Abstract
Germinal centres are areas of intense B lymphocyte proliferation inside primary B cell follicles in spleen and lymph nodes. Rearranged V genes from single human B cells, isolated from histological sections of two such structures by micromanipulation, were amplified and sequenced. Cells from the follicular mantle were clonally diverse and largely expressed germline V genes. Germinal centres were dominated by a few large B cell clones dispersed throughout these structures and exhibiting intraclonal diversity by ongoing somatic hypermutation. Pronounced counterselection of replacement mutations seen in one of the germinal centres may indicate a late phase of the germinal centre reaction. A polyclonal population of activated B cells expressing unmutated antibodies in the dark zone of the other germinal centre may represent the initial founder cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek C., Berger A., Apel M. Maturation of the immune response in germinal centers. Cell. 1991 Dec 20;67(6):1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- Brenner S., Milstein C. Origin of antibody variation. Nature. 1966 Jul 16;211(5046):242–243. doi: 10.1038/211242a0. [DOI] [PubMed] [Google Scholar]
- Brokaw J. L., Wetzel S. M., Pollok B. A. Conserved patterns of somatic mutation and secondary VH gene rearrangement create aberrant Ig-encoding genes in Epstein-Barr virus-transformed and normal human B lymphocytes. Int Immunol. 1992 Feb;4(2):197–206. doi: 10.1093/intimm/4.2.197. [DOI] [PubMed] [Google Scholar]
- Chen P. P. Structural analyses of human developmentally regulated Vh3 genes. Scand J Immunol. 1990 Mar;31(3):257–267. doi: 10.1111/j.1365-3083.1990.tb02767.x. [DOI] [PubMed] [Google Scholar]
- Chou C. L., Morrison S. L. A common sequence motif near nonhomologous recombination breakpoints involving Ig sequences. J Immunol. 1993 Jun 15;150(12):5350–5360. [PubMed] [Google Scholar]
- Clarke S., Rickert R., Wloch M. K., Staudt L., Gerhard W., Weigert M. The BALB/c secondary response to the Sb site of influenza virus hemagglutinin. Nonrandom silent mutation and unequal numbers of VH and Vk mutations. J Immunol. 1990 Oct 1;145(7):2286–2296. [PubMed] [Google Scholar]
- Coico R. F., Bhogal B. S., Thorbecke G. J. Relationship of germinal centers in lymphoid tissue to immunologic memory. VI. Transfer of B cell memory with lymph node cells fractionated according to their receptors for peanut agglutinin. J Immunol. 1983 Nov;131(5):2254–2257. [PubMed] [Google Scholar]
- Deane M., Norton J. D. Immunoglobulin heavy chain gene rearrangement involving V-V region recombination. Nucleic Acids Res. 1990 Mar 25;18(6):1652–1652. doi: 10.1093/nar/18.6.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny C. T., Hollis G. F., Hecht F., Morgan R., Link M. P., Smith S. D., Kirsch I. R. Common mechanism of chromosome inversion in B- and T-cell tumors: relevance to lymphoid development. Science. 1986 Oct 10;234(4773):197–200. doi: 10.1126/science.3092355. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Graninger W. B., Goldman P. L., Morton C. C., O'Brien S. J., Korsmeyer S. J. The kappa-deleting element. Germline and rearranged, duplicated and dispersed forms. J Exp Med. 1988 Feb 1;167(2):488–501. doi: 10.1084/jem.167.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Tarlinton D., Müller W., Rajewsky K., Förster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991 Jun 1;173(6):1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K., Yamagishi H. Lack of feedback inhibition of V kappa gene rearrangement by productively rearranged alleles. J Exp Med. 1991 Feb 1;173(2):409–415. doi: 10.1084/jem.173.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber C., Klobeck H. G., Zachau H. G. Ongoing V kappa-J kappa recombination after formation of a productive V kappa-J kappa coding joint. Eur J Immunol. 1992 Jun;22(6):1561–1565. doi: 10.1002/eji.1830220632. [DOI] [PubMed] [Google Scholar]
- Ichihara Y., Matsuoka H., Kurosawa Y. Organization of human immunoglobulin heavy chain diversity gene loci. EMBO J. 1988 Dec 20;7(13):4141–4150. doi: 10.1002/j.1460-2075.1988.tb03309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kassir R., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991 May 1;173(5):1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G., Rajewsky K., Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991 Dec 5;354(6352):389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- Kipps T. J., Duffy S. F. Relationship of the CD5 B cell to human tonsillar lymphocytes that express autoantibody-associated cross-reactive idiotypes. J Clin Invest. 1991 Jun;87(6):2087–2096. doi: 10.1172/JCI115239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobeck H. G., Bornkamm G. W., Combriato G., Mocikat R., Pohlenz H. D., Zachau H. G. Subgroup IV of human immunoglobulin K light chains is encoded by a single germline gene. Nucleic Acids Res. 1985 Sep 25;13(18):6515–6529. doi: 10.1093/nar/13.18.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C., Rajewsky K. Stable expression and somatic hypermutation of antibody V regions in B-cell developmental pathways. Annu Rev Immunol. 1989;7:537–559. doi: 10.1146/annurev.iy.07.040189.002541. [DOI] [PubMed] [Google Scholar]
- Kroese F. G., Timens W., Nieuwenhuis P. Germinal center reaction and B lymphocytes: morphology and function. Curr Top Pathol. 1990;84(Pt 1):103–148. doi: 10.1007/978-3-642-75519-4_5. [DOI] [PubMed] [Google Scholar]
- Kroese F. G., Wubbena A. S., Seijen H. G., Nieuwenhuis P. Germinal centers develop oligoclonally. Eur J Immunol. 1987 Jul;17(7):1069–1072. doi: 10.1002/eji.1830170726. [DOI] [PubMed] [Google Scholar]
- Lautner-Rieske A., Huber C., Meindl A., Pargent W., Schäble K. F., Thiebe R., Zocher I., Zachau H. G. The human immunoglobulin kappa locus. Characterization of the duplicated A regions. Eur J Immunol. 1992 Apr;22(4):1023–1029. doi: 10.1002/eji.1830220422. [DOI] [PubMed] [Google Scholar]
- Lederman S., Yellin M. J., Inghirami G., Lee J. J., Knowles D. M., Chess L. Molecular interactions mediating T-B lymphocyte collaboration in human lymphoid follicles. Roles of T cell-B-cell-activating molecule (5c8 antigen) and CD40 in contact-dependent help. J Immunol. 1992 Dec 15;149(12):3817–3826. [PubMed] [Google Scholar]
- Liu Y. J., Cairns J. A., Holder M. J., Abbot S. D., Jansen K. U., Bonnefoy J. Y., Gordon J., MacLennan I. C. Recombinant 25-kDa CD23 and interleukin 1 alpha promote the survival of germinal center B cells: evidence for bifurcation in the development of centrocytes rescued from apoptosis. Eur J Immunol. 1991 May;21(5):1107–1114. doi: 10.1002/eji.1830210504. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Johnson G. D., Gordon J., MacLennan I. C. Germinal centres in T-cell-dependent antibody responses. Immunol Today. 1992 Jan;13(1):17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Joshua D. E., Williams G. T., Smith C. A., Gordon J., MacLennan I. C. Mechanism of antigen-driven selection in germinal centres. Nature. 1989 Dec 21;342(6252):929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Zhang J., Lane P. J., Chan E. Y., MacLennan I. C. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991 Dec;21(12):2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C., Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986 Jun;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C., Johnson G. D., Liu Y. J., Gordon J. The heterogeneity of follicular reactions. Res Immunol. 1991 Mar-Apr;142(3):253–257. doi: 10.1016/0923-2494(91)90070-y. [DOI] [PubMed] [Google Scholar]
- Manser T. The efficiency of antibody affinity maturation: can the rate of B-cell division be limiting? Immunol Today. 1990 Sep;11(9):305–308. doi: 10.1016/0167-5699(90)90124-r. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams M. G., Nossal G. J., Lalor P. A. Molecular characterization of single memory B cells. Nature. 1991 Apr 11;350(6318):502–505. doi: 10.1038/350502a0. [DOI] [PubMed] [Google Scholar]
- McKean D., Huppi K., Bell M., Staudt L., Gerhard W., Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984 May;81(10):3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortari F., Newton J. A., Wang J. Y., Schroeder H. W., Jr The human cord blood antibody repertoire. Frequent usage of the VH7 gene family. Eur J Immunol. 1992 Jan;22(1):241–245. doi: 10.1002/eji.1830220135. [DOI] [PubMed] [Google Scholar]
- Pargent W., Schäble K. F., Zachau H. G. Polymorphisms and haplotypes in the human immunoglobulin kappa locus. Eur J Immunol. 1991 Aug;21(8):1829–1835. doi: 10.1002/eji.1830210808. [DOI] [PubMed] [Google Scholar]
- Pech M., Jaenichen H. R., Pohlenz H. D., Neumaier P. S., Klobeck H. G., Zachau H. G. Organization and evolution of a gene cluster for human immunoglobulin variable regions of the kappa type. J Mol Biol. 1984 Jun 25;176(2):189–204. doi: 10.1016/0022-2836(84)90420-0. [DOI] [PubMed] [Google Scholar]
- Pech M., Zachau H. G. Immunoglobulin genes of different subgroups are interdigitated within the VK locus. Nucleic Acids Res. 1984 Dec 21;12(24):9229–9236. doi: 10.1093/nar/12.24.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. F., Szubin R., Carson D. A., Kipps T. J. Molecular characterization of a supratypic cross-reactive idiotype associated with IgM autoantibodies. J Immunol. 1991 Sep 15;147(6):2041–2046. [PubMed] [Google Scholar]
- Radoux V., Chen P. P., Sorge J. A., Carson D. A. A conserved human germline V kappa gene directly encodes rheumatoid factor light chains. J Exp Med. 1986 Dec 1;164(6):2119–2124. doi: 10.1084/jem.164.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Rickert R., Clarke S. Low frequencies of somatic mutation in two expressed V kappa genes: unequal distribution of mutation in 5' and 3' flanking regions. Int Immunol. 1993 Mar;5(3):255–263. doi: 10.1093/intimm/5.3.255. [DOI] [PubMed] [Google Scholar]
- Sanz I., Kelly P., Williams C., Scholl S., Tucker P., Capra J. D. The smaller human VH gene families display remarkably little polymorphism. EMBO J. 1989 Dec 1;8(12):3741–3748. doi: 10.1002/j.1460-2075.1989.tb08550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittek B., Rajewsky K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 1990 Aug 23;346(6286):749–751. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- Scott M. G., Crimmins D. L., McCourt D. W., Chung G., Schäble K. F., Thiebe R., Quenzel E. M., Zachau H. G., Nahm M. H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type b polysaccharide. IV. The less frequently expressed VL are heterogeneous. J Immunol. 1991 Dec 1;147(11):4007–4013. [PubMed] [Google Scholar]
- Sharpe M. J., Milstein C., Jarvis J. M., Neuberger M. S. Somatic hypermutation of immunoglobulin kappa may depend on sequences 3' of C kappa and occurs on passenger transgenes. EMBO J. 1991 Aug;10(8):2139–2145. doi: 10.1002/j.1460-2075.1991.tb07748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si L., Roscoe G., Whiteside T. L. Selective distribution and quantitation of T-lymphocyte subsets in germinal centers of human tonsils. Definition by use of monoclonal antibodies. Arch Pathol Lab Med. 1983 May;107(5):228–231. [PubMed] [Google Scholar]
- Siebenlist U., Ravetch J. V., Korsmeyer S., Waldmann T., Leder P. Human immunoglobulin D segments encoded in tandem multigenic families. Nature. 1981 Dec 17;294(5842):631–635. doi: 10.1038/294631a0. [DOI] [PubMed] [Google Scholar]
- Stein H., Bonk A., Tolksdorf G., Lennert K., Rodt H., Gerdes J. Immunohistologic analysis of the organization of normal lymphoid tissue and non-Hodgkin's lymphomas. J Histochem Cytochem. 1980 Aug;28(8):746–760. doi: 10.1177/28.8.7003001. [DOI] [PubMed] [Google Scholar]
- Straubinger B., Huber E., Lorenz W., Osterholzer E., Pargent W., Pech M., Pohlenz H. D., Zimmer F. J., Zachau H. G. The human VK locus. Characterization of a duplicated region encoding 28 different immunoglobulin genes. J Mol Biol. 1988 Jan 5;199(1):23–34. doi: 10.1016/0022-2836(88)90376-2. [DOI] [PubMed] [Google Scholar]
- Tew J. G., DiLosa R. M., Burton G. F., Kosco M. H., Kupp L. I., Masuda A., Szakal A. K. Germinal centers and antibody production in bone marrow. Immunol Rev. 1992 Apr;126:99–112. doi: 10.1111/j.1600-065x.1992.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Tiegs S. L., Russell D. M., Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993 Apr 1;177(4):1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
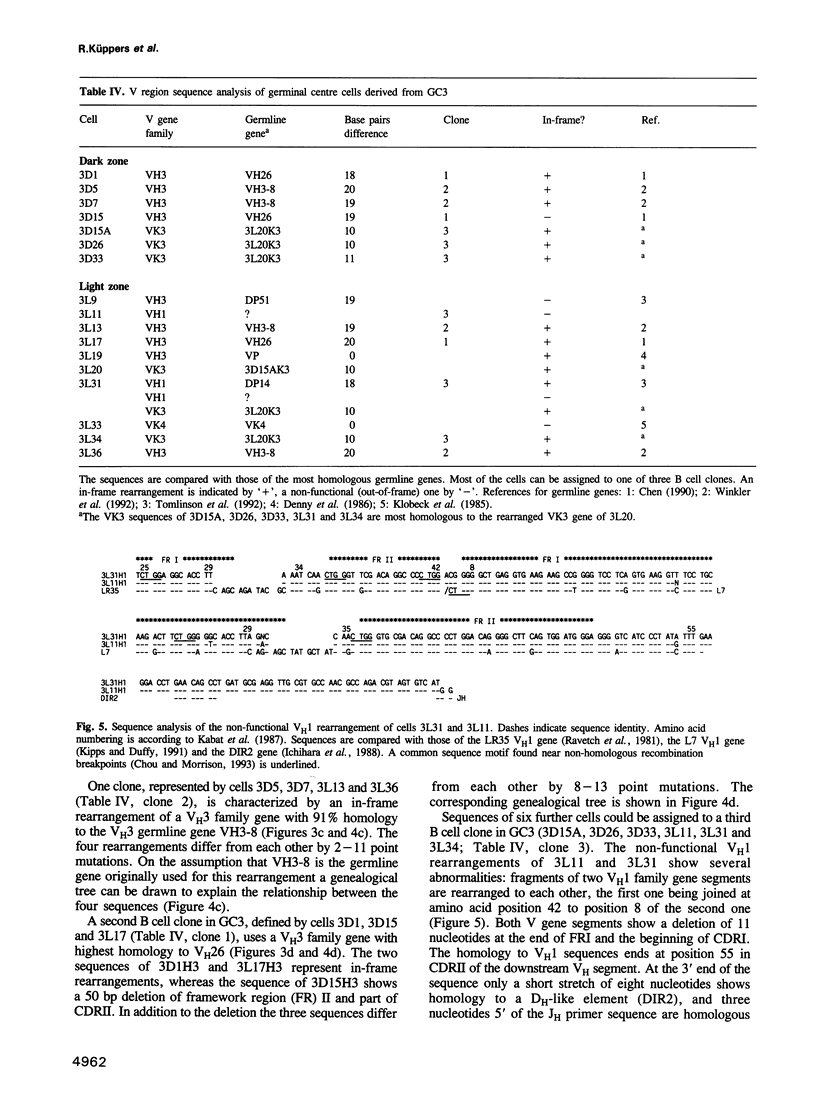
- Tomlinson I. M., Walter G., Marks J. D., Llewelyn M. B., Winter G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J Mol Biol. 1992 Oct 5;227(3):776–798. doi: 10.1016/0022-2836(92)90223-7. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A. The arrangement of immunoglobulin and T cell receptor genes in human lymphoproliferative disorders. Adv Immunol. 1987;40:247–321. doi: 10.1016/s0065-2776(08)60241-2. [DOI] [PubMed] [Google Scholar]
- Weichhold G. M., Klobeck H. G., Ohnheiser R., Combriato G., Zachau H. G. Megabase inversions in the human genome as physiological events. Nature. 1990 Sep 6;347(6288):90–92. doi: 10.1038/347090a0. [DOI] [PubMed] [Google Scholar]
- Weiss U., Rajewsky K. The repertoire of somatic antibody mutants accumulating in the memory compartment after primary immunization is restricted through affinity maturation and mirrors that expressed in the secondary response. J Exp Med. 1990 Dec 1;172(6):1681–1689. doi: 10.1084/jem.172.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss U., Zoebelein R., Rajewsky K. Accumulation of somatic mutants in the B cell compartment after primary immunization with a T cell-dependent antigen. Eur J Immunol. 1992 Feb;22(2):511–517. doi: 10.1002/eji.1830220233. [DOI] [PubMed] [Google Scholar]
- Weng N. P., Snyder J. G., Yu-Lee L. Y., Marcus D. M. Polymorphism of human immunoglobulin VH4 germ-line genes. Eur J Immunol. 1992 Apr;22(4):1075–1082. doi: 10.1002/eji.1830220430. [DOI] [PubMed] [Google Scholar]
- Winkler T. H., Fehr H., Kalden J. R. Analysis of immunoglobulin variable region genes from human IgG anti-DNA hybridomas. Eur J Immunol. 1992 Jul;22(7):1719–1728. doi: 10.1002/eji.1830220709. [DOI] [PubMed] [Google Scholar]
- Yamada M., Wasserman R., Reichard B. A., Shane S., Caton A. J., Rovera G. Preferential utilization of specific immunoglobulin heavy chain diversity and joining segments in adult human peripheral blood B lymphocytes. J Exp Med. 1991 Feb 1;173(2):395–407. doi: 10.1084/jem.173.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]





