Abstract
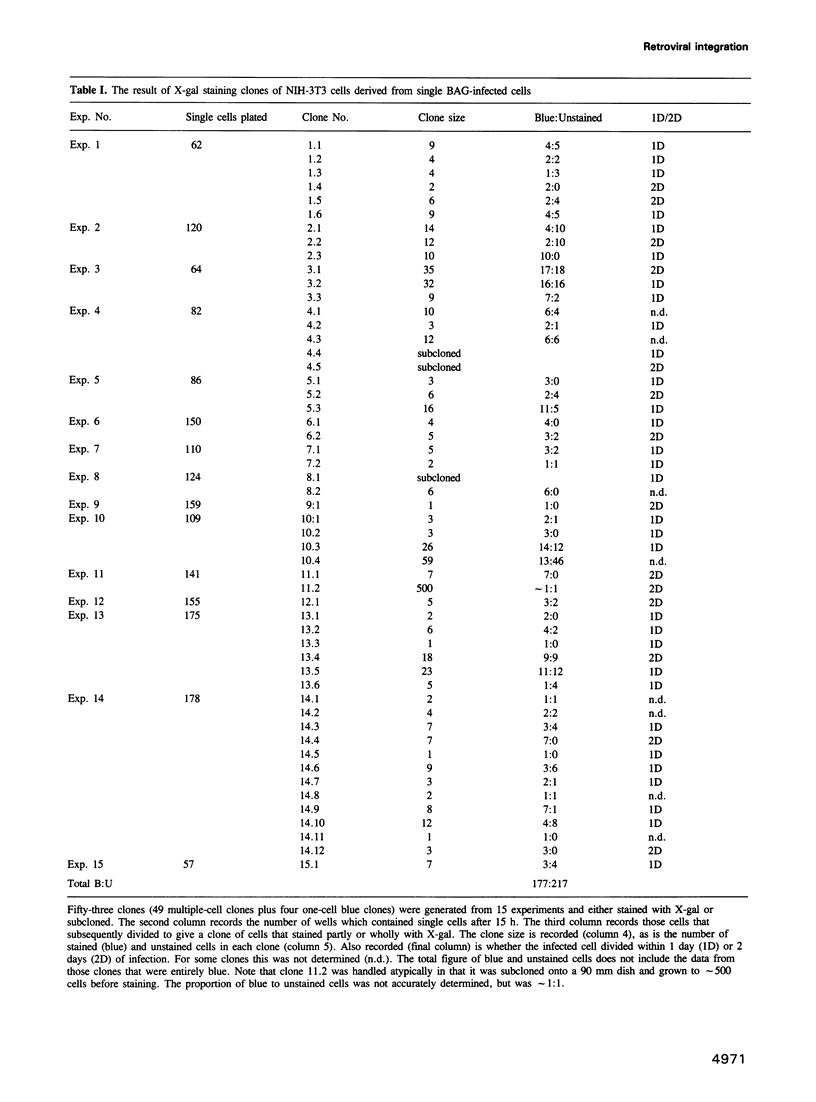
We have studied the question of whether a retrovirus integrates into the chromosomal DNA of the host cell before or after the DNA is replicated during the S phase of the cell cycle. We have infected single NIH-3T3 cells with BAG, a replication-incompetent retroviral vector which encodes the lacZ gene, then observed the clones derived from these cells to discover whether all the cells carry a copy of the proviral DNA. We have discovered that only half of the progeny of an infected cell carries a copy of the provirus. This indicates that the virus only integrates into post-replication DNA. We discuss the implications of this result for applications of retroviruses, such as gene therapy and cell lineage, which use them as vehicles for gene transfer into stem cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosch R. J., Woolf A. S., Fine L. G. Gene transfer into the mammalian kidney: direct retrovirus-transduction of regenerating tubular epithelial cells. Exp Nephrol. 1993 Jan-Feb;1(1):49–54. [PubMed] [Google Scholar]
- Bowerman B., Brown P. O., Bishop J. M., Varmus H. E. A nucleoprotein complex mediates the integration of retroviral DNA. Genes Dev. 1989 Apr;3(4):469–478. doi: 10.1101/gad.3.4.469. [DOI] [PubMed] [Google Scholar]
- Brown P. O., Bowerman B., Varmus H. E., Bishop J. M. Correct integration of retroviral DNA in vitro. Cell. 1987 May 8;49(3):347–356. doi: 10.1016/0092-8674(87)90287-x. [DOI] [PubMed] [Google Scholar]
- Brown P. O., Bowerman B., Varmus H. E., Bishop J. M. Retroviral integration: structure of the initial covalent product and its precursor, and a role for the viral IN protein. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2525–2529. doi: 10.1073/pnas.86.8.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. S., Temin H. M. Establishment of infection by spleen necrosis virus: inhibition in stationary cells and the role of secondary infection. J Virol. 1982 Jan;41(1):183–191. doi: 10.1128/jvi.41.1.183-191.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields-Berry S. C., Halliday A. L., Cepko C. L. A recombinant retrovirus encoding alkaline phosphatase confirms clonal boundary assignment in lineage analysis of murine retina. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):693–697. doi: 10.1073/pnas.89.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galileo D. S., Majors J., Horwitz A. F., Sanes J. R. Retrovirally introduced antisense integrin RNA inhibits neuroblast migration in vivo. Neuron. 1992 Dec;9(6):1117–1131. doi: 10.1016/0896-6273(92)90070-t. [DOI] [PubMed] [Google Scholar]
- Goff S. P. Integration of retroviral DNA into the genome of the infected cell. Cancer Cells. 1990 Jun;2(6):172–178. [PubMed] [Google Scholar]
- Handyside A. H., Lesko J. G., Tarín J. J., Winston R. M., Hughes M. R. Birth of a normal girl after in vitro fertilization and preimplantation diagnostic testing for cystic fibrosis. N Engl J Med. 1992 Sep 24;327(13):905–909. doi: 10.1056/NEJM199209243271301. [DOI] [PubMed] [Google Scholar]
- Harel J., Rassart E., Jolicoeur P. Cell cycle dependence of synthesis of unintegrated viral DNA in mouse cells newly infected with murine leukemia virus. Virology. 1981 Apr 15;110(1):202–207. doi: 10.1016/0042-6822(81)90022-2. [DOI] [PubMed] [Google Scholar]
- Hsu T. W., Taylor J. M. Effect of aphidicolin on avian sarcoma virus replication. J Virol. 1982 Nov;44(2):493–498. doi: 10.1128/jvi.44.2.493-498.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries E. H., Glover C., Reichmann M. E. Rous sarcoma virus infection of synchronized cells establishes provirus integration during S-phase DNA synthesis prior to cellular division. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2601–2605. doi: 10.1073/pnas.78.4.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Lewis P., Hensel M., Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992 Aug;11(8):3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. G., Adam M. A., Miller A. D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990 Aug;10(8):4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Price J. The distribution of clones of neurons in the rat somatosensory cortex. J Neurocytol. 1992 Oct;21(10):737–743. doi: 10.1007/BF01181588. [DOI] [PubMed] [Google Scholar]
- Panganiban A. T. Retroviral DNA integration. Cell. 1985 Aug;42(1):5–6. doi: 10.1016/s0092-8674(85)80092-1. [DOI] [PubMed] [Google Scholar]
- Price J., Thurlow L. Cell lineage in the rat cerebral cortex: a study using retroviral-mediated gene transfer. Development. 1988 Nov;104(3):473–482. doi: 10.1242/dev.104.3.473. [DOI] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A., Ozer H. L., DesGroseillers L., Jolicoeur P. An X-linked gene affecting mouse cell DNA synthesis also affects production of unintegrated linear and supercoiled DNA of murine leukemia virus. Mol Cell Biol. 1984 Jan;4(1):151–159. doi: 10.1128/mcb.4.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe T., Reynolds T. C., Yu G., Brown P. O. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993 May;12(5):2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springett G. M., Moen R. C., Anderson S., Blaese R. M., Anderson W. F. Infection efficiency of T lymphocytes with amphotropic retroviral vectors is cell cycle dependent. J Virol. 1989 Sep;63(9):3865–3869. doi: 10.1128/jvi.63.9.3865-3869.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C., Cepko C. L. Widespread dispersion of neuronal clones across functional regions of the cerebral cortex. Science. 1992 Jan 24;255(5043):434–440. doi: 10.1126/science.1734520. [DOI] [PubMed] [Google Scholar]
- Williams B. P., Read J., Price J. The generation of neurons and oligodendrocytes from a common precursor cell. Neuron. 1991 Oct;7(4):685–693. doi: 10.1016/0896-6273(91)90381-9. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Lemischka I. R., Nathan D. G., Mulligan R. C. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature. 1984 Aug 9;310(5977):476–480. doi: 10.1038/310476a0. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Orkin S. H., Mulligan R. C. Retrovirus-mediated transfer of human adenosine deaminase gene sequences into cells in culture and into murine hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2566–2570. doi: 10.1073/pnas.83.8.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beusechem V. W., Kukler A., Heidt P. J., Valerio D. Long-term expression of human adenosine deaminase in rhesus monkeys transplanted with retrovirus-infected bone-marrow cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7640–7644. doi: 10.1073/pnas.89.16.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]