Abstract
Guided by evolutionary game theory (Korte, Koolhaas, Wingfield, & McEwen, 2005), this study aimed to identify the genetic precursors and the psychosocial sequelae of inhibited temperament in a sociodemographically disadvantaged and racially diverse sample of 201 two-year-old children who experienced elevated levels of domestic violence. Using a multi-method, prospective design across three annual measurement occasions, SEM analyses indicated that trained observer ratings of inhibited temperament at age two were uniquely predicted by polymorphisms in dopamine and serotonin transporter genes. Children's inhibited temperament, in turn, indirectly predicted decreases in their externalizing problems at age four through its association with greater behavioral flexibility at three years of age. Results highlight the value of integrating evolutionary and developmental conceptualizations in more comprehensively charting the developmental cascades of inhibited temperament.
Children who exhibit a behaviorally inhibited temperament characterized by greater wariness and reticence in the context of novel stimuli and people are documented to exhibit a range of psychological adjustment difficulties (Degnan, Almas, & Fox, 2010). Problems are specifically reflected in vulnerability to a wide array of internalizing symptoms, peer difficulties, and negative cognitions (Degnan et al., 2010; Fox, Henderson, Marshall, Nichols, & Ghera, 2005). By the same token, the common yield of modest effect sizes in this work underscores the considerable heterogeneity in the sequelae of behaviorally inhibited children (Degnan et al., 2010; Prior, Smart, Sanson, & Oberklaid, 2000; Schwartz, Snidman, & Kagan, 1999). Although investigators have responded by successfully identifying several moderating conditions and mediational mechanisms that account for variability in the adjustment of inhibited children (e.g., Perez-Edgar et al., 2010; White, McDermott, Degnan, Henderson, & Fox, 2011), the central objective has been on characterizing the vulnerabilities of children with inhibited temperament (Aron, Aron, & Jagiellowicz, in press). Therefore, little is known about whether inhibited temperament may confer developmental advantages.
Part of this knowledge gap can be attributed to the paucity of conceptual models focusing on the potential benefits for children with high levels of temperamental inhibition. Although some temperament frameworks acknowledge that behavior inhibition may carry some limited developmental advantages, these modest benefits are noticeably outweighed by the conceptual focus on costs (e.g., Bates, Goodnight, & Fite, 2008; Henderson & Fox, 1998; Rothbart & Bates, 2006). For example, specific temperament theories posit that inhibition may reduce some children's vulnerability to developing externalizing symptoms through heightened sensitivity to punishment. However, any potential protective effects of sensitivity to punishment are eclipsed by its significant health tradeoffs as a precursor to an array of psychological (e.g., anxiety) difficulties (Bates et al., 2008; Rothbart, 1989). In more extraordinary circumstances (Rothbart & Bates, 2006; Rothbart, 2011), temperament scholars have noted that inhibition may be related to greater effortful control, but they question whether better performance on these tasks is truly a reflection of developmental competence or whether it may simply be a product of high sensitivity to punishment cues and a desire to be “good.” Moreover, emphasis is decidedly placed on conceptualizing behavior inhibition as a reactive, involuntary response pattern that undermines self-regulatory abilities, exploratory behavior, intrinsic task engagement, and perseverance in the face of cognitive challenge (Rothbart & Mauro, 1990; Rothbart & Bates, 1998; Rueda & Rothbart, 2009). Standing as a notable exception to the focus on pathological sequelae, research by Aksan and Kochanska (2004) supported a hypothesized pathway whereby children with inhibited temperament exhibited greater inhibitory control (i.e., refraining from a dominant response in favor of a subdominant, contextually-tailored response) by virtue of their lower impulsivity or speed of approach. However, additional research is needed to determine whether inhibited temperament is associated with self-regulatory capacities that extend beyond inhibitory control. Future progress also hinges on contextualizing these findings in larger process models that identify the genetic origins and mental health consequences of the underlying competencies of children with high temperamental inhibition to novelty.
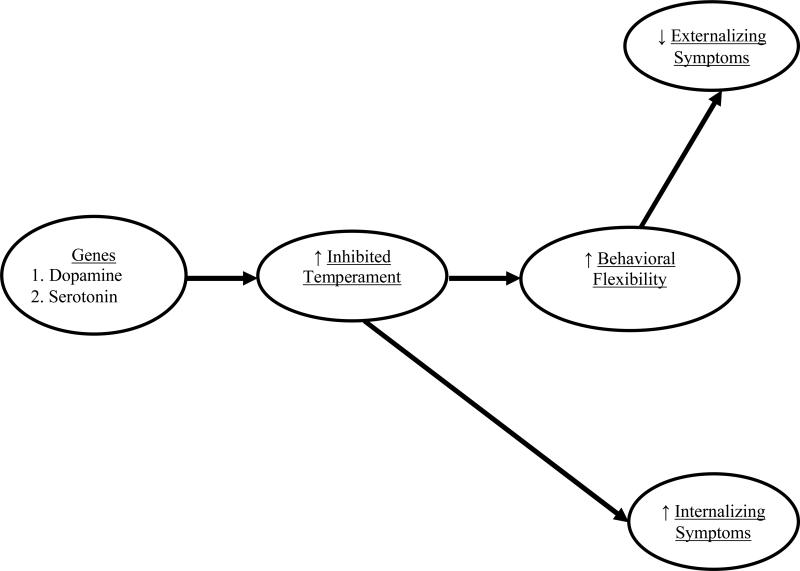
In building on the important work by Aksan and Kochanska (2004), a primary objective of this study is to utilize a developmental formulation of Evolutionary Game Theory (EGT) to identify the developmental benefits associated with inhibited temperament and its underlying genetic mechanisms during the preschool years (Ellis, Jackson, & Boyce, 2006; Korte, Koolhaas, Wingfield, & McEwen, 2005; Sih & Bell, 2008). Against the psychological landscape of predominantly cataloguing the burdens experienced by temperamentally inhibited children, the novelty of EGT is evident in its thesis that inhibited temperament is a precursor of an evolving personality syndrome of “Dove” attributes characterized by heightened behavioral flexibility and low propensity for experiencing externalizing symptoms. In further attempting to trace the origins of inhibition, EGT breaks further substantive ground by proposing that the personality constellation characterized by high temperamental inhibition, behavioral flexibility, and low aggressive tendencies is ultimately regulated by the activity of dopaminergic and serotonergic genes. However, little is known about how the relationships among inhibited temperament, behavioral flexibility, and psychological problems emerge over time in young children. Our study was designed to address this gap by integrating EGT within a developmental cascade model whereby genes involved in dopaminergic and serotonergic activity serve as precursors to inhibited temperament which, in turn, reduces children's subsequent risk for externalizing symptoms by increasing behavioral flexibility during the preschool years (see Figure 1).
Figure 1.
An evolutionary conceptualization of the genetic origins and developmental cascade of sequelae of children's inhibited temperament.
As the first pair of pathways in Figure 1 illustrate, EGT proposes that inhibited temperament is an endophenotype of genetic mechanisms organizing serotonergic and dopaminergic pathways in the brain. First, as a pivotal gene in regulating serotonin activity, the serotonin transporter gene (5-HTTLPR) encodes the serotonin transporter and its primary function of removing serotonin from the synaptic cleft and storing it in the presynaptic neuron (Bevilacqua & Goldman, 2011). Relative to the long (L) allele, the short (S) allele of the 5-HTTLPR gene is associated with lower transcriptional efficiency and diminished expression of the serotonin transporter. According to EGT, S allele carriers are more likely to experience heightened temperamental inhibition. Although inconsistency and complexity in results are evident in the literature (Schmidt, Fox, Rubin, Hu, & Hamer, 2002), studies support the hypothesis that carrying the S allele increases risk for fear, wariness, and sensitivity to aversive stimuli (Bevivacqua & Goldman, 2011).
Second, temperamental inhibition is further theorized to be a product of genetic polymorphisms regulating greater dopaminergic activity (Ellis et al., 2006; Korte et al., 2005). Consistent with this hypothesis, pharmacological and electrical manipulations to increase dopamine levels in the mesolimbic regions (e.g., ventral tegmental area, amygdala) of the brain have been shown to increase expressions of fear and sensitivity to aversive stimuli in animal and human studies (Pezze & Feldon, 2004). In contrast, pharmacological blockage of dopamine has resulted in diminished emotion processing and dampened fearful reactivity to stimuli (Mehta, Hinton, Montgomery, Bantick, & Grasby, 2005; Nader & LeDoux, 1999). Although EGT theory offers little guidance in the identification of specific dopamine genes underlying inhibited temperament (Korte et al., 2005), neurogenetic models have emphasized the critical role of dopamine transporter (DAT) genes in regulating dopamine in the mesolimbic pathway of the brain (Garcia-Garcia, Clemente, Dominguez-Borras, & Escera, 2010; Sharp, McQuillin, & Gurling, 2009). Because DAT is responsible for the reuptake of dopamine from the synapse and its subsequent transport to presynaptic neurons for storage, DAT alleles encoding for lower expression of DAT increase availability of synaptic dopamine (Congdon, Constable, Lesch, & Canli, 2009). In this study, we used a candidate gene approach to examine whether a composite of multiple DAT genes encoding for lower expression of DAT is associated with greater inhibited temperament (Van Gestel & Van Broeckhoven, 2003).
In the second component of the model, EGT specifically proposes that recurring challenges in ancestral environments sculpted the development of a suite of correlated behaviors consisting of inhibited temperamental traits and dimensions of behavioral flexibility. Behavioral flexibility is characterized as a repertoire of abilities that collectively function to alter behavioral strategies in response to specific cues and dynamic challenges in the environment. In evolutionary conceptualizations, flexibility is comprised of multiple facets including: (1) maintenance of an organized state of engagement in challenging tasks through regulation of negative affect, (2) persistent, intrinsic, and systematic exploration of the environment, and (3) the ability to devise, shift, and refine problem-solving strategies based on the careful processing of environmental cues and feedback from earlier unsuccessful approaches (Coppens, de Boer, & Koolhaas, 2010; Koolhaas, de Boer, & Buwalda, 2006; Korte et al., 2005). Thus, wary and cautious approach to novelty is regarded as one manifestation of sensitive, receptive, and reflective orientation toward the environment (Bell, 2007). In support of this thesis, research on a variety of animals has shown that greater temperamental inhibition is linked with thorough exploration of the environment, better problem-solving ability, and greater adaptation to changing cues (e.g., food location) in the environment (Sih & Bell, 2008).
If inhibited temperament is part of a broader pattern reflecting high sensitivity and reactivity to the environment as EGT proposes, then it begs the question of how temperamental inhibition and behavioral flexibility become nested together in a broader Dove phenotype over time. On the one hand, ethology research suggests that this personality profile is established very early in the lifespan. However, because the primary objective of these ethological studies has been on identifying the characteristics of the profile at a single point in time in relatively mature animals, the findings cannot readily trace the developmental emergence of associations between inhibited temperament and behavioral flexibility. On the other hand, developmental research underscores that skills supporting behavioral flexibility may not emerge until the toddler and early preschool period. For example, maternal reports of children's inhibited temperament at age three predicted subsequent increases in children's understanding of people's mental states two years later (Wellman, Lane, LaBounty, & Olson, 2011). Likewise, Aksan and Kochanska (2004) reported that the association between temperamental inhibition at 22 months of age and greater inhibitory control two years later was mediated by lower levels of impulsivity. Thus, from a developmental perspective, the early preschool years may be a sensitive period for the growth of behavioral flexibility. To test these two alternative predictions, we specifically examine whether temperamental inhibition to novelty when children are two years old predicts their behavioral flexibility both concurrently and prospectively over a one year period.
The proposed coupling of inhibited temperament and greater behavioral flexibility is further conceptualized to be part of a larger syndrome characterized by a lower proclivity to develop externalizing symptomatology (Ellis et al., 2006; Korte et al., 2005; Sih & Bell, 2008). In spite of calls in fields of evolution and ethology for a new generation of research designed to chart the expression, transformation, and change in the early emergence of temperamental and personality characteristics over time (Stamps & Groothuis, 2010), little attention has been devoted to understanding how the confluence of behavioral flexibility and diminished externalizing symptoms develops over time. Nevertheless, there is some conceptual precedence for proposing that behavioral flexibility is a precursor to lower levels of externalizing symptomatology. According to the asset protection principle, behavioral flexibility is specifically proposed to increase the acquisition of environmental resources (e.g., information) and, in turn, intensify conservative, future-oriented approaches that are manifested in lower tendencies to adopt risky, aggressive behaviors (e.g., Sih & Bell, 2008; Wolf, van Doorn, Leimar, & Weissing, 2007). Moreover, developmental research supports the hypothesis that children's abilities to engage in persistent, flexible coordination of multiple resources to address challenges may serve to successfully regulate disruptive impulses (e.g., Kochanska & Knaack, 2003). Thus, in the context of a developmental cascade model, individual differences in behavioral flexibility in the face of challenging tasks may help to further explain how and why children with heightened inhibited temperament may be buffered from experiencing externalizing symptoms.
In summary, this multi-method, multi-level prospective study was designed to delineate developmental precursors (i.e., serotonin and dopamine genes) and sequelae (i.e., behavioral flexibility, lower propensity for externalizing problems) of preschool children's inhibited temperament. Guided by EGT (Ellis et al., 2006; Korte et al., 2005; Sih & Bell, 2008), we extend prior knowledge by testing the novel hypothesis that children's inhibited temperament is associated with greater behavioral flexibility. Given the paucity of developmental models on how this linkage emerges over time, we specifically examine whether inhibited temperament is associated with greater flexibility both concurrently and over a one-year period. Breaking additional ground, we further test whether behavioral flexibility in a series of challenging cognitive tasks may be a key mechanism in explaining why inhibited temperament protects children from developing externalizing symptoms. Mediational models are specifically conducted using internalizing and externalizing symptoms as distinct outcomes to directly examine the hypothesis that behavioral flexibility is a specific mediator of children's externalizing, but not internalizing, symptoms. Towards the goal of identifying the architects of this ontogenetic process, another aim is to test polymorphisms in dopamine and serotonin genes as predictors of individual differences in inhibited temperament and their developmental consequences. As a strong test of our hypotheses, continuous observational ratings of children's inhibited temperament, behavioral flexibility, and internalizing and externalizing symptoms are nested within an autoregressive design to derive indices of change in the key constructs (Cole & Maxwell, 2003). Increasing the rigor of the analyses, we also investigate whether the stability of findings in the context of five potential covariates: children's sex, cognitive ability, pre-existing psychological problems, as well as interparental aggression and parenting difficulties.
Methods
Participants
The data for this study were drawn from a larger project focusing on children's coping and functioning in the context of interparental violence and conflict. Participants in this study included all of the 201 two-year-old children and their mothers who participated in the larger project. Due to the focus of the larger project on understanding differences in how children cope with significant family and social adversity, a two-step recruitment procedure was implemented. In the first step, we recruited participants through agencies serving disadvantaged families in a moderately-sized metropolitan area in the Northeast, including Women, Infants, and Children and Temporary Assistance to Needy Families rosters from the Department of Human and Health Services, and the county family court system. In the second step, we administered the brief version of the Physical Assault Subscale of the Conflict Tactics Scale 2 (CTS2; Straus, Hamby, Boney-McCoy, & Sugarman, 1996) to insure that roughly equal proportions of participating mothers experienced (a) no violence (i.e., 40%), (b) mild/moderate physical violence (i.e., 24%), and (c) severe physical violence (i.e., 36%) in the interpartner relationship. Additional inclusionary criteria consisted of: (a) the female caregiver is the biological mother; and (b) the male partner had maintained regular contact with the mother and toddler over the past year.
Median annual income for the family household was $18,300 (US) per year and a substantial minority of mothers (30%) and their partners (24%) did not complete high school. Most families received public assistance (95%) and were impoverished according to the US Federal Poverty Guidelines (99.5%). The mean age of the children was 26 months (SD = 1.69), with 44% of the sample consisting of girls (n = 92). The majority of participants were Black (56%), followed by smaller proportions of family members who identified as White (23%), Latino (11%), Multi-Racial (7%), and “Other” (3%). The cumulative retention rate across the three annual measurement occasions was 87%. To test for selective attrition, we conducted statistical comparisons between the mother-child dyads that participated through the third measurement occasion and dyads that dropped out during the longitudinal component of the study along 36 variables comprising the primary variables, covariates, and demographic characteristics at Wave 1 (e.g., family income, maternal education, maternal and child race). No significant differences were identified in the analyses.
Procedures and Measures
Mothers and children visited the laboratory at a research center on multiple occasions at each of three annual waves beginning when children were two years old. During the first wave, children participated in a set of tasks designed to assess their temperamental reactivity to novel stimuli and experimenters collected buccal cells from the children for subsequent DNA analysis. At the first and second waves, children engaged in a set of challenging problem-solving tasks designed to assess their success in resolving stage-salient tasks with their mothers in the room. Based on their experiences with the children during their multiple, lengthy visits to the laboratory, primary experimenters completed q-sort and questionnaire assessments of child internalizing and externalizing symptoms at the first and third measurement occasions. At the first wave, mothers also completed questionnaires and a semi-structured interview designed to assess children's exposure to interparental conflict. All research procedures were approved by the Institutional Review Board at the research site prior to conducting the study.
Serotonin and dopamine genotypes
Trained research assistants obtained DNA samples from children by collecting buccal cells with Epicentre Catch-All Collection Swabs. Using the conventional method, DNA was prepared for polymerase chain reaction (PCR) amplification through extraction with the Epicentre Buccal Amp DNA Extraction Kit. DNA was whole-genome amplified using the Repli-g kit (Qiagen, Chatsworth, CA., Catalog No. 150043) per the kit instructions to ensure availability of data over the long term for this sample. Amplified samples were then diluted to a working concentration. If a genotype for a gene could not be determined after the first run, then it was repeated up to four times. If the null result persisted, then a genotype was not assigned to that individual. Call rates, based on the 192 children who provided DNA samples, were 100% for the DAT rs 27072 and DAT rs40184, 97.4% for DAT 3’UTR VNTR, and 99.5% for 5-HTTLPR. All DNA samples were genotyped in duplicate for quality control. Additionally, human DNA from cell lines was purchased from Coriell Cell Repositories for all representative genotypes in duplicate, and genotypes were confirmed by sequencing using DTCS chemistry on an ABI 3130x1. These and a no template control were run alongside study samples representing 9% of the total data output. Any samples that were not able to be genotyped to a 95% or greater confidence level were repeated under the same conditions.
5-HTTLPR samples were genotyped for fragment length polymorphisms of 5-HTTLPR with Hot Star Taq PCR Mix (Qiagen, Catalog No. 203205) and previously described primers (Gelernter, Kranzler, & Cubells, 1997), followed by fragment analysis using a CEQ8000 (Beckman-Coulter, Inc., Fullerton, CA). Although genotypes with one or two short (S) alleles of the 5-HTTLPR gene are generally associated with lower transcription and function of 5-HTT protein in vitro (Bevilacqua & Goldman, 2011) than are genotypes with two long (L) alleles, research identifying an A>G substitution in a snp upstream from the promoter region has shown that LG functions more similarly to the S allele than the LA in its expression and binding potential (Praschak-Rieder et al., 2007; Reimold et al., 2007). Therefore, following established procedures (Mileva-Seitz et al., 2011), we used a triallelic approach to contrasting the genotypes with at least one copy of S or LG with genotypes with two copies of LA. Consequently, the LALA genotype (28%) was contrasted with an aggregated grouping of genotypes (72%) with at least one functional copy of the S allele (23% LALG, 26% LGS, 3% LGLG, 10% LGS, 9% SS). For quantification in analyses, the homozygote L and functional heterozygote S carriers were assigned values of 0 and 1, respectively. Genotype distribution for 5-HTTLPR was in Hardy-Weinberg equilibrium χ2 (1, N = 192) = 1.06, p = .30.
The three dopamine transporter genes were also assayed following published protocols. Samples were genotyped for DAT1 3’ UTR VNTR fragment length polymorphism using custom PCR primers and IDT (Forward 5’ FAM-TGTGGTGTAGGGAACGGCCTGAG3’ and Reverse 5’ CTTCCTGGAGGTCACGGCTCAAGG 3’) (Vandenbergh et al., 1992). These were amplified using the HotStarTaq DNA polymerase and dNTP mix (Qiagen, Items 203205 and 201900) on an ABI 9700 thermocycler. The PCR products were then run on an ABI 3130x1 sequencer against a GSLIZ 500 size standard (ABI, item 4322682) in POP-4 polymer (ABI, item 4352755). SNPs rs40184 and rs27072 were genotyped from Applied Biosystems, Inc. Individual allele determinations were made using TaqMan Genotyping Master Mix (Applied Biosystems, Catalog 4371357) with amplification on an ABI 9700 thermal cycler and analyzing the endpoint fluorescence using a Tecan M200 using JMP 8.0 (SAS, Inc.).
Guided by research indicating that the 10-repeat (10-R) allele of the DAT 3’UTR VNTR is associated with higher dopamine transporter expression and dopamine reduction in the synaptic cleft (Rommelse et al., 2008), we subsequently classified children into three groups: (0) two copies of 10-R, (1) one copy of 10-R, and (2) no copies of 10-R. Based on studies linking the C allele of the remaining two DAT genes with greater impulsivity, hyperactivity, and inattention (Gizer, Ficks, & Waldman, 2009; Soderqvist et al., in press), genotypes for rs27072 and rs40184 were quantified as (0) two copies of the C allele, (1) one copy of the C allele (C/T), and (2) no copies of the C allele (T/T). Because research has not definitively identified the dominant alleles for the DAT genes (Soderqvist et al., in press), we retained the ordinal rating for each gene. Following a candidate gene approach, we aggregated each of the three DAT variables into a single composite (range = 0 to 6) designed to more powerfully and parsimoniously index genetic variation in dopamine levels in the brain. Genotype distributions for the DAT 3’UTR VNTR (56% 37%, and 6% for two, one, and zero copies of the 10-repeat allele, respectively), rs27072 (66% C/C, 28% C/T, 6% T/T), and rs40841 (28% C/C, 46% C/T, 26% T/T) were in Hardy-Weinberg equilibrium, χ2 (2) = 4.87, p = .09, χ2 (2) = 4.44, p = .11, χ2 (2) = 0.34, p = .84.
Inhibited temperament
Following procedures for assessing temperament (e.g., Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Kagan, Reznick, & Snidman, 1987; Putnam & Stifter, 2005), children participated in a series of novel tasks and events. Mothers, who were in the same room, were instructed to complete questionnaires and only intervene with their children if they were concerned about their well-being. In the first episode (i.e., imitation task I), the experimenter escorted the child into the room containing a number of unusual objects (e.g., funnel, goggles, windshield cover). After a brief period in which the child was free to explore the room and the objects, the experimenter instructed the child to manipulate the objects in different ways (e.g., windshield cover: “Poke it!”). In the next episode (i.e., clown task), an unfamiliar female experimenter dressed as a clown introduced herself and then invited the child to play with a sack of toys for two minutes. Following the departure of the clown, the experimenter returned with a large robot holding an attractive toy. Operated surreptitiously by remote control from the control room, the robot alternated three times between periods of moving around the room and inactivity. Each time the robot stopped moving, the experimenter encouraged the child to approach the toy. In the final task (i.e., imitation task II), the primary female experimenter instructed the children to imitate the following events after first enacting them herself: (a) reach behind a black curtain to pull out a doll, (b) place a finger in glasses of water and prune juice, and (c) pick up a rubber snake and let it slide back onto the table.
Video records of children's behavioral response to each of the four unfamiliar episodes were subsequently assessed by a set of trained raters using three codes. Two molar ratings designed to index signs of inhibition (Davies, Sturge-Apple, & Cicchetti, 2011) included: (1) inhibition, characterized by the tendency to restrain behavior in order to assess the situation through signs of vigilance, hesitation, and apprehension; and (2) fearful distress, defined as vulnerable affective responses manifested in fear, freezing, distress, self-soothing, and comfort seeking. Coders rated each dimension along five-point scales ranging from 0 (none) to 4 (intense, frequent, and prolonged expression). The third code assessed latency (in seconds) to approach the novel stimuli in each of the four episodes. Two trained coders were randomly assigned to code 60% of the children in the unfamiliar episodes task, overlapping on approximately 25% of the tasks in order to calculate reliability. Values for each of the codes were standardized and aggregated across the four episodes to obtain composites of three dimensions of inhibited temperament for the primary analyses. Interrater reliability values, consisting of singe-rater intraclass correlation coefficients derived from one-way random effects ANOVA models (Choukalas, Melby, & Lorenz, 2000; McGraw & Wong, 1996), ranged from .85 to 1.0.
Children's behavioral flexibility
Children participated in a series of increasingly difficult problem-solving challenges in the first two waves to assess their behavioral flexibility. Adapted from a well-established paradigm for assessing individual differences in children's developmental competence (Matas, Arend, & Sroufe, 1978; Sroufe, Egeland, & Kreutzer, 1990), the procedural battery consisted of four tasks that were each five minutes in duration and designed to be difficult for the child to solve without the assistance of an adult. In Wave 1, the specific tasks included: (a) a puzzle, (b) a shape sorter task in which children must place plastic forms into holes corresponding to their shapes, (c) a large seesaw problem that requires the child to place a wooden block on one end in order to gain access to an attractive toy encased in a transparent box, and (d) a task in which the child must use a piece of balsa wood to retrieve a ball stuck in a plastic tube. For Wave 2, tasks were tailored to be challenging for the more advanced developmental level of the preschoolers. Tasks consisted of: (a) a more complicated puzzle, (b) task involving sorting shapes according to height, (c) a maze, and (d) a problem that required children to place spools onto correct colors of thread. The mother, who is available in the same room, is instructed to permit the child to try to first work on the problem independently before providing any assistance she thinks the child needs.
Trained coders, who did not serve as the primary experimenters on the visits, rated the videotaped records of the entire session (i.e., the collective series of four tasks). Molar rating scales originally developed by Matas and colleagues (1978) were used to assess three components of behavioral flexibility. First, maintaining organized engagement through regulation of negative affect was specifically indexed by the seven-point coping scale. Ratings ranged from (1) extremely low (i.e., becomes disorganized in the face of challenge even with support) to (7) high coping (i.e., child remains organized and regulated in spite of great challenge). Second, intrinsic and persistent exploration with the environment was assessed by the enthusiasm and persistence scales. For the enthusiasm code, judges utilized a seven-point scale, ranging from (7) persistent and enjoyable engrossment in the activities to (1) quickly developing signs of boredom and disengagement that persisted throughout the tasks. The persistence code gauged the ability of the children to maintain a goal-oriented approach to the problem, with values ranging from (1) little persistence in which the child quickly disengages without any effort to (5) high persistence characterized by high levels of goal-oriented behavior throughout the activities. Third, the ability to shift problem-solving strategies based on the careful processing of environmental cues was indexed by the problem-solving scale. Problem-solving ratings ranged from (0) the absence of any goal-directed, resourceful activity (e.g., complete passivity) to (4) innovative approaches to solving the problem that use a wide range of resources and flexibility in the face of setbacks. Two coders independently rated 20% of the video records at each wave to assess interrater reliability. Single-rater intraclass correlation coefficients based on one-way way random effects ANOVA models ranged from .55 for Wave 1 persistence to .93 for Wave 2 enthusiasm (M = .82).
Children's psychological symptoms
Two primary experimenters who were responsible for keeping contact with the families, visiting families at their homes, providing transportation to our laboratory, and overseeing the activities and tasks during the visits completed ratings of child adjustment at the first and third waves. Ratings were based on close observations of the children for approximately eight hours during each wave, encompassing multiple visits to our laboratory and, in most cases, transportation of families to and from the research center. To increase validity of the observations, experimenters retained a written record of their observations of the children after each visit using a form organized around dimensions of personality and psychopathology.
Both experimenters for the visit completed q-set ratings using the California Child Q-Set (CCQ; Block, 2008). The CCQ requires raters to sort 100 descriptors of children's behaviors, personality characteristics, and psychological symptoms into nine different piles ranging from “extremely uncharacteristic” to “extremely characteristic.” To assess child psychological problems, we utilized expert ratings of prototypical attributes of a child exhibiting externalizing symptoms and internalizing symptoms based on established guidelines for the CCQ (Block, 2008). Consistent with previous research (e.g., Shields & Cicchetti, 1997), we selected q-set items for scale development that were, on average, rated in the top two piles by the experts. The resulting externalizing scale consisted of 15 items that reflected high levels of hostility, opposition, and impulsivity (i.e., “Is aggressive physically or verbally,” “Is unable to delay gratification,” “characteristically pushes and tries to stretch limits”). Reliabilities, as indexed by internal consistencies, ranged from .83 to .87 across the two experimenters and measurement occasions. The 12 items comprising the internalizing scale were designed to assess anxiety (e.g., “Is fearful and anxious”), social withdrawal (e.g., “likes to be by him/herself, enjoys solitary activities”), and depressive difficulties (e.g., “appears to feel unworthy; thinks of self as ‘bad’”). Internal consistencies across two waves and experimenters ranged from α = .65 to .76.
The experimenter who completed the q-set ratings and was primarily responsible for overseeing the children's activities during family visits and contacts also filled out the Internalizing and Externalizing scales of the Caregiver-Teacher Report Form (C-TRF; Achenbach & Rescorla, 2006). The Externalizing scale indexes aggressive, oppositional, and attention problems, whereas the Internalizing scale assesses anxious, depressive, withdrawn, and somatic symptoms. Response alternatives for the items on the scales range from “Not True” (0) to “Very or Often True” (2). Internal consistency estimates for the Internalizing and Externalizing Symptoms scales ranged from .84 to .94 across the two measurement occasions.
Covariates: Child Attributes
Sex and cognitive ability were included as covariates in the model. The measurement of children's cognitive ability was derived from experimenter administration of the Cognitive Scale of the Battelle Developmental Inventory Screening Test at Wave 2 (BDIST; Newborg, Stock, Wnek, Guidubaldi, & Svinicki, 1984). The BDIST utilizes a combination of a structured testing format, parent interview, and observations of the child during the visit to measure cognitive ability across several domains including attention, perceptual discrimination, memory, reasoning, and academic and conceptual skills. As a widely used measure, the reliability and validity of BDIST is supported by previous research (Aylward, 2004).
Covariates: Family Characteristics
In addition, three measures of interparental aggression and parenting difficulties at Wave 1 were also specified as covariates. The first two assessments of interparental aggression were derived from maternal reports on the Physical Assault and Psychological Aggression Subscales of the Revised Conflict Tactics Scale (CTS2; Straus et al., 1996). The Physical Assault Subscale contains 24 items designed to assess maternal and partner acts of physical attacks toward each other in the interpartner relationship, ranging from relatively mild (e.g., “I pushed or shoved my partner”) to severe (e.g., “I used a knife or gun on my partner”) forms of assault. The Psychological Aggression Subscale is comprised of 14 items that assess maternal and partner forms of psychological hostility directed toward one another (e.g., “shouted or yelled at my partner”). Following scoring guidelines, prevalence scores were calculated for each of the two scales based on the sum of the occurrences of specific aggressive acts (1 = act occurred one or more times; 0= specific act did not occur) over the past year. Internal consistencies were .92 and .88 for the Physical Assault (M = 1.36, SD = 1.36) and Psychological Aggression (M = 3.22, SD = 3.22) Subscales, respectively.
The remaining indicator of interparental aggression was derived from the Interparental Conflict Characteristics (ICC) Module of the Interparental Disagreement Interview (IDI). The ICC module of the IDI is a semi-structured, narrative interview with the mother designed to assess the nature and course of interpartner conflicts witnessed by child participants (Davies, Sturge-Apple, Cicchetti, & Manning, 2012). Video records of the interview were subsequently coded for interparental aggression. Coders specifically rated the level of maternal and partner aggression during the conflicts along seven-point scales. Aggression was operationally defined as the level of hostility and aggression directed toward the partner in the interparental relationship. At one extreme, no aggression (0 = none) was characterized by no evidence of any overt form of aggression or hostility directed toward the partner. At the other extreme, high (6) aggression reflects high levels of intense and threatening forms of aggression (e.g., pushing, hitting). Consistent with the prior procedures, ratings of mother and partner scores of aggression were averaged together to form a single interpartner aggression scale (M = 1.98, SD = 1.85). The intraclass correlation coefficient, based on a second coder independently rating 25% of the interviews, was .84. Support for the validity of the IDI code is reflected in its associations with established measures of interparental discord and child psychological functioning (e.g., Davies et al., 2012).
Observational assessments of maternal parenting difficulties were obtained from coder ratings of mother-child interactions during the problem-solving task at Wave 1 using the Insensitive/Parent-Centered and Warmth/Support Scales from the Iowa Family Interaction Rating Scales (IFIRS, Melby & Conger, 2001) and the Quality of Assistance Scale from the Minnesota Study of Risk and Adaptation (Sroufe, Egeland, Carlson, & Collins, 2005). The IFIRS scales range from (1) not at all characteristic to (9) mainly characteristic. The Insensitive Scale is designed to assess individual differences in parental awareness of the children's needs, emotional states, and abilities, whereas the Warmth Scale indexes parental support and affection toward the child. Quality of Assistance was rated on a seven-point scale ranging from 1 (“mother distracts her child, frustrates him/her, or simply provides no assistance”) to 7 (offers “helpful, well-timed instructions in a clear, orderly, and understandable manner.”). To evaluate interrater reliabilities, a second coder independently rated a randomly generated subsample (24%) of the parent-child interactions. Intraclass correlation coefficients for the three codes ranged from .80 and .86.
Results
Descriptive and Preliminary Analyses
Table 1 provides the means, standard deviations, and intercorrelations for the variables used in the main constructs for the primary analyses. Missing data analyses for primary variables and covariates (Median = 7%, Range = 0 – 26%) indicated that data were missing completely at random (MCAR) based on Little's MCAR test (Schlomer, Bauman, & Card, 2010), χ2 = 730.97, df = 697, p = .18. Therefore, missing data were estimated using full-information maximum likelihood (FIML) to retain the full sample for primary analyses (Enders, 2001).
Table 1.
Means, standard deviations, and correlations of the primary variables in the analyses.
| M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genetic Polymorphism | |||||||||||||
| 1. Dopamine | 1.91 | 1.28 | -- | ||||||||||
| 2. Serotonin | 0.72 | 0.45 | .06 | -- | |||||||||
| Inhibited Temperament | |||||||||||||
| 3. Inhibition | 0.15 | 0.61 | .25* | .14 | -- | ||||||||
| 4. Fearful Distress | 0.13 | 0.63 | .16* | .09 | .73* | -- | |||||||
| 5. Latency to Appr. | 0.17 | 0.60 | .14 | .10 | .73* | .62* | -- | ||||||
| Wave 1 Behavioral Flexibility in Stage-Salient Tasks | |||||||||||||
| 6. Coping | 3.75 | 1.46 | −.03 | .05 | .02 | .01 | .06 | -- | |||||
| 7. Enthusiasm | 3.88 | 1.23 | −.02 | .07 | .05 | −.11 | −.02 | .62* | -- | ||||
| 8 Persistence | 3.16 | 0.93 | .00 | .03 | .11 | .02 | .10 | .62* | .77* | -- | |||
| 9. Problem-Solve | 1.90 | 0.78 | −.10 | −.06 | −.03 | −.15 | −.07 | .38* | .56* | .41* | -- | ||
| Wave 2 Behavioral Flexibility in Stage-Salient Tasks | |||||||||||||
| 10. Coping | 3.66 | 1.30 | .18* | −.04 | .20* | .15 | .06 | .25* | .38* | .39* | .27* | -- | |
| 11. Enthusiasm | 3.68 | 1.23 | .18* | .00 | .21* | .19* | .06 | .06 | .29* | .31* | .20* | .78* | -- |
| 12. Persistence | 3.19 | 1.00 | .15 | −.04 | .26* | .23* | .09 | .16* | .33* | .31* | .24* | .80* | .86* |
| 13. Problem-Solve | 1.78 | 0.85 | .17* | .06 | .21* | .15 | .10 | .08 | .31* | .29* | .30* | .59* | .72* |
| Wave 1 Externalizing Symptoms | |||||||||||||
| 14. Exp. 1 Q-set | 71.57 | 16.31 | −.11 | .01 | −.23* | −.06 | −.10 | −.09 | −.10 | −.12 | −.07 | −.32* | −.22* |
| 15. Exp. 2 Q-set | 73.88 | 17.19 | −.19* | −.01 | −.23* | −.16* | −.12 | −.26* | −.13 | −.16* | −.10 | −.30* | −.27* |
| 16. Exp 1 CTRF | 5.72 | 7.17 | −.11 | −.07 | −.16* | −.04 | −.05 | −.06 | −.10 | −.06 | −.06 | −.24* | −.25* |
| Wave 3 Externalizing Symptoms | |||||||||||||
| 17. Exp. 1 Q-set | 74.83 | 17.93 | −.14 | −.11 | −.29* | −.16 | −.12 | −.14 | −.17* | −.12 | −.17* | −.30* | −.26* |
| 18. Exp. 2 Q-set | 75.10 | 17.70 | −.09 | −.14 | −.30* | −.17* | −.20* | −.19* | −.15 | −.15 | −.14 | −.33* | −.27* |
| 19. Exp 1 CTRF | 9.29 | 10.64 | −.05 | −.10 | −.26* | −.14 | −.06 | −.18* | −.17* | −.12 | −.21* | −.32* | −.30* |
| Wave 1 Internalizing Symptoms | |||||||||||||
| 20. Exp. 1 Q-set | 47.83 | 11.60 | −.02 | −.04 | .26* | .18* | .22* | .00 | −.07 | .05 | −.14* | .03 | .00 |
| 21. Exp. 2 Q-set | 47.24 | 12.09 | .04 | .01 | .34* | .23* | .30* | −.05 | −.16* | −.02 | −.11 | .05 | .04 |
| 22. Exp 1 CTRF | 3.85 | 4.74 | .07 | .02 | .19* | .15* | .21* | −.02 | −.17* | −.04 | −.16* | −.10 | −.14 |
| Wave 3 Internalizing Symptoms | |||||||||||||
| 23. Exp. 1 Q-set | 43.70 | 10.04 | .16* | .01 | .24* | .11 | .29* | .09 | −.08 | −.05 | −.11 | .12 | .04 |
| 24. Exp. 2 Q-set | 43.17 | 9.62 | .03 | .03 | .17* | .17* | .25* | .09 | −.04 | .06 | −.08 | .19* | .12 |
| 25. Exp 1 CTRF | 3.70 | 5.16 | .05 | −.04 | .02 | .01 | .13 | −.08 | −.14 | −.10 | −.20* | −.09 | −.11 |
| 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wave 2 Behavioral Flexibility in Stage-Salient Tasks | |||||||||||||
| 12 Persistent | -- | ||||||||||||
| 13. Problem-Solve | .65* | -- | |||||||||||
| Wave 1 Externalizing Symptoms | |||||||||||||
| 14. Exp. 1 Q-set | −.23* | −.20* | -- | ||||||||||
| 15. Exp. 2 Q-set | −.27* | −.28* | .65* | -- | |||||||||
| 16. Exp 1 CTRF | −.19* | -.27* | .73* | .51* | -- | ||||||||
| Wave 3 Externalizing Symptoms | |||||||||||||
| 17. Exp. 1 Q-set | −.29* | −.30* | .30* | .35* | .21* | -- | |||||||
| 18. Exp. 2 Q-set | −.25* | −.26* | .28* | .32* | .23* | .67* | -- | ||||||
| 19. Exp 1 CTRF | −.32* | −.28* | .26* | .26* | .23* | .82* | .66* | -- | |||||
| Wave 1 Internalizing Symptoms | |||||||||||||
| 20. Exp. 1 Q-set | .01 | −.07 | −.27* | −.18* | −.09 | −.03 | −.10 | .07 | -- | ||||
| 21. Exp. 2 Q-set | −.01 | −.02 | −.08 | −.20* | −.03 | −.12 | −.21* | −.04 | .58* | -- | |||
| 22. Exp 1 CTRF | −.12 | −.22* | .15* | .02 | .38* | −.06 | −.08 | .01 | .45* | .52* | -- | ||
| Wave 3 Internalizing Symptoms | |||||||||||||
| 23. Exp. 1 Q-set | .11 | .03 | −.13 | −13* | −.10 | −.26* | −25* | .01 | .33* | .29* | .26* | -- | |
| 24. Exp. 2 Q-set | .20* | .14 | −.10 | −13* | −.07 | −.08 | −.27* | .00 | .30* | .21* | .13 | .45* | -- |
| 25. Exp 1 CTRF | −.10 | −.12 | .09 | .07 | .09 | .39* | .25* | .64* | .25* | .14 | .13 | .50* | .37* |
Note.
p ≤ .05.
Primary Analyses
The hypothesized evolutionary model of the precursors and sequelae of children's inhibited temperament was tested using structural equation modeling (SEM) with the Amos 19.0 computer software package. To conservatively test the proposed prospective associations, we utilized an autoregressive approach in which: (a) children's inhibited temperament at Wave 1 was specified as a predictor of Wave 2 behavioral flexibility after controlling for Wave 1 behavioral flexibility, and (b) Wave 2 behavioral flexibility was estimated as a predictor of Wave 3 child psychological problems controlling for Wave 1 psychological problems. Inclusion of repeated measures of both latent endogenous measures (i.e., internalizing and externalizing) within the same model resulted in substantial increases in the number of estimated parameters and placed considerable demands on the model estimation. Accordingly, separate models were specified for internalizing and externalizing problems. In delineating precursors of temperament, serotonin and dopamine polymorphisms were further specified as predictors of children's behavior inhibition. As a more rigorous analysis of the distinctive nature of the hypothesized pathways among children's serotonin and dopamine genotypes, inhibition, behavioral flexibility, and internalizing and externalizing symptoms, we also estimated additional pathways (see Figures 2 and 3), including: the two genotypes as predictors of children's behavioral flexibility and their internalizing and externalizing symptoms at each time point, children's inhibition as a predictor of their internalizing and externalizing symptoms at each measurement occasion, and Wave 1 child internalizing and externalizing symptoms as predictors of Wave 2 behavioral flexibility. Finally, additional covariates were specified as predictors of each of the endogenous variables in the models, including: (1) child sex, (2) child cognitive ability, (3) parenting difficulties, and (4) exposure to interparental aggression.
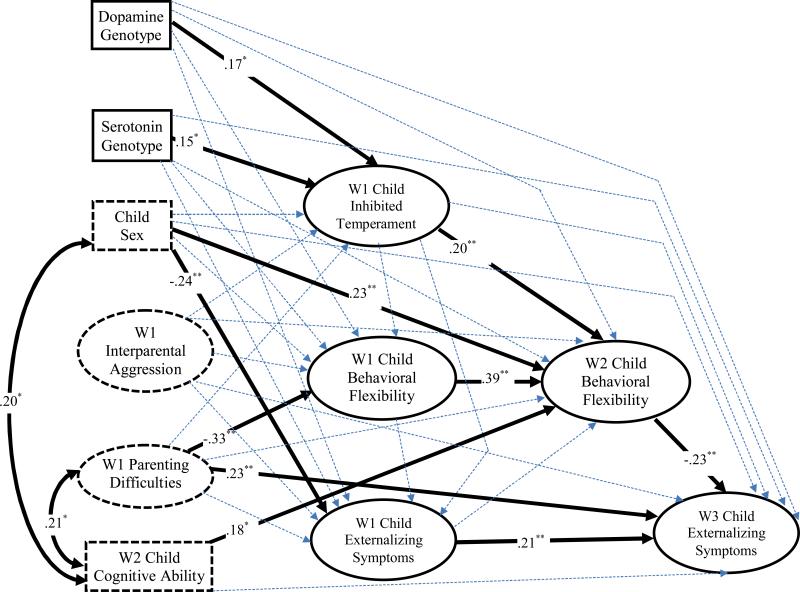
Figure 2.
Structural equation model examining the genetic predictors and developmental consequences of inhibited temperament in the context of children's externalizing symptoms. Bolded arrows denote significant paths and dotted arrows signify nonsignificant paths. Standardized path coefficients are shown for significant structural paths only. Correlations were estimated among all predictors, but only significant associations are presented for clarity. ** p < .01; * p < .05.
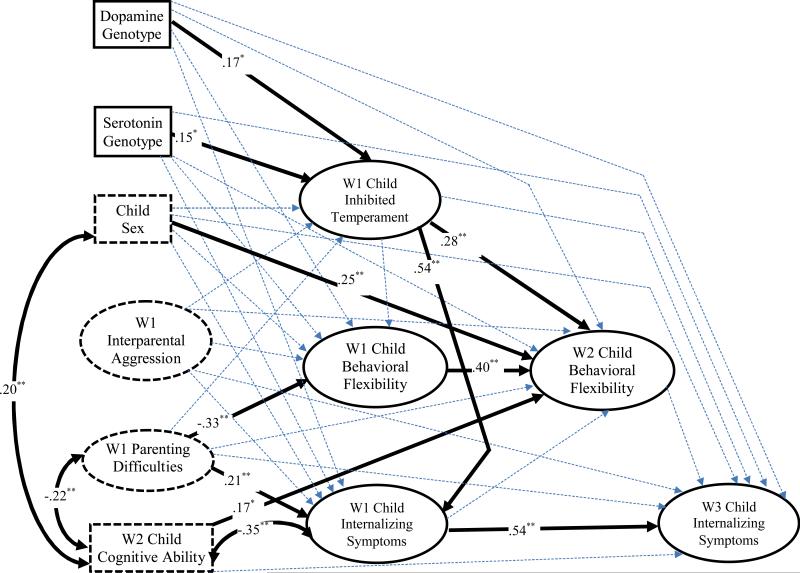
Figure 3.
Structural equation model examining the genetic predictors and developmental consequences of inhibited temperament in the context of children's internalizing symptoms. Bolded arrows denote significant paths and dotted arrows signify nonsignificant paths. Standardized path coefficients are shown for significant structural paths only. Correlations were estimated among all predictors, but only significant associations are presented for clarity. ** p < .01; * p < .05.
Externalizing Model
The model for children's externalizing symptoms, which is depicted in Figure 2, provided a good representation of the data, χ2 (278, N = 201) = 416.03, p < .001, RMSEA = .05, and χ2/df ratio = 1.58, CFI = .93, and TLI = .91. Lending support to the measurement model, the standardized loadings of the manifest indicators onto their respective latent constructs were all significant (p < .001) and high in magnitude (range .51 to .96). In accord with the hypothesized genetic precursors of temperament, dopamine and serotonin genotypes each predicted children's inhibited temperament at Wave 1, β = .17, p < .05 and β = .15, p < .05, respectively. Consistent with the second link in our proposed model, children's inhibited temperament at Wave 1 predicted their behavioral flexibility at Wave 2 even after controlling for Wave 1 behavioral flexibility, β = .20, p < .01. Wave 2 behavioral flexibility, in turn, predicted decreases in Wave 3 child externalizing symptoms after controlling for W1 externalizing symptoms, β = −.23, p < .01. These findings remained significant with the simultaneous estimation of multiple significant pathways, including: (a) Wave 1 parenting difficulties as predictors of lower behavioral flexibility at Wave 1, β = −.33, p < .01, and greater externalizing symptoms at Wave 3, β = .23, p < .01; (b) child sex as a predictor of externalizing symptoms at Wave 1, β = −.24, p < .01, and behavioral flexibility at Wave 2, β = .23, p < .01; (c) Wave 2 child cognitive ability as a correlate of behavioral flexibility at Wave 2, β = .18, p < .05, and (d) autoregressive paths from Wave 1 to Wave 2 measures of behavioral flexibility, β = .39, p < .01 and Wave 1 to Wave 3 assessments of externalizing symptoms, β = .21, p < .05.
Our findings on the hypothesized cascade of processes can be further examined as two interlocking mediational chains. In the first part of the cascade, Wave 1 inhibited temperament is proposed to mediate associations between serotonin and dopamine genotypes and Wave 2 behavioral flexibility. Using bootstrapping tests of mediation with the PRODCLIN software program (MacKinnon, Fritz, Williams, & Lockwood, 2007), the results indicated that the indirect paths involving genotype, Wave 1 inhibited temperament, and Wave 2 behavioral flexibility were significantly different from 0 for serotonin, 95% CI = .004 to .19, and dopamine, 95% CI = .003 to 0.07. In the subsequent part of the cascade, the results in Figure 1 supported a pathway whereby children's inhibited temperament at Wave 1 predicted lower levels of their externalizing symptoms at Wave 3 by increasing their behavioral flexibility at Wave 2. The bootstrapping test of this mediational chain indicated that behavioral flexibility mediated the path between inhibition and children's subsequent externalizing symptoms, 95% CI = 0.17 to 2.68.
Internalizing model
The results of the SEM analyses of inhibited temperament and its underlying processes in the prediction of internalizing symptoms are depicted in Figure 3. The model fit the data well, χ2 (278, N = 201) = 416.95, p < .001, RMSEA = .05, and χ2/df ratio = 1.50, CFI = .94, and TLI = .91. Moreover, loadings of all manifest indicators on the latent composites were significant (ps < .001) and, on average, high in magnitude (M = .78). Consistent with the externalizing symptoms model, the serotonin and dopamine genotypes were related to greater inhibited temperament at Wave 1, with inhibited temperament, in turn predicting better behavioral flexibility at Wave 2. However, no support was found for the second part of the cascade model, as Wave 2 behavioral flexibility failed to predict children's internalizing symptoms at Wave 3. Therefore, subsequent increases in behavioral flexibility did not decrease the likelihood of experiencing internalizing symptoms. Consistent with prior research, inhibited temperament at Wave 1 was a significant predictor of higher levels of concurrent internalizing symptoms, β = .54, p < .01.
Tests of alternative explanations
Because our dimensional assessment of behavior inhibition also captures uninhibited temperament at the other end of the scale, one plausible alternative interpretation for our findings is that temperamentally uninhibited children largely account for the mediational role of behavioral flexibility. In other words, the findings may simply be a spurious result of the tendency for uninhibited children to exhibit poor behavioral flexibility and high levels of externalizing symptoms. Although EGT does propose that temperamentally uninhibited individuals should exhibit greater aggression and poor behavioral flexibility, it does not posit that the mediational role of behavioral flexibility is primarily or exclusively due to the small proportion of uninhibited children on the continuum. Therefore, to test this alternative explanation, we conducted our SEM model of externalizing symptoms again (i.e., Figure 2) after eliminating the subsample of children who were identified as temperamentally uninhibited. Between 10 and 15% of children are commonly identified as being uninhibited in previous samples (Henderson & Fox, 1998; Kagan, Reznick, & Snidman, 1987). Thus, consistent with prior procedures for identifying children who are very high in uninhibited temperament, our re-analysis excluded children who scored in the top 15% on our standardized latency to approach measure. The findings produced an identical pattern of significant findings for the hypothesized pathways with one exception: the serotonin genotype was no longer associated with children's inhibition at Wave 1, β = .08, ns. Notably, the dopamine genotype continued to predict Wave 1 inhibition, β = .19, p < .05, with inhibition, in turn, predicting greater behavioral flexibility at Wave 2, β = .20, p < .05. Behavioral flexibility at Wave 2 also remained a significant predictor of Wave 3 externalizing symptoms, β = −.19, p < .05. Therefore, the findings support the notion that the mediational role of behavioral flexibility in the pathway between inhibited temperament and diminished externalizing problems is not primarily attributable to the poor flexibility and behavior problems of children who are highly uninhibited
Another alternative interpretation is that our findings may be an artifact of an underlying curvilinear relationship between inhibition and behavioral flexibility such that the relatively large proportion of children who exhibit moderate levels of temperamental inhibition may evidence the greatest behavioral flexibility relative to highly inhibited or uninhibited children. To test this possibility, we re-ran our SEM model of externalizing after including a latent construct consisting of curvilinear terms (X2) for each of the three inhibition indicators. In further support of our hypotheses, the curvilinear term did not add unique explanatory power as a predictor. In addition, our original latent construct reflecting linear inhibition at Wave 1 continued to significantly predict Wave 2 behavioral flexibility, β = .20, p < .01.
Discussion
Evaluated in relation to the predominant efforts in psychological research to characterize the burdens of growing up with an inhibited temperament, Evolutionary Game Theory (EGT) is novel in its proposal that inhibited temperament confers developmental advantages and disadvantages within a lawful developmental cascade of processes. Although findings from animal studies support some of the proposed precursors and sequelae of inhibited temperament outlined within EGT, these pathways have yet to be systematically explored in human populations. As a first step in addressing this gap, the current study was designed to test hypotheses derived from EGT on the origins and consequences of inhibited temperament in a sample of racially diverse children who were exposed to elevated socioeconomic impoverishment and family aggression. First, in support of the proposed precursors of EGT, allelic variation in dopaminergic and serotonergic genes each uniquely predicted individual differences in a dimensional index of children's inhibited temperament. Second, consistent with predictions regarding developmental advantages, indices of behavioral flexibility over a one year period mediated links between their inhibited temperament at two years of age and subsequent decreases in externalizing symptoms over a two year period. Replicating the developmental disadvantages of inhibited temperament, findings also showed that children who were more inhibited experienced higher levels of concurrent internalizing symptoms.
In the context of the novel hypotheses generated by EGT, our preliminary objective was to provide a basis for comparing findings from an evolutionary approach with the existing literature on associations between inhibited temperament and child psychopathology. Although findings are not always replicated (e.g., Stifter, Putman, & Jahromi, 2008), previous research has shown that inhibition is a relatively consistent predictor of higher levels of internalizing symptoms (Degnan et al., 2010). In accord with these earlier results, observational measures of inhibited temperament in our study were concurrently associated with greater internalizing symptoms. Thus, the present findings corresponded well with earlier research on the psychological vulnerabilities experienced by children with inhibited temperaments.
Extending this knowledge base, we further examined whether behavioral flexibility served as a mediating mechanism in the link between behavior inhibition and lower externalizing symptoms. Consistent with the first link in the mediational pathway, EGT conceptualizes inhibition as part of a broader Dove phenotype characterized by thorough engagement in exploratory activities and resourceful and flexible problem-solving strategies that are exquisitely designed to address environmental challenges. Although the SEM analyses did not identify a concurrent association between inhibited temperament and behavioral flexibility at age 2, inhibition significantly predicted children's greater behavioral flexibility one year later. Thus, in the early preschool period, a single snapshot approach was insufficient in identifying putative adaptive behaviors associated with an inhibited temperament. Rather, in underscoring the value of a developmental framework, the results indicated that dimensions of inhibition and behavioral flexibility only emerged as a correlated suite of behaviors over time.
These findings raise the key question of why inhibited temperament appears to have a sleeper effect in subsequently increasing behavioral flexibility. Informed by an evolutionary perspective, the sensory process sensitivity model proposes that emotional reactivity may be an operative mechanism through which inhibited temperament may engender flexible planning and problem-solving (Aron et al., in press). A primary assumption is that inhibited temperament is part of a larger tendency to experience elevated emotional reactivity to both positive and negative stimuli. Heightened emotional reactivity, in turn, is specifically theorized to stimulate and regulate deeper processing, planning, and problem-solving strategies. Consistent with this assumption, research indicates that children with behavior inhibition exhibited heightened neurocognitive sensitivity to both threat and reward cues (e.g., Bar-Haim et al., 2009; Guyer et al., 2006; Hardin et al., 2006; Perez-Edgar et al., 2007). Although this pattern of sensitivity has been commonly construed as a “perturbation” (Bar-Haim et al., 2009), the interpretation of our findings within sensory processing sensitivity theory suggests it may have long-term adaptive advantages of increasing behavioral flexibility.
Consistent with calls to integrate developmental and evolutionary frameworks (Stamps & Groothuis, 2010), a developmental framework also offers a theoretical base for understanding why inhibited temperament is transformed into a newly organized pattern of behavioral flexibility during early preschool years. As key building blocks for behavioral flexibility, emotion regulation, persistent and intrinsic exploration, and resourceful problem solving are conceptualized as prominent stage-salient challenges during the toddler period (Cummings & Davies, 2010). Because these stage-salient tasks are formidable challenges for even the most developmentally advantaged toddlers, behavioral flexibility in the Dove phenotype may not fully emerge until children have had ample opportunity to master and integrate these skills. Thus, it is possible that temperamental inhibition to novelty may only be lawfully related to greater flexibility once children reach the preschool period when the underlying developmental capacities for behavioral flexibility are in place.
In addressing the second link of the mediational cascade, results of the SEM analyses revealed that children's behavioral flexibility predicted lower levels of externalizing symptoms one year later when children were four years old. These findings are consistent with the general developmental thesis that characteristics of behavioral flexibility may guide children toward more adaptive developmental trajectories. In keeping with this perspective, effective emotion regulation, intrinsic engagement in exploration, and use of flexible problem-solving strategies may serve as tools for effectively mastering subsequent socio-emotional and behavioral challenges as children grapple with the transition to formal schooling (Bierman, Torres, Domitrovich, Welsh, & Gest, 2009). However, the general focus on mastering developmental challenges as an explanatory mechanism does not readily account for the finding that behavioral flexibility predicted decreases in externalizing symptoms but not lower levels of internalizing problems. As an explanation for the specificity in the findings, evolutionary models have proposed that the resources acquired from behavioral flexibility may increase children's subsequent tendencies to adopt a risk-averse strategy characterized by specific decreases in bold and aggressive behavior rather than more general decreases in psychopathology (Sih & Bell, 2008; Wolf et al., 2007). Thus, the cautious and reflective responsiveness to environmental cues of the Dove phenotype may become increasingly canalized into a guarded, vigilant strategy for avoiding the costs of the risky, audacious behaviors encompassing externalizing symptoms.
Confidence in the identification of behavioral flexibility as a mediator of the relationship between behavior inhibition and lower levels of externalizing symptoms is strengthened by the estimation of autoregressive paths, inclusion of several covariates, and specification of additional analyses. In highlighting the role of sex as a potential third variable, research has shown that girls exhibit greater inhibited temperament and behavioral flexibility and lower levels of externalizing symptoms (e.g., Zahn-Waxler, Shirtcliff, & Marceau, 2008). Likewise, previous research on linkages among family discord and children's self-regulatory and externalizing problems raised the possibility that interparental and parenting difficulties may be common etiological mechanisms underlying some of the findings (Bernier, Carlson, & Whipple, 2010). Furthermore, if evolutionary models are correct in the assumption that the operative mechanism underlying the link between inhibited temperament and behavioral flexibility is heightened reflectiveness and sensitivity (Sih & Bell, 2008), then the results should remain significant after including intellectual ability and externalizing problems as predictors. Findings indicated that many of the covariates did serve as predictors of behavioral flexibility and externalizing problems. Nevertheless, behavioral flexibility continued to mediate the association between behavior inhibition and externalizing symptoms with all of the covariates included as predictors. Furthermore, because it is possible that findings of our linear analytic models may be artifacts of curvilinear relationships in mediational pathways among inhibited temperament, behavioral flexibility, and child externalizing problems, we explored these possibilities by conducting additional analyses that: (a) included a curvilinear inhibition term and (b) excluded uninhibited children from the analytic models. Notably, the mediational role of behavioral flexibility in our original analyses remained significant with these additional restrictions. Thus, this more authoritative analysis of EGT predictions suggests that behavior inhibition, in itself, carries unique advantages that cannot be attributed to children's sex, cognitive ability, earlier experiences with behavior problems and family discord, or the difficulties experienced by the highly uninhibited children in our sample
In an effort to identify the origins of the multiple developmental pathways involving inhibited temperament, another aim of this study was to test the EGT predictions that behavior inhibition is an endophenotype of polymorphisms in dopaminergic and serotonergic genes (Ellis et al., 2006; Korte et al., 2005). Our findings indicated that polymorphisms in genes regulating the expression of DAT and 5-HTT each uniquely predicted behavior inhibition when children were two years old. Alleles with lower transcriptional efficiency in the production of dopamine (i.e., absence of the 10-R allele of DAT 3’UTR VNTR and the C allele of DAT rs27072 and rs40184) and serotonin (i.e., S and LG alleles of 5-HTTLPR) transporters were associated with greater behavior inhibition. Examining dopamine and serotonin genotypes as predictors of multiple forms of functioning offered a level of precision in testing the hypothesis that inhibited temperament served as the specific endophenotype. The results indicated that polymorphisms in the dopamine and serotonin genes were only indirectly related to children's behavioral flexibility, externalizing problems, and internalizing symptoms through their association with inhibited temperament. Thus, by virtue of their stronger constitutional bases, early emerging temperamental attributes may serve as more proximal products of genetic and neurobiological variability than measures of personality and psychopathology (Van Gestel & Van Broeckhoven, 2003). On a more general level, these findings further highlight the value of increasing precision in the search for intermediate phenotypes to more closely approximate the specific actions of genes (Bellgrove & Mattingly, 2008). In the context of previous inconsistencies in the dopaminergic and serotonergic genetic underpinnings of temperament, personality, and adjustment, our significant findings may also reflect that the predictive role of genes may be more pronounced by virtue of the heightened demographic and family adversity experienced by children in our sample. For example, G × E research has revealed that the short 5-HTTLPR allele was the strongest predictor of behavior inhibition with peers during elementary school when family support was low (Fox, Nichols, et al., 2005).
Discussion of the limitations of our study is also necessary for a balanced interpretation of the findings. First, given that our constructs were all derived from measures during the children's visits to the laboratory, expanding assessments beyond the laboratory context (e.g., home assessments, parent reports) will be valuable in future efforts to replicate findings. In addressing a related measurement theme, our results should not be misconstrued as suggesting that children with more inhibited traits necessarily enjoy greater flexibility and protection from externalizing problems across multiple contexts and conditions. For example, in highlighting the significance of examining environmental characteristics as potential moderators of inhibited temperament, evolutionary frameworks underscore that the coupling of temperamental sensitivity and indices of developmental competence may breakdown under highly adverse proximal conditions (Ellis et al., 2006) or following protracted exposure to environmental threat (Belsky & Pluess, 2009). Thus, although it is important to note that our findings were obtained from a sample of children who experienced histories of psychosocial and economic adversity, our assessment of children's behavioral flexibility in a relatively benign context (e.g., no significant threat cues, presence of their mothers) may have maximized our power to identify developmental strengths associated with inhibited temperament.
Second, although our sample is more racially diverse and impoverished than many of the samples in previous studies, tests of the generalizability of these findings in other samples are an important direction for future research. Additional care should be exercised in generalizing the findings in light of our behavioral flexibility measures. Although the availability of mothers during the assessment of behavioral flexibility was designed to increase ecological validity by approximating young children's common experiences of coping with cognitive challenges in family contexts (e.g., Sroufe et al., 1990), we cannot rule out the possibility that indices of children's behavioral flexibility were partly a product of maternal caregiving quality. However, inclusion of parenting difficulties during the behavioral flexibility task as a covariate in the analyses did not alter the findings in our study. Thus, the likelihood that the mediational role of behavioral flexibility is an artifact of initial individual differences in parenting quality during the problem-solving tasks is reduced.
Third, although identifying even modest pathways involving the genetic underpinnings and advantageous outcomes of children with inhibited temperament is regarded as substantively and theoretically significant (Ellis et al., 2006), associations in the proposed cascade of processes were modest to moderate in magnitude (absolute βs ranging from .15 to .23). In accord with our findings, evolutionary models acknowledge that many children who are high in wariness and inhibition are not necessarily endowed with greater behavioral flexibility (Belsky & Pluess, 2009). For example, if greater sensitivity to stimuli is a key mechanism underlying the greater behavioral flexibility of children who exhibit high levels of temperamental inhibition, the tradeoff is that this same process may have anxiogenic effects that undermine planning and problem solving for some children under certain developmental conditions. Thus, inclusion of additional explanatory pathways and moderating conditions is an important next step in accounting for individual differences in the mediational pathways.
Despite these limitations, our study is the first attempt to systematically test the EGT conceptualization of the precursors and sequelae of inhibited temperament in a sample of children (Ellis et al., 2006; Korte et al., 2005). In the context of a developmental literature that has predominantly focused on identifying risks associated with behavior inhibition, our study was designed to break new ground by delineating the developmental advantages of growing up with an inhibited temperament. Consistent with EGT, results of our multi-level, multi-method study showed that children's behavior inhibition at two years of age was predicted by allelic variation in both dopamine transporter and serotonin transporter genes. Inhibited temperament, in turn, was associated with both disadvantages expressed through heightened internalizing symptoms and advantages characterized by increases in behavioral flexibility and ultimately decreases in externalizing symptoms. As part of a more complex developmental chain of processes, behavioral flexibility mediated the protective effects of inhibited temperament on the development of externalizing symptoms. Consistent with prior scientific calls (Stamps & Groothuis, 2010), our results highlight the value of integrating evolutionary models within a developmental framework to achieve a more comprehensive understanding of the sequelae of inhibited temperament.
Table 2.
Measurement Models for the SEM analyses in Figures 2 and 3: Standardized Loadings of the Manifest Indicators for Each Latent Construct
| Latent Construct | Manifest Indicators | Model 1 Loadings | Model 2 Loadings |
|---|---|---|---|
| T1 Behavior Inhibition | Inhibition | .96 | .95 |
| Fearful Distress | .82 | .82 | |
| Latency to Approach | .83 | .83 | |
| T1 Behavioral Flexibility | Coping | .69 | .69 |
| Enthusiasm | .90 | .90 | |
| Persistence | .86 | .86 | |
| Problem-Solving | .63 | .63 | |
| T2 Behavioral Flexibility | Coping | .85 | .85 |
| Enthusiasm | .94 | .94 | |
| Persistence | .92 | .92 | |
| Problem-Solving | .70 | .70 | |
| T1 Externalizing Problems | Experimenter 1 Q-Sort | .87 | -- |
| Experimenter 2 Q-Sort | .66 | -- | |
| Experimenter 1 C-TRF | .84 | -- | |
| T3 Externalizing Problems | Experimenter 1 Q-Sort | .95 | -- |
| Experimenter 2 Q-Sort | .75 | -- | |
| Experimenter 1 C-TRF | .84 | -- | |
| T1 Internalizing Problems | Experimenter 1 Q-Sort | -- | .77 |
| Experimenter 2 Q-Sort | -- | .71 | |
| Experimenter 1 C-TRF | -- | .67 | |
| T3 Internalizing Problems | Experimenter 1 Q-Sort | -- | .76 |
| Experimenter 2 Q-Sort | -- | .70 | |
| Experimenter 1 C-TRF | -- | .55 | |
| T1 Interparental Aggression | CTS Physical Assault | .79 | .79 |
| CTS Psychological Aggression | .86 | .85 | |
| IDI Aggression | .51 | .51 | |
| T2 Parenting Difficulties | Insensitivity | .89 | .88 |
| Warmth | −.80 | −.80 | |
| Quality Assistance | −.84 | −.85 |
Acknowledgments
This research was supported by the National Institute of Mental Health (R01 MH071256) awarded to Patrick T. Davies and Dante Cicchetti and the Spunk Fund, Inc. The project was conducted at Mt. Hope Family Center. The authors are grateful to the children, parents, and community agencies who participated in this project and to Michael Ripple, Crista Crittenden, and the rest of the Mt. Hope Family Center staff for their assistance on the project.
Contributor Information
Patrick T. Davies, University of Rochester
Dante Cicchetti, University of Minnesota and Mt. Hope Family Center, University of Rochester.
Rochelle F. Hentges and, University of Rochester.
Melissa L. Sturge-Apple, University of Rochester
References
- Achenbach TM, Rescorla LA. The Achenbach System of Empirically Based Assessment. In: Archer RP, editor. Forensic uses of clinical assessment instruments. Erlbaum; Mahwah, NJ: 2006. pp. 229–262. [Google Scholar]
- Aksan N, Kochanska G. Links between systems of inhibition from infancy to preschool years. Child Development. 2004;75:1477–1490. doi: 10.1111/j.1467-8624.2004.00752.x. doi: 10.1111/j.1467-8624.2004.00752.x. [DOI] [PubMed] [Google Scholar]
- Aron EN, Aron A, Jagiellowicz J. Sensory processing sensitivity: A review in the light of the evolution of biological responsivity. Personality and Social Psychology Review. doi: 10.1177/1088868311434213. in press. [DOI] [PubMed] [Google Scholar]
- Aylward GP. Measures of infant and early childhood development. In: Goldstein G, Beers SR, Hersen M, editors. Comprehensive handbook of psychological assessment, Vol. 1: Intellectual and neuropsychological assessment. Wiley; Hoboken, NJ: 2004. pp. 87–97. [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, Ernst M. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychological Science. 2009;20:1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. doi: 10.1111/j.1467-9280.2009.02401x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JE, Goodnight JA, Fite JE. Temperament and emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of emotions. 3rd ed. Guilford; New York: 2008. pp. 485–496. [Google Scholar]
- Bell AM. Future directions in behavioural syndromes research. Proceedings of the Royal Society. 2007;274:755–761. doi: 10.1098/rspb.2006.0199. doi: 10.1098/rspb.2006.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Mattingly JB. Molecular genetics of attention. Annals of the New York Academy of Sciences. 2008;1129:200–212. doi: 10.1196/annals.1417.013. doi: 10.1196/annals.1417.013. [DOI] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, Whipple N. From external regulation to self-regulation: Early parenting precursors of young children's executive functioning. Child Development. 2010;81:326–339. doi: 10.1111/j.1467-8624.2009.01397.x. doi: 10.1111/j.1467-8624.2009.01397.x. [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, Goldman D. Genetics of emotion. Trends in Cognitive Sciences. 2011;15:401–408. doi: 10.1016/j.tics.2011.07.009. doi: 10.1016/j.tics.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierman KL, Torres MM, Domitrovich CE, Welsh JA, Gest SD. Behavioral and cognitive readiness for school: Cross-domain associations for children attending Head Start. Social Development. 2009;18:305–323. doi: 10.1111/j.1467-9507.2008.00490.x. doi: 10.1111/j.1467-9507.2008.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block J. The Q-sort in character appraisal: Encoding subjective impressions of persons quantitatively. American Psychological Association; Washington, D.C.: 2008. [Google Scholar]
- Choukalas CG, Melby JN, Lorenz FO. Technical report: Intraclass correlation coefficients in SPSS. Institute for Social and Behavioral Research; Ames, IA: 2000. [Google Scholar]
- Cole DA, Maxwell SE. Testing mediational models with longitudinal data: Questions and tips in the use of structural equation modeling. Journal of Abnormal Psychology. 2003;112:558–577. doi: 10.1037/0021-843X.112.4.558. doi: 10.1037/0021-843X.112.4.558. [DOI] [PubMed] [Google Scholar]
- Congdon E, Constable RT, Lesch KP, Canli T. Influence of SLC6A3 and COMT variation on neural activation during response inhibition. Biological Psychology. 2009;81:144–152. doi: 10.1016/j.biopsycho.2009.03.005. doi: 10.1016/j.biopsycho.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens CM, de Boer SF, Koolhaas JM. Coping styles and behavioral flexibility: Towards underlying mechanisms. Philosophical Transactions of the Royal Society B. 2010;365:4021–4028. doi: 10.1098/rstb.2010.0217. doi: 10.1098/rstb.2010.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings EM, Davies PT. Children and marital conflict: The impact of family dispute and resolution. Guilford; New York: 2010. [Google Scholar]
- Davies PT, Sturge-Apple M, Cicchetti D. Interparental aggression and children's adrenocortical reactivity: Testing an evolutionary model of allostatic load. Development and Psychopathology. 23:801–814. doi: 10.1017/S0954579411000319. doi:10.1017/S0954579411000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D, Manning LG, Vonhold SE. Pathways and processes of risk in associations among maternal antisocial personality symptoms, interparental aggression, and preschooler's psychopathology. Development and Psychopathology. 2012;24:807–832. doi: 10.1017/S0954579412000387. doi:10.1017/S0954579412000387. [DOI] [PubMed] [Google Scholar]
- Degnan KA, Almas AN, Fox NA. Temperament and the environment in the etiology of childhood anxiety. Journal of Child Psychology and Psychiatry. 2010;51:497–517. doi: 10.1111/j.1469-7610.2010.02228.x. doi: 10.1111/j.1469-7610.2010.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Jackson JJ, Boyce WT. The stress response systems: Universality and adaptive individual differences. Developmental Review. 2006;26:175–212. doi: 10.1016/j.dr.2006.02.004. [Google Scholar]
- Enders CK. A primer on maximum likelihood algorithms available for use with missing data. Structural Equation Modeling. 2001;8:128–141. doi: 10.1207/S15328007SEM0801_7. [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. doi:10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Nichols KE, Henderson HA, Rubin K, Schmidt L, Hamer D, Ernst M, Pine D. Evidence for a gene-environment interaction in predicting behavioral inhibition in middle childhood. Psychological Science. 2005;16:921–926. doi: 10.1111/j.1467-9280.2005.01637.x. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia M, Clemente I, Dominguez-Borras J, Escera C. Dopamine transporter regulates the enhancement of novelty processing by a negative emotional context. Neuropsychologia. 2010;48:1483–1488. doi: 10.1016/j.neuropsychologia.2010.01.018. doi: 10.1016/j.neuropsychologia.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Human Genetics. 1997;101:243–246. doi: 10.1007/s004390050624. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Gizer IA, Ficks C, Waldman ID. Candidate gene studies of ADHD: A meta-analytic review. Human Genetics. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, Ernst M. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. The Journal of Neuroscience. 2006;26:6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Perez-Edgar K, Guyer AE, Pine DS, Fox NA, Ernst M. Reward and punishment sensitivity in shy and non-shy adults: Relations between social and motivated behavior. Personality and Individual Differences. 2006;40:699–711. doi: 10.1016/j.paid.2005.08.010. doi: 10.1016/j.paid.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA, Fox NA. Inhibited and uninhibited children: Challenges in school settings. School Psychology Review. 1998;27:492–505. [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Development. 1987;58:1459–1473. [PubMed] [Google Scholar]
- Kochanska G, Knaack A. Effortful control as a personality characteristic of young children: Antecedents, correlates, and consequences. Journal of Personality. 2003;71:1087–1112. doi: 10.1111/1467-6494.7106008. doi: 10.1111/1467-6494.7106008. [DOI] [PubMed] [Google Scholar]
- Koolhass JM, de Boer SF, Buwalda B. Stress and adaptation: Toward ecologically relevant animal models. Current Directions in Psychological Science. 2006;15:109–112. doi: 10.1111/j.0963-7214.2006.00417.x. [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neuroscience and Biobehavioral Reviews. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychological Methods. 1996;1:30–46. doi: 10.1037/1082-989X.1.1.30. [Google Scholar]
- MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: Program PRODCLIN. Behavior Research Methods. 2007;39:384–389. doi: 10.3758/bf03193007. doi: 10.3758/BF03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matas L, Arend RA, Sroufe LA. Continuity of adaptation in the second year: The relationship between quality of attachment and later competence. Child Development. 1987;49:547–556. [Google Scholar]
- Mehta MA, Hinton EC, Montgomery AJ, Bantick RA, Grasby PM. Sulpiride and mnemonic function: Effects of a dopamine D2 receptor antagonist on working memory, emotional memory and long-term memory in healthy volunteers. Journal of Psychopharmacology. 2005;19:29–38. doi: 10.1177/0269881105048889. doi: 10.1177/0269881105048889. [DOI] [PubMed] [Google Scholar]
- Melby JN, Conger RD. The Iowa Family Interaction Rating Scales: Instrument summary. In: Kerig PK, Lindahl KM, editors. Family observational coding systems: Resources for systemic research. Erlbaum; Mahwah, NJ: 2001. pp. 33–58. [Google Scholar]
- Mileva-Seitz V, Kennedy J, Atkinson L, Steiner M, Levitan R, Matthews SG, Fleming AS. Serotonin transporter allelic variation in mothers predicts maternal sensitivity behavior and attitudes toward 6-month-old infants. Genes, Brain and Behavior. 2011;10:325–333. doi: 10.1111/j.1601-183X.2010.00671.x. doi: 10.1111/j.1601-183X.2011.00753.x. [DOI] [PubMed] [Google Scholar]
- Nader K, LeDoux J. Inhibition of the mesoamygdala dopaminergic pathway impairs the retrieval of conditioned fear associations. Behavioral Neuroscience. 1999;113:891–901. doi: 10.1037//0735-7044.113.5.891. doi: 10.1037/0735-7044.113.5.891. [DOI] [PubMed] [Google Scholar]
- Newborg J, Stock JR, Wnek L, Guidubaldi J, Svinicki J. Battelle Developmental Screening Inventory Test. DLM-Teaching Resources; Allen, TX: 1984. [Google Scholar]
- Perez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, Ernst M. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Progress in Neurobiology. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Praschak-Rieder N, Kennedy J, Wilson AA, Hussey D, Boovariwala A, Willeit M, Meyer JH. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: A [11C] DASB positron emission topography study. Biological Psychiatry. 2007;62:327–331. doi: 10.1016/j.biopsych.2006.09.022. doi: 10.1016/j.biopsych.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Prior M, Smart D, Sanson A, Oberklaid F. Does shy-inhibited temperament in childhood lead to anxiety problems in adolescence? Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:461–468. doi: 10.1097/00004583-200004000-00015. doi: 10.1097/00004583-200004000-00015. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Stifter CA. Behavioral approach-inhibition in toddlers: Prediction from infancy, positive and negative affect components, and relations with behavior problems. Child Development. 2005;76:212–226. doi: 10.1111/j.1467-8624.2005.00840.x. doi: 10.1111/j.1467-8624.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- Reimold M, Smolka MN, Schumann G, Zimmer A, Wrase J, Mann K, Heniz A. Midbrain serotonin transporter binding potential measured with [11C]DASB is affected by serotonin transporter genotype. Journal of Neural Transmission. 2007;114:635–639. doi: 10.1007/s00702-006-0609-0. doi: 10.1007/s00702-006-0609-0. [DOI] [PubMed] [Google Scholar]
- Rommelse NNJ, Altink ME, Arias-Vasquez A, Buschgens CJM, Fliers E, Faraone SV, Oosterlaan J. A review and analysis of the relationship between neuropsychological measures and DAT1 in ADHD. American Journal of Medical Genetics. 2008;147:1536–1546. doi: 10.1002/ajmg.b.30848. doi: 10.1002/ajmg.b.30848. [DOI] [PubMed] [Google Scholar]
- Rothbart MK. Temperament in childhood: A framework. In: Kohnstamm GA, Bates JE, Rothbart MK, editors. Temperament and development. Wiley; Chichester: England: 1989. pp. 59–73. [Google Scholar]
- Rothbart MK. Becoming who we are: Temperament and personality in development. Guilford; New York: 2011. [Google Scholar]
- Rothbart MK, Mauro JA. Temperament, behavioral inhibition, and shyness in childhood. In: Leitenberg H, editor. Handbook of social and evaluation anxiety. Plenum; New York: 1990. pp. 139–160. [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Eisenberg N, editors. Handbook of child psychology: Vol. 3, Social, emotional, and personality development. 5th ed. Wiley; Hoboken, NJ: 1998. pp. 105–176. [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Eisenberg N, Damon W, Learner RM, editors. Handbook of child psychology: Vol. 3, Social, emotional, and personality development. 6th ed Wiley; Hoboken, NJ: 2006. pp. 99–166. [Google Scholar]
- Rueda MR, Rothbart MK. The influence of temperament on the development of coping: The role of maturation and experience. In: Skinner EA, Gembeck MJ, editors. Coping and the development of regulation. Jossey-Bass; San Francisco: 2009. pp. 19–31. [DOI] [PubMed] [Google Scholar]
- Schlomer GL, Bauman S, Card NA. Best practices for missing data management in counseling psychology. Journal of Counseling Psychology. 2010;57:1–10. doi: 10.1037/a0018082. doi: 10.1037/a0018082. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Rubin KH, Hu S, Hamer DH. Molecular genetics of shyness and aggression in preschoolers. Personality and Individual Differences. 2002;33:227–238. doi: 10.1016/S0191-8869(01)00147-7. [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Sharp SI, McQuillin A, Gurling HMD. Genetics of attention-deficit hyperactivity disorder (ADHD). Neuropharmacology. 2009;57:590–600. doi: 10.1016/j.neuropharm.2009.08.011. doi: 10.1016/j.neuropharm.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Shields A, Cicchetti D. Emotion regulation among school-age children: The development and validation of a new criterion Q-sort scale. Developmental Psychology. 1997;33:906–916. doi: 10.1037//0012-1649.33.6.906. doi: 10.1037/0012-1649.33.6.906. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell AM. Insights for behavioral ecology from behavioral syndromes. Advances in the Study of Behavior. 2008;38:227–281. doi: 10.1016/S0065-3454(08)00005-3. doi: 10.1016/S0065-3454(08)00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderqvist S, Bergman Nutley S, Peyrard-Janvid M, Matsson H, Humphreys K, Kere J, Klingberg T. Dopamine, working memory, and training induced plasticity: Implications for developmental research. Developmental Psychology. doi: 10.1037/a0026179. in press. [DOI] [PubMed] [Google Scholar]
- Sroufe LA, Egeland B, Kreutzer T. The fate of early experience following developmental change: Longitudinal approaches to individual adaptation in childhood. Child Development. 1990;61:1363–1373. doi: 10.1111/j.1467-8624.1990.tb02867.x. doi: 10.1111/j.1467-8624.1990.tb02867.x. [DOI] [PubMed] [Google Scholar]
- Sroufe LA, Egeland B, Carlson EA, Collins WA. The development of the person: The Minnesota study of risk and adaptation from birth to adulthood. Guilford; New York: 2005. [Google Scholar]
- Stamps JA, Groothuis TGG. Developmental perspectives on personality: Implications for ecological and evolutionary studies of individual differences. Philosophical Transactions of the Royal Society B. 2010;365:4029–4041. doi: 10.1098/rstb.2010.0218. doi: 10.1098/rstb.2010.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifter CA, Putnam S, Jahromi L. Exuberant and inhibited toddlers: Stability of temperament and risk for problem behavior. Development and Psychopathology. 2008;20:401–421. doi: 10.1017/S0954579408000199. doi: 10.1017/S0954579408000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The revised conflict tactics scale (CTS2): Development and preliminary psychometric data. Journal of Family Issues. 1996;17:283–316. doi: 10.1177/019251396017003001. [Google Scholar]
- Van Gestel S, Van Broeckhoven C. Genetics of personality: Are we making progress? Molecular Psychiatry. 2003;8:840–852. doi: 10.1038/sj.mp.4001367. doi: 10.1038/sj.mp.4001367. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. doi: 10.1016/S0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Wellman HM, Lane JD, LaBounty J, Olson SL. Observant, nonaggressive temperament predicts theory-of-mind development. Developmental Science. 2011;14:319–326. doi: 10.1111/j.1467-7687.2010.00977.x. doi: 10.1111/j.1467-7687.2010.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LK, McDermott JM, Degnan KA, Henderson HA, Fox NA. Behavioral inhibition and anxiety: The moderating roles of inhibitory control and attention shifting. Journal of Abnormal Child Psychology. 2011;39:735–747. doi: 10.1007/s10802-011-9490-x. doi: 10.1007/s10802-011-9490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, van Doorn GS, Leimar O, Weissing FJ. Life-history trade-offs favor evolution of animal personalities. Nature. 2007;447:581–584. doi: 10.1038/nature05835. doi: 10.1038/nature05835. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: Gender and Psychopathology. Annual Review of Clinical Psychology. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]