Abstract
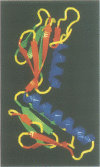
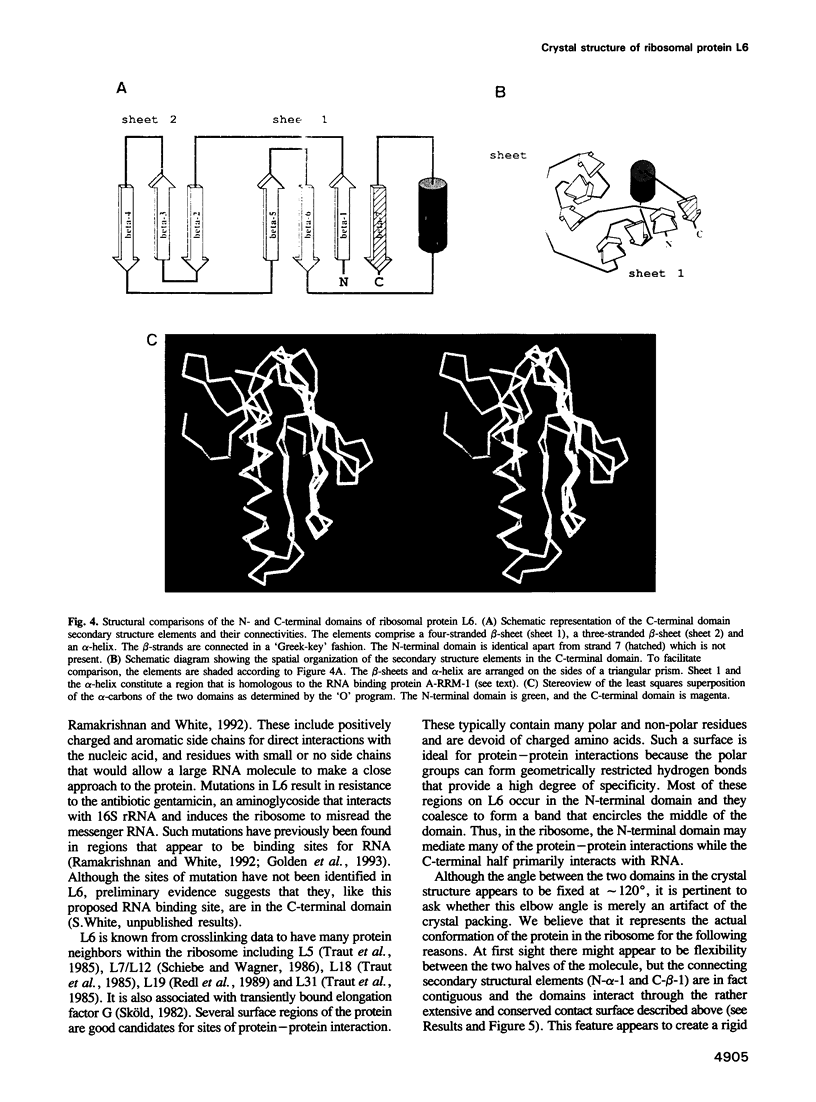
In all cells, protein synthesis is coordinated by the ribosome, a large ribonucleoprotein particle that is composed of > 50 distinct protein molecules and several large RNA molecules. Here we present the crystal structure of ribosomal protein L6 from the thermophilic bacterium Bacillus stearothermophilus solved at 2.6 A resolution. L6 contains two domains with almost identical folds, implying that it was created by an ancient gene duplication event. The surface of the molecule displays several likely sites of interaction with other components of the ribosome. The RNA binding sites appear to be localized in the C-terminal domain whereas the N-terminal domain contains the potential sites for protein-protein interactions. The domain structure is homologous with several other ribosomal proteins and to a large family of eukaryotic RNA binding proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelt K., Dijk J., White S. W., Wilson K. S. Proteins of the Bacillus stearothermophilus ribosome. Crystallization of protein L6. FEBS Lett. 1983 Aug 22;160(1-2):75–77. doi: 10.1016/0014-5793(83)80939-9. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Cerretti D. P., Dean D., Davis G. R., Bedwell D. M., Nomura M. The spc ribosomal protein operon of Escherichia coli: sequence and cotranscription of the ribosomal protein genes and a protein export gene. Nucleic Acids Res. 1983 May 11;11(9):2599–2616. doi: 10.1093/nar/11.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackl W., Stöffler-Meilicke M. Immunoelectron microscopic localisation of ribosomal proteins from Bacillus stearothermophilus that are homologous to Escherichia coli L1, L6, L23 and L29. Eur J Biochem. 1988 Jun 1;174(2):431–435. doi: 10.1111/j.1432-1033.1988.tb14116.x. [DOI] [PubMed] [Google Scholar]
- Hoffman D. W., Query C. C., Golden B. L., White S. W., Keene J. D. RNA-binding domain of the A protein component of the U1 small nuclear ribonucleoprotein analyzed by NMR spectroscopy is structurally similar to ribosomal proteins. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2495–2499. doi: 10.1073/pnas.88.6.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kühberger R., Piepersberg W., Petzet A., Buckel P., Böck A. Alteration of ribosomal protein L6 in gentamicin-resistant strains of Escherichia coli. Effects on fidelity of protein synthesis. Biochemistry. 1979 Jan 9;18(1):187–193. doi: 10.1021/bi00568a028. [DOI] [PubMed] [Google Scholar]
- Leijonmarck M., Appelt K., Badger J., Liljas A., Wilson K. S., White S. W. Structural comparison of the prokaryotic ribosomal proteins L7/L12 and L30. Proteins. 1988;3(4):243–251. doi: 10.1002/prot.340030405. [DOI] [PubMed] [Google Scholar]
- Leijonmarck M., Eriksson S., Liljas A. Crystal structure of a ribosomal component at 2.6 A resolution. Nature. 1980 Aug 21;286(5775):824–826. doi: 10.1038/286824a0. [DOI] [PubMed] [Google Scholar]
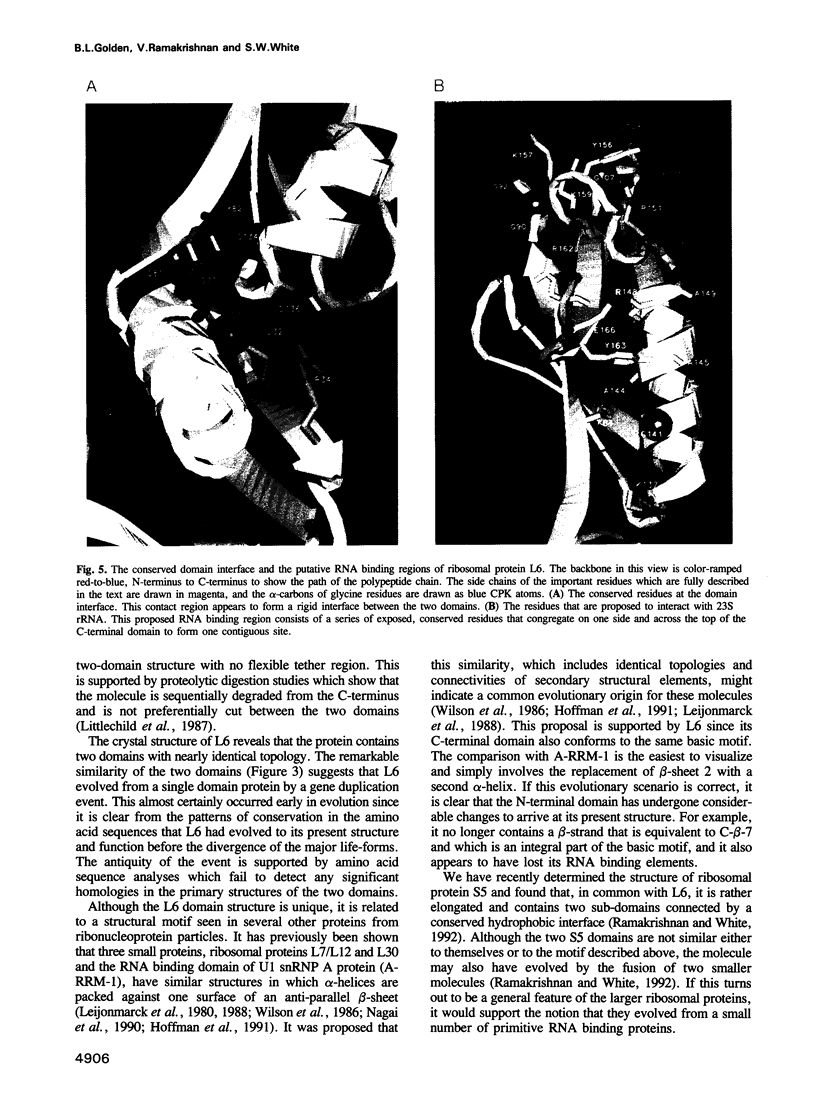
- Littlechild J., Malcolm A., Paterakis K., Ackermann I., Dijk J. The tertiary structure of salt-extracted ribosomal proteins from Escherichia coli as studied by proton magnetic resonance spectroscopy and limited proteolysis experiments. Biochim Biophys Acta. 1987 Jun 17;913(2):245–255. doi: 10.1016/0167-4838(87)90336-0. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Osswald M., Schueler D., Brimacombe R. Selective isolation and detailed analysis of intra-RNA cross-links induced in the large ribosomal subunit of E. coli: a model for the tertiary structure of the tRNA binding domain in 23S RNA. Nucleic Acids Res. 1990 Aug 11;18(15):4325–4333. doi: 10.1093/nar/18.15.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Oubridge C., Jessen T. H., Li J., Evans P. R. Crystal structure of the RNA-binding domain of the U1 small nuclear ribonucleoprotein A. Nature. 1990 Dec 6;348(6301):515–520. doi: 10.1038/348515a0. [DOI] [PubMed] [Google Scholar]
- Nelson R. M., Long G. L. A general method of site-specific mutagenesis using a modification of the Thermus aquaticus polymerase chain reaction. Anal Biochem. 1989 Jul;180(1):147–151. doi: 10.1016/0003-2697(89)90103-6. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Hoffarth V., Zimniak L. Unusual resistance of peptidyl transferase to protein extraction procedures. Science. 1992 Jun 5;256(5062):1416–1419. doi: 10.1126/science.1604315. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Ribosomal RNA and translation. Annu Rev Biochem. 1991;60:191–227. doi: 10.1146/annurev.bi.60.070191.001203. [DOI] [PubMed] [Google Scholar]
- Ollis D., White S. Protein crystallization. Methods Enzymol. 1990;182:646–659. doi: 10.1016/0076-6879(90)82050-c. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan V., Gerchman S. E. Cloning, sequencing, and overexpression of genes for ribosomal proteins from Bacillus stearothermophilus. J Biol Chem. 1991 Jan 15;266(2):880–885. [PubMed] [Google Scholar]
- Ramakrishnan V., White S. W. The structure of ribosomal protein S5 reveals sites of interaction with 16S rRNA. Nature. 1992 Aug 27;358(6389):768–771. doi: 10.1038/358768a0. [DOI] [PubMed] [Google Scholar]
- Redl B., Walleczek J., Stöffler-Meilicke M., Stöffler G. Immunoblotting analysis of protein-protein crosslinks within the 50S ribosomal subunit of Escherichia coli. A study using dimethylsuberimidate as crosslinking reagent. Eur J Biochem. 1989 May 1;181(2):351–356. doi: 10.1111/j.1432-1033.1989.tb14731.x. [DOI] [PubMed] [Google Scholar]
- Richardson J. S., Richardson D. C. Amino acid preferences for specific locations at the ends of alpha helices. Science. 1988 Jun 17;240(4859):1648–1652. doi: 10.1126/science.3381086. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. beta-Sheet topology and the relatedness of proteins. Nature. 1977 Aug 11;268(5620):495–500. doi: 10.1038/268495a0. [DOI] [PubMed] [Google Scholar]
- Scheibe U., Wagner R. Identification of neighbouring proteins by cross-linking of intact 70 S ribosomes from Escherichia coli. Biochim Biophys Acta. 1986 Jan 17;869(1):1–7. doi: 10.1016/0167-4838(86)90302-x. [DOI] [PubMed] [Google Scholar]
- Scholzen T., Arndt E. Organization and nucleotide sequence of ten ribosomal protein genes from the region equivalent to the spectinomycin operon in the archaebacterium Halobacterium marismortui. Mol Gen Genet. 1991 Aug;228(1-2):70–80. doi: 10.1007/BF00282450. [DOI] [PubMed] [Google Scholar]
- Sköld S. E. Chemical cross-linking of elongation factor G to both subunits of the 70-S ribosomes from Escherichia coli. Eur J Biochem. 1982 Oct;127(2):225–229. doi: 10.1111/j.1432-1033.1982.tb06859.x. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Olvera J., Wool I. G. The primary structure of rat ribosomal protein L9. Gene. 1990 Sep 14;93(2):297–300. doi: 10.1016/0378-1119(90)90239-n. [DOI] [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]
- Wilson K. S., Appelt K., Badger J., Tanaka I., White S. W. Crystal structure of a prokaryotic ribosomal protein. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7251–7255. doi: 10.1073/pnas.83.19.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- Wower I., Wower J., Meinke M., Brimacombe R. The use of 2-iminothiolane as an RNA-protein cross-linking agent in Escherichia coli ribosomes, and the localisation on 23S RNA of sites cross-linked to proteins L4, L6, L21, L23, L27 and L29. Nucleic Acids Res. 1981 Sep 11;9(17):4285–4302. doi: 10.1093/nar/9.17.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]