, but typically a small percentage (~5–10%) survive to further mature into memory CD8+ T cells. This process of selecting out the memory T cell pool is not entirely random, as originally proposed4, because memory cell potential is not inherited equivalently by all effector cells (in other words, they are not equipotent). Rather, some CD8+ T cells are intrinsically better able than others to persist and populate the memory CD8+ T cell pool (FIG. 1). In certain well-characterized model systems of infection, such as with lymphocytic choriomeningitis virus (LCMV) or Listeria monocytogenes, a small subset of effector T cells that is enriched for memory precursor cells has been distinguished based on the increased expression of IL-7 receptor subunit-α (IL-7Rα), CD27 and B cell lym-phoma 2 (BCL-2), and decreased expression of killer cell lectin-like receptor G1 (KLRG1)5–9. These pheno-typic distinctions are not exclusive criteria for forming memory T cells nor do they represent universal markers for memory precursor cells across all types of immune response, because death is also observed in the IL-7Rαhi effector T cell compartment following infection and the frequency of KLRG1hiIL-7Rαlow cells can vary widely across different types of infection and vaccina-tion6,10,11. Moreover, many long-lived KLRG1hiIL-7Rαhi memory CD8+ T cells are observed following secondary infections12–14, and these cells are discussed below. Importantly, however, these markers do offer a means of determining the relative memory cell potential and lifespan of effector CD8+ T cells in several circumstances, in particular during primary infections, and they have become valuable for identifying pathways that regulate these crucial cell fate decisions.
After an acute infection, memory CD8+ T cells are maintained in an antigen-independent, cytokine-dependent manner mainly through the actions of IL-7 and IL-15, which promote memory CD8+ T cell survival and self-renewal (through homeostatic (basal) prolifera-tion)15. A distinguishing feature of memory CD8+ T cells is their ability to rapidly generate effector functions and to produce a ‘burst’ of secondary CTLs that can rapidly contain a secondary infection. However, it is now evident that these properties can vary according to the pheno-type, function and location of the memory CD8+ T cells (as recently reviewed in REF. 16) (BOX 1). For example, central memory T cells (TCM cells) residing in secondary lymphoid organs have greater proliferative potential than effector memory T cells (TEM cells), but TEM cells (in contrast to TCM cells) constitutively display certain effector functions (such as cytotoxicity)17. Interestingly, repetitive reactivation of memory CD8+ T cells through vaccine boosters or successive infections cumulatively augments the effector-like properties of memory CD8+ T cells and the frequency of TEM cells in the resulting memory T cell pool13,14,16. Given that there have been major mechanistic advances in our understanding of the formation of diverse types of effector and memory CD8+ T cells over the past few years, in this Review we discuss how several factors — including antigens, cytokines and other environmental cues — influence CD8+ T cell transcription, metabolism and differentiation during acute infection. We comment only briefly on the effects of chronic viral infection on CD8+ T cell function and differentiation (BOX 2), as this topic has been covered recently in other excellent reviews18,19.
Memory T cell subsets.
Conventionally, two broad subsets of memory T cell — central memory T (T CM) cells and effector memory T (T EM) cells — have been the best characterized. Early studies defined these subsets based mainly on their phenotypic markers, anatomical locations and functions. That is, TCM cells are mainly CD62LhiCCR7hi and home to secondary lymphoid organs and bone marrow. TEM cells are defined based on a CD62LlowCCR7low phenotype and are most commonly found in non-lymphoid tissues. Functionally, there are some notable differences: TCM cells tend to mount more robust recall responses and produce interleukin-2 (IL-2), whereas CD4+ TEM cells are immediate producers of cytokines such as interferon-γ and tumour necrosis factor and CD8+ TEM cells are immediate producers of cytotoxic proteins16,17,122–125. Both TCM and TEM cell populations are thought to continuously circulate through blood vessels, and there is evidence that they might interconvert as they pass through lymphoid and non-lymphoid tissues16,124–129.
Emerging evidence indicates that other memory T cells reside long-term in the brain and mucosal tissues (such as the lungs, gut and skin) and show only limited levels of egress and recirculation (in particular, this is the case for CD8+ memory T cells in the skin109,114–118). Such memory T cells have been referred to as tissue-resident memory T (TRM) cells125,130–134. These cells have a characteristic CD103hiCD69hiCD27low phenotype and, in certain cases, they also express high levels of granzyme B106,111–115.
It is likely that the generation of diverse memory T cell subsets ensures optimal protective immunity through the division of labour. Following secondary infection, TEM and TRM cells normally confer immediate effector functions and first-line defence at the portal of pathogen entry, whereas the recall response of TCM cells generates a larger number of secondary effector cells more rapidly than during a primary response to control pathogens that breach the initial containment. For example, TRM cells formed in the lungs or skin confer better protection than other memory T cell subsets in response to secondary influenza or vaccinia virus infections, whereas TCM cells are better at restraining chronic systemic lymphocytic choriomeningitis virus (LCMV) infections13,127,130,132.
| Cell type | Phenotype | Location | Functional properties |
|---|---|---|---|
| TCM cell | CD62LhiCCR7hi | Lymph nodes, spleen, blood |
↑ Proliferative potential ↑IL-2 production ↑ Migration ↓Effector functions and cytotoxicity |
| TEM cell | CD62LlowCCR7low | Spleen, blood, liver | ↓- Proliferative potential ↓IL-2 production ↑ Migration ↑Effector functions and cytotoxicity |
| TRM cell | CD103hiCD69hiCD62LlowCD27low; expression of tissue-specific chemokine receptors and integrins* |
Skin, lung, gut, brain | ↓- Proliferative potential ↓IL-2 production ↓-Migration ↑Effector functions and cytotoxicity |
Following localized infection, tissue-specific chemokine and adhesion molecule signals direct the migration of effector and memory T cells to the sites of infection. For example, CC-chemokine receptor 9 (CCR9) and integrin α4β7 are required for homing to the gut, whereas CCR4, CCR10 and cutaneous lymphocyte antigen (CLA) are required for homing to the skin.
T cell exhaustion.
During chronic infections — for example with HIV, hepatitis B virus and hepatitis C virus in humans and with lymphocytic choriomeningitis virus (LCMV) clone 13 in mice — the persistent encounter with antigen alters the function and gene expression of virus-specific CD8+ T cells18,19,124. Such changes have been referred to as T cell ‘exhaustion’ because the antigen-specific T cells display a hierarchical loss of interleukin-2 (IL-2), tumour necrosis factor and interferon-γ production, impaired proliferation and, to some degree, decreased cytotoxic activity18,19,124. The maintenance of antigen-specific T cells during chronic viral infection seems to be more dependent on the presence of the antigen and less dependent on IL-7 and IL-15, in stark contrast to the maintenance of the functional memory CD8+ T cells that form after acute infection or in response to most vaccines135. These functional changes have been associated with increased expression of inhibitory receptors such as programmed cell death protein 1 (PD1), lymphocyte activation gene 3 (LAG3), CD244 and CD160 (REF. 18).
Gene expression profiling studies have revealed that exhausted T cells develop a transcriptional state distinct from that of functional effector and memory CD8+ T cells. A few transcription factors have been found to be functionally important in this process, including B lymphocyte-induced maturation protein 1 (BLIMP1), T-bet, eomesodermin and basic leucine zipper transcriptional factor ATF-like (BATF)18,73,136,137. BLIMP1 expression in virus-specific CD8+ T cells is increased during chronic LCMV infection compared with acute infection and promotes the expression of the inhibitory receptors mentioned above, which suppress the function and proliferation of cytotoxic T lymphocytes (CTLs) 73. However, deletion of Blimp1 does not rescue CTL ‘exhaustion’, owing to the fact that BLIMP1 also enhances granzyme B expression and CTL function73. Interestingly, rendering virus-specific CD8+ T cells haploinsufficient for BLIMP1 provided a healthier balance between effector cell function and exhaustion, and improved viral control73,138. Similarly to its role in driving CTL differentiation during acute infections, T-bet sustains CTLs during persistent infection. In fact, T-bet expression decays over time in antiviral CD8+ T cells as the degree of dysfunction and PD1 expression increase during chronic viral infection137. T-bet directly binds to and represses the Pd1 gene, and consequently T-bet deficiency leads to increased PD1 expression and decreased CD8+ T cell function and survival137. Eomesodermin is also necessary for the persistence of virus-specific CD8+ T cells, but in contrast to T-bet it seems to promote the generation of PD1hi (perhaps terminally exhausted) CD8+ T cells (E. John Wherry, personal communication). BATF is another transcription factor that is upregulated in exhausted CD8+ T cells; it is induced by PD1 activation (in response to the engagement of PD1 ligand 1) and impairs CD8+ T cell proliferation and cytokine production138. These data underscore the versatility of transcription factors in regulating multiple differentiation and functional states in CD8+ T cells. Understanding how their activity, binding partners and regulation of target gene expression are adjusted in each situation will be crucial for a thorough understanding of CD8+ T cell differentiation.
Models of T cell diversification
Several potential mechanisms have been put forward to explain how heterogeneous pools of effector and memory CD8+ T cells arise during infection (FIG. 2). This raises the more general question of how effector CD8+ T cell differentiation is balanced to enable the formation of cells with various phenotypes, functions and short- or long-term fates.
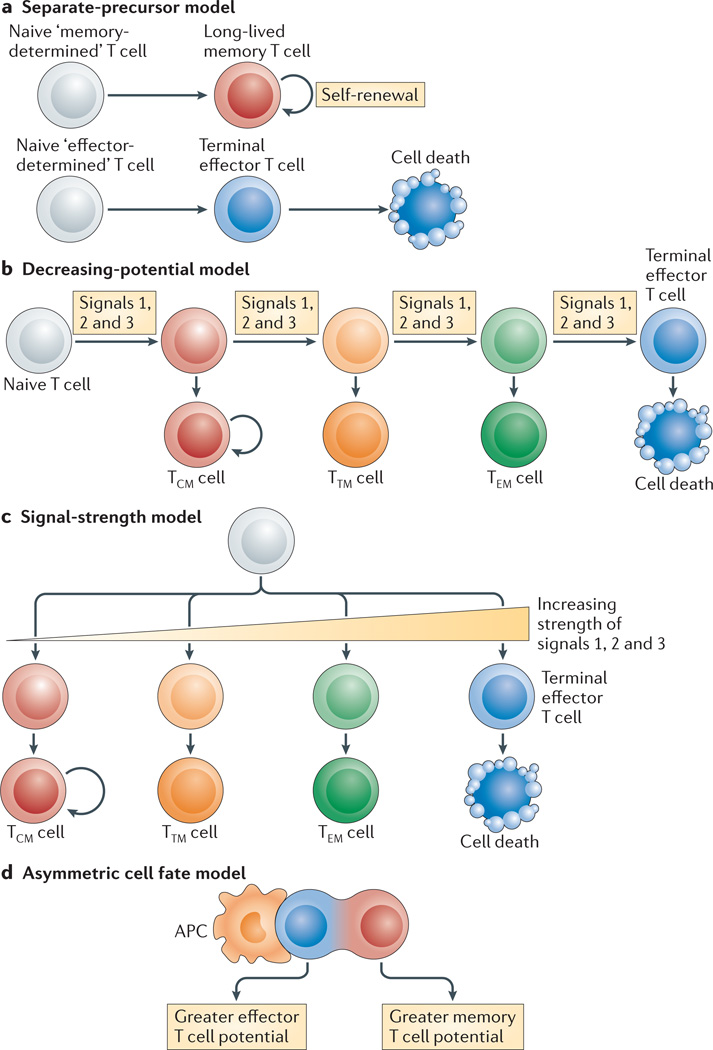
Figure 2. Models for generating effector and memory T cell heterogeneity.
a | In the separate-precursor model, naive T cells are ‘pre-programmed’ to adopt certain differentiation states following activation based on information received during thymic development. However, currently, little evidence exists to support this model. b | The decreasing-potential model suggests that effector T cells adopt various differentiation states according to the cumulative history of signals that they encounter during infection. Repetitive stimulation with antigen and other signals, such as interleukin-2 (IL-2) and IL-12, drives greater effector cell proliferation and terminal differentiation. As the cells acquire terminally differentiated states they remain functional and cytolytic but lose memory cell properties, such as enhanced longevity and proliferative potential. c | In the signal-strength model, the formation of heterogeneous effector cell populations is dependent on the overall ‘strength’ of the signals (signals 1, 2 and 3 denote antigen, co-stimulatory molecules and cytokines, respectively) that are encountered early during T cell priming. In combination, a strong signal may drive greater clonal expansion and be important for selecting out T cells that are competent to form memory cells, but when delivered in excess these signals may also cause terminal effector T cell differentiation. d | The asymmetric cell fate model suggests that effector and memory T cell fates can arise from a single precursor T cell through asymmetric cell division that occurs as early as the first cell division after antigen stimulation. Evidence suggests that the proximal daughter cell (which is closer to the antigen-presenting cell (APC)) adopts an effector cell fate, whereas the distal daughter cell (which is further from the APC) adopts a memory cell fate. TCM, central memory T; TEM, effector memory T; TTM, transitional memory T.
Separate-precursor model
It is unlikely that naive T cells are ‘pre-programmed’ during thymic development to adopt certain differentiation states following activation (FIG. 2a), because elegant studies using adoptive transfer of single CD8+ T cells or cellular barcoding have demonstrated that a single naive T cell is multipotent and can give rise to both effector and memory T cells, including both TCM and TEM cells20,21. However, it is important to note that these studies were carried out with T cell receptor (TCR)-transgenic CD8+ T cells and, therefore, potential effects of different TCR signalling strengths have not been thoroughly examined.
Decreasing-potential model
One idea is that repetitive stimulation of T cells with antigens and pro-inflammatory cytokines drives greater proliferation and terminal effector cell differentiation (FIG. 2b). As the T cells acquire terminally differentiated states they retain their effector functions and cytolytic capacity, but lose memory cell properties, such as enhanced longevity, proliferative potential and IL-7Rα expression. Such a linear progression model, often referred to as the decreasing-potential model, provides a mechanism for creating a spectrum of effector T cells at various differentiation states according to the cumulative history of signals that were encountered during infection4. Supporting this model are studies showing that truncating the duration of antigen exposure and decreasing inflammation through antibiotic treatment or dendritic cell immunization accelerates memory T cell formation, and that latecomers in a T cell response (when antigen availability is decreased) preferentially acquire TCM cell properties6,8,22–24.
Signal-strength model
Another model also allows for the formation of heterogeneous effector cell populations in a manner dependent on the overall ‘strength’ of the signals delivered by the antigen (signal 1), co-stimulation (signal 2) and pro-inflammatory cytokines (signal 3) encountered early during T cell priming25 (FIG. 2c). In combination, a strong signal can drive greater clonal expansion and be important for selecting out T cells that are competent to form memory cells, but when delivered in excess, a strong signal can also cause terminal effector T cell differentiation. This model differs from the decreasing-potential hypothesis in that different cell fates can be specified early during the response, in a more divergent manner, according to the intensity of the signals received, rather than in a linear, stepwise manner driven by successive rounds of stimulation.
Asymmetric cell fate model
The signal-strength model is also conceptually similar to the asymmetric cell fate model, which supports the notion that memory and effector T cell fates can arise from a single precursor T cell through asymmetric cell division that occurs as the activated T cells clonally expand and might even begin as early as the first cell division26 (FIG. 2d). Evidence indicates that the daughter cell that inherits the immunological synapse receives stronger TCR and co-stimulatory signals — in addition to signals mediated by pro-inflammatory cytokines, such as IL-12 and IFNγ — owing to its proximity to the antigen-presenting cell (APC). When integrated within the decreasing-potential model, the unequal inheritance of ‘effector cell differentiation factors’ (in the form of TCR and cytokine signalling) through asymmetric cell division following repetitive encounters with antigen-bearing APCs enables some activated T cells to collectively amass a stronger set of differentiation signals, driving them towards terminal differentiation. In addition, this process simultaneously preserves a population of less-differentiated cells that have greater memory cell potential and stem cell-like qualities.
The above models are not mutually exclusive and, ultimately, they achieve the same net result — the formation of effector T cells with a spectrum of differentiation states. At one end of the spectrum are effector T cells with greater memory cell potential and longevity, and at the other end are terminal effector T cells. In between these two extremes are effector T cells with intermediate differentiation states (FIG. 3). Such a ‘gradient model’ provides plasticity within an effector T cell lineage to allow cells to transit between differentiation states according to the net signal input. This flexibility might be important for keeping the T cell response in sync with the infection, which can vary in burden, duration and tropism.
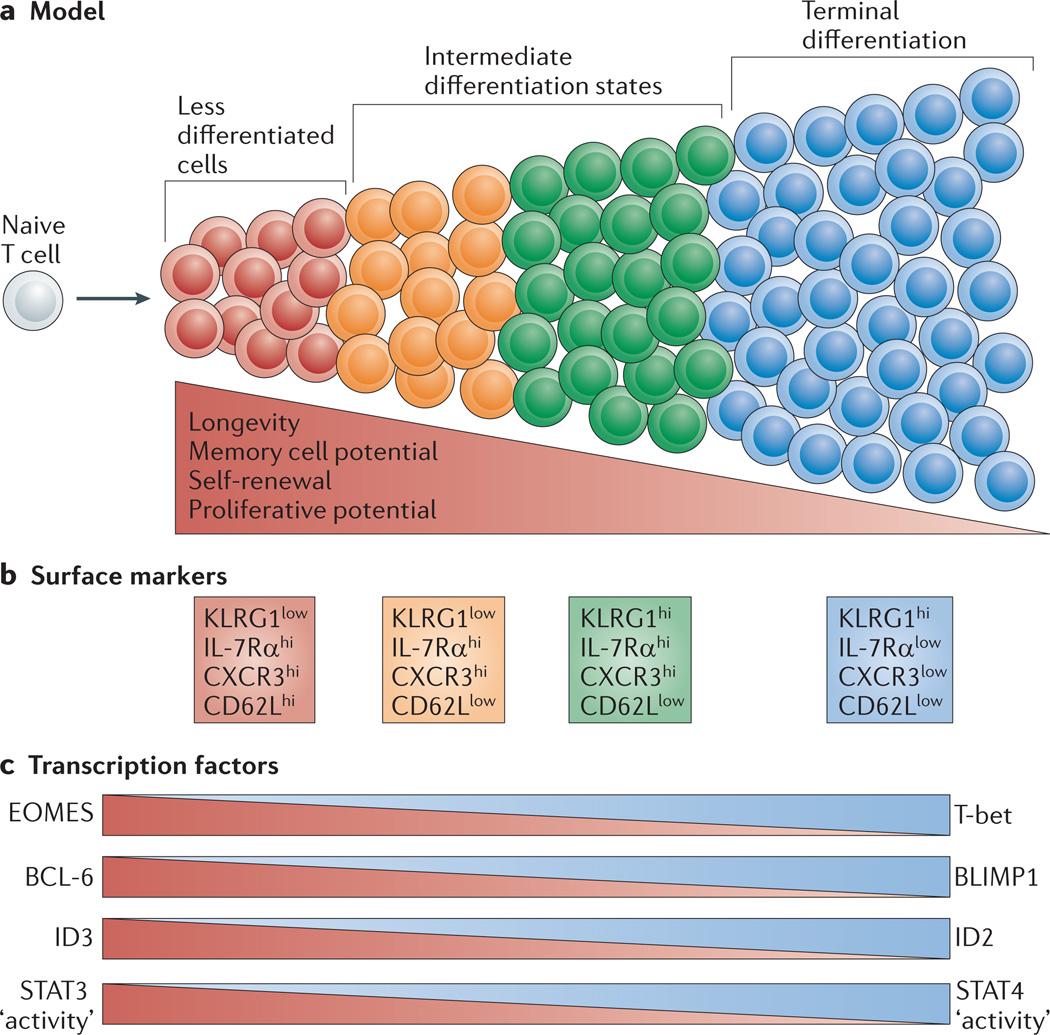
Figure 3. Graded activity of transcriptional programmes that control effector and memory T cell differentiation.
a | This model postulates that, in response to different levels of signal input, the differentiation of antigen-specific effector CD8+ T cells occurs along a continuum. Cells that have greater memory cell potential, longevity and proliferative potential are at one end of the spectrum, and terminally differentiated effector T cells are present at the other end of the spectrum. In between these two extreme end points are effector T cells that exist in intermediate differentiation states. b | The heterogeneous differentiation states of effector CD8+ T cells can be distinguished by the expression of several surface markers, such as killer cell lectin-like receptor G1 (KLRG1), IL-7 receptor subunit-α (IL-7Rα), CD27, CXC-chemokine receptor 3 (CXCR3) and CD62L. c | The transcriptional programmes that control terminal effector cell differentiation and memory cell potential seem to be based on the graded expression or activity of certain competing sets of transcription factors. As the relative expression or activity of T-bet, B lymphocyte-induced maturation protein 1 (BLIMP1), inhibitor of DNA binding 2 (ID2) and signal transducer and activator of transcription 4 (STAT4) increases and surpasses a given threshold during infection, effector CD8+ T cells acquire more terminally differentiated phenotypes that are associated with a reduction in proliferative capacity and longevity. These factors counter-regulate, and can also interact with, an opposing set of transcriptional regulators, including eomesodermin (EOMES), B cell lymphoma 6 (BCL-6), ID3 and STAT3, that prevent terminal differentiation of effector T cells and help to maintain memory cell properties. Note that to some extent this figure is a conceptual diagram, as the exact amounts of the different transcription factors or their activities have not been accurately measured in all cases.
Signal strength influences effector T cell fate
The CD8+ T cell response to infection conceptually has two primary goals. The first is an immediate goal of generating large numbers of CTLs to help to eliminate the present infection. The second is a long-term goal of retaining a cellular subset with enhanced longevity and regenerative capacity to protect against future encounters with the same pathogen. What are the signals and transcriptional programmes that control effector CD8+ T cell differentiation to successfully achieve both of these outcomes
The three major classes of signals that modulate T cell proliferation and effector and memory CD8+ T cell differentiation are delivered by antigens (signal 1), co-stimulation (signal 2) and inflammation (signal 3). During infections, crucial co-stimulatory receptors for CD8+ T cells (and their interacting ligands, which are written in parentheses) include 4-1BB (4-1BBL) and CD27 (CD70), but CD28 (CD80 and CD86), CD40 (CD40L) and OX40 (OX40L) are also important in certain infectious settings27–32. The main pro-inflammatory cytokines produced during viral and other intracellular infections are type I IFNs, IFNγ, IL-2, IL-12, IL-27 and IL-33, which enhance the proliferation, differentiation and survival of CTLs11,22,23,33–41. Potential mechanisms by which these cytokines promote CTL survival include increasing the expression of cytokine and co-stimulatory receptors (such as CD25, OX40 and 4-1BB), of cysteine and serine protease inhibitors (such as the granzyme B inhibitor SPI6 (encoded by Serpinb9)) and of BCL-3, an NF-κB inhibitor (IκB) family member that is thought to limit the transcription of nuclear factor-κB (NF-κB)-dependent genes42.
Signals 1, 2 and 3 are closely linked and inseparable in vivo during infection, and the duration or relative amount of these signals can affect the number, pheno-type, function and long-term fate of effector CD8+ T cells. For example, the extent of CD8+ T cell clonal expansion is typically in direct proportion to antigen abundance and/or affinity43–45. Furthermore, clonal expansion can be decreased by reducing the overall exposure to antigens and inflammation by shortening the duration of the infection, by recruiting naive T cells at the tail-end of the infection or by increasing the precursor frequency of antigen-specific naive CD8+ T cells (which increases intraclonal competition for cognate antigen and cytokines). However, these interventions can also hasten the differentiation of memory precursor cells and/ or TCM cells6,8,22,24,46–49. In addition, experimentally increasing the amount of inflammation, when the levels of antigen presentation are held relatively constant, augments the ratio of terminally differentiated or shorter-lived CTLs to memory precursor cells6,22,47. Similarly, deficiency of IFNγ or IL-12 increases the proportion of IL-7Rαhi memory precursor cells and CD8+ TCM cells following L. monocytogenes infection23,47,50,51, and a similar effect is observed during infection with LCMV or vesicular stomatitis virus (VSV) when CD8+ T cells have impaired IFNα/β receptor or IL-27 receptor signalling, respectively11,52. Together, these results indicate that the clonal expansion and differentiation of effector CD8+ T cells are tightly coordinated with the environment during an immune response. As the intensity of inflammation and T cell signalling (through the TCR and co-stimulatory receptors) increases, more CTLs and memory T cells can be generated (up to a point), but in some cases this decreases memory cell formation because it can also cause more of the activated T cells to adopt terminally differentiated states.
In physiological situations, how then does a CD8+ T cell sense the level or duration of signals 1, 2 and 3 in vivo to adopt diverse differentiation states during an infection? Certainly, an activated T cell’s microenviron-ment and the cell types that it interacts with are key — a point that is nicely demonstrated by recent data showing that the early migratory patterns of activated T cells influence their commitment to particular cell fates. In the case of effector CD8+ T cells, CXC-chemokine receptor 3 (CXCR3)-dependent trafficking to virus-infected cells enhances terminal differentiation, probably owing to increased exposure to antigens and type I IFNs53–55.
In the case of CD4+ T cells, CXCR5-dependent trafficking to B cell follicles enforces TFH cell development owing to interactions with antigen-presenting B cells. Supporting these data, our laboratory found that effector and memory T cell subsets are differentially localized to distinct splenic compartments during LCMV infection. The KLRG1lowIL-7Rαhi memory precursor cells were found in both the red pulp and T cell zones, whereas KLRG1hiIL-7Rαlow terminal effector CTLs were found exclusively in the red pulp56. In addition, it is interesting to speculate how exposure to anti-inflammatory signals or molecules that suppress TCR activation might offset pro-effector signals that drive terminal effector T cell differentiation and instead promote memory cell potential. For example, increasing the number of regulatory T (TReg) cells present at the time of CD8+ T cell priming increases the frequency of IL-7Rαhi memory precursor cells that form following vaccination with modified vaccinia virus Ankara57. Moreover, in the absence of the regulatory cytokine IL-10, fewer IL-7Rαhi memory precursor cells and memory CD8+ T cells develop following an L. monocytogenes infection58 (W.C. and S.M.K., unpublished observations), and transforming growth factor-β (TGFβ) receptor deficiency increases the frequency of KLRG1hi terminal effector T cells. However, the effect of TGFβ might have more to do with its selective effect on the apoptosis of KLRG1hi cells than their differentiation59. Thus, it is likely that the balance of pro- and anti-inflammatory signals in the micro-environment of a CD8+ T cell influences the degree to which it differentiates.
Transcriptional regulation of T cell differentiation
The past decade represents the dawn of a molecular revolution in the field of effector and memory CD8+ T cell development as the transcriptional circuitry that underlies this process has started to be elucidated. Several transcription factors that regulate effector and memory CD8+ T cel l development have been identified. Interestingly, several of them function in pairs that form counter-regulatory axes to simultaneously produce effector T cells that provide both short- and long-term protection — in other words, to regulate effector and memory cell potential (FIG. 3).
T-bet and eomesodermin
T-bet and eomesodermin (EOMES), two T-box transcription factors, have crucial roles in the formation and function of effector and memory CD8+ T cells. In early activated CD8+ T cells, T-bet and EOMES cooperate through partially redundant activities to create CTLs by inducing the expression of IFNγ, granzyme B, perforin, CXCR3 and CXCR4 (REFS 6,38,60– 62). CD8+ T cells lacking both T-bet and EOMES lose CTL identity and abnormally differentiate into IL-17-producing CD8+ T cells that cause excessive neutro-phil infiltration and a lethal inflammatory syndrome during LCMV infection63. T-bet expression is induced initially by TCR signalling and amplified by IL-12-mediated signals and mammalian target of rapamycin (mTOR) activity in effector CD8+ T cells6,64,65. The expression of EOMES seems to be induced subsequently to that of T-bet in a RUNX3-dependent manner and can be amplified by IL-2, but repressed by IL-12 and mTOR, in part through inhibition of the transcriptional activator forkhead box O1 (FOXO1)38,64,66,67. In memory CD8+ T cells, T-bet and EOMES cooperate to sustain memory CD8+ T cell homeostasis through expression of IL-2Rβ (also known as CD122), which enables IL-15-mediated signalling and the homeostatic proliferation of memory cells6,38,61,62. CD8+ T cells lacking T-bet are impaired in forming KLRG1hiIL-7Rαlow terminal effector cells and, when overexpressed, T-bet is sufficient to induce the formation of these effector cells6. This finding supported a model whereby accumulating amounts of T-bet in effector CD8+ T cells could drive their terminal differ-entiation6. By contrast, EOMES-deficient effector CD8+ T cells efficiently generate KLRG1lowIL-7Rαhi memory precursor cells, but are unable to generate memory cells with normal expression of CD122, CD62L, CXCR3 and CXCR4, which are involved in IL-15-mediated signalling and homing to lymph nodes and bone marrow. Thus, populations of memory CD8+ T cells lacking EOMES contain fewer TCM cells and have impaired homeostatic turnover and long-term persistence62.
Interestingly, although T-bet and EOMES cooperate in many regards, their expression is somewhat reciprocal. For example, T-bet expression is highest in early effector CD8+ T cells, but progressively declines as memory cells form14. Conversely, EOMES is upregulated in early effector CD8+ T cells by IL-2, but in keeping with its role in memory T cell homeostasis its expression increases further during the effector to memory cell transition, possibly in response to WNT signalling and the transcription factor TCF1 (also known as TCF7; discussed further below)14,38,62,68. Thus, the ratio of T-bet to EOMES is highest at effector cell stages and lowest at memory cell stages. Together, these data indicate that the phenotype, function and long-term fate of effector CD8+ T cells are acutely sensitive to the relative ratio of T-bet and EOMES6,14,60,62. Currently, we do not have a thorough understanding of how this ratio is regulated, or how T-bet and EOMES might direct each other’s expression, but the balance of FOXO1 activity and exposure to pro-inflammatory cytokines (such as IL-12, type I IFNs and IL-2) and other factors (such as IL-4, IL-10, IL-21 and WNT signalling) during infection is certainly involved52,64,65,67,68.
BLIMP1 and BCL-6
B lymphoc yte-induce d maturation protein 1 (BLIMP1; also known as PRDM1) and BCL-6 are a pair of antagonistic transcription factors that function as genetic switches for cell fate decisions in B and T cells69. BLIMP1 is a transcriptional repres-sor that is robustly expressed by effector CD8+ T cells and is primarily induced by IL-2, IL-12 and IL-21 (REFS 37,38,70,71). Following pathogen clearance, the expression of BLIMP1 declines as memory CD8+ T cells mature, and it is expressed at the lowest levels in TCM cells. In effector CD8+ T cells, BLIMP1 is part of a tran-scriptional programme that increases the formation of KLRG1hiIL-7Rαlow terminal effector cells and enhances CTL functions such as migration to sites of inflammation and the expression of IFNγ and granzyme B72–74. As such, animals lacking BLIMP1 in CD8+ T cells are impaired in their ability to clear influenza virus owing to poor recruitment of virus-specific CD8+ T cells to the lungs74. BLIMP1 also suppresses certain aspects of the memory cell transcriptional programme, as in the absence of BLIMP1 greater numbers of CD62LhiIL-7Rαhi memory CD8+ T cells and their precursors form following LCMV infection72. However, over time, differences between the populations of wild-type and BLIMP1-deficient memory CD8+ T cells become marginal, probably owing to homeostatic pressure and the fact that BLIMP1 expression naturally declines as wild-type memory CD8+ T cells mature72. Characterizing the target genes and transcriptional cofactors with which BLIMP1 interacts will be important for understanding how effector and memory CD8+ T cell differentiation states are regulated.
BCL-6 is a well-characterized antagonist of BLIMP1 activity, and its expression is inversely correlated with that of BLIMP1 in effector and memory CD8+ T cells75. Our recent study showed that, during LCMV infection, IL-7Rαhi memory precursor T cells express slightly more BCL-6 than do KLRG1hiIL-7Rαlow effector cells, but as the virus-specific memory CD8+ T cells mature (and BLIMP1 expression decreases) BCL-6 progressively accumulates75. IL-10 and IL-21, signalling through signal transducer and activator of transcription 3 (STAT3), are likely candidates for sustaining or possibly even increasing BCL-6 expression in memory CD8+ T cells following acute infection75. BCL-6 is crucial for the formation of mature self-renewing TCM cells76,77. CD8+ T cells that overexpress BCL-6 generate increased numbers of memory cells and, in particular, TCM cells78. BCL-6 functions in association with various co-repressors (such as NCOR1, SMRT (also known as NCOR2) and BCOR), and recent data from CD4+ T cells have shown that BCL-6 and T-bet interact directly, which can have two consequences. First, the recruitment of BCL-6 to T-bet target genes can cause repression of these genes; and, second, this interaction with T-bet blocks the DNA-binding domain of BCL-6, thereby interfering with its ability to repress BCL-6 target genes79,80. These data suggest that the relative concentrations of BCL-6 and T-bet in a cell ultimately determine whether or not a complex is formed; it is probable that these factors only operate independently of one another when they surpass a certain expression threshold or ratio79. Incorporating into this model the respective reciprocal expression patterns of EOMES with T-bet and BCL-6 with BLIMP1, one can begin to envision more clearly the complexity of the transcription factor-based rheostats that regulate effector and memory cell transcriptomes in CD8+ T cells.
ID2 and ID3
Inhibitor of DNA binding 2 (ID2) and ID3 are another pair of transcription factors that have important roles in effector and memory CD8+ T cell development. They operate in part by inhibiting the DNA-binding activity of E protein transcription factors. Both ID2 and ID3 are expressed by effector CD8+ T cells, but their activities seem to be separated somewhat temporally. For example, ID2 supports the survival of effector CD8+ T cells early during the naive to effector cell transition, whereas ID3 supports their survival later during the effector to memory cell transition81–83. In addition to supporting clonal expansion, ID2 is important for the formation of terminal KLRG1hiIL-7Rαlow effector T cells83. ID3, by contrast, is required for the generation of long-lived memory CD8+ T cells, and its overex-pression sustains the survival of effector CD8+ T cells that normally die during the contraction phase of the response81,83. Moreover, within a few days of infection, increased ID3 expression can distinguish those effector CD8+ T cells that are starting to acquire a memory precursor cell genetic signature83. ID3 is a direct target of BLIMP1-mediated repression81, and therefore the ratio of ID2 to ID3 levels differs between effector cell subsets. The ID2 to ID3 ratio is higher in KLRG1hiIL-7Rαlow terminal effector T cells than in KLRG1lowIL-7Rαhi memory precursor T cells81–83. Interestingly, the pro-survival effects of ID2 and ID3 seem to be distinct. For example, ID2 mediates the expression of pro- and anti-apoptotic genes, including Bcl2, Serpinb9 and Bcl2l11 (which encodes BIM), whereas ID3 induces the expression of ‘caretakers’ of DNA replication and genome stability, including FOXM1, NEK2 and members of the minichro-mosome maintenance and kinesin complexes81,83. Thus, despite their common ability to bind E proteins, ID2 and ID3 function in a non-redundant manner to modulate the differentiation and survival of effector CD8+ T cells.
STAT3, STAT4 and STAT5
JAK–STAT signalling pathways are, arguably, the most central signalling pathways in effector T cell differentiation and in the survival and homeo-stasis of memory CD8+ T cells. There are seven members of the STAT family in mammals (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B and STAT6), which are mainly known for their function as transcription factors, although non-nuclear STAT functions have also been described84–86. Although most JAK–STAT-associated cytokine receptors function through a dominant STAT protein, it is clear that multiple STATs can be activated downstream of a single receptor. Therefore, it remains a challenge to understand how different pro-inflammatory cytokines — such as type I IFNs, IFNγ, IL-2, IL-12, IL-21 and IL-27, which enhance CTL proliferation and differentiation — regulate gene expression in effector CD8+ T cells in cooperative and distinct manners.
Various mechanisms, however, do exist to differentially modulate STAT activity in various subtypes of T cell. The most direct mechanism is to alter the expression of the cytokine receptors themselves to influence the range of cytokines that a T cell can sense. In CD4+ T cells, for example, inverse expression levels of the receptors for IL-12 and IFNγ versus IL-4 have a role in TH 1 versus TH2 cell specification. In CD8+ T cells, a higher level of expression of IL-2Rα is associated with effector CTLs that acquire increased expression of BLIMP1 and terminally differentiated states, which indicates that intense or prolonged IL-2-mediated signalling promotes proliferation and terminal CTL differentiation37,38,71. A higher level of expression of IL-2Rα is also observed in TH1 cell progenitors compared with TFH cell progenitors2,87.
Other mechanisms that modulate STAT activity in CTLs have been described. For example, STAT1 levels decline in effector CD8+ T cells as these cells proliferate during viral infection88. Given that type I IFNs activate both STAT1 and STAT4 in effector CD8+ T cells89, this suggests that type I IFN-mediated signalling qualitatively changes from a STAT1-dominated to a STAT4-dominated response over time. Moreover, increased levels of STAT4 activity — resulting from IL-12- and possibly type I IFN-mediated signalling — enhance T-bet expression and the terminal differentiation of KLRG1hiIL-7Rαlow T cells in L. monocytogenes infection6,90 (W.C. and S.M.K., unpublished observations). Lastly, a recent study elegantly showed that, in addition to dimerization, tetramerization of STAT5 A and STAT5B is crucial for the normal gene expression and function both of virus-specific CD8+ T cells during LCMV infection and of TReg cells91.
Suppressor of cytokine signalling (SOCS) proteins are an important set of inhibitors that refine the quality and quantity of JAK-STAT signalling in T cells92. The expression of SOCS5 by TH1 cells, SOCS3 by TH2 cells and SOCS1 by TH17 cells helps to insulate these cell subsets from cytokines that direct alternative CD4+ T cell fates92–94. Our recent work found an important role for STAT3 and its target gene Socs3 in memory CD8+ T cell development. In the absence of STAT3, LCMV-specific CD8+ T cells were unable to mature into a protective population of self-renewing memory cells but, instead, maintained a highly activated terminal effector cell phenotype. STAT3 was required to sustain the expression of SOCS3, BCL-6, EOMES, IL-7Rα and CD62L during the effector to memory cell transition, and the decreased amount of SOCS3 in STAT3-deficient CD8+ T cells caused them to become hyperresponsive to IL-12 (REF. 75). Another study found a similar role for STAT3 in human memory CD8+ T cells, as patients with hyper-IgE syndrome, who produce a dominant-negative form of STAT3, had decreased numbers of memory T cells and a paucity of TCM cells95. It is unclear whether the expression or function of the different STAT factors in T cells is as diametrically opposed as the other transcription factor pairs that we have described (that is, BLIMP1-BCL-6, T-bet-EOMES and ID2-ID3), but evidence suggests that such a reciprocal interaction might occur to a certain degree. For example, in CD8+ T cells, STAT4 and STAT3 largely promote distinct cell fates (terminal effector versus memory cell fates) and, in CD4+ T cells, STAT3 and STAT5 compete with one another for binding to the Il17 locus, thereby influencing TH17 versus TReg cell fates6,65,75,96.
Collectively, these findings support a contemporary model wherein the transcriptional programmes that control effector CD8+ T cell differentiation and memory cell potential are based on the graded expression of competing sets of transcription factors. As the expression or activity of T-bet, BLIMP1, ID2 and STAT4 increases and surpasses a given threshold during infection, effector CD8+ T cells acquire more terminally differentiated phenotypes that are associated with reductions in proliferative capacity and longevity. These factors counter-regulate, and may also interact with, an opposing set of transcriptional regulators, including EOMES, BCL-6, ID3, TCF1 and STAT3, that prevent terminal differentiation and/or help to maintain memory cell properties, such as long-term survival, proliferative potential, developmental plasticity and the ability to self-renew (FIG. 3). Such a gradient model also provides a flexible way to manage the size and quality of the CD8+ T cell population during infection, particularly when one considers the unpredictable properties of an infection, which can vary in intensity, tropism and duration. Most importantly, this model achieves the primary goal of a T cell response: to simultaneously generate an expendable pool of effector cells to combat the present infection and a pool of long-lived progenitors to combat future infections. As the discovery of signalling pathways and transcription factors involved in effector and memory CD8+ T cell development continues, it will be essential to identify how these factors cooperate or impede each other’s function, the target genes that they bind to and the epigenetic marks that they create, in a similar manner to the pioneering efforts in CD4+ T cells79,80,96–98.
Metabolic regulation of T cell differentiation
Another burgeoning area of research on T cells is immuno-metabolism — the interconnection between cellular metabolism and T cell differentiation, effector function and lifespan. Resting naive or memory T cells primarily generate cellular energy in the form of ATP through fatty acid oxidation and mitochondrial oxidative phosphoryl-ation. However, following activation, T cells undergo a metabolic switch to aerobic glycolysis and lipid synthesis to meet the tremendous bioenergetic and biosynthetic demands for rapid clonal expansion and the production of effector molecules99–101. It is thought that, following pathogen clearance, effector CD8+ T cells reduce their dependence on glycolysis and are gradually ‘reset’ back to a more catabolic state of mitochondrial oxida-tive phosphorylation to survive mitogen and growth factor withdrawal and further develop into memory CD8+ T cells100,101 (FIG. 4a). Although the mechanistic details supporting this model are currently sparse, this switch from glycolysis to fatty acid oxidation during the effector to memory cell transition probably involves the transition from a metabolic state governed by phospho-inositide 3-kinase (PI3K)–AKT–mTOR signalling to a metabolic state governed by AMP-activated protein kinase (AMPK), as described below100–102.
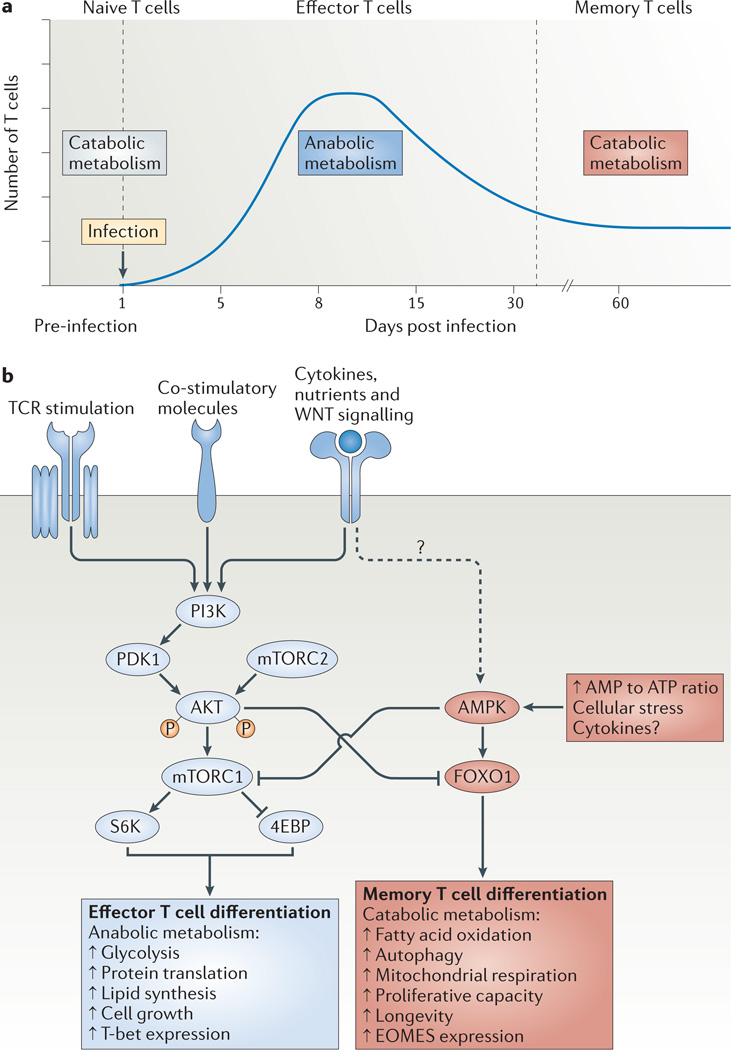
Figure 4. Model for the metabolic regulation of effector and memory T cell differentiation.
a | Resting naive or memory T cells primarily generate ATP through fatty acid oxidation and mitochondrial respiration (which are catabolic processes), but following activation the T cells undergo a metabolic switch to lipid synthesis (an anabolic process) and aerobic glycolysis to meet the bioenergetic and biosynthetic demands for rapid clonal expansion and the production of effector molecules. Following pathogen clearance, it has been proposed that effector CD8+ T cells reduce their dependence on glycolysis and are gradually ‘reset’ back to a more catabolic state to survive and further develop into memory CD8+ T cells. b | In response to T cell receptor (TCR), co-stimulatory and cytokine signals, the activity of the phosphoinositide 3-kinase (PI3K)–AKT–mammalian target of rapamycin (mTOR) pathway has a key role in regulating effector CD8+ T cell metabolism and differentiation by orchestrating nutrient uptake, protein translation and lipid synthesis in rapidly proliferating effector T cells. Typically AKT functions upstream of mTOR complex 1 (mTORC1) and downstream of mTORC2, but a recent study110 has shown that S6 kinase (S6K) can be activated independently of AKT in CD8+ T cells. By contrast, cellular stress and ATP deprivation (that is, an increased AMP to ATP ratio) activate AMP-activated protein kinase (AMPK), which in turn inhibits mTOR and enhances fatty acid oxidation to cope with limiting resources. In addition, these pathways can modulate effector T cell fate decisions through transcriptional regulation. Sustained PI3K–AKT– mTOR activity inhibits forkhead box O1 (FOXO1), which acts as a molecular switch to simultaneously induce T-bet and repress eomesodermin (EOMES) expression, and thereby promotes the clonal expansion and terminal differentiation of effector T cells. 4EBP, eIF4E–binding protein; PDK1, 3-phosphoinositide-dependent protein kinase 1.
The nutrient-sensing kinase mTOR signals through two complexes, mTORC1 and mTORC2, and promotes AKT activation (through mTORC2), glycolysis, clonal expansion and the generation of effector T cells102. The PI3K–AKT-mTOR pathway is activated by TCR signalling and sustained by pro-inflammatory cytokines such as IL-2 and IL-12 (REFS 65,103–105). By contrast, cellular stress and ATP deprivation activate AMPK, which in turn inhibits mTOR and enhances fatty acid oxidation, autophagy and other stress responses to cope with limiting resources100,101,104,106. Although widely known for its immunosuppressive effects, surprisingly the mTOR inhibitor rapamycin enhances the formation of memory CD8+ T cells and their precursors when administered at low doses during LCMV infection65,67,103,104. AMPK activity is thought to promote memory CD8+ T cell development, because treatment with metformin (an AMPK activator) or overexpression of carnitine palmitoyltransferase 1A (which increases mitochondrial fatty acid transport and oxidation) augments memory CD8+ T cell development during L. monocytogenes infection104,106. Together, these studies suggest that a metabolic switch from glycolysis to fatty acid oxidation or other catabolic processes is required for effector CD8+ T cells to survive and develop into memory CD8+ T cells. A prediction of this model would be that, compared with other effector CTLs, IL-7Rαhi memory precursor cells more efficiently undergo this metabolic switch to persist long-term.
In addition to the direct effects of mTOR on T cell metabolism, several recent studies have outlined a crucial role for mTOR in regulating STAT activity and the expression of several key transcription factors that control effector and memory T cell development. For example, mTORC1 helps to specify TH1 and TH17 cells, whereas mTORC2 has a greater role in TH2 cell development, by differentially regulating the activity of STAT4, STAT3 and STAT6, respectively94,107. A deficiency of both mTORC1 and mTORC2 in T cells impairs the development of all three effector CD4+ T cell types (TH1, TH2 and TH17 cells), but not that of TReg cells; this is in keeping with the notion that effector T cells primarily use glucose for fuel, whereas TReg cells primarily use fatty acids94,107,108. Other reports have extended this paradigm to CD8+ T cells by showing that IL-12-STAT4 signalling sustains PI3K–AKT-mTOR activity and inhibits the transcription factor FOXO1, which functions as a molecular switch to simultaneously induce T-bet expression and repress EOMES expression65,67,109 (FIG. 4b). Given that increased T-bet expression drives the formation of terminally differentiated KLRG1hiIL-7Rαlow effector CD8+ T cells during viral infection6, this finding helps to explain why the inhibition of mTOR activity with rapamycin or metformin promotes memory cell formation in the above studies. In addition to T-bet and EOMES, FOXO1 regulates the expression of other key genes involved in effector and memory CD8+ T cell migration and survival, such as those encoding IL-7Rα, CC-chemokine receptor 7 (CCR7), CD62L, BCL-2, Krüppel-like factor 2 (KLF2) and IFNγ67,109,110.
The effector to memory T cell transition
Although the effector to memory T cell transition seems to run on ‘autopilot’ (that is, it is a passive process that proceeds independently of instructions), gene and protein expression data suggest that the surviving cells must continue to differentiate to some degree to acquire ‘fully fledged’ memory cell properties, such as increased proliferative responses to their cognate antigen and to the homeostatic cytokines IL-15 and IL-7 (REFS 22,75,111). These functional changes are accompanied by phenotypic changes that enrich the diversity of the memory T cell pool (BOX 1). For example, in many cases, the proportion of IL-7RαhiCD27hiCXCR3hi CD62LhiKLRG1low memory T cells increases over time, and this directly correlates with increased expression of BCL-6, EOMES, TCF1 and BCL-2 and decreased expression of T-bet, granzyme B and KLRG1 in the memory T cells68,75,95. These longitudinal studies demonstrate that, following an acute infection, TCM cells tend to accumulate over time. However, this process can be altered by latent or persistent infection, which shows that the composition of the memory CD8+ T cell pool is influenced by the nature of the pathogen or the course of infection112.
It is unclear how this progressive maturation after acute infections is regulated, but recent work has shown that the process involves STAT3-activating cytokines, such as IL-10 and IL-21, and WNT signalling path-ways68,75,95,113. Deprivation of both IL-10 and IL-21 or the loss of STAT3 during LCMV infection severely affected memory T cell maturation; indeed, as mentioned above, very few KLRG1lowIL-7RαhiCD62LhiEOMEShi BCL-6hi CD8+ TCM cells accumulated after infection in these experiments68,75,95. Work by others has shown that TCF1 and EOMES are also coordinately involved in generating populations of mature memory CD8+ T cells that contain TCM cells and respond well to secondary infection or IL-15 (owing to increased IL-2Rβ expres-sion)68. In vitro, WNT3A stimulates EOMES expression through TCF1, which is a downstream transcription factor of WNT signalling, and overexpression of EOMES can compensate for TCF1 function to a certain degree68. Moreover, the combined deletion of the genes encoding β-catenin and γ-catenin (which presumably function downstream of WNT proteins) impairs the development of memory CD8+ T cells, whereas the ectopic expression of TCF1 and stabilized β-catenin in antigen-specific CD8+ T cells enhances memory cell development68,114,115. WNT3A also seems to promote the development of CD44lowCD62LhiSCA1hiCD122hiBCL-2hi memory ‘stem cells’116. Together, these reports strongly suggest that the canonical WNT signalling pathway is important for the maturation and homeostasis of memory T cells in vivo, although the actual WNT factors or receptors involved in this process have yet to be identified. Lastly, the fact that STAT3-deficient memory CD8+ T cells also have decreased EOMES expression indicates that the IL-10–STAT3 or IL-21–STAT3 pathway and the WNT– β-catenin–TCF1 pathway cooperate to maintain the survival, plasticity and proliferative fitness of memory CD8+ T cells.
Effects of re-infection on memory T cells
Most of the above discussion focuses on our understanding of memory CD8+ T cell development during an acute primary infection or following a primary vaccination, but over the course of an individual’s lifetime memory T cells will be repeatedly, or in some cases persistently, re-exposed to the same pathogen. A greater discussion of the effects of chronic antigen exposure on CD8+ T cell differentiation can be found in BOX 2. After successive infections, memory CD8+ T cells persist in an extended TEM cell state in which they have increased cytotoxicity and higher levels of expression of granzyme B and KLRG1, but decreased expression of CD62L, CCR7 and CD27 (REFS 12–14,117,118). In general, secondary and tertiary memory cells also express IL-7Rα and can persist long-term despite a reduced ability to undergo homeostatic proliferation12–14,117,118, indicating that KLRG1 expression is not a strict indicator of CD8+ T cells with a shortened lifespan in memory CD8+ T cell populations.
Compared with primary memory CD8+ T cells, secondary memory CD8+ T cells are more responsive to inflammation and offer greater protection against certain types of infection. However, their reduced trafficking to lymph nodes and decreased proliferative capacity could limit their ability to control pathogens that target lymphoid tissues or replicate extremely rapidly, such as LCMV clone 13 (REFS 12–14,117,119). Similarly to the phenotype of primary memory CD8+ T cells, the phe-notype of secondary memory T cells can be affected by the pre-existing memory cell precursor frequency, the amount or type of inflammation and their level of T-bet expression14,119. Indeed, secondary and tertiary memory CD8+ T cells have a more effector-like gene expression signature, as they have increased levels of the mRNAs encoding granzyme B, CCR5, IL-2Rα, T-bet, BLIMP1 and ID2, and decreased levels of the mRNAs encoding CCR7, CD62L, TCF1, ID3 and MYC compared with primary memory T cells118. However, repetitive stimulation of memory CD8+ T cells does not induce hallmarks of T cell exhaustion, such as expression of the inhibitory receptors programmed cell death protein 1 (PD1), lymphocyte activation gene 3 (LAG3), CD160, cytotoxic T lymphocyte antigen 4 (CTLA4) and CD244 (REF. 118).
The progressive loss of TCM cells in secondary and tertiary memory populations implies that, over successive infections, the pool of memory T cells might reach a Hayflick limit through the accumulation of senescent TEM cells, and this has been observed in serial adoptive transfer experiments using memory CD8+ T cells12–14,120. However, in contrast to the results from the adoptive transfer of small numbers of memory CD8+ T cells, the serial infection of immune animals has shown that the memory CD8+ T cell population can be repeatedly boosted to yield larger numbers of antigen-specific memory CD8+ T cells12,121. Moreover, our studies have suggested that the increased precursor frequency of antigen-specific memory CD8+ T cells that naturally exists after infection might function, in essence, as a cellular form of ‘herd immunity’ by reducing the re-exposure of memory T cells to signals that drive terminal differentiation (such as the cognate antigen and inflammation).
This could help to preserve the proliferative fitness of the pool of memory CD8+ T cells14. Although the correlates of immune protection for several life-threatening infectious pathogens are still unclear, the above studies outline several key factors that need to be considered in the design of future prime–boost vaccines.
Concluding remarks and perspective
This Review has focused on the recent advances over the past decade in our understanding of the mechanisms by which CD8+ T cells coordinate a large number of signals during infection to create diverse types of effector and memory T cell. As we begin to uncover the signals and transcriptional programmes that regulate memory T cell potential, phenotype and function, an important question that arises is how this information can be used to enhance protection against infectious diseases and cancer or, alternatively, to suppress T cell function to prevent autoimmunity or transplant rejection. Currently, several clinically relevant biologics are aimed at directly blocking T cell-derived cytokines or receptors; however, the modulation of memory T cell differentiation states could be another potential therapeutic tactic that might have more stable results. Moreover, we are discovering a more intricate relationship between our immune system and the metabolic fitness and function of organs and tissues. The tissue environment can affect the phenotype, function and longevity of memory B and T cells, but much remains to be learnt about how the function of an organ is, conversely, affected by the memory T cells that dwell within. This could have important implications for tissue homeostasis, inflammatory disease, obesity and ageing. The availability of innovative techniques such as intravi-tal imaging, epigenomic profiling and metabolic analyses should provide more opportunities for us to further our understanding of these issues, and this will ultimately help to improve vaccine design and the treatment of infectious and inflammatory diseases and tumours.
Acknowledgements
We thank the members of the Kaech laboratory for helpful comments and discussions. This work was supported by grants to S.M.K. from the US National Institutes of Health (grants R01AI074699, RO1AI066232, R21AI097767 and R21AI081150) and from the Howard Hughes Medical Institute.
Glossary
- Type 1 responses
Coordinated immune responses that occur following viral or intracellular bacterial infection. They are usually characterized by the rapid induction of innate cytokines such as interleukin-12 and interferons and the development of T helper 1 (TH1) cells and cytotoxic T cells.
- T follicular helper cells
(TFH cells). A distinct subset of CD4+ helper T cells that are CXCR5hiPD1hi. These cells primarily migrate into germinal centres following immunization, where they regulate the development of antigen specific B cell immune responses.
- Central memory T cells
(TCM cells). A subset of memory T cells that are normally CD62LhiCCR7hi and that home to the secondary lymphoid organs.
- Effector memory T cells
(TEM cells). A subset of memory T cells that are normally CD62LlowCCR7low and that reside in non-lymphoid tissues.
- Cellular barcoding
A tool for clonal analysis. Retroviral vectors with random sequences (that are referred to as ‘barcodes’) are transduced into progenitor cells. On integration, each vector introduces a unique, identifiable and heritable mark into the host cell genome, allowing the clonal progeny of each cell to be tracked over time.
- Asymmetric cell division
A process that produces two daughter cells with different cellular fates. This is in contrast to symmetric cell division, which gives rise to daughter cells of equivalent fates.
- T-box transcription factors
A family of transcription factors characterized by their homologous T-box DNA-binding domain.
- Mammalian target of rapamycin
(mTOR). A serine/threonine protein kinase that regulates cell growth, cell proliferation, cell motility, cell survival, protein synthesis and transcription. mTOR belongs to the phosphoinositide 3-kinase (PI3K)-related kinase protein family. The PI3K–AKT–mTOR pathway is activated by T-cell receptor signalling and sustained by pro-inflammatory cytokines such as IL-2 and IL-12.
- WNT
A family of glycoproteins related to the Drosophila melanogaster protein Wingless, a ligand that regulates the temporal and spatial development of the embryo. WNT-mediated signalling has been shown to regulate cell fate determination, proliferation, adhesion, migration and polarity during development. In addition to their crucial role in embryogenesis, WNT proteins and their downstream signalling molecules have been implicated in tumorigenesis and have causative roles in human colon cancers. WNT signalling activates TCF and LEF family transcription factors by stabilizing their co-activator, β-catenin, and mobilizing this factor from the cytoplasm to the nucleus.
- E protein transcription factors
Key transcriptional regulators that control many aspects of lymphocyte development. E proteins bind as homodimers or heterodimers to DNA at their canonical E box sites, where they function as transcriptional activators or repressors. There are four E proteins in mammals, namely E47, E12, HEB and E2-2.
- JAK–STAT signaling
A signalling pathway that transmits information from cell-surface receptors for specific chemical signals outside the cell to gene promoters in the DNA in the cell nucleus, which causes DNA transcription and activity in the cell.
- Hyper-IgE syndrome
Also known as Job’s syndrome). A heterogeneous group of immune disorders caused by autosomal dominant mutation of STAT3. It is characterized by recurrent infections and very high concentrations of the serum antibody IgE.
- mTORC1
(mTOR complex 1). A complex composed of mTOR, regulatory associated protein of mTOR (RAPTOR), LST8 (also known as GβL), RAS40 and DEPTOR. This complex is characterized by the classic features of mTOR, in that it functions as a nutrient, energy and redox sensor and controls protein synthesis.
- mTORC2
(mTOR complex 2). A complex composed of mTOR, rapamycin-insensitive companion of mTOR (RICTOR), LST8 and SAPK-interacting protein 1 (SIN1). mTORC2 phosphorylates the serine/ threonine protein kinase AKT at a serine residue (S473). mTORC2 has also been shown to function as an important regulator of the cytoskeleton.
- Hayflick limit
The number of times that a normal cell population will divide before it stops and enters a phase of senescence.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Susan M. Kaech’s homepage: http://immunobiology.yale.edu/ people/susan
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Marshall HD, et al. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4+ cell properties during viral infection. Immunity. 2011;35:633–646. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roman E, et al. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J. Exp. Med. 2002;196:957–968. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 5.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 6. Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010.This study shows that pro-inflammatory cytokines (such as IL-12) induce the terminal differentiation of effector CD8+ T cells through the induction of increased T-bet expression.
- 7.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J. Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar S, et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J. Exp. Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo . Nature Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 10.Croom HA, et al. Memory precursor phenotype of CD8+ T cells reflects early antigenic experience rather than memory numbers in a model of localized acute influenza infection. Eur. J. Immunol. 2011;41:682–693. doi: 10.1002/eji.201040625. [DOI] [PubMed] [Google Scholar]
- 11.Obar JJ, et al. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J. Immunol. 2011;187:4967–4978. doi: 10.4049/jimmunol.1102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J. Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 13.Nolz JC, Harty JT. Protective capacity of memory CD8+ T cells is dictated by antigen exposure history and nature of the infection. Immunity. 2011;34:781–793. doi: 10.1016/j.immuni.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi NS, et al. Increased numbers of preexisting memory CD8 T cells and decreased T-bet expression can restrain terminal differentiation of secondary effector and memory CD8 T cells. J. Immunol. 2011;187:4068–4076. doi: 10.4049/jimmunol.1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 18.Wherry EJ. T cell exhaustion. Nature Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 19.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 20.Stemberger C, et al. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 21. Gerlach C, et al. One naive T cell, multiple fates in CD8+ T cell differentiation. J. Exp. Med. 2010;207:1235–1246. doi: 10.1084/jem.20091175. References 20 and 21 provide evidence that a single naive CD8+ T cell can give rise to both terminal effector and memory T cells.
- 22.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nature Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 23.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nature Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 24.D’Souza WN, Hedrick SM. Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program. J. Immunol. 2006;177:777–781. doi: 10.4049/jimmunol.177.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nature Rev. Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 26. Chang JT, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393.This paper illustrates that memory and effector T cell fates can arise from a single precursor T cell through asymmetric cell division.
- 27.Mousavi SF, et al. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J. Immunol. 2008;181:5990–6001. doi: 10.4049/jimmunol.181.9.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendriks J, et al. CD27 is required for generation and long-term maintenance of T cell immunity. Nature Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 29.Hendriks J, et al. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J. Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 30.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu. Rev. Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 31.Pulle G, Vidric M, Watts TH. IL-15-dependent induction of 4-1BB promotes antigen-independent CD8 memory T cell survival. J. Immunol. 2006;176:2739–2748. doi: 10.4049/jimmunol.176.5.2739. [DOI] [PubMed] [Google Scholar]
- 32.Salek-Ardakani S, et al. The TNFR family members OX40 and CD27 link viral virulence to protective T cell vaccines in mice. J. Clin. Invest. 2011;121:296–307. doi: 10.1172/JCI42056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mescher MF, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 34.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J. Immunol. 2003;171:5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 35.Haring JS, Badovinac VP, Olson MR, Varga SM, Harty J. T; In vivo generation of pathogen-specific Th1 cells in the absence of the IFN-γ receptor. J. Immunol. 2005;175:3117–3122. doi: 10.4049/jimmunol.175.5.3117. [DOI] [PubMed] [Google Scholar]
- 36.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalia V, et al. Prolonged interleukin-2Rα expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo . Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 38. Pipkin ME, et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012.References 37 and 38 indicate that IL-2-mediated signalling promotes the terminal differentiation of effector CD8+ T cells.
- 39.Whitmire JK, Eam B, Benning N, Whitton JL. Direct interferon-γ signaling dramatically enhances CD4+ and CD8+ T cell memory. J. Immunol. 2007;179:1190–1197. doi: 10.4049/jimmunol.179.2.1190. [DOI] [PubMed] [Google Scholar]
- 40.Whitmire JK, Tan JT, Whitton JL. Interferon-γ acts directly on CD8+ T cells to increase their abundance during virus infection. J. Exp. Med. 2005;201:1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonilla WV, et al. The alarmin interleukin-33 drives protective antiviral CD8 T cell responses. Science. 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal P, et al. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J. Immunol. 2009;183:1695–1704. doi: 10.4049/jimmunol.0900592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wherry EJ, Puorro KA, Porgador A, Eisenlohr LC. The induction of virus-specific CTL as a function of increasing epitope expression: responses rise steadily until excessively high levels of epitope are attained. J. Immunol. 1999;163:3735–3745. [PubMed] [Google Scholar]
- 44.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nature Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marzo AL, et al. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nature Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui W, Joshi NS, Jiang A, Kaech SM. Effects of signal 3 during CD8 T cell priming: bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J. Immunol. 2007;179:53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 49.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J. Exp. Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J. Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 51.Keppler SJ, Theil K, Vucikuja S, Aichele P. Effector T-cell differentiation during viral and bacterial infections: role of direct IL-12 signals for cell fate decision of CD8+ T cells. Eur. J. Immunol. 2009;39:1774–1783. doi: 10.1002/eji.200839093. [DOI] [PubMed] [Google Scholar]
- 52.Wiesel M, et al. Type-I IFN drives the differentiation of short-lived effector CD8+ T cells in vivo . Eur. J. Immunol. 2012;42:320–329. doi: 10.1002/eji.201142091. [DOI] [PubMed] [Google Scholar]
- 53.Hu JK, Kagari T, Clingan JM, Matloubian M. Expression of chemokine receptor CXCR3 on T cells affects the balance between effector and memory CD8 T-cell generation. Proc. Natl Acad. Sci. USA. 2011;108:e118–e127. doi: 10.1073/pnas.1101881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurachi M, et al. Chemokine receptor CXCR3 facilitates CD8+ T cell differentiation into short-lived effector cells leading to memory degeneration. J. Exp. Med. 2011;208:1605–1620. doi: 10.1084/jem.20102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kohlmeier JE, et al. Inflammatory chemokine receptors regulate CD8+ T cell contraction and memory generation following infection. J. Exp. Med. 2011;208:1621–1634. doi: 10.1084/jem.20102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung YW, Rutishauser RL, Joshi NS, Haberman AM, Kaech SM. Differential localization of effector and memory CD8 T cell subsets in lymphoid organs during acute viral infection. J. Immunol. 2010;185:5315–5325. doi: 10.4049/jimmunol.1001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kastenmuller W, et al. Regulatory T cells selectively control CD8+ T cell effector pool size via IL-2 restriction. J. Immunol. 2011;187:3186–3197. doi: 10.4049/jimmunol.1101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foulds KE, Rotte MJ, Seder RA. IL-10 is required for optimal CD8 T cell memory following Listeria monocytogenes infection. J. Immunol. 2006;177:2565–2574. doi: 10.4049/jimmunol.177.4.2565. [DOI] [PubMed] [Google Scholar]
- 59.Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-β and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31:131–144. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Intlekofer AM, et al. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J. Exp. Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 62.Banerjee A, et al. Cutting edge: the transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J. Immunol. 2010;185:4988–4992. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Intlekofer AM, et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J. Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 65.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cruz-Guilloty F, et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 2009;206:51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rao RR, Li Q, Gubbels Bupp MR, Shrikant PA. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8+T cell differentiation. Immunity. 2012;36:374–387. doi: 10.1016/j.immuni.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou X, et al. Differentiation and persistence of memory CD8+ T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nature Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwon H, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gong D, Malek TR. Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J. Immunol. 2007;178:242–252. doi: 10.4049/jimmunol.178.1.242. [DOI] [PubMed] [Google Scholar]
- 72.Rutishauser RL, et al. Transcriptional repressor Blimp-1 promotes CD8+ T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shin H, et al. A role for the transcriptional repressor Blimp-1 in CD8+ T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8+ T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. References 72–74 collectively demonstrate that BLIMP1 promotes the terminal differentiation or exhaustion of CD8+ T cells in acute and chronic viral infections.
- 75.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+T cells. Immunity. 2011;35:792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ichii H, et al. Bcl6 is essential for the generation of long-term memory CD4+ T cells. Int. Immunol. 2007;19:427–433. doi: 10.1093/intimm/dxm007. [DOI] [PubMed] [Google Scholar]
- 77.Ichii H, et al. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nature Immunol. 2002;3:558–563. doi: 10.1038/ni802. [DOI] [PubMed] [Google Scholar]
- 78.Ichii H, Sakamoto A, Kuroda Y, Tokuhisa T. Bcl6 acts as an amplifier for the generation and proliferative capacity of central memory CD8+ T cells. J. Immunol. 2004;173:883–891. doi: 10.4049/jimmunol.173.2.883. [DOI] [PubMed] [Google Scholar]
- 79.Oestreich KJ, Huang AC, Weinmann AS. The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. J. Exp. Med. 2011;208:1001–1013. doi: 10.1084/jem.20102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oestreich KJ, Mohn SE, Weinmann AS. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nature Immunol. 2012;13:405–411. doi: 10.1038/ni.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ji Y, et al. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nature Immunol. 2011;12:1230–1237. doi: 10.1038/ni.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cannarile MA, et al. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nature Immunol. 2006;7:1317–1325. doi: 10.1038/ni1403. [DOI] [PubMed] [Google Scholar]
- 83.Yang CY, et al. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nature Immunol. 2011;12:1221–1229. doi: 10.1038/ni.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gough DJ, et al. Mitochondrial STAT3 supports Ras- dependent oncogenic transformation. Science. 2009;324:1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wegrzyn J, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choi YS, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gil MP, Salomon R, Louten J, Biron CA. Modulation of STAT1 protein levels: a mechanism shaping CD8 T-cell responses in vivo . Blood. 2006;107:987–993. doi: 10.1182/blood-2005-07-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nguyen KB, et al. Critical role for STAT4 activation by type 1 interferons in the interferon-γ response to viral infection. Science. 2002;297:2063–2066. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- 90.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J. Immunol. 2009;182:2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin JX, et al. Critical role of STAT5 transcription factor tetramerization for cytokine responses and normal immune function. Immunity. 2012;36:586–599. doi: 10.1016/j.immuni.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palmer DC, Restifo NP. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol. 2009;30:592–602. doi: 10.1016/j.it.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seki Y, et al. Expression of the suppressor of cytokine signaling-5 (SOCS5) negatively regulates IL-4-dependent STAT6 activation and Th2 differentiation. Proc. Natl Acad. Sci. USA. 2002;99:13003–13008. doi: 10.1073/pnas.202477099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nature Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Siegel AM, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35:806–818. doi: 10.1016/j.immuni.2011.09.016.This paper, together with reference 75, demonstrates that cytokine signalling through the STAT3 pathway is required for the functional maturation and maintenance of memory CD8+ T cells.
- 96.Yang XP, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nature Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O’Shea JJ, Lahesmaa R, Vahedi G, Laurence A, Kanno Y. Genomic views of STAT function in CD4+ T helper cell differentiation. Nature Rev. Immunol. 2011;11:239–250. doi: 10.1038/nri2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weng NP, Araki Y, Subedi K. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nature Rev. Immunol. 2012;12:306–315. doi: 10.1038/nri3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nature Rev. Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pearce EL. Metabolism in T cell activation and differentiation. Curr. Opin. Immunol. 2010;22:314–320. doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Michalek RD, Rathmell JC. The metabolic life and times of a T-cell. Immunol. Rev. 2010;236:190–202. doi: 10.1111/j.1600-065X.2010.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Finlay D, Cantrell DA. Metabolism, migration and memory in cytotoxic T cells. Nature Rev. Immunol. 2011;11:109–117. doi: 10.1038/nri2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097.References 103 and 104 were the first studies to describe the role of metabolic regulation in the differentiation of memory and effector CD8+ T cells.
- 105.Sinclair LV, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nature Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van der Windt GJ, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Delgoffe GM, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Michalek RD, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kerdiles YM, et al. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Macintyre AN, et al. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34:224–236. doi: 10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 112.van Lier RA, ten Berge IJ, Gamadia LE. Human CD8+ T-cell differentiation in response to viruses. Nature Rev. Immunol. 2003;3:931–939. doi: 10.1038/nri1254. [DOI] [PubMed] [Google Scholar]
- 113.Xue HH, Zhao DM. Regulation of mature T cell responses by the Wnt signaling pathway. Ann. NY Acad. Sci. 2012;1247:16–33. doi: 10.1111/j.1749-6632.2011.06302.x. [DOI] [PubMed] [Google Scholar]
- 114.Zhao DM, et al. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J. Immunol. 2010;184:1191–1199. doi: 10.4049/jimmunol.0901199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jeannet G, et al. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc. Natl Acad. Sci. USA. 2010;107:9777–9782. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gattinoni L, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nature Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J. Exp. Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wirth TC, et al. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8+ T cell differentiation. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wirth TC, Martin MD, Starbeck-Miller G, Harty JT, Badovinac VP. Secondary CD8+ T-cell responses are controlled by systemic inflammation. Eur. J. Immunol. 2011;41:1321–1333. doi: 10.1002/eji.201040730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Voehringer D, et al. Viral infections induce abundant numbers of senescent CD8 T cells. J. Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- 121.Vezys V, et al. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–199. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- 122.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Tw o subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 123.Mazo IB, et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22:259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 124.Kaech SM, Wherry EJ. Heterogeneity and cell- fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sheridan BS, Lefrancois L. Regional and mucosal memory T cells. Nature Immunol. 2011;12:485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo . J. Exp. Med. 2005;201:579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J. Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nature Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 129.Marzo AL, Yagita H, Lefrancois L. Cutting edge: migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. J. Immunol. 2007;179:36–40. doi: 10.4049/jimmunol.179.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Teijaro JR, et al. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jiang X, et al. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gebhardt T, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 134.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl Acad. Sci. USA. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 137.Kao C, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nature Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Quigley M, et al. Transcriptional analysis of HIV- specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nature Med. 2010;16:1147–1151. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]