Abstract
Diabetes mellitus is associated with dysregulation of adipose tissue metabolism and increased level of serum lipids. In our previous work we found that Securigera securidaca decreases cholesterol level in blood of diabetic rats. The present study was carried out to further investigate the effects of this plant on lipid metabolism, lipolysis, and adipogenesis, in diabetic rats. Female Wistar rats were rendered diabetic by intraperitoneal injection of streptozotocin. Retroperitoneal adipose tissue was removed from diabetic animals after seven days of streptozotocin injection. Effect of hydroalcoholic extract of S. securidaca seeds (100–800 μg/mL) on adipose tissue lipolysis was evaluated in ex vivo condition. Also, to evaluate adipogenesis, preadipocytes were isolated from adipose tissue and differentiated to adipocytes in the presence of the extract. The extract at concentration of 800 μg/mL decreased both basal and catecholamine-stimulated lipolysis (P < 0.05). Incubation of differentiating preadipocytes with 800 μg/mL of S. securidaca extract decreased intracellular lipid droplet accumulation as evaluated with Oil Red O staining (P < 0.001). The extract even at high concentrations had no effect on viability of preadipocytes. In conclusion, S. securidaca decreases lipolysis and adipogenesis without cytotoxicity, which makes it a good candidate for management of dyslipidemia and reduction of cardiovascular risks in diabetes.
1. Introduction
Diabetes mellitus is a major cause of hospitalization and still is one of the main diseases causing death and disability. The number of diabetic patients is markedly increasing in the world. According to the World Health Organization reports (October, 2013), 347 million people suffer from diabetes worldwide and without urgent action, it will be the 7th cause of mortality in 2030. Diabetes is associated with impaired glucose and lipid metabolism and over time leads to microvascular and macrovascular complications such as cardiovascular diseases [1]. Dyslipidemia, a main risk factor of cardiovascular diseases, is often present in diabetic patients. Diabetic dyslipidemia is characterized by increased serum triglyceride and low density lipoprotein and decreased high density lipoprotein [2]. Patients with type-1 diabetes also undergo dysregulation of adipose tissue metabolism (lipolysis and lipogenesis) due to insulin deficiency.
Currently, statins, fibrates, niacin, and bile acid binding sequestrants are the most widely used medications for dyslipidemia. However, the clinical uses of these drugs are accompanied with unpleasant side effects such as myopathy and hepatic toxicity [3, 4]. Moreover, despite aggressive drug therapy, a number of diabetic patients still experience coronary heart disease events [5]. Therefore, finding new hypolipidemic agents with better efficacy and lesser side effects is promising approach for management of diabetic dyslipidemia.
Several studies have shown beneficial effects of natural agents on diabetes associated hyperglycemia and dyslipidemia [6, 7]. Securigera securidaca, an annual herb belonging to the Fabaceae family, is used in Iranian folk medicine for treatment of diabetes. Experimental studies have revealed that administration of S. securidaca seeds decreases blood glucose in normal and diabetic subjects [8–10]. Also this plant reduces the level of triglyceride and cholesterol in serum of high-fat fed rats [11]. In our previous work we showed that hydroalcoholic extract of Securigera securidaca seeds decreases serum cholesterol in diabetic rats [12]. This study was carried out to investigate effects of this extract on adipose tissue lipolysis and adipogenesis in diabetic rats.
2. Materials and Methods
2.1. Chemicals and Reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Carlsbad, CA). Streptozotocin (STZ) was obtained from Enzo Life Science (USA). Fatty acid-free bovine serum albumin fraction V, glycerol assay reagent, isoproterenol, penicillin-streptomycin, type-II collagenase, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), and 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid sodium salt (HEPES) were provided from Sigma (USA). Dimethyl sulfoxide and 3-isobutyl-1-methylxanthine (IBMX) were purchased from Fluka Chemical Co. Indomethacin and human insulin were kindly provided by EXIR Company (Iran).
2.2. Preparation of Extract
The S. securidaca seeds were powdered with a blender and 860 g of the powder was suspended in 3200 mL of 70% ethanol. The mixture was left in dark at room temperature for 72 h under gentle shaking. The hydroalcoholic extract was then filtered and dried on a water bath.
2.3. Animals
Female albino Wistar rats (230 ± 30 g) were housed in a room with controlled lighting (12 h light/12 h darkness) and temperature (22 ± 2°C). All animal studies were carried out in accordance with the ethical guidelines of the animal care of the Mashhad University of Medical Sciences, Iran. Diabetes was induced by a single intraperitoneal injection of STZ (50 mg/kg). Three days after STZ injection, induction of diabetes was confirmed by measuring fasting blood glucose (FBG). Animals were considered to be diabetic if they had FBG of 250 mg/dL or higher [13, 14].
2.4. Lipolysis Studies
The effect of S. securidaca extract on adipose tissue lipolysis was evaluated in ex vivo condition [15, 16]. Retroperitoneal adipose tissues were removed from diabetic animals after seven days of STZ injection. The tissues were minced into uniform small slices of about 5 mg. The tissue slices were washed with phosphate-buffered saline, dried on the gauze, and weighted precisely. Then, tissue slices were distributed into 24-well plate (100 mg/well) and bathed with 1 mL Krebs-Ringer bicarbonate buffer supplemented with 25 mM HEPES, 5.5 mM glucose, and 2% (w/v) bovine serum albumin. The tissues were treated with vehicle (basal lipolysis) or isoproterenol (stimulated lipolysis) in the absence or presence of S. securidaca extract under constant shaking for 90 min at 37°C. At the end of the treatment, glycerol concentration was measured in the buffer by an enzymatic method.
2.5. Preadipocyte Preparation and Adipogenesis Assay
On day seven of diabetes inception, the rats were anesthetized with ether and retroperitoneal adipose tissue was excised through a sterile laparotomy procedure. The tissue sample was sliced into small pieces and washed with phosphate-buffered saline (PBS). The tissue pieces were then digested in PBS containing 2 mg/mL collagenase under shaking (60 cycles/min) at 37°C. The cellular pellet was isolated via centrifugation (2000 rpm for 5 min) and suspended in DMEM medium supplemented with 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin. The cells were plated in culture flask and incubated in a humidified 5% CO2 incubator until they reached confluence. The cells were then trypsinized and seeded in 12-well plates (104 cells/well). After 24 h of incubation, the culture medium was changed into differentiation medium (DMEM supplemented with 3% FBS, 250 μM IBMX, 34 μM d-pantothenate, 1 μM dexamethasone, 0.2 μM insulin, and 5 μM indomethacin). After 3 days, the cells were exposed to the adipocyte maintenance medium (DMEM supplemented with 3% FBS, 34 μM d-pantothenate, 1 μM dexamethasone, and 0.2 μM insulin). The cells were further cultured in this medium for 6 days and the medium was changed every 3 days [17]. To evaluate the effects of S. securidaca extract on adipogenesis, both differentiation and adipocyte maintenance media were supplemented with varying concentrations of the extract or vehicle (1% DMSO).
2.6. Oil Red O Staining
To examine the effect of S. securidaca on adipogenesis, Oil Red O was used to stain accumulated intracellular triglycerides in differentiated adipocytes. After 9 days of differentiation, the cells were fixed using 10% formalin and then stained by 200 μL Oil Red O solution. After three times washing with distilled water, the stain was eluted from cells using 200 μL isopropanol and its optical density was read at 545 nm [18, 19].
2.7. Cell Viability Assay
Effect of S. securidaca extract on viability of isolated rat preadipocytes and L929 mouse fibroblast cells was determined using MTT colorimetric assay. The cells were seeded (5000/well) in 96-well culture plates containing DMEM medium supplemented with antibiotic and 10% FBS. After 24 h, the medium was changed to a fresh one containing various concentrations of S. securidaca extract and the cells were further incubated for 48 h. At the end of incubation, the MTT solution was added to each well at final concentration of 0.5 mg/mL and the plate was placed for 2 h at 37°C [20, 21]. Then, the supernatant was discarded and the resulting formazan was dissolved by adding 200 μL dimethyl sulfoxide to each well. The absorbance of formazan dye was read at 545 nm against 630 nm as background.
2.8. Statistical Analysis
The values were compared using the one-way analysis of variance followed by Tukey's post hoc test. P value < 0.05 was considered to be statistically significant. The results are presented as mean ± SEM.
3. Results
3.1. Effect of S. securidaca on Lipolysis
Figure 1 demonstrates the effect of S. securidaca extract on basal and ISO-stimulated lipolysis. In basal condition, the extract at concentration of 800 μg/mL significantly decreased glycerol release to 47 ± 8% of control (P < 0.05). To examine the effect of S. securidaca on stimulated lipolysis, the lipolytic activity was tested in the presence of isoproterenol, a nonselective beta adrenergic receptor agonist. As expected, 1 μM isoproterenol led to significant elevation in glycerol release (205 ± 25% of control, P < 0.001). The extract at concentration of 800 μg/mL significantly reduced the stimulated lipolysis to 135 ± 7% of control (P < 0.05).
Figure 1.
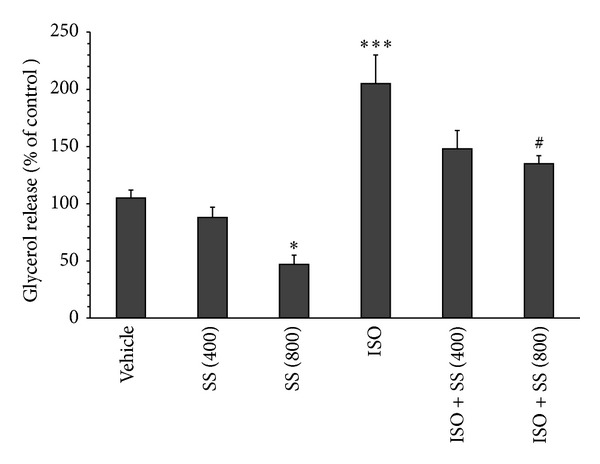
Effects of hydroalcoholic extract of Securigera securidaca on lipolysis in diabetic rats. Retroperitoneal adipose tissues were treated with vehicle or Securigera securidaca (SS) in the absence (basal lipolysis) or presence of 1 μM isoproterenol (ISO) for 90 min. Concentration of SS is shown in parentheses (μg/mL). Data are presented as means ± SEM of 6 independent experiments. *P < 0.05 versus vehicle; ***P < 0.001 versus control; # P < 0.05 versus ISO.
3.2. Effects of S. securidaca on Adipogenesis
Incubation of differentiating cells with S. securidaca extract decreased intracellular lipid droplet accumulation as evaluated with Oil Red O staining (Figure 2). The presence of 100, 200, 400, and 800 μg/mL of the extract in the culture medium decreased the lipid droplet content from 100 ± 4% (untreated cells) to 75 ± 9%, 86 ± 8%, 78 ± 15%, and 51 ± 3% (P < 0.001), respectively.
Figure 2.
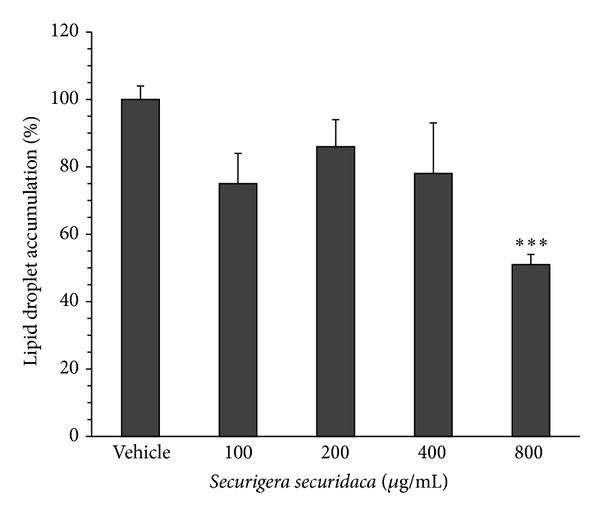
Effect of Securigera securidaca on lipid droplet accumulation in differentiating preadipocyte isolated from diabetic rats. The lipid accumulation was estimated by measuring the optical density of Oil Red O stain eluted from cells. Data are mean ± SEM (n = 5). *P < 0.001 versus vehicle.
3.3. Effect of S. securidaca on Viability of Preadipocytes
None of S. securidaca concentrations decreased proliferation of preadipocytes. In the presence of 50, 100, 200, 400, and 800 μg/mL of S. securidaca extract, viability of the cells was 97 ± 6%, 100 ± 7%, 109 ± 4%, 112 ± 3%, and 105 ± 3%, respectively (Figure 3).
Figure 3.
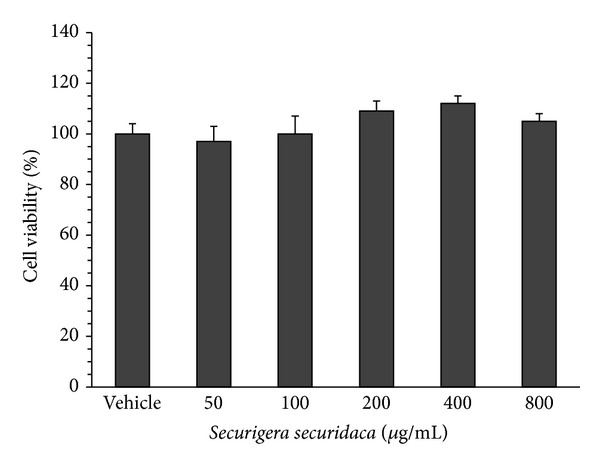
Effect of Securigera securidaca on viability of preadipocytes isolated from diabetic rats. The cells were cultured in the presence of hydroalcoholic extract of S. securidaca for 24 h. The bars show percent of cell viability as compared with untreated cells (vehicle). Data are mean ± SEM (n = 8).
4. Discussion
Dysregulation of lipid metabolism is a key feature of some pathological conditions including diabetes mellitus, insulin resistance, obesity, and fatty liver [22, 23]. In diabetes, a spectrum of abnormalities including increased serum lipids, uncontrolled lipolysis, and dysregulation of adipogenesis and lipogenesis was involved in development of atherosclerosis and cardiovascular diseases [2, 24]. It has been shown that S. securidaca reduces the serum level of triglyceride and cholesterol in hypercholesterolemic animals [11]. Also, in our previous work we showed that hydroalcoholic extract of this plant decreases serum cholesterol in diabetic rats [12]. In the present study, we further evaluated the effects of S. securidaca on adipose tissue in STZ-induced diabetes. Our data showed that this extract significantly decreases adipocyte lipolysis and differentiation of preadipocytes.
It has been well documented that hypercholesterolemia is associated with an increase in risks of atherogenesis in diabetes mellitus [2, 25]. Because of hypocholesterolemic action, consumption of S. securidaca may improve the management of diabetic dyslipidemia and reduce the incidence of cardiovascular events in diabetic patients. Atherogenic dyslipidemia is caused by different metabolic abnormalities including (1) increased cholesterol synthesis, (2) increased production of triglyceride-rich lipoproteins, and (3) increased HDL catabolism. It is believed that among these abnormalities, the pivotal role is played by increased hepatic production of lipoproteins [2]. Availability of triglycerides within hepatocytes is an important factor influencing synthesis of very low density lipoprotein (VLDL) [26]. Triglycerides are provided from de novo synthesized or extrahepatic fatty acids. Adipose tissue-derived free fatty acid is the largest extrahepatic source of fatty acid for triglyceride synthesis [2, 27]. Fatty acids are released from adipose tissue through a highly regulated process named lipolysis. Although a variety of factors are involved in regulation of lipolysis, in physiologic situation insulin and catecholamines are the main antilipolytic and prolipolytic agents, respectively [28]. Our results showed that S. securidaca extract inhibits both basal and catecholamine-stimulated lipolysis. Therefore, the antilipolytic action of S. securidaca may be responsible in part for its effect in decreasing blood cholesterol. In diabetes, deficiency of insulin in conjunction with glucagon- or catecholamine-stimulated lipolysis increases fatty acid delivery to liver which may lead to ketoacidosis, a life-threatening condition [29]. It is reasonable to conclude that S. securidaca can decrease the risk of ketoacidosis through inhibition of lipolysis. One limitation of our study is that we could not test more concentrations of S. securidaca on lipolysis because a limited amount of adipose tissue can be obtained from diabetic rats (the animals show rigorous weight loss).
The mass of adipose tissue is determined by the number and size of adipocytes. Number of adipocytes is dependent on the rate of formation of new adipocytes from precursor cells (adipogenesis) and the rate of adipocyte apoptosis. Size of adipocytes is increased by lipogenesis and decreased by lipolysis [30]. Our data showed that S. securidaca extract inhibits differentiation of preadipocyte to adipocyte. This effect in conjunction with its antilipolytic action makes S. securidaca a good candidate for management of diabetic dyslipidemia in obese diabetic patients. Because the S. securidaca extract had no effect on viability of preadipocytes, most probably its beneficial actions on lipid metabolism are not accompanied by cytotoxic effect.
In conclusion, the present study demonstrated that extract of S. securidaca seed inhibits lipolysis and adipogenesis in diabetic animals. These effects make this plant a good candidate for management of diabetic dyslipidemia and reduction of cardiovascular risk in diabetic patients.
Acknowledgment
This work is part of a Ph.D. thesis of one of the authors and is supported by a grant from Research Council of Mashhad University of Medical Sciences, Mashhad, Iran.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Physical Therapy. 2008;88(11):1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arca M, Pigna G, Favoccia C. Mechanisms of diabetic dyslipidemia: relevance for atherogenesis. Current Vascular Pharmacology. 2012;10(6):684–686. doi: 10.2174/157016112803520864. [DOI] [PubMed] [Google Scholar]
- 3.Harper CR, Jacobson TA. Avoiding statin myopathy: understanding key drug interactions. Clinical Lipidology. 2011;6(6):665–674. [Google Scholar]
- 4.Sorrentino MJ. An update on statin alternatives and adjuncts. Clinical Lipidology. 2012;7(6):721–730. [Google Scholar]
- 5.Solano MP, Goldberg RB. Management of dyslipidemia in diabetes. Cardiology in Review. 2006;14(3):125–135. doi: 10.1097/01.crd.0000188034.76283.5e. [DOI] [PubMed] [Google Scholar]
- 6.Ghorbani A. Phytotherapy for diabetic dyslipidemia: evidence from clinical trials. Clinical Lipidology. 2013;8(3):311–319. [Google Scholar]
- 7.Ghorbani A. Best herbs for managing diabetes: a review of clinical studies. Brazilian Journal of Pharmaceutical Sciences. 2013;49(3):413–422. [Google Scholar]
- 8.Ghiasi I, Nikbakht MR, Sadeghi HE, Sabzali V, Sabzali S, Shahrani M. The hypoglycemic effects of a hydro-alcoholic extract from Securigera securidaca seeds on induced diabetic in male rats. Journal of Shahrekord University of Medical Sciences. 2007;8:68–73. [Google Scholar]
- 9.Hosseinzadeh H, Ramezani M, Danaei AR. Antihyperglycaemic effect and acute toxicity of Securigera Securidaca L. seed extracts in mice. Phytotherapy Research. 2002;16(8):745–747. doi: 10.1002/ptr.1020. [DOI] [PubMed] [Google Scholar]
- 10.Porchezhian E, Ansari SH. Effect of Securigera securidaca on blood glucose levels of normal and alloxan-induced diabetic rats. Pharmaceutical Biology. 2001;39(1):62–64. [Google Scholar]
- 11.Garjani A, Fathiazad F, Zakheri A, et al. The effect of total extract of Securigera securidaca L. seeds on serum lipid profiles, antioxidant status, and vascular function in hypercholesterolemic rats. Journal of Ethnopharmacology. 2009;126(3):525–532. doi: 10.1016/j.jep.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Rajaei Z, Hadjzadeh MR, Moradi R, Ghorbani A, Saghebi A. Antihyperglycemic and antihyperlipidemic effects of hydroalcoholic extract of Securigera securidaca seeds in streptozotocin-induced diabetic rats. doi: 10.4103/2277-9175.150427. Advanced Biomedical Research. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghorbani A, Omrani GR, Hadjzadeh MA, Varedi M. Proinsulin C-peptide inhibits lipolysis in diabetic rat adipose tissue through phosphodiestrase-3B enzyme. Hormone and Metabolic Research. 2013;45(3):221–225. doi: 10.1055/s-0032-1323764. [DOI] [PubMed] [Google Scholar]
- 14.Shafiee-Nick R, Ghorbani A, Vafaee Bagheri F, Rakhshandeh H. Chronic administration of a combination of six herbs inhibits the progression of hyperglycemia and decreases serum lipids and aspartate amino transferase activity in diabetic rats. Advances in Pharmacological Sciences. 2012;2012:6 pages. doi: 10.1155/2012/789796.789796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghorbani A, Omrani GR, Hadjzadeh MAR, Varedi M. Effects of rat C-peptide-II on lipolysis and glucose consumption in cultured rat adipose tissue. Experimental and Clinical Endocrinology and Diabetes. 2011;119(6):343–347. doi: 10.1055/s-0031-1275662. [DOI] [PubMed] [Google Scholar]
- 16.Ghorbani A, Abedinzade M. Comparison of in vitro and in situ methods for studying lipolysis. ISRN Endocrinology. 2013;2013:6 pages. doi: 10.1155/2013/205385.205385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghorbani A, Hadjzadeh MR, Rajaei Z, Zendehbad SB. Effects of fenugreek seeds on adipogenesis and lipolysis in normal and diabetic rat. Pakistan Journal of Biological Sciences. 2014;17(4):523–528. doi: 10.3923/pjbs.2014.523.528. [DOI] [PubMed] [Google Scholar]
- 18.Ghorbani A, Jalali SA, Varedi M. Isolation of adipose tissue mesenchymal stem cells without tissue destruction: a non-enzymatic method. Tissue & Cell. 2014;46(1):54–58. doi: 10.1016/j.tice.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Yu G, Floyd ZE, Wu X, et al. Adipogenic differentiation of adipose-derived stem cells. Methods in Molecular Biology. 2011;702:193–200. doi: 10.1007/978-1-61737-960-4_14. [DOI] [PubMed] [Google Scholar]
- 20.Mortazavian SM, Ghorbani A, Hesari TG. Effect of hydro-alcoholic extracts of viola tricolor and its fractions on proliferation of cervix carcinoma cells. Iranian Journal of Obstetrics, Gynecology and Infertility. 2012;15(22):9–16. [Google Scholar]
- 21.Hadjzadeh MAIR, Tavakol Afshari J, Ghorbani A, Shakeri MT. The effects of aqueous extract of garlic (Allium sativum L.) on laryngeal cancer cells (Hep-2) and L929 cells in vitro. Journal of Medicinal Plants. 2006;5(18):41–48. [Google Scholar]
- 22.Arner P, Langin D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends in Endocrinology and Metabolism. 2014;25:255–262. doi: 10.1016/j.tem.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134(2):424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lafontan M. Adipose tissue and adipocyte dysregulation. Diabetes & Metabolism. 2014;40:16–28. doi: 10.1016/j.diabet.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Soehnlein O, Swirski FK. Hypercholesterolemia links hematopoiesis with atherosclerosis. Trends in Endocrinology and Metabolism. 2013;24(3):129–136. doi: 10.1016/j.tem.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher EA, Ginsberg HN. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. The Journal of Biological Chemistry. 2002;277(20):17377–17380. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- 27.Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends in Endocrinology and Metabolism. 2011;22(9):353–363. doi: 10.1016/j.tem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Large V, Peroni O, Letexier D, Ray H, Beylot M. Metabolism of lipids in human white adipocyte. Diabetes & Metabolism. 2004;30(4):294–309. doi: 10.1016/s1262-3636(07)70121-0. [DOI] [PubMed] [Google Scholar]
- 29.Perilli G, Saraceni C, Daniels MN, Ahmad A. Current Emergency and Hospital Medicine Reports. Vol. 1. 1: 2013. Diabetic ketoacidosis: a review and update; pp. 10–17. [Google Scholar]
- 30.Ghorbani A, Varedi M, Hadjzadeh MR, Omrani GH. Type-1 diabetes induces depot-specific alterations in adipocyte diameter and mass of adipose tissues in the rat. Experimental and Clinical Endocrinology and Diabetes. 2010;118(7):442–448. doi: 10.1055/s-0030-1247566. [DOI] [PubMed] [Google Scholar]


