Abstract
This review is focused on the expression and regulation of amiloride-sensitive sodium channels in the epithelial cells of the aldosterone-sensitive distal nephron (ENaC) and amiloride-sensitive sodium channel activity in vascular endothelial and smooth muscle cells. Guyton’s hypothesis stated that blood pressure control is critically dependent on vascular tone and fluid handling by the kidney. With the study of Mendelian forms of hypertension and their corresponding transgenic mouse models, the main components of the aldosterone- and angiotensin-dependent sodium transporters have been identified over the past 20 years. Proteolytic processing of the ENaC external domain, and inhibition by increased sodium concentrations are important features of the ENaC complexes expressed in the distal nephron. In contrast, amiloride-sensitive sodium channels expressed in the vascular system are activated by increased external sodium concentrations, resulting in changes in the mechanical properties and function of endothelial cells. Mechano-sensitivity and shear stress affect both epithelial and vascular sodium channel activity. The synergistic effects and complementary regulation of the epithelial and vascular systems are consistent with the Guytonian model of volume and blood pressure regulation, and may reflect sequential evolution of the two systems. The integration of vascular tone, renal perfusion and regulation of renal sodium reabsorption is the central underpinning of the Guytonian model. We summarize the recent evidence in this review that describes the central role of amiloride-sensitive sodium channels in the efferent (e.g., vascular) and afferent (e.g., epithelial) arms of this homeostatic system.
Introduction
Sodium (Na+) transport in the distal nephron is mediated by epithelial Na+ channels (ENaCs), which are expressed in the apical cellular membranes.1–3 The regulated reabsorption of filtered Na+ by the nephron has a key role in the regulation of extracellular fluid volume and blood pressure.4 The role of these channels in the control of blood pressure is highlighted by gain-of-function mutations (Liddle syndrome) and loss-of-function mutations (pseudohypoaldosteronism type I) that are associated with increases or decreases in blood pressure, respectively.5–10
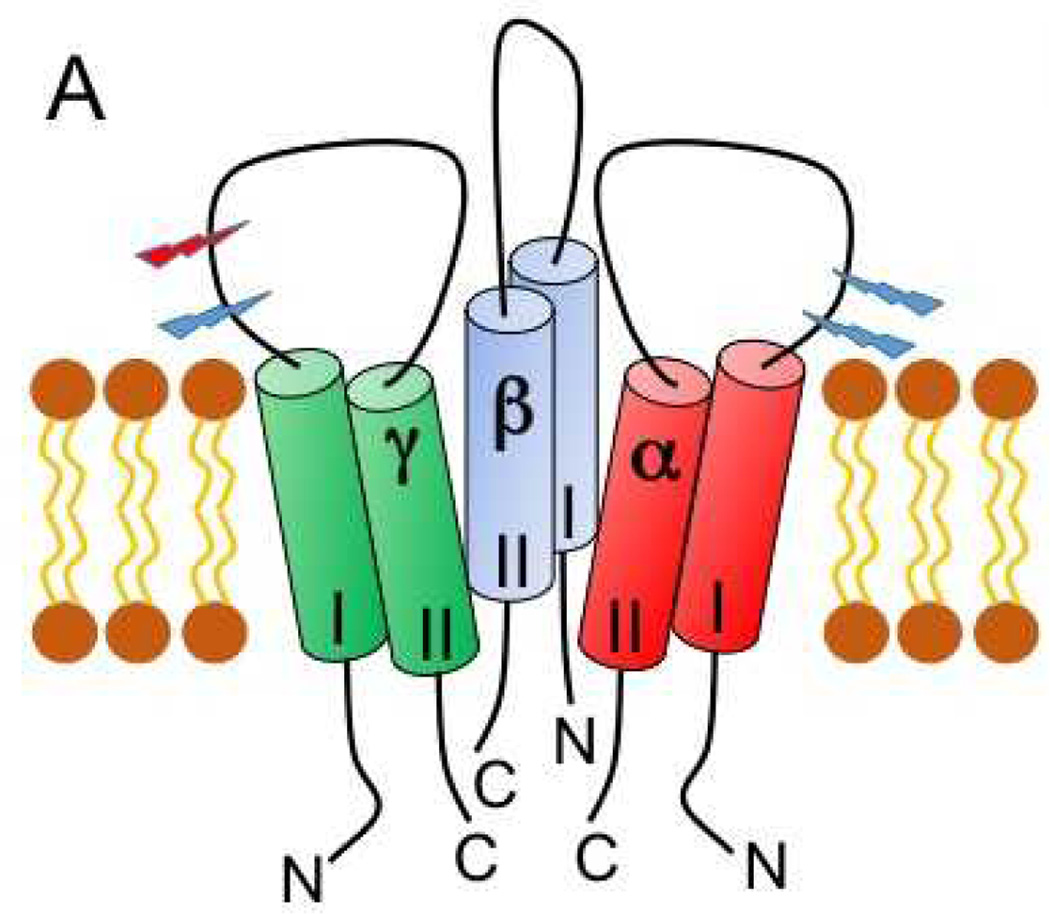
ENaCs also facilitate Na+ transport across airway and alveolar epithelia, where they have roles in modulating the volume of fluids in airway and alveoli.11–13 These channels are found in other epithelia and other cell types, including vascular endothelial cells and vascular smooth muscle.14, 15 ENaCs are members of the ENaC/Degenerin family of cation-selective ion channels.16, 17 As shown in Figure 1, they are comprised of three subunits, termed α, β and γ, which share similar structural features with two transmembrane domains separated by a large extracellular region, and short cytoplasmic amino- and carboxyl-terminal tails.3, 17–20
Figure 1. Heteromeric architecture and subunit structure of ENaC.
A topology model of the trimeric architecture of ENaC. The second transmembrane domain (II) line of each subunit lines the pore, and the first transmembrane domain (I) faces the membrane. Extracellular regions are shown as loops. Intracellular amino- (N) and carboxyl- (C) termini are indicated. Light blue stepped arrowheads are placed where furin cleaves the α and γ subunits, whereas a red arrowhead identifies the location of γ subunit cleavage by other proteases. Defined extracellular domains are labeled and differentially colored.
This review will focus on ENaCs expressed in the kidney and amiloride-sensitive Na+ channels expressed in vascular endothelial and smooth muscle cells. Our primary goal is to place the regulation of vascular tone into context with regulation of vascular Na+ channel activity, with a focus on the similarities and differences between ENaCs and the vascular amiloride-sensitive Na+ channel activity, and consideration of the complementary roles both systems play in the regulation of vascular tone, volume homeostasis and blood pressure.
Expression and Regulation of ENaC in the Distal Nephron
The cells of the distal convoluted tubule, connecting tubule and collecting duct are the main sites involved in hormonal regulation of ENaC activity. Aldosterone plays a critical role in achieving Na+ and potassium (K+) balance by controlling Na+ reabsorption and K+ secretion in the distal nephron. Glucocorticoid receptors are ubiquitously expressed in the glomerulus and the entire nephron whereas mineralocorticoid receptors are expressed in specific segments of the distal nephron. Studies using highly specific antibodies demonstrated that mineralocorticoid receptors are co-expressed with glucocorticoid receptors in the distal nephron.21 Mineralocorticoid specificity is insured by the co-expression of 11-beta-hydroxysteroid dehydrogenase, type 2 (11β-HSD2), which metabolizes cortisol and corticosterone to inactive metabolites, preventing the illicit occupation of mineralocorticoid receptors by glucocorticoids. There may also be important differences, based on cell culture models22 as well as in vivo23, 24 studies in acute responses to aldosterone mediated via mineralocorticoid receptors compared to later or more tonic effects of aldosterone that could even be mediated via glucocorticoid receptors. Based on the criteria of co-expression of mineralocorticoid receptors and 11β-HSD2, the Aldosterone Sensitive Distal Nephron (ASDN) has been defined as the distal part of the distal convoluted tubule, connecting tubule and collecting tubule.4, 25
Na+ and K+ balance and regulation of blood pressure are achieved through the final regulation of transport in the distal nephron and the collecting duct under hormonal control (aldosterone, vasopressin, angiotensin, insulin, bradykinin, endothelin, etc.).4 Mechanisms by which these hormones regulate renal ENaCs have been the focus of numerous reviews.2, 3, 9, 26–29 Aldosterone increases expression of the α subunit as well as many other proteins, including the serum and glucocorticoid regulated kinase (SGK1), and the adaptors glucocorticoid-induced leucine zipper 1 (GILZ1) and connector enhancer of kinase suppressor of Ras isoform 3, which enhance surface expression of channels.2, 30–35
The renin-angiotensin is well described as the primary regulator of aldosterone secretion in response to volume challenges. Recent work has described all of the components of this system along the nephron,36, 37 and the regulation of ENaC activity in the ASDN via apically located angiotensin II, type 1 receptors.38 Another potentially important feature would be “cross-talk” between the apically expressed AT1 receptor and the cytoplasmic mineralocorticoid receptor, mediated by kinase phosphorylation cascades.39–42 The responsiveness of the luminal renin activity expressed in the connecting tubule to changes in dietary Na+ intake is a notable aspect of the intra-renal renin-angiotensin system.36
ENaC regulation: Internalization and degradation
Ubiquitination of ENaCs at the plasma membrane targets channels for internalization and degradation.43, 44 Protein interacting modules, “WW” domains within the E3 ubiquitin ligase NEDD4-2 and PY motifs in the carboxyl termini of ENaC subunits, facilitate interactions between these two protein complexes.44–46 Both SGK1 and GILZ1 reduce the extent of channel ubiquitination, enhancing channel surface expression.2, 44 Phosphorylation of NEDD4-2 by SGK1 or 14-3-3 adaptor proteins recruited to NEDD4-2 by vasopressin, hinders interactions between NEDD4-2 and ENaC subunits, thus prolonging the dwell time of ENaCs in the apical membrane.47–51 Mitogen activated protein (MAP) kinase signaling via ERK1/2 enhances interactions between NEDD4-2 and ENaC subunits, which is thought to be due to ERK1/2 dependent phosphorylation of carboxyl terminus of the β and γ subunits of ENaC.2, 52, 53 GILZ1 inhibits Raf early in the MAP kinase signaling pathway,54 and also recruits SGK1 to a complex with ENaC and other regulatory components.2 Cell-based studies suggest that internalized ENaCs are modified by a number of de-ubiquitinating enzymes, which enhances the recycling of internalized channel to the plasma membrane.55–62
Other cytoplasmic factors have important roles in regulating channel activity. The interaction of specific inositol phospholipids (phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylinositol 3,4,5-triphosphate (PIP3)) with basic cytoplasmic residues enhances channel activity, and regulatory cascades that alter expression of these inositol phospholipids affect channel activity.63–66 For example, purinergic P2Y receptor activation inhibits ENaCs in association with the activation of phospholipase C and reduction in membrane PIP2.67–69 Modification of channel subunits by palmitoylation activates the channel, possibly by conformation changes induced by the interaction of palmitate with the plasma membrane.70, 71
Extracellular domains and Proteolytic processing of ENaC
The resolved crystal structure of an acid sensing ion channel (ASIC1),72–75 a member of this family, revealed that the large extracellular region is a highly organized structure. Recent studies suggest that the extracellular regions of ASICs and ENaCs share similar structural features.17, 76, 77 ENaC activity is regulated by extracellular factors that modulate channel gating through interactions at sites within the extracellular region of ENaC subunits.78, 79 For example, channels that have not undergone proteolytic processing have very low activity at the cell surface.80, 81 Specific proteases activate ENaCs by cleaving the α or γ subunits at defined sites in the extracellular domains.17, 77, 78, 80, 82 Furin is a serine protease that resides primarily in the trans-Golgi network and processes proteins moving through the biosynthetic pathway.83 The α subunit is cleaved twice by furin leading to moderate channel activation.84 The γ subunit is cleaved only once by furin, and must be cleaved by a second protease to further activate the channel.82 A number of proteases, including prostasin, TMPRSS4, matriptase, elastase, kallikrein, chymotrypsin and plasmin have been shown to activate the channel by cleaving the γ subunit.78, 82, 85–90 The αW493R variant,91 the γ L511Q variant, 3 and the βV348M variant92 show less activation by of chymotrypsin, and increased baseline channel activity. Cysteine, alkaline and metaloproteases also activate ENaC.88, 93–95
At present, the key proteases that cleave and activate the channel in different physiologic states remain to be defined. Proteases often work in functional cascades, and it will be important to determine which functional protease cascades are involved in channel activation. Prostasin and kallikrein may have roles in activating the channel in the setting of volume depletion or in response to aldosterone.96 Plasminogen is filtered by damaged glomeruli and is cleaved by tubular urokinase to plasmin, which directly cleaves and activates ENaCs.87, 97, 98 In addition, plasmin can activate ENaCs by cleaving and activating prostasin.99 Protease activation of ENaCs provides a potential explanation for the renal Na+ retention that occurs in some individuals with nephrotic syndrome despite suppression of the renin angiotensin system due to activation of distal Na+ transport mechanisms.97, 100
Extracellular and intracellular Na+ inhibit ENaC activity
Inhibition of ENaC activity by increased extracellular Na+ is referred to as Na+ self-inhibition, and reflects low affinity interactions between Na+ and sites in the extracellular region of ENaC.17, 101–103 ENaC activity is also inhibited by increased intracellular Na+ concentrations, with a reduction in open probability and surface expression, referred to as “feedback inhibition” or “channel run-down”.104–106 These mechanisms play a role in modulating channel activity in the distal nephron, where reductions in the urinary Na+ concentration to low millimolar ranges facilitate channel activation, and increases in extracellular Na+ with increased Na+ entry inactivates ENaCs and reduces Na+ reabsorption. A loss of Na+ self-inhibition has been observed with some channel-activating human ENaC variants. For example, the γL511Q variant3 and the aW493R variant91 show increased baseline channel activity, reflecting increased open probability, and reduced Na+ self-inhibition response, as well as reduced activation by proteases. These inhibitory effects of Na+ suggest that reduced ENaC activity by increased urinary Na+ concentration facilitates urinary Na+ excretion in response to a high Na+ diet, and suggests that a loss of Na+ self-inhibition could increase the risk of developing salt-sensitive hypertension.
Shear Stress and Mechano-sensitivity
Several members of the ENaC/Degenerin family, including ENaC, are mechano-sensitive channels.16, 107, 108 ENaCs are exposed to varying rates of tubular flow and laminar shear stress. Isolated perfused tubules respond to increases in the rate of tubular perfusion with an increase in amiloride-sensitive Na+ absorption mediated by ENaCs.109 Channel activation by shear stress has also been shown in heterologous systems, such as Xenopus oocytes, and at a single channel level with an outside-out patch configuration.102, 110 Shear stress appears to be sensed by the large extracellular region, where it induces a conformational change that is transmitted to the channel gate leading to an increase in channel activity.108, 111–113 While shear stress directly activates ENaCs, increases in distal flow leads to a release of cellular ATP that likely dampens flow-dependent channel activation through stimulation of P2Y2 receptors.69, 114 It has been suggested that the flow-induced increase in ENaC activity facilities K+ secretion that is also activated by increases in tubular flow rates.115
Role of ENaCs in the development of salt-sensitive hypertension
Mutations in the β or γ subunit genes (SCNN1B and SCNN1G) that either truncate the cytoplasmic carboxyl terminus with a loss of the PY motif, or result in a missense mutation of a key residue in the PY motif have been described in individuals with Liddle syndrome.5, 6, 8 As discussed above, a loss of the PY motif disrupts the binding of NEDD4-2 to the channel, leading to a reduction of channel ubiquitination and an increase in channel surface expression and activity.43, 44 These mutations result in robust channel activation and early onset hypertension that is notably impacted by dietary salt intake.8, 116, 117 An important question is whether there are other ENaC gene variants that activate the channel and increase the risk of salt-sensitive hypertension. There has been a dramatic increase in the number of sequenced genes that are available in databases. Currently there are close to 4,000 variants in human ENaC genes, including single nucleotide polymorphisms (SNPs), insertions and deletions. Some SNPs change an amino acid, and several of these have been shown to alter ENaC activity when expressed in heterologous systems.3, 92, 118–121 Several variants in the extracellular region, including αW493R, γL511Q and βV348M led to large increases in channel activity.3, 91, 92 Functional studies have shown the enhanced activity of the γL511Q and αW493R variants is due, in part, to a loss of Na+ self-inhibition.3, 91 There are other sites where mutations will likely increase channel activity, such as sites in the transmembrane domain that are in the vicinity of the channel gate. Important questions that have not yet been addressed are whether specific channel activating variants, in addition to those described in Liddle syndrome, increase the risk of salt-sensitive hypertension, and whether hypertension in individuals with the variants would respond to an ENaC inhibitor such as amiloride. In addition to ENaC variants that affect channel activity, it is likely that selected variants in genes whose products regulate ENaC activity may increase the risk of salt-sensitive hypertension.4, 122, 123
A SNP variant has been described in the first exon of human NEDD4 that corresponds to a cryptic splice site, which form a frame-shifted transcript.124. This SNP has been associated with hypertension in Japanese males,125 and Swedish males and females.126 This variant leads to 2 isoforms of NEDD4 (with or without the C2 domain) that affect the subcellular targeting and interactions between ENaC and NEDD4.127
Expression and Regulation of Amiloride-Sensitive Na+ Channels in the Vasculature
Besides the ASDN, the mineralocorticoid hormone aldosterone targets the vascular endothelium and smooth muscle cells, regulating vaso-reactivity through several processes involving ion channel modulation. In the endothelium, it swells,128 stiffens129, 130 and renders endothelial cells sensitive to increases in extracellular Na+ concentration.15, 131 These endothelial effects of aldosterone action appear to be mediated by amiloride-sensitive Na+ channels.129 Several groups have shown that vascular endothelial cells express amiloride-sensitive Na+ channel activity.130, 132, 133 There is not yet sufficient molecular evidence to conclude that these channels are different from ENaC, but because of the striking differences in response to changes in external Na+ concentrations, we will refer to the endothelial cell amiloride-sensitive Na+ channel activity as “EnNaC”. Furthermore, endothelial cells express mineralocorticoid receptors,134–136 as well as 11-βHSD2, which confers mineralocorticoid specificity,137 although the relative activity of 11-βHSD2 at the local tissue level in vascular cells is an important issue that needs to be further developed.138 Endothelial expression and membrane insertion of EnNaCs is enhanced by aldosterone.139–141 Amiloride is associated with inactivation of EnNaC activity at the cell surface.140 Thus, vascular endothelial cells possess all the components for aldosterone-mediated Na+ transport across the plasma membrane.
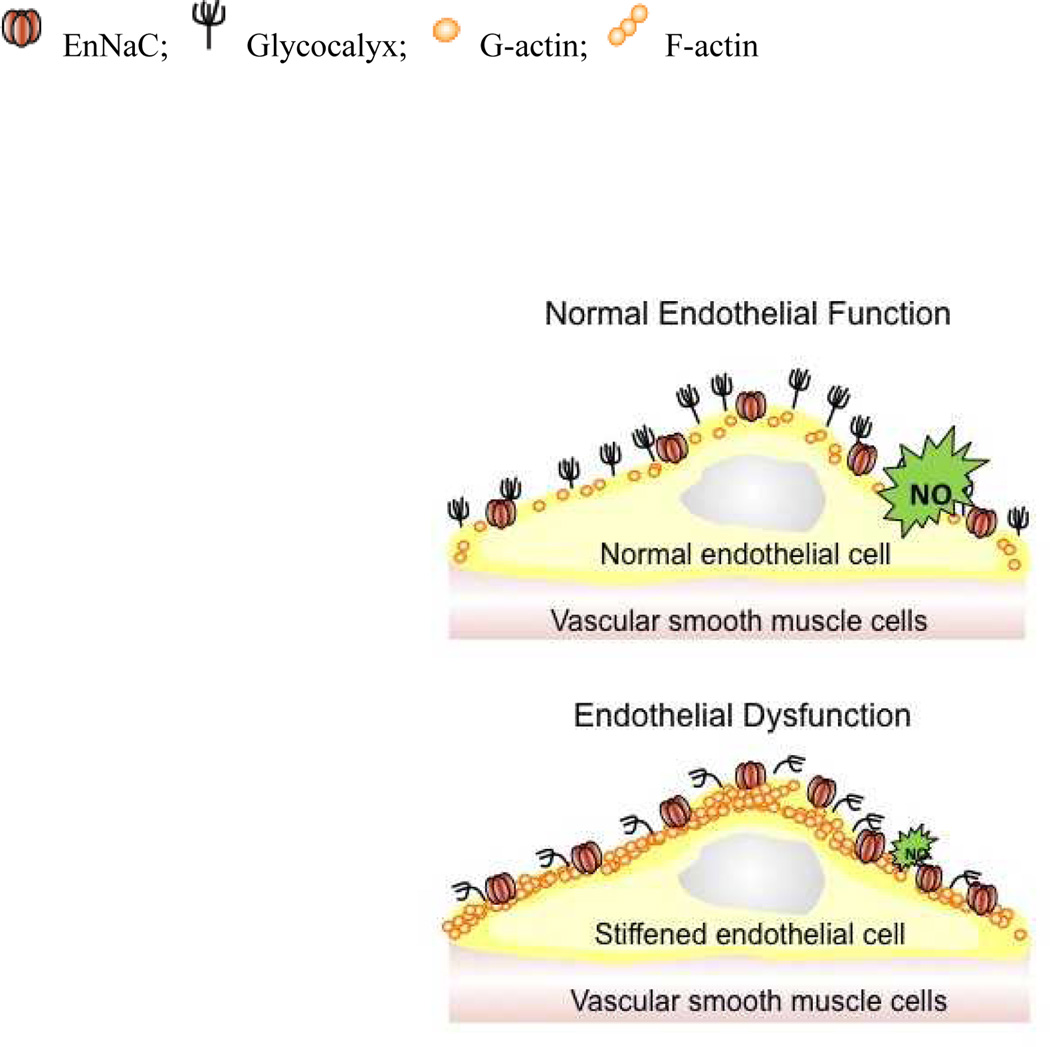
In contrast to the “tight” epithelia in the ASDN, 1, 2, 4, 142, 143 the vascular endothelium is a “leaky” barrier, with well described fenestrae.144 Therefore, passive electrochemical gradients and Starling forces drive most of the net Na+ transport across the vascular wall via paracellular pathways. While EnNaCs may not play any role in net Na+ transport across the vascular wall, they appear to have an important role in controlling the mechanical properties (i.e., stiffness) of endothelial cells (Figure 2). There is a surface layer, the endothelial glycocalyx, which is a negatively charged biopolymer known to function as an effective Na+ buffering system.14 It was shown that acute increases in ambient Na+ concentrations derange the glycocalyx in parallel to the increased EnNaC abundance in the plasma membrane and increased Na+ entry.15 Additionally, this layer could dampen shear-stress modulation of EnNaC activity, proving another mechanisms by which acutely increased ambient Na+ concentrations and deranged glycocalyx could modulate plasma membrane EnNaC activity.
Figure 2. Model of EnNaC-dependent transition from endothelial function to dysfunction.
Normally, the endothelial cell is protected by a well-developed glycocalyx and EnNaC membrane expression is low. Thus, the access of Na+ into the endothelial cell is limited, NO is released and vasodilation is maintained. Increased EnNaC membrane abundance together with deranged glycocalyx,14, 15 facilitate Na+ entry into endothelial cells and triggers the polymerization of G-actin to F-actin. As a result, NO release is reduced and the plasma membrane and immediate sub-membrane compartment “stiffens”.
Shortly after ingestion of a meal with 6 grams of added salt, normal volunteers have acute increases in blood pressure that correlates with acute increases in plasma Na+ concentration: a 1 mM increase was associated with a 1.9 mmHg increase in systolic blood pressure.145, 146 Plasma Na+ concentrations are chronically increased approximately 1–3 mM in hypertensive individuals and patients with primary aldosteronism.145, 147 Patients with moderately severe chronic kidney disease may have volume expansion as evidenced by left atrial enlargement in association with serum Na+ concentrations >140 mM.148 Using an atomic force microscope (AFM) as a nanosensor, the mechanical properties of endothelial cells have been probed in the presence of aldosterone and acute changes in extracellular Na+ concentrations within the physiological range.131 Within minutes, acute elevation of Na+ concentrations from 135 mM to 145 mM rapidly increased endothelial cell stiffness, and this response was blocked by amiloride. Recent observations of Na+-mediated regulation of surface expression of EnNaCs showed that exposure to 150 mM Na+ doubled the expression of EnNaCs at the endothelial cell surface. Although these changes in ambient Na+ concentration are far greater than the usual variations in serum Na+ concentration, these findings have been interpreted as showing that endothelial EnNaC activity is controlled by a “feed-forward mechanism”, presumably by some sort of extracellular Na+ sensor.149 This phenomena is completely opposite to what is observed with ENaC activity in the ASDN where increased Na+ concentrations (“self-inhibition” and “feed-back regulation”) reduce ENaC activity.104–106, 150 An important caveat is that the electrophysiologic properties of EnNaC have not been characterized. It will be important to define their cation selectivity and amiloride sensitivity in the activated state.
The underlying mechanism of Na+-induced EnNaC membrane insertion has not been defined. The combination of aldosterone and acute increases in ambient Na+ concentrations from 135 to 145 mM appear to trigger the rapid membrane insertion of preformed EnNaC complexes residing in the membrane of vesicles underneath the plasma membrane.141 By analogy to the regulation of ENaC surface expression and activity,2, 26, 30–33 increased expression of αEnNaC subunits as well as other proteins, including SGK1, and GILZ1 that inhibit Nedd4 activity, would enhance surface expression of EnNaC complexes. This is an important area for further work; at present, there is only a single report describing Nedd4 in endothelial cells.151
Role of amiloride-sensitive Na+ channels in shear stress and mechano-sensitivity
There is accumulating evidence that endothelial ENaCs may play a physiological role in the control of cellular mechanics and peripheral vascular resistance.131, 152, 153 By studying the mechanical properties of endothelial cells in vitro and ex vivo with AFM, a positive correlation has been observed between expression of EnNaCs and mechanical stiffness of the 50 to 200 nm cytoplasmic zone directly beneath the apical plasma membrane of endothelial cells (“cellular cortex”).154
Mechanical stiffness, i.e. the resistance of a structure against deformation, reflects the physiological state of a vascular endothelium. Stiffness, in quantitative terms, both controls and reflects cellular responses to biochemical and biophysical signals. Of note, the activity of the endothelial nitric oxide synthase is tightly coupled to shear stress exerted by the pulsatile blood flow.155 It was found that nitrous oxide (NO) release is dramatically decreased152 when endothelial cells stiffen;131 decreased NO release is a hallmark of endothelial dysfunction.156 Na+ entry via EnNaCs influences endothelial nitric oxide synthase activity and NO production, possibly via the PI3K/Akt signaling pathway.157, 158 The effects of reactive oxygen species on NO activity has not been explored when EnNaC activity is stimulated by increased extracellular Na+. Long-term treatment with mineralocorticoid receptor antagonists ameliorates the endothelial dysfunction159 that has been associated with EnNaC activation.160
Up to now it is not clear if endothelial stiffness as described above is directly linked to peripheral arterial stiffness. However, it was shown that the vascular endothelium plays a crucial role in vascular tone and consequently in arterial stiffness. Endothelium-dependent relaxation or dilation in response to mechanical forces such as shear stress caused by blood flow is due to the release of EDRF (endothelial-derived relaxing factor) or EDHF (endothelial-hyperpolarizing factor). In this context, local inhibition of EDRF and EDHF pathways in humans prevented the decrease in in smooth muscle tone and wall stiffness of the radial artery.161
There are well described interactions between ENaC with F-actin, and other cytoskeletal proteins such as spectrin or cortactin.45, 162–164 Endothelial stiffening mediated by increased EnNaC activity appears to be related to the interaction of the C-terminus of the α subunit with F-actin in the sub-membraneous cortical cytoskeleton of endothelial cells.163 It has been postulated that interaction of EnNaC with F-actin and other proteins of the cortical cytoskeleton leads to the development of a sub-membraneous web, which determines the mechanical stiffness of this subcellular compartment that immediately underlies the surface of the plasma membrane. In this context it was shown that F-actin (forming a gel-like cell cortex) stiffens, while G-actin (forming a sol-like cortex) softens the cortex.165 Increased EnNaC activity in the endothelial plasma membrane enhances the Na+ influx into the cell, and may stabilize F-actin through strengthening of the inter-subunit contacts of the protein.166 Therefore, it is not just the presence of ENaCs in the endothelial membrane but rather their activity that influences cell mechanics and determines endothelial stiffness (Figure 2).
Amiloride-sensitive Na+ channels in vascular smooth muscle cells
ENaC subunits have also been described in vascular smooth muscle cells, suggesting that amiloride-sensitive Na+ channels could have a role in mechano-sensing and control of peripheral vascular resistance.167 These channels are not the prototypic ENaCs observed in epithelia. While vascular smooth muscle cells express β and γ subunits of ENaC, alpha ENaC subunit expression is debated: while α subunit expression was shown by western-blot and immunocytochemistry in rat mesenteric arteries,157 but not in rat renal and cerebral arterial segments.165. It is apparent that more complete characterization of the subunits of the amiloride-sensitive Na+ channel complexes is a matter of high priority. For example, mechano-activated whole cell Na+ currents have been described in vascular smooth muscle cells.168 These currents were reduced when β subunit expression was reduced, but it is not known if these mechano-activated whole cell currents are amiloride sensitive. Vascular smooth muscle cells also express ASIC subunits, and it has been suggested that the mechano-sensory channel complexes might be composed of a combination of ENaC and ASIC subunits.14, 169 Mineralocorticoid receptors are also expressed in smooth muscle cells and have been proposed to have an important role in regulating myogenic tone.14, 170, 171 Whether amiloride-sensitive Na+ channels are regulated by aldosterone in vascular smooth muscle cells has not been defined.
Physiological role of vascular amiloride-sensitive Na+ channels in vivo
Pharmacological approaches have been used to assess the role of amiloride-sensitive Na+ channels in vascular reactivity. Contractility studies of ex vivo rat mesenteric arteries have shown that acute treatment with amiloride (or benzamil) decreases the vaso-constrictive response to phenylephrine and serotonin, suggesting a role for amiloride-sensitive Na+ channels in the vaso-constrictor response.157 However chronic amiloride administration did affect concentration response curve to phenylephrine in renal interlobar arteries of hypertensive rat overexpressing murine renin.172 Pressure-induced constriction is an important response in certain blood vessels that is referred to as “myogenic tone” or “autoregulation”. It is mediated by vascular smooth muscle cells and in small arteries and arterioles in the cerebral, mesenteric, cardiac, and renal circulation. Benzamil or amiloride abolishes the myogenic constriction in renal interlobar arteries in a concentration-dependent manner.173
Additional insights into the contribution of EnNaCs to vascular tone have been obtained in genetically modified mouse models. Increased EnNaC surface expression and endothelial stiffness have been observed in the aorta of mice expressing a truncated βENaC (similar to the Liddle mutation).,141, 154 This recapitulates the ex vivo experiments in cultured endothelial cells describe above. Mice expressing low levels of β ENaC in the lung, kidney, and vascular smooth muscle cells,174 demonstrated a 50% reduction in pressure-induced vasoconstriction in middle cerebral arteries.175 The use of mouse models with conditional, cell-specific inactivation or activation of the different amiloride-sensitive Na+ channel subunits in the vasculature would help to dissect the cell specific role of EnNaC subunits.
Consequences of altered EnNaC function in the renal vasculature
Impaired autoregulation of renal hemodynamics, marked by impaired natriuresis in response to increases in systemic blood pressure have been associated with chronic angiotensin II infusion,176 and increased activity of the renin-angiotensin system.177, 178 Endothelin A receptor activation is pro-inflammatory and pro-hypertensive.179 Medullary endothelin B receptor activation is associated with increased renal Na+ excretion, an effect that is blunted by Angiotensin II.180 Impaired salt excretion would thus occur in the setting of over-activity of the renin-angiotensin-aldosterone system, and an imbalance between endothelin A and endothelin B receptor activation. Salt-sensitive hypertension is associated with impaired sodium excretion and inflammatory infiltrates in the tubulo-interstitium.181
Global β ENaC inactivation is associated with defective myogenic autoregulation of renal blood flow.182 This loss of autoregulation was also associated with increased inflammatory markers and increased systemic blood pressure in the mouse model with reduced βENaC activity,183 suggesting a linkage between vascular amiloride-sensitive Na+ activity and inflammation in several pathophysiological disorders. It has also been proposed that loss of baroreflex sensitivity in the βENaC-deficient mouse model can increase blood pressure variability and pressure swings. The loss of the myogenic constrictor response (due to loss of vascular smooth muscle βENaC activity) blunts autoregulation, and permits arterial pressure fluctuations to be transmitted to the microvasculature, thus increasing the susceptibility to renal injury and hypertension.184 .
Do vascular amiloride-sensitive Na+ channels play a role in hypertension?
Recent pharmacologic efforts have focused on development of amiloride analogues,185 non-steroidal mineralocorticoid receptor antagonists,186 and lower dose studies of mineralocorticoid receptor blockers that may preferentially inhibit extra-renal ENaC and mineralocorticoid receptors.187, 188 The hope of providing the well-described beneficial effects of mineralocorticoid receptor antagonists189–191 to patients with chronic kidney disease without adverse effects associated with ENaC inhibition is well worth pursuing.
Clinical studies show that aldosterone and/or high salt intake may be responsible to a large extent for the development of arterial hypertension and harmful effects on the cardiovascular system, even independent of any significant rise in blood pressure.145, 192–194 Competitive inhibition of aldosterone receptors by spironolactone or eplerenone,189, 190 blockade of ENaC-mediated Na+ entry by amiloride and recent data derived from animal models154, 195 support the view that endothelial EnNaCs contributes to the development of cardiovascular disease. ENaCs are well known to be a key player in the pathogenesis of Liddle's syndrome.5, 8 In this disease, mutations in the ENaC β- or γ-subunit lead to “gain-of-function” via an increase in surface density and activity of the channel.6 Recent data indicate that not only the altered kidney function but also abnormal vascular function could be contributes to hypertension in a mouse model of Liddle’s syndrome.154 The sensitivity of endothelial stiffness to modest changes in extracellular Na+ concentration, especially in the presence of aldosterone, and the subsequent dampening of NO-mediated vasodilation (Figure 3) suggests that the vascular amiloride-sensitive Na+ channels may also participates in the pathogenesis of salt-sensitive hypertension in humans.196
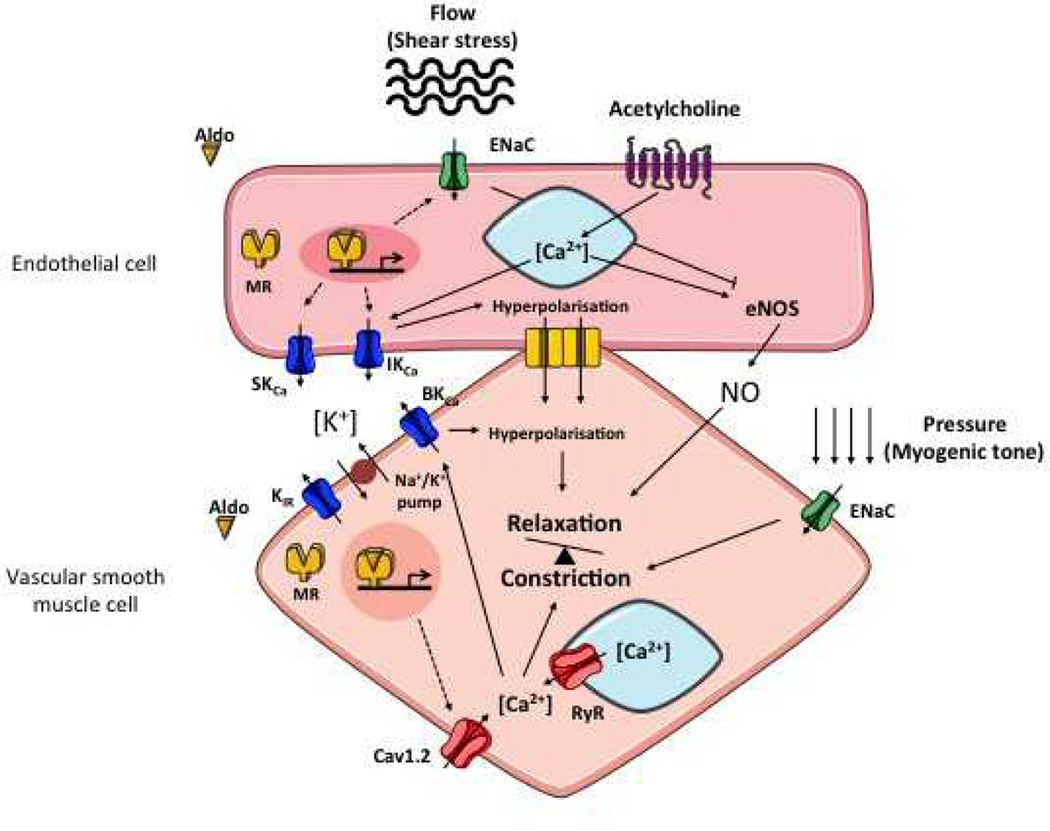
Figure 3. Interactions between vascular endothelial cells and smooth muscle cells.
Aldosterone binds the mineralocorticoid receptor in cytosol and leads to the translocation of the complex into the nucleus. In endothelial cells, MR induces the transcription of two calcium activated potassium channels SKca and IKca201 These channels are responsible for an outward current of potassium and therefore a hyperpolarization of the endothelial cells. When transduced to vascular smooth muscle cell (by gap junctions), hyperpolarization triggers relaxation.202 MR also increases EnNaC at the apical membrane of endothelial cells, where Na+ entry (and/or shear stress) mediates cortical cell stiffness and eNOS phosphorylation.141, 154 In vascular smooth muscle cells, MR increases the transcription of calcium channel Cav1.2 which increase intracellular calcium concentration,170 and decreases expression of the BKCa, calcium-activated potassium channel.203 In vascular smooth muscle cells, ENaC is part of the transduction pathway of constriction response to pressure (myogenic tone).14, 170 Whether ENaC is regulated by aldosterone in vascular smooth muscle cells has not yet been defined.
Aldo, aldosterone; BKCa, potassium big conductance calcium-activated channel; [Ca++], calcium concentration; Cav1.2, calcium channel, voltage-dependent, L type; ENaC, Epithelial sodium channel; eNOS, endothelial nitric oxide synthase; IKCa, potassium intermediate conductance calcium-activated channel; [K+], potassium concentration; MR, mineralocorticoid receptor; NO, nitric oxide; RyR, ryanodine receptor; SKCa, potassium small conductance calcium-activated channel
Evolution of Volume Homeostasis and Pressure Natriuresis
The emergence of Na, K-ATPase, together with the ENaC/Degenerin family appear to be linked to the development of multi-cellularity in the Metazoan kingdom. The establishment of multi-cellularity and the associated extracellular compartment ("internal milieu") precedes the emergence of other key elements of the aldosterone-signaling pathway.197 In the primordial high salt environment, it seems likely that the constant extracellular Na+ concentration maintained vascular tone through tonic activation of the EnNaCs. In that environment, variations in external Na+ concentrations were not an issue; as a consequence, tonic EnNaC activation supported maintenance of vascular tone rather than responding to variations in external Na+. Once the transition to estuarine environments and dry land began, two important forces shaped the evolutionary development of the EnNaC and ENaC systems; the need to conserve Na+ to develop other pressor systems to maintain systemic perfusion (e.g., angiotensin II) and salt-conserving hormones (e.g., aldosterone) when there was not a surfeit of salt in the environment. The EnNaC system appears to be a positive-feed back loop, and subsequent development of self-inhibition and feedback inhibition for ENaCs expressed in the ASDN closed the feedback loop in the Guytonian sense.9 What has been termed “pressure-natriuresis” integrates the activity of EnNaCs and ENaCs to maintain systemic blood pressure and volume homeostasis,198 and refers to increased renal perfusion pressure associated decreased Na+ reabsorption and increased Na+ excretion. Part of the intra-renal mechanisms for decreased Na+ reabsorption appears to be related to increases in medullary blood flow and interstitial hydrostatic pressure, as a result of nitric oxide-induced reductions in renal medullary vascular resistance.199 The contributions of increased vascular amiloride-sensitive Na+ channel activity to the increased medullary blood flow, and reduced ENaC activity to increased Na+ excretion could play central roles in pressure-natriuresis phenomena. The absolute increases in excretion that maintain Na+ balance in responses to volume-mediated increases in systemic blood pressure are greater than can be explained by decreases in Na+ reabsorption in the distal nephron and imply more proximal effects on Na+ reabsorption that could be explained by redistribution of Na+ transporters out of the apical membranes of the proximal tubule,200 as well as increases in interstitial hydrostatic pressure.199
Summary and Clinical Implications.
The balance between renal perfusion pressure, glomerular filtration and net renal Na+ reabsorption achieves maintenance of volume homeostasis.
Amiloride-sensitive Na+ channels and mineralocorticoid receptors are expressed in the aldosterone-sensitive distal nephron, vascular endothelial cells and vascular smooth muscle cells.
In the presence of aldosterone and normal Na+ concentrations in the arterial circulation, peripheral vascular resistance is maintained by the combination of endothelial cell stiffness and myogenic tone. A number of systems contribute to this maintenance, including hormones, and sympathetic nervous system as well as the intrinsic properties of the vascular cells themselves.
Increased perfusion pressure, increased glomerular filtration, increased interstitial pressure and decreased proximal reabsorption delivery more Na+ to the aldosterone-sensitive distal nephron. Inhibition of distal Na+ reabsorption occurs with increased delivery of Na+ to these sites, closing the loop on what is referred to as pressure natriuresis.
While there appear to be structural similarities between the amiloride-sensitive Na+ channels in the epithelial, endothelial and smooth muscle cells, endothelial cell Na+ channels have not been sufficiently characterized at the molecular and structural level to conclude that prototypic ENaC complexes are expressed in endothelia. It is clear that prototypic ENaC complexes are not expressed in smooth muscle cells.
Distinctions based on their functional differences, especially in response to changes in extracellular Na+ concentrations, may permit pharmacologic approaches that could distinguish between the epithelial and other amiloride-sensitive Na+ channels. Agents that preferentially inhibit the vascular channels could lower systemic blood pressure without the attendant inhibition of parallel K+ secretion that accompanies the currently available Na+ channel blockers and mineralocorticoid receptor antagonists.
Research Agenda BLOCK.
- Endothelial EnNaC need much better definition; full length cloning, sequence and expression studies of all of the subunits, especially in light of SNPs that have been shown to affect Epithelial Na+ Channel activity in aldosterone responsive distal nephron.
-
○Increased extracellular Na increases Endothelial Na+ Channel expression in endothelial cells. Determination of temporal course of response would help define optimal time to harvest endothelial cells for harvesting informative RNA.
-
○Are there alternate splice sites that could cause differences in the external loops and thereby affect the subunit structure and activity?
-
○Detailed electrophysiologic characterization of the Endothelial Na+ Channel is needed before and after aldosterone-dependent Na+ activation.
-
○A molecular basis for Na+-self-inhibition of the Epithelial Na+ Channel, and Na+-activation of the Endothelial Na+ Channel needs to be defined.
-
○
The link between increased Na+ entry and reduced production NO and constriction needs to be defined. The role of reactive oxygen species and free radicals need to be considers
Detailed comparisons of the dose-response curves for mineralocorticoid receptor blockers in the vascular and epithelial systems may yield important insights. Effects of pressors like Angiotensin II and Endothelin-1 need to be incorporated in to the vascular model of blood pressure regulation
Regulation of Endothelial Na+ Channel Nedd-4. SGK and other pathways needs to be explored
- Mouse Models of Disease:
-
○The expression of different subunits (α, β, γ and δ) and their localization in vascular cells (endothelium and/or smooth muscle).
-
○Mouse models with conditional, cell-specific inactivation or inactivation of the different Na+ Channel subunits would be very instructive.
-
▪Conditional knock-out of the α subunit would be an important proof of principle that would place the experimental basis for existence of the Endothelial Na+ Channel on much firmer ground.
-
▪A true-gain-of-function mutation of the β or γ Epithelial Na+ Channel subunits would be an important step forward
-
▪Parallel in vitro and in vitro studies of cells and vascular functions in these models would be very illuminating.
-
▪Tissue-specific conditional expression models, and cross-transplantation models could be used to tease apart the vascular from the renal phenotypes in these model systems.
-
▪
-
○Mouse models with conditional, cell-specific inactivation or inactivation of the mineralocorticoid receptors in endothelial and smooth muscle cells would also be very instructive.
-
○
- Human Models of disease
-
○Do variants that reduce ENaC Na+ self-inhibition increase the likelihood of developing salt-sensitive hypertension that would be responsive to amiloride? While these have been described with in vitro expression systems, the link to human hypertension needs to be explored in clinical studies
-
○Is there a vascular phenotype (flow mediated dilation, central aortic pressure, etc.) in patients with Liddle’s syndrome?
-
○Is there any acute effect of changes in dietary Na+ intake on the vascular phenotype in patients with Liddle’s syndrome and appropriately matched controls?
-
○What are the acute effects of Na+ channel blockade on the vascular phenotype in patients with Liddle’s syndrome and appropriately matched controls?
-
○
Acknowledgments
We thank Bernard C. Rossier (University of Lausanne, Lausanne Switzerland) for insightful comments made during the preparation of this review.
Supported by grants from the National Institutes of Health (DK051391, DK065161 and DK079307: TRK); Institut National pour la Santé et Recherche Médicale and the Agence Nationale pour la Recherche (ANR09-BLAN-0156-01; FJ); Deutsche Forschungsgemeinschaft (1496/4-1; KKU) and Koselleck grant (63/18: HO); and the UAB/UCSD O’Brien Center for Kidney Research (P30 DK079337; DGW) are acknowledged. HO and KK gratefully acknowledge the networking activities of EU-COST Action TD 1002 (AFM4NANOMED&BIO). T. R. Kleyman and F. Jaisser contributed equally as senior authors of the manuscript.
References
- 1.Palmer LG, Patel A, Frindt G. Regulation and dysregulation of epithelial Na+ channels. Clin Exp Nephrol. 2012;16:35–43. doi: 10.1007/s10157-011-0496-z. [DOI] [PubMed] [Google Scholar]
- 2.Soundararajan R, Pearce D, Ziera T. The role of the ENaC-regulatory complex in aldosterone-mediated sodium transport. Mol Cell Endocrinol. 2012;350:242–247. doi: 10.1016/j.mce.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Kleyman TR, Sheng S. Gain-of-function variant of the human epithelial sodium channel. Am J Physiol Renal Physiol. 2013;304:F207–F213. doi: 10.1152/ajprenal.00563.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossier BC, Staub O, Hummler E. Genetic dissection of sodium and potassium transport along the aldosterone-sensitive distal nephron: Importance in the control of blood pressure and hypertension. FEBS Lett. 2013;587:1929–1941. doi: 10.1016/j.febslet.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Shimkets RA, et al. Liddle's syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell. 1994;79:407–414. doi: 10.1016/0092-8674(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 6.Snyder PM, et al. Mechanism by which Liddle's syndrome mutations increase activity of a human epithelial Na+ channel. Cell. 1995;83:969–978. doi: 10.1016/0092-8674(95)90212-0. [DOI] [PubMed] [Google Scholar]
- 7.Chang SS, et al. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet. 1996;12:248–253. doi: 10.1038/ng0396-248. [DOI] [PubMed] [Google Scholar]
- 8.Warnock DG. Liddle syndrome: an autosomal dominant form of human hypertension. Kidney Int. 1998;53:18–24. doi: 10.1046/j.1523-1755.1998.00728.x. [DOI] [PubMed] [Google Scholar]
- 9.Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol. 2002;64:877–897. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- 10.Soundararajan R, Pearce D, Hughey RP, Kleyman TR. Role of epithelial sodium channels and their regulators in hypertension. J Biol Chem. 2010;285:30363–30369. doi: 10.1074/jbc.R110.155341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631–1636. doi: 10.1378/chest.07-0288. [DOI] [PubMed] [Google Scholar]
- 12.Davis IC, Matalon S. Epithelial sodium channels in the adult lung--important modulators of pulmonary health and disease. Adv Exp Med Biol. 2007;618:127–140. doi: 10.1007/978-0-387-75434-5_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu Rev Physiol. 2009;71:403–423. doi: 10.1146/annurev.physiol.010908.163250. [DOI] [PubMed] [Google Scholar]
- 14.Drummond HA, Jernigan NL, Grifoni SC. Sensing tension: epithelial sodium channel/acid-sensing ion channel proteins in cardiovascular homeostasis. Hypertension. 2008;51:1265–1271. doi: 10.1161/HYPERTENSIONAHA.107.093401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kusche-Vihrog K, Callies C, Fels J, Oberleithner H. The epithelial sodium channel (ENaC): Mediator of the aldosterone response in the vascular endothelium? Steroids. 2010;75:544–549. doi: 10.1016/j.steroids.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 17.Kashlan OB, Kleyman TR. ENaC structure and function in the wake of a resolved structure of a family member. Am J Physiol Renal Physiol. 2011;301:F684–F696. doi: 10.1152/ajprenal.00259.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canessa CM, Merillat AM, Rossier BC. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol Cell Physiol. 1994;267:C1682–C1690. doi: 10.1152/ajpcell.1994.267.6.C1682. [DOI] [PubMed] [Google Scholar]
- 19.Canessa CM, et al. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 20.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 21.Ackermann D, et al. In vivo nuclear translocation of mineralocorticoid and glucocorticoid receptors in rat kidney: differential effect of corticosteroids along the distal tubule. Am J Physiol Renal Physiol. 2010;299:F1473–F1485. doi: 10.1152/ajprenal.00437.2010. [DOI] [PubMed] [Google Scholar]
- 22.Gaeggeler HP, et al. Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol. 2005;16:878–891. doi: 10.1681/ASN.2004121110. [DOI] [PubMed] [Google Scholar]
- 23.Bailey MA, Mullins JJ, Kenyon CJ. Mineralocorticoid and glucocorticoid receptors stimulate epithelial sodium channel activity in a mouse model of Cushing syndrome. Hypertension. 2009;54:890–896. doi: 10.1161/HYPERTENSIONAHA.109.134973. [DOI] [PubMed] [Google Scholar]
- 24.Craigie E, Evans LC, Mullins JJ, Bailey MA. Failure to downregulate the epithelial sodium channel causes salt sensitivity in Hsd11b2 heterozygote mice. Hypertension. 2012;60:684–690. doi: 10.1161/HYPERTENSIONAHA.112.196410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loffing J, et al. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Renal Physiol. 2001;280:F675–F682. doi: 10.1152/ajprenal.2001.280.4.F675. [DOI] [PubMed] [Google Scholar]
- 26.Pearce D, Kleyman TR. Salt, sodium channels, and SGK1. J Clin Invest. 2007;117:592–595. doi: 10.1172/JCI31538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol. 2008;19:1845–1854. doi: 10.1681/ASN.2008020225. [DOI] [PubMed] [Google Scholar]
- 28.Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol. 2010;6:261–273. doi: 10.1038/nrneph.2010.30. [DOI] [PubMed] [Google Scholar]
- 29.Frindt G, Palmer LG. Regulation of epithelial Na+ channels by adrenal steroids: mineralocorticoid and glucocorticoid effects. Am J Physiol Renal Physiol. 2012;302:F20–F26. doi: 10.1152/ajprenal.00480.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999;104:R19–R23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, et al. Aldosterone-induced Sgk1 relieves Dot1a-Af9-mediated transcriptional repression of epithelial Na+ channel alpha. J Clin Invest. 2007;117:773–783. doi: 10.1172/JCI29850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen SY, et al. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci U S A. 1999;96:2514–2519. doi: 10.1073/pnas.96.5.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soundararajan R, Zhang TT, Wang J, Vandewalle A, Pearce D. A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel-mediated sodium transport. J Biol Chem. 2005;280:39970–39981. doi: 10.1074/jbc.M508658200. [DOI] [PubMed] [Google Scholar]
- 34.Soundararajan R, et al. Scaffold protein connector enhancer of kinase suppressor of Ras isoform 3 (CNK3) coordinates assembly of a multiprotein epithelial sodium channel (ENaC)-regulatory complex. J Biol Chem. 2012;287:33014–33025. doi: 10.1074/jbc.M112.389148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pao AC. SGK regulation of renal sodium transport. Curr Opin Nephrol Hypertens. 2012;21:534–540. doi: 10.1097/MNH.0b013e32835571be. [DOI] [PubMed] [Google Scholar]
- 36.Rohrwasser A, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 37.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 38.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 39.Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96:643–650. doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- 40.Di Zhang A, et al. Cross-talk between mineralocorticoid and angiotensin II signaling for cardiac remodeling. Hypertension. 2008;52:1060–1067. doi: 10.1161/HYPERTENSIONAHA.108.117531. [DOI] [PubMed] [Google Scholar]
- 41.Jain G, Campbell RC, Warnock DG. Mineralocorticoid receptor blockers and chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1685–1691. doi: 10.2215/CJN.01340209. [DOI] [PubMed] [Google Scholar]
- 42.Rautureau Y, Paradis P, Schiffrin EL. Cross-talk between aldosterone and angiotensin signaling in vascular smooth muscle cells. Steroids. 2011;76:834–839. doi: 10.1016/j.steroids.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Staub O, et al. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. Embo J. 1997;16:6325–6336. doi: 10.1093/emboj/16.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rotin D, Staub O. Role of the ubiquitin system in regulating ion transport. Pflugers Arch. 2011;461:1–21. doi: 10.1007/s00424-010-0893-2. [DOI] [PubMed] [Google Scholar]
- 45.Rotin D, et al. An SH3 binding region in the epithelial Na+ channel (alpha rENaC) mediates its localization at the apical membrane. EMBO J. 1994;13:4440–4450. doi: 10.1002/j.1460-2075.1994.tb06766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staub O, et al. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. Embo J. 1996;15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- 47.Snyder PM, Olson DR, Thomas BC. Serum and glucocorticoid-regulated kinase modulates Nedd4-2-mediated inhibition of the epithelial Na+ channel. J Biol Chem. 2002;277:5–8. doi: 10.1074/jbc.C100623200. [DOI] [PubMed] [Google Scholar]
- 48.Debonneville C, et al. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na(+) channel cell surface expression. Embo J. 2001;20:7052–7059. doi: 10.1093/emboj/20.24.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snyder PM, Olson DR, Kabra R, Zhou R, Steines JC. cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na(+) channel through convergent phosphorylation of Nedd4-2. J Biol Chem. 2004;279:45753–45758. doi: 10.1074/jbc.M407858200. [DOI] [PubMed] [Google Scholar]
- 50.Bhalla V, et al. Serum- and glucocorticoid-regulated kinase 1 regulates ubiquitin ligase neural precursor cell-expressed, developmentally down-regulated protein 4-2 by inducing interaction with 14-3-3. Mol Endocrinol. 2005;19:3073–3084. doi: 10.1210/me.2005-0193. [DOI] [PubMed] [Google Scholar]
- 51.Ichimura T, et al. 14-3-3 proteins modulate the expression of epithelial Na+ channels by phosphorylation-dependent interaction with Nedd4-2 ubiquitin ligase. J Biol Chem. 2005;280:13187–13194. doi: 10.1074/jbc.M412884200. [DOI] [PubMed] [Google Scholar]
- 52.Shi H, et al. Interactions of beta and gamma ENaC with Nedd4 Can Be Facilitated by an ERK-mediated Phosphorylation. J Biol Chem. 2002;277:13539–13547. doi: 10.1074/jbc.M111717200. [DOI] [PubMed] [Google Scholar]
- 53.Yang LM, Rinke R, Korbmacher C. Stimulation of the epithelial sodium channel (ENaC) by cAMP involves putative ERK phosphorylation sites in the C termini of the channel's beta- and gamma-subunit. J Biol Chem. 2006;281:9859–9868. doi: 10.1074/jbc.M512046200. [DOI] [PubMed] [Google Scholar]
- 54.Soundararajan R, Melters D, Shih IC, Wang J, Pearce D. Epithelial sodium channel regulated by differential composition of a signaling complex. Proc Natl Acad Sci U S A. 2009;106:7804–7809. doi: 10.1073/pnas.0809892106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Butterworth MB, et al. The deubiquitinating enzyme UCH-L3 regulates the apical membrane recycling of the epithelial sodium channel. J Biol Chem. 2007;282:37885–37893. doi: 10.1074/jbc.M707989200. [DOI] [PubMed] [Google Scholar]
- 56.Fakitsas P, et al. Early aldosterone-induced gene product regulates the epithelial sodium channel by deubiquitylation. J Am Soc Nephrol. 2007;18:1084–1092. doi: 10.1681/ASN.2006080902. [DOI] [PubMed] [Google Scholar]
- 57.Ke Y, Butt AG, Swart M, Liu YF, McDonald FJ. COMMD1 downregulates the epithelial sodium channel through Nedd4-2. Am J Physiol Renal Physiol. 2010;298:F1445–F1456. doi: 10.1152/ajprenal.00257.2009. [DOI] [PubMed] [Google Scholar]
- 58.Chang T, Ke Y, Ly K, McDonald FJ. COMMD1 regulates the delta epithelial sodium channel (δENaC) through trafficking and ubiquitination. Biochem Biophys Res Commun. 2011;411:506–511. doi: 10.1016/j.bbrc.2011.06.149. [DOI] [PubMed] [Google Scholar]
- 59.Zhou R, et al. Ubiquitin-specific peptidase 8 (USP8) regulates endosomal trafficking of the epithelial Na+ channel. J Biol Chem. 2013;288:5389–5397. doi: 10.1074/jbc.M112.425272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pouly D, et al. Mice carrying ubiquitin-specific protease 2 (Usp2) gene inactivation maintain normal sodium balance and blood pressure. Am J Physiol Renal Physiol. 2013;305:F21–F30. doi: 10.1152/ajprenal.00012.2013. [DOI] [PubMed] [Google Scholar]
- 61.Liu YF, Swart M, Ke Y, Ly K, McDonald FJ. Functional interaction of COMMD3 and COMMD9 with the epithelial sodium channel. Am J Physiol Renal Physiol. 2013;305:F80–F89. doi: 10.1152/ajprenal.00158.2013. [DOI] [PubMed] [Google Scholar]
- 62.Ly K, et al. Regulation of the delta and alpha epithelial sodium channel (ENaC) by ubiquitination and Nedd8. J Cell Physiol. 2013;228:2190–2201. doi: 10.1002/jcp.24390. [DOI] [PubMed] [Google Scholar]
- 63.Yue G, Malik B, Yue G, Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem. 2002;277:11965–11969. doi: 10.1074/jbc.M108951200. [DOI] [PubMed] [Google Scholar]
- 64.Helms MN, et al. Phosphatidylinositol 3,4,5-trisphosphate mediates aldosterone stimulation of epithelial sodium channel (ENaC) and interacts with gamma-ENaC. J Biol Chem. 2005;280:40885–40891. doi: 10.1074/jbc.M509646200. [DOI] [PubMed] [Google Scholar]
- 65.Tong Q, Gamper N, Medina JL, Shapiro MS, Stockand JD. Direct activation of the epithelial Na(+) channel by phosphatidylinositol 3,4,5-trisphosphate and phosphatidylinositol 3,4-bisphosphate produced by phosphoinositide 3-OH kinase. J Biol Chem. 2004;279:22654–22663. doi: 10.1074/jbc.M401004200. [DOI] [PubMed] [Google Scholar]
- 66.Pochynyuk O, Tong Q, Staruschenko A, Stockand JD. Binding and direct activation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. J Physiol. 2007;580:365–372. doi: 10.1113/jphysiol.2006.127449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pochynyuk O, et al. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem. 2008 doi: 10.1074/jbc.M807129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pochynyuk O, et al. Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone. Faseb J. 2010;24:2056–2065. doi: 10.1096/fj.09-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vallon V, Rieg T. Regulation of renal NaCl and water transport by the ATP/UTP/P2Y2 receptor system. Am J Physiol Renal Physiol. 2011;301:F463–F475. doi: 10.1152/ajprenal.00236.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mueller GM, et al. Cys palmitoylation of the beta subunit modulates gating of the epithelial sodium channel. J Biol Chem. 2010;285:30453–30462. doi: 10.1074/jbc.M110.151845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mueller GM, et al. Multiple residues in the distal C terminus of the α-subunit have roles in modulating human epithelial sodium channel activity. Am J Physiol Renal Physiol. 2012;303:F220–F228. doi: 10.1152/ajprenal.00493.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 73.Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature. 2009;460:599–604. doi: 10.1038/nature08218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baconguis I, Gouaux E. Structural plasticity and dynamic selectivity of acid-sensing ion channel-spider toxin complexes. Nature. 2012;489:400–405. doi: 10.1038/nature11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dawson RJ, et al. Structure of the acid-sensing ion channel 1 in complex with the gating modifier Psalmotoxin 1. Nat Commun. 2012;3:936. doi: 10.1038/ncomms1917. [DOI] [PubMed] [Google Scholar]
- 76.Stockand JD, Staruschenko A, Pochynyuk O, Booth RE, Silverthorn DU. Insight toward epithelial Na+ channel mechanism revealed by the acid-sensing ion channel 1 structure. IUBMB Life. 2008;60:620–628. doi: 10.1002/iub.89. [DOI] [PubMed] [Google Scholar]
- 77.Kashlan OB, et al. Constraint-based, homology model of the extracellular domain of the epithelial Na+ channel α subunit reveals a mechanism of channel activation by proteases. J Biol Chem. 2011;286:649–640. doi: 10.1074/jbc.M110.167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem. 2009;284:20447–20451. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kashlan OB, Kleyman TR. Epithelial Na(+) channel regulation by cytoplasmic and extracellular factors. Exp Cell Res. 2012;318:1011–1019. doi: 10.1016/j.yexcr.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hughey RP, et al. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem. 2004;279:18111–18114. doi: 10.1074/jbc.C400080200. [DOI] [PubMed] [Google Scholar]
- 81.Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol. 2004;286:C190–C194. doi: 10.1152/ajpcell.00342.2003. [DOI] [PubMed] [Google Scholar]
- 82.Bruns JB, et al. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J Biol Chem. 2007;282:6153–6160. doi: 10.1074/jbc.M610636200. [DOI] [PubMed] [Google Scholar]
- 83.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carattino MD, et al. The epithelial Na+ channel is inhibited by a peptide derived from proteolytic processing of its alpha subunit. J Biol Chem. 2006;281:18901–18907. doi: 10.1074/jbc.M604109200. [DOI] [PubMed] [Google Scholar]
- 85.Rossier BC, Stutts MJ. Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu Rev Physiol. 2009;71:361–379. doi: 10.1146/annurev.physiol.010908.163108. [DOI] [PubMed] [Google Scholar]
- 86.Passero CJ, et al. TMPRSS4-dependent activation of the epithelial sodium channel requires cleavage of the γ-subunit distal to the furin cleavage site. Am J Physiol Renal Physiol. 2012;302:F1–F18. doi: 10.1152/ajprenal.00330.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Passero CJ, et al. Plasmin activates epithelial Na+ channels by cleaving the gamma subunit. J Biol Chem. 2008;283:36586–36591. doi: 10.1074/jbc.M805676200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adebamiro A, Cheng Y, Rao US, Danahay H, Bridges RJ. A segment of gamma ENaC mediates elastase activation of Na+ transport. J Gen Physiol. 2007;130:611–629. doi: 10.1085/jgp.200709781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haerteis S, et al. Plasmin and chymotrypsin have distinct preferences for channel activating cleavage sites in the γ subunit of the human epithelial sodium channel. J Gen Physiol. 2012;140:375–389. doi: 10.1085/jgp.201110763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kota P, et al. Energetic and structural basis for activation of the epithelial sodium channel by matriptase. Biochemistry. 2012;51:3460–3469. doi: 10.1021/bi2014773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rauh R, et al. A mutation of the epithelial sodium channel associated with atypical cystic fibrosis increases channel open probability and reduces Na+ self inhibition. J Physiol. 2010;588:1211–1225. doi: 10.1113/jphysiol.2009.180224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rauh R, et al. A mutation in the β-subunit of ENaC identified in a patient with cystic fibrosis-like symptoms has a gain-of-function effect. Am J Physiol Lung Cell Mol Physiol. 2013;304:L43–L55. doi: 10.1152/ajplung.00093.2012. [DOI] [PubMed] [Google Scholar]
- 93.Alli AA, et al. Phosphatidylinositol phosphate-dependent regulation of Xenopus ENaC by MARCKS protein. Am J Physiol Renal Physiol. 2012;303:F800–F811. doi: 10.1152/ajprenal.00703.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Butterworth MB, Zhang L, Heidrich EM, Myerburg MM, Thibodeau PH. Activation of the epithelial sodium channel (ENaC) by the alkaline protease from Pseudomonas aeruginosa. J Biol Chem. 2012;287:32556–32565. doi: 10.1074/jbc.M112.369520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haerteis S, et al. Proteolytic activation of the epithelial sodium channel (ENaC) by the cysteine protease cathepsin-S. Pflugers Arch. 2012;464:353–365. doi: 10.1007/s00424-012-1138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Patel AB, Chao J, Palmer LG. Tissue kallikrein activation of the epithelial Na channel. Am J Physiol Renal Physiol. 2012;303:F540–F550. doi: 10.1152/ajprenal.00133.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Passero CJ, Hughey RP, Kleyman TR. New role for plasmin in sodium homeostasis. Curr Opin Nephrol Hypertens. 2010;19:13–19. doi: 10.1097/MNH.0b013e3283330fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Svenningsen P, et al. Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol. 2009;20:299–310. doi: 10.1681/ASN.2008040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Svenningsen P, et al. Prostasin-dependent activation of epithelial Na+ channels by low plasmin concentrations. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1733–R1741. doi: 10.1152/ajpregu.00321.2009. [DOI] [PubMed] [Google Scholar]
- 100.Svenningsen P, Skott O, Jensen BL. Proteinuric diseases with sodium retention: Is plasmin the link? Clin Exp Pharmacol Physiol. 2012;39:117–124. doi: 10.1111/j.1440-1681.2011.05524.x. [DOI] [PubMed] [Google Scholar]
- 101.Chraibi A, Horisberger JD. Na self inhibition of human epithelial Na channel: temperature dependence and effect of extracellular proteases. J Gen Physiol. 2002;120:133–145. doi: 10.1085/jgp.20028612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carattino MD, Sheng S, Kleyman TR. Epithelial Na+ channels are activated by laminar shear stress. J Biol Chem. 2004;279:4120–4126. doi: 10.1074/jbc.M311783200. [DOI] [PubMed] [Google Scholar]
- 103.Kashlan OB, et al. Allosteric inhibition of the epithelial Na+ channel through peptide binding at peripheral finger and thumb domains. J Biol Chem. 2010;285:35216–35223. doi: 10.1074/jbc.M110.167064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abriel H, Horisberger JD. Feedback inhibition of rat amiloride-sensitive epithelial sodium channels expressed in Xenopus laevis oocytes. J Physiol. 1999;516(Pt 1):31–43. doi: 10.1111/j.1469-7793.1999.031aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Knight KK, Wentzlaff DM, Snyder PM. Intracellular sodium regulates proteolytic activation of the epithelial sodium channel. J Biol Chem. 2008;283:27477–27482. doi: 10.1074/jbc.M804176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Patel AB, Frindt G, Palmer LG. Feedback inhibition of ENaC during acute sodium loading in vivo. Am J Physiol Renal Physiol. 2013;304:F222–F232. doi: 10.1152/ajprenal.00596.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arnadottir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- 108.Shi S, Carattino MD, Hughey RP, Kleyman TR. ENaC Regulation by Proteases and Shear Stress. Curr Mol Pharmacol. 2013;6:28–34. doi: 10.2174/18744672112059990027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na(+) channels are regulated by flow. Am J Physiol Renal Physiol. 2001;280:F1010–F1018. doi: 10.1152/ajprenal.2001.280.6.F1010. [DOI] [PubMed] [Google Scholar]
- 110.Althaus M, Bogdan R, Clauss WG, Fronius M. Mechano-sensitivity of epithelial sodium channels (ENaCs): laminar shear stress increases ion channel open probability. FASEB J. 2007;21:2389–2399. doi: 10.1096/fj.06-7694com. [DOI] [PubMed] [Google Scholar]
- 111.Shi S, Blobner BM, Kashlan OB, Kleyman TR. Extracellular finger domain modulates the response of the epithelial sodium channel to shear stress. J Biol Chem. 2012;287:15439–15444. doi: 10.1074/jbc.M112.346551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shi S, Carattino MD, Kleyman TR. Role of the wrist domain in the response of the epithelial sodium channel to external stimuli. J Biol Chem. 2012;287:44027–44035. doi: 10.1074/jbc.M112.421743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shi S, et al. Base of the thumb domain modulates epithelial sodium channel gating. J Biol Chem. 2011;286:14753–14761. doi: 10.1074/jbc.M110.191734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Toney GM, Vallon V, Stockand JD. Intrinsic control of sodium excretion in the distal nephron by inhibitory purinergic regulation of the epithelial Na(+) channel. Curr Opin Nephrol Hypertens. 2012;21:52–60. doi: 10.1097/MNH.0b013e32834db4a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Satlin LM, Carattino MD, Liu W, Kleyman TR. Regulation of cation transport in the distal nephron by mechanical forces. Am J Physiol Renal Physiol. 2006;291:F923–F931. doi: 10.1152/ajprenal.00192.2006. [DOI] [PubMed] [Google Scholar]
- 116.Liddle GW, Bledsoe T, Coopage WS. A familial renal disorder simlating primary aldosteronism but with negligible aldosterone secretion. Trans Assoc Am Physicians. 1963;76:199–213. [Google Scholar]
- 117.Rossier BC. 1996 Homer Smith Award Lecture. Cum grano salis: the epithelial sodium channel and the control of blood pressure. J Am Soc Nephrol. 1997;8:980–992. doi: 10.1681/ASN.V86980. [DOI] [PubMed] [Google Scholar]
- 118.Cui Y, et al. Loss of protein kinase C inhibition in the beta-T594M variant of the amiloride-sensitive Na+ channel. Proc Natl Acad Sci U S A. 1997;94:9962–9966. doi: 10.1073/pnas.94.18.9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Samaha FF, et al. Functional polymorphism in the carboxyl terminus of the alpha-subunit of the human epithelial sodium channel. J Biol Chem. 2004;279:23900–23907. doi: 10.1074/jbc.M401941200. [DOI] [PubMed] [Google Scholar]
- 120.Tong Q, Menon AG, Stockand JD. Functional polymorphisms in the alpha-subunit of the human epithelial Na+ channel increase activity. Am J Physiol Renal Physiol. 2006;290:F821–F827. doi: 10.1152/ajprenal.00312.2005. [DOI] [PubMed] [Google Scholar]
- 121.Azad AK, et al. Mutations in the amiloride-sensitive epithelial sodium channel in patients with cystic fibrosis-like disease. Hum Mutat. 2009;30:1093–1103. doi: 10.1002/humu.21011. [DOI] [PubMed] [Google Scholar]
- 122.Rao AD, et al. Polymorphisms in the serum- and glucocorticoid-inducible kinase 1 gene are associated with blood pressure and renin response to dietary salt intake. J Hum Hypertens. 2013;27:176–180. doi: 10.1038/jhh.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lang F, Voelkl J. Therapeutic potential of serum and glucocorticoid inducible kinase inhibition. Expert Opin Investig Drugs. 2013;22:701–714. doi: 10.1517/13543784.2013.778971. [DOI] [PubMed] [Google Scholar]
- 124.Dunn DM, et al. Common variant of human NEDD4L activates a cryptic splice site to form a frameshifted transcript. J Hum Genet. 2002;47:665–676. doi: 10.1007/s100380200102. [DOI] [PubMed] [Google Scholar]
- 125.Ishigami T, et al. NEDD4L protein truncating variant (v13[G/A]: rs4149601) is associated with essential hypertension in a sample of the Japanese population. Geriac Gerontol Int. 2007;7:114–117. [Google Scholar]
- 126.Fava C, et al. 24-h ambulatory blood pressure is linked to chromosome 18q21-22 and genetic variation of NEDD4L associates with cross-sectional and longitudinal blood pressure in Swedes. Kidney Int. 2006;70:562–569. doi: 10.1038/sj.ki.5001590. [DOI] [PubMed] [Google Scholar]
- 127.Garrone NF, Blazer-Yost BL, Weiss RB, Lalouel JM, Rohrwasser A. A human polymorphism affects NEDD4L subcellular targeting by leading to two isoforms that contain or lack a C2 domain. BMC Cell Biol. 2009;10:26. doi: 10.1186/1471-2121-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oberleithner H, et al. Human endothelium: target for aldosterone. Hypertension. 2004;43:952–956. doi: 10.1161/01.HYP.0000123572.45556.a5. [DOI] [PubMed] [Google Scholar]
- 129.Oberleithner H, Riethmuller C, Ludwig T, Hausberg M, Schillers H. Aldosterone remodels human endothelium. Acta Physiol (Oxf) 2006;187:305–312. doi: 10.1111/j.1748-1716.2006.01574.x. [DOI] [PubMed] [Google Scholar]
- 130.Oberleithner H, et al. Differential action of steroid hormones on human endothelium. J Cell Sci. 2006;119:1926–1932. doi: 10.1242/jcs.02886. [DOI] [PubMed] [Google Scholar]
- 131.Oberleithner H, et al. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci U S A. 2007;104:16281–16286. doi: 10.1073/pnas.0707791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Golestaneh N, et al. Mineralocorticoid receptor-mediated signaling regulates the ion gated sodium channel in vascular endothelial cells and requires an intact cytoskeleton. Biochem Biophys Res Commun. 2001;280:1300–1306. doi: 10.1006/bbrc.2001.4275. [DOI] [PubMed] [Google Scholar]
- 133.Wang S, Meng F, Mohan S, Champaneri B, Gu Y. Functional ENaC channels expressed in endothelial cells: a new candidate for mediating shear force. Microcirculation. 2009;16:276–287. doi: 10.1080/10739680802653150. [DOI] [PubMed] [Google Scholar]
- 134.Caprio M, et al. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res. 2008;102:1359–1367. doi: 10.1161/CIRCRESAHA.108.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wildling L, Hinterdorfer P, Kusche-Vihrog K, Treffner Y, Oberleithner H. Aldosterone receptor sites on plasma membrane of human vascular endothelium detected by a mechanical nanosensor. Pflugers Arch. 2009;458:223–230. doi: 10.1007/s00424-008-0615-1. [DOI] [PubMed] [Google Scholar]
- 136.Nguyen Dinh Cat A, et al. The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB J. 2010;24:2454–2463. doi: 10.1096/fj.09-147926. [DOI] [PubMed] [Google Scholar]
- 137.Lombes M, Farman N, Bonvalet JP, Zennaro MC. Identification and role of aldosterone receptors in the cardiovascular system. Ann Endocrinol (Paris) 2000;61:41–46. [PubMed] [Google Scholar]
- 138.Hadoke PW, Kipari T, Seckl JR, Chapman KE. Modulation of 11beta-hydroxysteroid dehydrogenase as a strategy to reduce vascular inflammation. Curr Atheroscler Rep. 2013;15:320. doi: 10.1007/s11883-013-0320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen W, et al. Aldosterone signaling modifies capillary formation by human bone marrow endothelial cells. Vascul Pharmacol. 2004;40:269–277. doi: 10.1016/j.vph.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 140.Kusche-Vihrog K, et al. Aldosterone and amiloride alter ENaC abundance in vascular endothelium. Pflugers Arch. 2008;455:849–857. doi: 10.1007/s00424-007-0341-0. [DOI] [PubMed] [Google Scholar]
- 141.Kusche-Vihrog K, Jeggle P, Oberleithner H. The role of ENaC in vascular endothelum. Pfulgers Arch. 2013 doi: 10.1007/s00424-013-1356-3. [DOI] [PubMed] [Google Scholar]
- 142.Loffing J, Korbmacher C. Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC) Pflugers Arch. 2009;458:111–135. doi: 10.1007/s00424-009-0656-0. [DOI] [PubMed] [Google Scholar]
- 143.Sheng S, Hallows KR, Kleyman TR. In: Seldin and Giebisch's The Kidney: Physiology & Pathophysiology. Alpern RJ, Caplan MJ, Moe OW, editors. New York, NY: Academic Press; 2012. pp. 983–1017. [Google Scholar]
- 144.Stan RV, et al. The diaphragms of fenestrated endothelia: Gatekeepers of vascular permeability and blood composition. Developmental cell. 2012;23:1203–1218. doi: 10.1016/j.devcel.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.He FJ, Markandu ND, Sagnella GA, de Wardener HE, MacGregor GA. Plasma sodium: ignored and underestimated. Hypertension. 2005;45:98–102. doi: 10.1161/01.HYP.0000149431.79450.a2. [DOI] [PubMed] [Google Scholar]
- 146.Suckling RJ, He FJ, Markandu ND, MacGregor GA. Dietary salt influences postprandial plasma sodium concentration and systolic blood pressure. Kidney Int. 2012;81:407–411. doi: 10.1038/ki.2011.369. [DOI] [PubMed] [Google Scholar]
- 147.de Wardener HE, He FJ, MacGregor GA. Plasma sodium and hypertension. Kidney Int. 2004;66:2454–2466. doi: 10.1111/j.1523-1755.2004.66018.x. [DOI] [PubMed] [Google Scholar]
- 148.Yee-Moon Wang A, Lu Y, Cheung S, Hiu-Shuen Chan I, Wai-Kei Lam C. Plasma sodium and subclinical left atrial enlargement in chronic kidney disease. Nephrol Dial Transplant. 2013;28:2319–2328. doi: 10.1093/ndt/gfs588. [DOI] [PubMed] [Google Scholar]
- 149.Korte S, et al. Firewall function of the endothelial glycocalyx in the regulation of sodium homeostasis. Pflugers Arch. 2012;463:269–278. doi: 10.1007/s00424-011-1038-y. [DOI] [PubMed] [Google Scholar]
- 150.Cook DI, Dinudom A, Komwatana P, Kumar S, Young JA. Patch-clamp studies on epithelial sodium channels in salivary duct cells. Cell Biochem Biophys. 2002;36:105–113. doi: 10.1385/cbb:36:2-3:105. [DOI] [PubMed] [Google Scholar]
- 151.Murdaca J, et al. Grb10 prevents Nedd4-mediated vascular endothelial growth factor receptor-2 degradation. J Biol Chem. 2004;279:26754–26761. doi: 10.1074/jbc.M311802200. [DOI] [PubMed] [Google Scholar]
- 152.Oberleithner H, et al. Potassium softens vascular endothelium and increases nitric oxide release. Proc Natl Acad Sci U S A. 2009;106:2829–234. doi: 10.1073/pnas.0813069106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kliche K, Jeggle P, Pavenstadt H, Oberleithner H. Role of cellular mechanics in the function and life span of vascular endothelium. Pflugers Arch. 2011;462:209–217. doi: 10.1007/s00424-011-0929-2. [DOI] [PubMed] [Google Scholar]
- 154.Jeggle P, et al. Epithelial sodium channel stiffens the vascular endothelium in vitro and in liddle mice. Hypertension. 2013;61:1053–1059. doi: 10.1161/HYPERTENSIONAHA.111.199455. [DOI] [PubMed] [Google Scholar]
- 155.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 156.Lang F. Stiff endothelial cell syndrome in vascular inflammation and mineralocorticoid excess. Hypertension. 2011;57:146–147. doi: 10.1161/HYPERTENSIONAHA.110.164558. [DOI] [PubMed] [Google Scholar]
- 157.Perez FR, et al. Endothelial epithelial sodium channel inhibition activates endothelial nitric oxide synthase via phosphoinositide 3-kinase/Akt in small-diameter mesenteric arteries. Hypertension. 2009;53:1000–1007. doi: 10.1161/HYPERTENSIONAHA.108.128520. [DOI] [PubMed] [Google Scholar]
- 158.Li J, et al. Salt inactivates endothelial nitric oxide synthase in endothelial cells. J Nutr. 2009;139:447–451. doi: 10.3945/jn.108.097451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Abiose AK, et al. Effect of spironolactone on endothelial function in patients with congestive heart failure on conventional medical therapy. Am J Cardiol. 2004;93:1564–1566. doi: 10.1016/j.amjcard.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 160.Druppel V, et al. Long-term application of the aldosterone antagonist spironolactone prevents stiff endothelial cell syndrome. FASEB J. 2013;27:3652–3659. doi: 10.1096/fj.13-228312. [DOI] [PubMed] [Google Scholar]
- 161.Bellien J, et al. Arterial stiffness is regulated by nitric oxide and endothelium-derived hyperpolarizing factor during changes in blood flow in humans. Hypertension. 2010;55:674–680. doi: 10.1161/HYPERTENSIONAHA.109.142190. [DOI] [PubMed] [Google Scholar]
- 162.Smith PR, Saccomani G, Joe EH, Angelides KJ, Benos DJ. Amiloride-sensitive sodium channel is linked to the cytoskeleton in renal epithelial cells. Proc Natl Acad Sci U S A. 1991;88:6971–6975. doi: 10.1073/pnas.88.16.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mazzochi C, Bubien JK, Smith PR, Benos DJ. The carboxyl terminus of the alpha-subunit of the amiloride-sensitive epithelial sodium channel binds to F-actin. J Biol Chem. 2006;281:6528–6538. doi: 10.1074/jbc.M509386200. [DOI] [PubMed] [Google Scholar]
- 164.Ilatovskaya DV, Pavlov TS, Levchenko V, Negulyaev YA, Staruschenko A. Cortical actin binding protein cortactin mediates ENaC activity via Arp2/3 complex. FASEB J. 2011;25:2688–2699. doi: 10.1096/fj.10-167262. [DOI] [PubMed] [Google Scholar]
- 165.Fels J, Jeggle P, Kusche-Vihrog K, Oberleithner H. Cortical actin nanodynamics determines nitric oxide release in vascular endothelium. PLoS One. 2012;7:e41520. doi: 10.1371/journal.pone.0041520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Oda T, Makino K, Yamashita I, Namba K, Maeda Y. Distinct structural changes detected by X-ray fiber diffraction in stabilization of F-actin by lowering pH and increasing ionic strength. Biophys J. 2001;80:841–851. doi: 10.1016/S0006-3495(01)76063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Drummond HA, Gebremedhin D, Harder DR. Degenerin/epithelial Na+ channel proteins: components of a vascular mechanosensor. Hypertension. 2004;44:643–648. doi: 10.1161/01.HYP.0000144465.56360.ad. [DOI] [PubMed] [Google Scholar]
- 168.Chung WS, Weissman JL, Farley J, Drummond HA. βENaC is required for whole cell mechanically gated currents in renal vascular smooth muscle cells. Am J Physiol Renal Physiol. 2013;304:F1428–F1437. doi: 10.1152/ajprenal.00444.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Drummond HA, Grifoni SC, Jernigan NL. A New Trick for an Old Dogma: ENaC Proteins as Mechanotransducers in Vascular Smooth Muscle. Physiology (Bethesda) 2008;23:23–31. doi: 10.1152/physiol.00034.2007. [DOI] [PubMed] [Google Scholar]
- 170.McCurley A, et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18:1429–1433. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McCurley A, Jaffe IZ. Mineralocorticoid receptors in vascular function and disease. Mol Cell Endocrinol. 2012;350:256–265. doi: 10.1016/j.mce.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schluter T, et al. Amiloride lowers arterial pressure in cyp1a1ren-2 transgenic rats without affecting renal vascular function. J Hypertens. 2010;28:2267–2277. doi: 10.1097/HJH.0b013e32833d77b4. [DOI] [PubMed] [Google Scholar]
- 173.Jernigan NL, Drummond HA. Vascular ENaC proteins are required for renal myogenic constriction. Am J Physiol Renal Physiol. 2005;289:F891–F901. doi: 10.1152/ajprenal.00019.2005. [DOI] [PubMed] [Google Scholar]
- 174.Pradervand S, et al. Salt restriction induces pseudohypoaldosteronism type 1 in mice expressing low levels of the beta-subunit of the amiloride-sensitive epithelial sodium channel. Proc Natl Acad Sci U S A. 1999;96:1732–1737. doi: 10.1073/pnas.96.4.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.VanLandingham LG, Gannon KP, Drummond HA. Pressure-induced constriction is inhibited in a mouse model of reduced betaENaC. Am J Physiol Regul Integr Comp Physiol. 2009;297:R723–F728. doi: 10.1152/ajpregu.00212.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol Renal Physiol. 2000;279:F319–F325. doi: 10.1152/ajprenal.2000.279.2.F319. [DOI] [PubMed] [Google Scholar]
- 177.van Paassen P, de Zeeuw D, de Jong PE, Navis G. Renin inhibition improves pressure natriuresis in essential hypertension. J Am Soc Nephrol. 2000;11:1813–1818. doi: 10.1681/ASN.V11101813. [DOI] [PubMed] [Google Scholar]
- 178.Erbanova M, et al. Impairment of the autoregulation of renal hemodynamics and of the pressure-natriuresis relationship precedes the development of hypertension in Cyp1a1-Ren-2 transgenic rats. J Hypertens. 2009;27:575–586. doi: 10.1097/hjh.0b013e32831cbd5a. [DOI] [PubMed] [Google Scholar]
- 179.Speed JS, Pollock DM. Endothelin, kidney disease, and hypertension. Hypertension. 2013;61:1142–1145. doi: 10.1161/HYPERTENSIONAHA.113.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kittikulsuth W, Pollock JS, Pollock DM. Loss of renal medullary endothelin B receptor function during salt deprivation is regulated by angiotensin II. Am J Physiol Renal Physiol. 2012;303:F659–F666. doi: 10.1152/ajprenal.00213.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Franco M, et al. Impaired pressure natriuresis resulting in salt-sensitive hypertension is caused by tubulointerstitial immune cell infiltration in the kidney. Am J Physiol Renal Physiol. 2013;304:F982–F990. doi: 10.1152/ajprenal.00463.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Grifoni SC, Chiposi R, McKey SE, Ryan MJ, Drummond HA. Altered whole kidney blood flow autoregulation in a mouse model of reduced beta-ENaC. Am J Physiol Renal Physiol. 2010;298:F285–F292. doi: 10.1152/ajprenal.00496.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Drummond HA, et al. Renal inflammation and elevated blood pressure in a mouse model of reduced {beta}-ENaC. Am J Physiol Renal Physiol. 2011;301:F443–F449. doi: 10.1152/ajprenal.00694.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Drummond HA. βENaC is a molecular component of a VSMC mechanotransducer that contributes to renal blood flow regulation, protection from renal injury, and hypertension. Front Physiol. 2012;3:341. doi: 10.3389/fphys.2012.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.O'Riordan TG, et al. Acute hyperkalemia associated with Inhalation of a potent ENaC antagonist: Phase 1 trial of GS-9411. J Aerosol Med Pulm Drug Deliv. 2013 doi: 10.1089/jamp.2013.1037. [DOI] [PubMed] [Google Scholar]
- 186.Kolkhof P, Borden SA. Molecular pharmacology of the mineralocorticoid receptor: prospects for novel therapeutics. Mol Cell Endocrinol. 2012;350:310–317. doi: 10.1016/j.mce.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 187.Ori Y, et al. Regression of left ventricular hypertrophy in patients with primary aldosteronism/low-renin hypertension on low-dose spironolactone. Nephrol Dial Transplant. 2013;28:1787–1793. doi: 10.1093/ndt/gfs587. [DOI] [PubMed] [Google Scholar]
- 188.Funder JW. Primary aldosteronism and low-renin hypertension: a continuum? Nephrol Dial Transplant. 2013;28:1625–1627. doi: 10.1093/ndt/gft052. [DOI] [PubMed] [Google Scholar]
- 189.Pitt B, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 190.Pitt B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 191.Zannad F, et al. Mineralocorticoid receptor antagonists for heart failure with reduced ejection fraction: integrating evidence into clinical practice. Eur Heart J. 2012;33:2782–2795. doi: 10.1093/eurheartj/ehs257. [DOI] [PubMed] [Google Scholar]
- 192.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 193.Titze J, Ritz E. Salt and its effect on blood pressure and target organ damage: new pieces in an old puzzle. J Nephrol. 2009;22:177–189. [PubMed] [Google Scholar]
- 194.DuPont JJ, et al. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens. 2013;31:530–536. doi: 10.1097/HJH.0b013e32835c6ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Blasi ER, et al. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 196.Oberleithner H. A physiological concept unmasking vascular salt sensitivity in man. Pflugers Arch. 2012;464:287–293. doi: 10.1007/s00424-012-1128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Studer RA, Person E, Robinson-Rechavi M, Rossier BC. Evolution of the epithelial sodium channel and the sodium pump as limiting factors of aldosterone action on sodium transport. Physiol Genomics. 2011;43:844–854. doi: 10.1152/physiolgenomics.00002.2011. [DOI] [PubMed] [Google Scholar]
- 198.Weder AB. Evolution and hypertension. Hypertension. 2007;49:260–265. doi: 10.1161/01.HYP.0000255165.84684.9d. [DOI] [PubMed] [Google Scholar]
- 199.Granger JP, Alexander BT, Llinas M. Mechanisms of pressure natriuresis. Curr Hypertens Rep. 2002;4:152–159. doi: 10.1007/s11906-002-0040-3. [DOI] [PubMed] [Google Scholar]
- 200.Zhang Y, et al. Rapid redistribution and inhibition of renal sodium transporters during acute pressure natriuresis. Am J Physiol. 1996;270:F1004–F1014. doi: 10.1152/ajprenal.1996.270.6.F1004. [DOI] [PubMed] [Google Scholar]
- 201.Zhao M, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122:2672–2679. doi: 10.1172/JCI61427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Garland CJ, Hiley CR, Dora KA. EDHF: spreading the influence of the endothelium. Br J Pharmacol. 2011;164:839–852. doi: 10.1111/j.1476-5381.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ambroisine ML, et al. Aldosterone-induced coronary dysfunction in transgenic mice involves the calcium-activated potassium (BKCa) channels of vascular smooth muscle cells. Circulation. 2007;116:2435–2443. doi: 10.1161/CIRCULATIONAHA.107.722009. [DOI] [PubMed] [Google Scholar]