Abstract
Introduction
Chronic unimanual motor practice increases the motor output not only in the trained but also in the non-exercised homologous muscle in the opposite limb. We examined the hypothesis that adaptations in motor cortical excitability of the non-trained primary motor cortex (iM1) and in interhemispheric inhibition (IHI) from the trained to the non-trained M1 mediate this inter-limb cross education.
Methods
Healthy, young volunteers (n = 12) performed 1000 submaximal voluntary contractions (MVC) of the right first dorsal interosseus (FDI) at 80% MVC over 20 sessions.
Results
Trained FDI’s MVC increased 49.9% and the untrained FDI’s MVC increased 28.1%. Although corticospinal excitability in iM1, measured with magnetic brain stimulation (TMS) before and after every 5th session, increased 6% at rest, these changes, as those in intracortical inhibition and facilitation, did not correlate with cross education. When weak and strong TMS of iM1 were, respectively, delivered on a background of a weak and strong muscle contraction of the right FDI, excitability of iM1 increased dramatically after 20 sessions. IHI decreased 8.9% acutely within sessions and 30.9% chronically over 20 sessions and these chronic reductions progressively became more strongly associated with cross education. There were no changes in force or TMS measures in the trained group’s left ADM nor were there changes in a non-exercising control group (n = 8).
Conclusions
The findings provide the first evidence for plasticity of interhemispheric connections to mediate cross education produced by a simple motor task.
Keywords: muscle, force, motor cortex, excitability, motor control
Introduction
Historical and recent observations suggest that unimanual motor practice increases the motor output not only in the practiced but also in the unpracticed homologous muscle (3, 9, 29). Neural mechanisms, which are unknown, must mediate such cross education because it occurs in the absence of muscle hypertrophy. One possibility is that unimanual practice leads to adaptive changes in the primary motor cortex ipsilateral to the practicing side (iM1) that is willfully not involved in the practice and this increased excitability mediates the ensuing cross education. Indeed, EEG (38), fMRI (7), MEG (35), PET (19), and TMS studies (16, 26, 27, 30) show that activation of iM1 and the excitability of the corticospinal path targeting the resting hand increase during unilateral voluntary isometric contractions. The increase in iM1 excitability increases with increasing contraction intensity of hand and wrist muscles (16, 27, 30) and the increase in neural drive from iM1 to the untrained muscles after unilateral training (23) all strongly point to the possibility that iM1 is a prime mediator of cross education.
Execution of a motor task with one hand also modifies inhibition that controls the balance of excitability between the task and the non-task hemispheres. In early experiments interhemispheric inhibition (IHI) deepened with a slight unimanual muscle contraction (11) but recent experiments with careful adjustments of the conditioning and test pulse size found that IHI actually decreased from the active to the non-active M1 (30). In addition, in cross sectional experiments unimanual serial reaction-time task transferred implicit knowledge and unimanual pinching task transferred speed and accuracy to the non-practiced hand so that the transferred motor behaviors were associated with the magnitude of decrease in IHI (2, 32). It thus appears reasonable to hypothesize that chronic unimanual practice involving, as in numerous studies before (3, 9, 29), effortful isometric contractions would produce cross education and IHI would mediate this inter-limb transfer of the ability to exert maximal force. Therefore, the purpose of the present study was to determine if increases in excitability of the non-task M1 or reductions in IHI from the task to the non-task M1 contribute to cross education produced by chronic unimanual motor practice comprising serial effortful isometric contractions of a finger muscle.
Methods
Participants and study design. Twenty healthy adults volunteered for the study (12 males, 8 females; mean age 30.9 y ±SE 1.4, height 1.75 m ±0.02, mass 72.4 kg ±3.3. Participants gave their informed consent for the experimental procedures that were approved by the local ethics committee. The study was performed in accordance with the Declaration of Helsinki. Participants were interviewed about their general health on the telephone and were included only if they were right handed and, based on a medical examination, had no history of neurological disorders, including no head or hand injuries. After medical and neurological screening, volunteers visited the laboratory for a 1-hour session and were familiarized with the equipment and testing and training procedures. At the time of the study, none of the subjects was practicing any activity that involved strengthening of the fingers. Volunteers were randomly assigned to an experimental (n = 12, eight men, four women) and a control group (n = 8, six men, two women).
Interventions. The stated goal of the program to the subjects was to increase muscle strength in the right first dorsal interosseus (FDI) without providing explicit details about a potential contralateral effect. Participants attended twenty, 15-minute-long training sessions on non-consecutive days over an 8-week period. At the start of each exercise session, subjects performed 1 trial of 5 seconds long force production at what they perceived was 50%, 75%, and 90% MVC as a warm-up then performed one 100% MVC to assess strength gains and used this value as a base to set the 80% target of training intensity. Subjects in the experimental group exercised the right FDI by performing 5 blocks of 10 isometric index finger abduction at an intensity of 80% of MVC, set as a target on an oscilloscope (Model TDS220, Tektronix, Richardson, TX). The duration of each contraction and the inter-contraction rest interval were both 5-s long. Participants ramped force production to the target in 0.5 s, held it for 4.0 s, and reduced force in 0.5 s. The inter-block rest period was 60 s. To minimize mirror activity in the contralateral left, FDI (14, 33, 40) we used 80% instead of 100% exercise intensity and subjects received the following standardized instruction before each block of contractions: “Get ready to contract on the right and completely relax the left side.” In addition, the investigator reminded the subject to relax the arm and shoulder muscles and checked this by palpation. Auditory feedback of EMG activity from the contralateral, left, FDI also helped subjects to maintain relaxation in the left arm throughout each block of exercise. Preliminary experiments showed that the exercise program caused minimal fatigue because the MVC taken before and 1 minute after the end of a session were not significantly different. Members of the control group did not exercise and participated in testing only. They came to the laboratory, placed their right hand in the dynamometer, and quietly sat or read a newspaper.
Sessions 1, 5, 10, 15, and 20 were longer, about 1.5 hours, than the other sessions because maximal voluntary force MVC of the right FDI and left FDI and left abductor minimi digiti (ADM) was measured and peripheral nerve stimulation and TMS experiments were conducted before and after the training bouts. ADM was used to determine the spatial specificity of cross education. In all experiments subjects were comfortably seated in a reclining padded chair, equipped with an armrest on each side and an adjustable head support.
Testing procedures. Voluntary force. A custom-designed hand dynamometer, described in details previously (15), was used for strength training and testing of the right FDI and it was re-configured for the testing of the left FDI and ADM. Briefly, the dynamometer’s plexiglass base was affixed to the chair’s armrest, supporting the hand and forearm. With the right wrist pronated, the palm rested on the plexiglass base. The index finger was isolated with the thumb extended and abducted and the 3rd, 4th, and 5th fingers extended and, with a Velcro strap, abducted. The center of the proximal interphalangeal joint of the extended index finger was aligned with the center of the load cell (Model 31, Honeywell-Sensotec, Columbus, OH). The wrist was stabilized with a Velcro strap, wrapped around the chair’s armrest and the dynamometer’s plexiglass base. The optimal position of the load cell was determined for each subject and adjusted individually. Such a set-up insured that the sole source of force production was index finger abduction. In separate measurements with the same dynamometer re-configured, we measured MVCs in the left FDI and ADM. Subjects were familiarized with the procedure by performing 1 trial of 5-s-long force production at 50%, 75%, and 90% MVC with each finger. Subjects were then instructed to perform 2 trials of 5-s-long MVC with 1 minute of inter-trial rest and the force was recorded from these trials on the computer. Participants received biofeedback of their force production from an oscilloscope. The order of testing of the right FDI, left FDI, and left ADM was systematically rotated between subjects. MVC of the right FDI was measured in every session in the experimental group and it was also measured in the left FDI and ADM in sessions 1, 5, 10, 15, and 20. MVCs of the three fingers in the control group were measured in sessions 1, 5, 10, 15, and 20. Table 1 summarizes the schedule of measurements.
Table 1.
Measurement schedule.
| Before | RC | • | • | • | |||||||||||||||||
| Cond MEPs | • | • | • | • | • | ||||||||||||||||
| SICI, ICF, IHI | • | • | • | • | • | ||||||||||||||||
| rMT | • | • | • | • | • | ||||||||||||||||
| Mmax | • | • | • | • | • | ||||||||||||||||
| MVC, LADM | • | • | • | • | • | ||||||||||||||||
| MVC, L FDI | • | • | • | • | • | ||||||||||||||||
| MVC, R FDI | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | |
| Session | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| After | MVC, R FDI | ||||||||||||||||||||
| MVC, LFDI | |||||||||||||||||||||
| MVC, LADM | |||||||||||||||||||||
| Mmax | • | • | • | • | • | ||||||||||||||||
| rMT | • | • | • | • | • | ||||||||||||||||
| SICI, ICF, IHI | • | • | • | • | • | ||||||||||||||||
| Cond MEPs | |||||||||||||||||||||
| RC | • | • | • |
denotes that a measurement was administered
Before and After refer to a measurement taken before and after a session. RC: recruitment curve; Cond MEPs: TMS at 120% rMT conditioned with 20% MVC and TMS at 160% rMT conditioned with 80% MVC; SICI: short intracortical inhibition; ICF: intracortical facilitation; IHI: interhemispheric inhibition; rMT: resting motor threshold; Mmax: maximal compound action potential evoked by peripheral nerve stimulation; MVC, L ADM: maximal voluntary contraction of the abductor minimi digiti; MVC, L FDI: maximal voluntary contraction of the left first dorsal interosseus; MVC, R FDI: maximal voluntary contraction of the right first dorsal interosseus.
Electromyography (EMG). The EMG activity of the right FDI and left FDI and ADM was recorded using silver-silver chloride surface EMG electrodes (2 cm center-to-center inter-electrode distance), placed over these muscles in a belly-tendon montage. EMG signals were amplified using a Nicolet Viking electromyography system (Madison, WI) and band-pass filtered between 10 and 2,000 Hz. Signals were digitized at a frequency of 5 kHz and fed into a laboratory computer for further off-line analysis. The EMG activity was recorded during cortical and peripheral nerve stimulation. EMG activity was also recorded from the left FDI during the training contractions in sessions 1, 5, 10, 15, and 20 to determine the magnitude of mirror EMG activity as a percent of MVC.
Transcranial magnetic stimulation (TMS) and peripheral nerve stimulation. Before TMS, a cap (Electro Caps International, Inc., Eaton, OH) was placed on the subject’s head. For each subject we marked individual anatomical landmarks on the cap that allowed us to place the cap on the head in the same position within and across sessions. The optimal coil position was also marked in a coordinate system drawn on surface of the cap. Thus the coil was placed on the surface of the cap in the same position within and across sessions. These procedures made it possible to stimulate the same area of the cortex within and across sessions. MEPs were elicited by TMS delivered from a Magstim 200 stimulator (Magstim company, Dyfed, UK) through a figure-of-eight coil (external loop diameter, 8 cm; type number, SP15602) with a monophasic current waveform. The coil was moved in 0.5-cm steps over M1 to identify the optimal scalp position, i.e., hot spot, for activation of the target muscle for the right FDI overlying left M1 and left FDI and ADM overlying the right M1. The hot spot correlates well with stimulation of Brodmann’s area 4. The intersection of the coil was placed tangentially to the scalp with the handle pointing backward and laterally at 45° angle away from the midline over the hot spot for the target muscle to activate the corticospinal system preferentially trans-synaptically via horizontal corticocortical connections. The direction of the current flow was posterior-to-anterior across M1. The coil was secured with a coil holder to ensure that the same area of the cortex was stimulated within a session and across sessions. Single pulse stimuli were delivered at an inter-stimulus interval varying between 5 to 6 s. During experiments, MEPs were displayed on the monitor of the data collection computer, visually inspected, and stored on a computer for offline analysis. TMS measures included resting motor threshold (rMT), MEP amplitudes using recruitment curves (RC), short intracortical inhibition (SICI) and facilitation (ICF), IHI from left M1 to right M1, and MEPs at 120% and 160% rMT conditioned with the contraction of the right FDI at 20 and 80% MVC.
rMT was determined with 1% increment in stimulator output as the minimal stimulus intensity required to produce MEPs of at least >50 µV in peak-to-peak amplitude in at least 5 out of 10 consecutive trials.
RC. RC was measured in the left FDI and ADM with both hands at rest before and after sessions 1, 10, and 20 (Table 1). For the RC, stimulation started at 10% below rMT and increased in 10% steps of maximal stimulator output until the MEP amplitude did not experience additional increases or the maximal stimulator output was reached. The stimulation intensities were administered in a pseudo-random order, with the highest intensity always presented last. There were 7 trials to determine MEP amplitude at each stimulation intensity. The amplitude of MEPs was measured peak-to-peak, averaged off-line, and expressed as a percentage of the maximal motor response (Mmax). To determine Mmax, the ulnar nerve was stimulated (1 ms rectangular pulse, model Viking IV, Nicolet Biomedical, Madison, WI) with supramaximal intensity using bipolar surface electrodes placed at the wrist with the subject’s hand in the dynamometer. The stimulating electrode was placed the intersection of the two lines representing the flexor carpi ulnaris tendon and the ulnar styloid. The intensity of stimulation was increased from a sub-threshold level until there was no further increase in the peak-to-peak amplitude of the M-wave with increasing stimulation intensity. At this final stimulation intensity, we recorded the twitch-evoked force in three trials. We normalized each participant’s TMS data to the individual Mmax, making it possible to compare MEP amplitudes between different test sessions. Stimulation intensity was expressed as a percent of each participant’s rMT. For the sake of group comparisons, all stimulus intensities were normalized to rMT of a stimulus-response curve determined before an intervention during the first training session. Such a normalization process makes it possible to reliably determine shifts in RCs due to a specific intervention.
SICI, ICF, and IHI. SICI and ICF, in one block and IHI, in another block, were measured in the left FDI and ADM with both hands at rest before and after sessions 1, 5, 10, 15, and 20 (Table 1). We used the methods described by Kujirai et al. (21). The conditioning stimulus was set at ∼80% of rMT, an intensity that does not affect spinal excitability (8). The test stimulus was delivered 2 and 10 ms after the conditioning stimulus for SICI and ICF, respectively. Because there are two distinct phases of inhibition (12), we used the 2-ms inter-stimulus (21) interval to avoid a mixture of the two phases. Under these conditions, the conditioning stimulus produced ∼50% inhibition and ∼30% facilitation at 2 and 10 ms inter-stimulus interval. The intensity used for the conditioning stimulus ranged between 29 to 56% of stimulator output, corresponding to 78.9% of the rMT and was kept constant within and between sessions. The size of the test stimulus was set at ∼1.0 mV and was also kept constant. Seven test stimuli, 7 SICIs, and 7 ICFs were presented at random at rest and repeated 2 times to establish the stimulation parameters. There was 5 s of rest between trials. We also assessed ICF with a conditioning stimulus set at 90% of rMT as such a stimulus intensity is less likely to produce changes in excitability of motoneurons at the spinal level (27) and found similar results at 80 and 90% conditioning stimulus (n = 6, session 1, 10, and 20, data not shown).
In a separate block administered in an alternating order with the SICI and ICF block, we measured IHI from left to right M1 in the left FDI and ADM using two custom-built figure-of-eight coils (loop diameter: 8 cm, type number SP15602, SP15001). The two coils were positioned at the optimal location for activating the left and right FDI, respectively and in a separate run for the left ADM. The handles of the coils pointed 45° backward and laterally relative to the midsagittal line. A suprathreshold conditioning stimulus was set at an intensity that elicited inhibition of ∼75% to allow its up- or down-regulation. The intensity used for the conditioning stimulus ranged from 51 to 82% (69.9 ±8.3 of stimulator output), corresponding to 126.4% of the rMT. The conditioning stimulus was delivered to the left M1 10 ms before the test stimulus given to the right M1. The size of the test stimulus was set at ∼1.0 mV within and between sessions. Twelve test stimuli and 12 IHIs were presented at random with 5 s of rest between each trial. In these double-pulse experiments, the control MEPs and the conditioned MEPs were normalized to Mmax, averaged, and the conditioned MEPs expressed relative to the control MEPs, yielding one data point per subject for SICI, ICF, and IHI before and after sessions 1, 5, 10, 15, and 20 in the left FDI and ADM.
MEPs conditioned with muscle contraction. We evaluated the effect of exercise training of the right FDI on the size of MEPs measured in the left FDI conditioned with a voluntary contraction of the right FDI before sessions 1, 5, 10, 15, and 20 (Table 1). In these experiments the left FDI, from which the MEPs were recorded, was always at rest. Block 1 consisted of TMS given at 120% of the rMT at rest and conditioned with 20% MVC. Block 2 consisted of TMS given at 160% of the rMT at rest and conditioned with 80% MVC. The order of the two blocks and the 5 trials at rest and 5 trials with contraction within each block, respectively, was rotated between subjects but the order was kept the same in each subject across the five testing sessions. There was 5 s of rest between trials. TMS was delivered at 2.5 s of the 5-s-long conditioning contractions. Within each block, the control and conditioned MEPs were normalized to Mmax and then, respectively, averaged and the conditioned MEPs were expressed relative to the MEPs at rest, yielding one data point per block per subject before sessions 1, 5, 10, 15, and 20 in the left FDI.
Statistical analysis
Data in the text and figures are reported as mean ±SE. We tested for normal distribution with the Kolmogorov–Smirnov tests for each variable. Mauchly’s test of sphericity, conducted for each repeated measures analysis, was followed by a group (experimental, control) by session (1, 5, 10, 15, and 20) ANOVA with repeated measures on session to determine changes in MVC of each of the 3 muscles and in mirror EMG activity. The amplitude of the maximal compound action potentials, Mmax, and the associated evoked twitch forces were measured at the start and end of sessions 1, 5, 10, 15, and 20 and were analyzed with a group (2) by session (5) by time (2) ANOVA in the right FDI and left FDI and ADM.
Acute changes in rMT, SICI, ICF, and IHI, using the before and after data measured within session 1, 5, 10, 15, and 20, respectively, were analyzed with a group (2) by time (2) ANOVA with repeated measures on time. Acute changes MEP size (RCs), using the before and after data measured within session 1, 10, and 20, respectively, were analyzed with a group (2) by time (2) by stimulation intensity (8) ANOVA with repeated measures on time and intensity.
Chronic changes in rMT, SICI, ICF, and IHI measured before sessions 1, 5, 10, 15, and 20 in the two groups’ left FDI and ADM muscle, respectively, were analyzed with a group (2) by session (5) ANOVA with repeated measures on session. Chronic changes in MEP size measured at 8 intensities before sessions 1, 10, and 20 in the two groups’ left FDI and ADM muscle, respectively, were analyzed with a group (2) by session (3) by intensity (8) ANOVA with repeated measures on session and intensity. In case of significant interaction effects, we used Tukey’s posthoc to determine the means that were different at p < 0.05. Pearson correlation analysis was used to determine correlations as needed.
Results
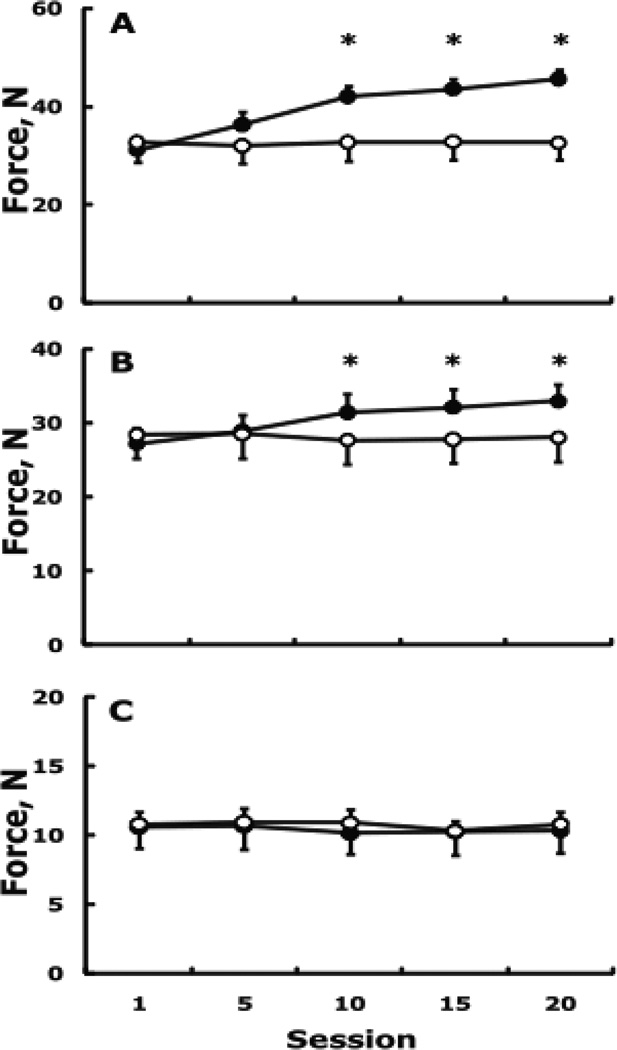
Voluntary force and EMG. Fig. 1A shows the group by session interaction in the right, trained, FDI (F4,68 = 27.0, p = 0.001). The training program increased maximal voluntary force from baseline to session 20 by 14.5 ±1.5 N or 49.9 ±6.3% without changes in the control group. Fig. 1B shows the group by session interaction in the left, untrained, FDI (F4,68 = 18.9, p = 0.001). The time course of adaptation revealed 15.6 ±1.7% cross education at session 10 (p < 0.05) that further increased 6.2 ±2.0% by session 20 (p < 0.05), with a total amount of cross education of 5.7 ±0.6 N or 21.8 ± 2.3% (both p < 0.05) from baseline to session 20. There were no changes in MVC of the control group’s untrained FDI (−1.9 ±3.5). The changes in maximal voluntary force of the ADM were not significant in the experimental (−0.8 ±3.8%) and control group (−0.5 ±3.5%) (group by time interaction, F4,68 = 0.5, p = 0.747, Fig. 1C). There was a group by session interaction in the left, untrained, FDI’s EMG activity normalized for Mmax (F4,68 = 3.9, p = 0.006) with an increase of 27.6 ±2.9% in the experimental group and 6.1 ±3.0% in the control group (n.s.). The changes in surface EMG activity were not significant in the experimental (3.1 ±2.6%) and control group’s (3.2 ±3.4%) left ADM.
Figure 1.
Changes in maximal voluntary force in the right, trained, FDI (A), left, untrained, FDI (B), and the control muscle, left ADM (C). Filled and open symbols, respectively, indicate the experimental and control group. Vertical bars are 1 + or − SE. * higher than control and session 1 (p < 0.05).
Responses to peripheral nerve stimulation. There were no group by session by time or other two-way interactions for Mmax or twitch forces in the right FDI and left FDI and ADM. For example, the pre-to-post changes in Mmax of the right, trained, FDI were 2.0 ±1.7% (p = 0.258) and the changes in the evoked forces were 3.6 ±4.5% (p = 0.482).
Responses to TMS. rMT. There were no significant acute (within session) and chronic (across sessions 1, 5, 10, 15, 20) changes in rMT. The largest pre-to-post change in rMT of all groups and muscles was a reduction from 50.3 ±4.1% in session 1 to 47.5 ±4.6% in session 20 in the trained, right FDI (p = 0.111).
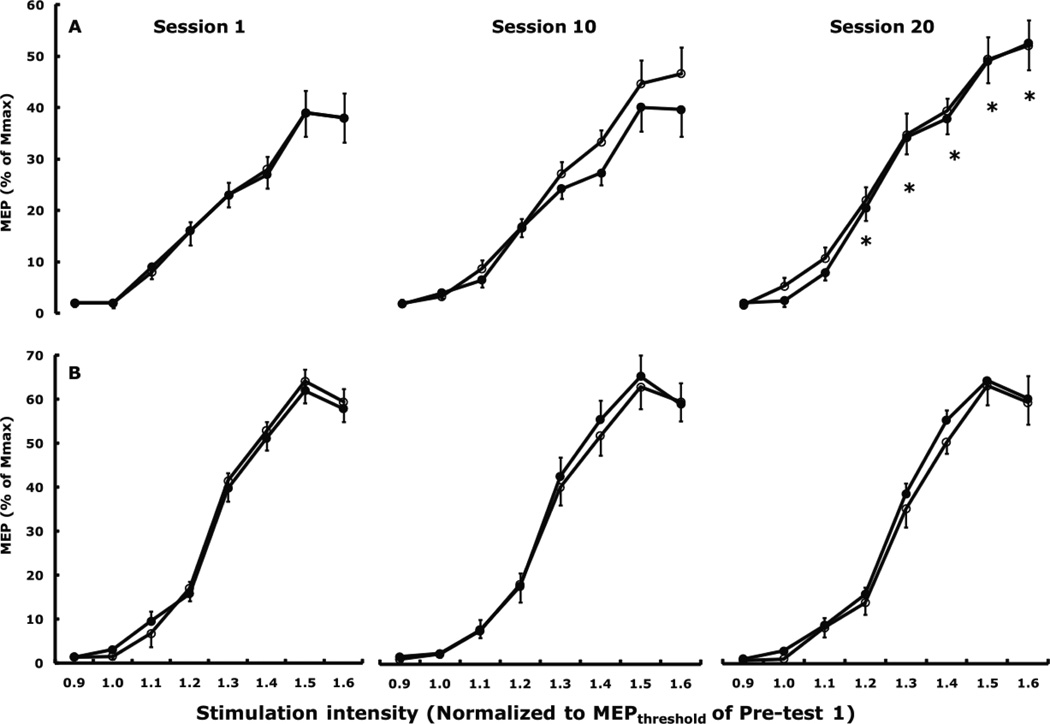
Cortical excitability. For the acute changes in MEP size measured at 8 stimulation intensities before and after session 1 and 20, respectively, in the two groups’ left FDI and ADM muscle there were no significant main, 2- or 3-way interactions. For the chronic changes in MEP size measured at 8 intensities before sessions 1, 10, and 20 in the two groups’ untrained, left FDI, there were a group by session (F2,34 = 7.4, p = 0.002) and a group by session by stimulation intensity interaction effects (F14,238 = 2.4, p = 0.004). Fig. 2A shows that MEP size increased 6% (p = 0.010) from the 1st to the 20th session. In the control group’s left FDI, MEP size did not change between the 1st (41.2% ±4.7) and the 20th session (43.1% ±5.1). None of main and interaction effects were significant in the ADM (Fig. 2B).
Figure 2.
Acute (within session) and chronic (across session) changes in MEP sizes in the left, untrained, FDI, (A) and left ADM (B) following exercise training of the right FDI. There were no significant acute effects but there was a significant chronic increase in MEP size from Session 1 to Session 20 in the left FDI. No acute or chronic changes occurred in the left ADM. Open symbols denote recruitment curves measured at the start of a session before exercise training and filled symbols denote the recruitment curves measured immediately after the training was completed. * p < 0.05 relative to Session 1.
SICI and ICF. There were no significant acute (within session) and chronic (across sessions) changes in SICI and ICF in the experimental and control groups’ FDI and ADM. As a percent of the test MEP, the level of SICI across all sessions was 52.4 ±2.9% and 53.9 ±4.1% in the experimental and control group’s left FDI. The largest change in SICI occurred in the experimental group’s FDI in session 5 where SICI diminished from 48.0 ±2.4% before exercise to 57.1 ±3.9% of the test MEP after exercise without the group by time interaction reaching significance (F1,17 = 1.4, p = 0.246). The overall level of ICF across all sessions was 137.0 ±9.1% and 134.1 ±6.2% of the test MEP in the experimental and control group’s left FDI. The largest change in ICF occurred in session 5 where ICF increased from 133.0 ±8 to 149.0 ±11.0% of the test MEP in the experimental group’s left FDI (group by time interaction, F1,17 = 3.9, p = 0.064). In the SICI and ICF experiments the size of the test pulse was constant within and across sessions. For example, the size of the test pulse in the experimental group’s left FDI was 1.02 ±0.06, 1.01 ±0.05, 1.06 ±0.06, 1.05 ±0.07, and 1.10 ±0.08 mV in session 1, 5, 10, 15, and 20, respectively (session effect, F4,72 = 3.72, p = 0.071), with similar values in the control group (group by session interaction F4,72 = 0.9, p = 0.457). The size of the test pulse was similar (∼1.0 mV) in the experimental and control group’s left ADM and was also stable across sessions.
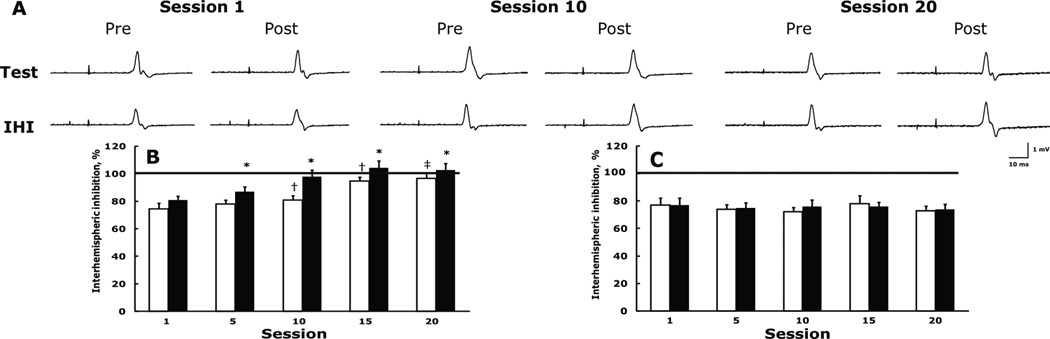
IHI. Fig. 3A shows a single-subject example for IHI in the left FDI and Fig. 3B shows the experimental group’s data. In session 1, the magnitude of IHI in the experimental and control group’s left FDI was statistically similar 74.3 ±2.4% and 79.8 ±3.2% of the test MEP. IHI in the two group’s ADM was 76.9 ±3.3% and 75.5 ±3.9% of the test MEP. In the experimental but not in the control group’s left FDI IHI acutely diminished 8.8 ±3.9% in session 1 (Tukey post-hoc, p = 0.159), 11.6 ±2.9% in session 5 (p = 0.016), 7.6 ±4.5% in session 10 (p = 0.254), 10.4 ±2.6% in session 15 (p = 0.048), and 6.3 ±3.8% in session 20 (p = 0.141). The average acute within session diminishment in IHI was 8.9%. In the control group, the average acute within session change in IHI was 1.8%. Relative to session 1, in the experimental but not in the control group’s left FDI IHI chronically diminished (group by session interaction F4,72 = 8.2, p = 0.010) 5.4 ±2.8% by session 5 (Tukey post-hoc p = 0.111), 23.4 ±5.1% by session 10 (p = 0.002), 28.1 ±3.8% by session 15 (p = 0.001), and 30.9 ±3.8% by session 20 (p = 0.001). %. In the control group, the average chronic change in IHI between session 1 and session 20 was 3.5%. No significant acute and chronic changes occurred in IHI in the experimental group’s ADM (Fig. 3C). In the IHI experiments the size of the test pulse was constant within and across sessions. For example, the size of the test pulse in the experimental group’s left FDI was 1.06 ±0.13, 0.99 ±0.18, 1.12 ±0.18, 1.15 ±0.27, and 1.11 ±0.28 mV in session 1, 5, 10, 15, and 20, respectively (session effect, F4,72 = 3,72, p = 0.268), with similar values in the control group (group by session interaction F4,72 = 1.9, p = 0.222). The size of the test pulse was similar (∼1.0 mV) in the experimental and control groups’ left ADM and was also stable across sessions.
Figure 3.
Representative example in one subject for interhemispheric plasticity as evidenced by a diminishment of interhemispheric inhibition measured at rest in the untrained, left, FDI before (pre) and after (post) exercise of the right FDI in sessions 1, 10, and 20 (A). Test, motor evoked potentials produced by TMS of the right primary motor cortex in left FDI. IHI, interhemispheric inhibition produced by a conditioning TMS pulse given to the left primary motor cortex followed 10 ms later by a test pulse. Each tracing is an average of 7 trials. Note the stability of the size of the test pulse across sessions and the reduction in IHI (indicated by the increase in size of the motor evoked potentials). Interhemispheric inhibition in the experimental group’s left, untrained, FDI (B) and ADM (C). Open and filled columns, respectively, denote data collected immediately before and after exercise of the right FDI. The horizontal line at 100% in each panel represents control value, i.e., absence of inhibition and facilitation. * p < 0.05 before vs. after exercise (acute changes) and † relative to the before value in session 1 (chronic changes) (p < 0.05).
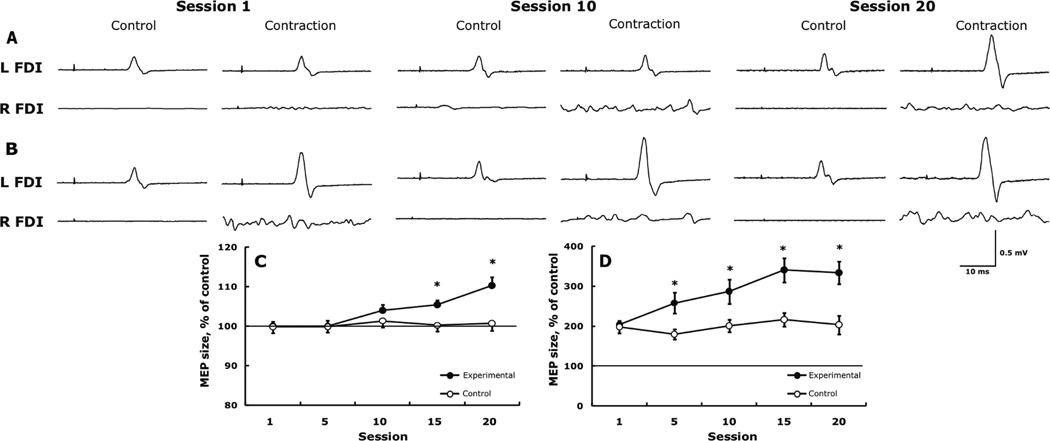
Cortical excitability during muscle contraction. Fig. 4 shows representative trials in one subject (A, B) and also the experimental and control groups’ data for changes in cortical excitability measured in the left FDI while the right M1 received TMS at an intensity of 120% (C) and 160% (D) of the rMT during rest and the right FDI was contracted at 20 and 80% MVC. At 120% TMS and 20% MVC, cortical excitability remained unchanged initially but, relative to session 1, increased 5.4 ±1.5% by session 15 (p = 0.002) and 10.3 ±2.3% by session 20 (p = 0.001) (group by session interaction, F4,68 = 9.0, p = 0.008) (Fig. 4A and C). At 160% TMS and 80% MVC, cortical excitability, relative to session 1, increased 26.6 ±3.8% by session 5 (p = 0.001), 40.7 ±5.7% by session 10, 67.0 ±7.2% (p = 0.001) by session 15, and 63.9 ±8.4% by session 20 (p = 0.001) (group by session interaction, F4,68 = 3.8, p = 0.012) (Fig. 4B and D).
Figure 4.
Representative example in one subject for changes in the facilitation of motor evoked potentials (MEPs) produced by TMS and muscle contraction. MEPs produced by TMS 20% above resting motor threshold (rMT) in the left FDI at rest (control) and combined with contraction of the right FDI at 20% MVC (A) and by TMS 160% above rMT and 80% MVC (B). Note the facilitation of MEPs in panel B compared with panel A at rest and due to the stronger contraction and TMS. Note also how the facilitation of ipsilateral MEPs becomes increasingly larger across sessions. Each tracing is the average of 7 trials. L and R FDI, left and right first dorsal interosseus. Group data for the weak TMS combined with weak contraction (C) and for strong TMS combined with strong contraction (D) in the experimental, trained (filled symbols) and control, untrained group (open symbols). In panels C and D, data are expressed as a percent of the control MEP. * p < 0.05 between the two groups and relative to session 1.
Correlation analyses. The percent changes in cross education in the untrained, left FDI were unrelated to the percent changes in strength gains in the trained, right FDI in session 5 and 10 but by session 15 (r = 0.55, p = 0.021) and session 20 (r = 0.81, p = 0.001) cross education became strongly associated with strength gains. Subjects were instructed to contract the right FDI and keep the left FDI completely relaxed to avoid mirror EMG activity. The mirror EMG activity, expressed as a percent of the EMG during an MVC, in the left FDI measured in sessions 1 (0.8% ±0.8), 5 (2.0% ±1.0), 10 (1.2% ±0.9), 15 (1.2% ±1.3) , and 20 (1.5% ±0.9), averaging 1.3% (±1.0) did not change and this mirror activity did not correlate with the magnitude of cross education (y = −0.15x + 28.6, r = −0.052, p = 0.873).
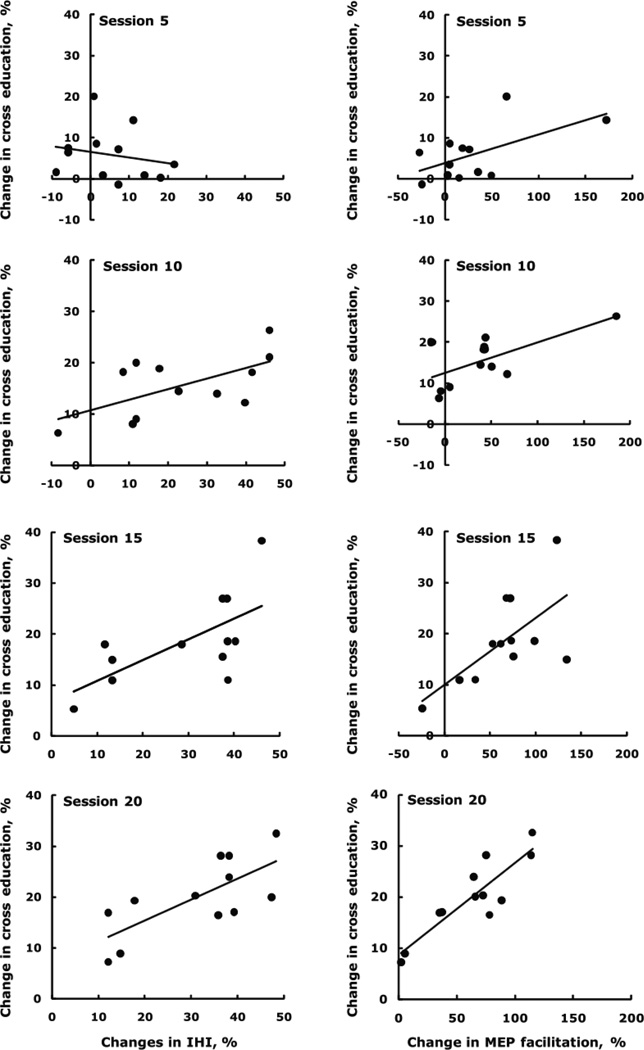
The changes in MEP size measured with RCs at rest did not correlate with changes in cross education at session 10 (r = 0.33, p = 0.172) and this relationship remained unchanged by session 20 (r = 0.20, p = 0.293). The changes in the amount of facilitation of the MEPs when the right FDI contracted weakly (20% MVC) and the right M1 was stimulated with low intensity TMS (120% rMT), did not correlate with the changes in cross education at session 5 (r = 0.19, p = 0.549), session 10 (r = 0.43, p = 0.167), session 15 (r = 0.49, p = 0.105), but the correlation was significant at session 20 (r = 0.68, p = 0.014). Fig. 5 shows that the changes in the amount of facilitation of the MEPs when the right FCR contracted strongly (80% MVC) and the right M1 was stimulated with high intensity TMS (160% rMT), correlated significantly with the changes in cross education r = 0.58 (p = 0.047) already at session 5, r = 0.66 (p = 0.020) at session 10, r = 0.65 (p = 0.022) at session 15, and r = 0.88 (p = 0.001) at session 20.
Figure 5.
Time course of correlations between changes in cross education and changes in interhemispheric inhibition at rest (left column), showing the evolution and consolidation of interhemispheric plasticity in relation to cross education. The relationships became stronger across session 5: R2 = 0.04, y = −0.14x + 6.5; session 10: R2 = 0.37, y = 0.21x + 10.7; session 15: R2 = 0.43, y = 0.41x + 6.8; session 20: R2 = 0.52, y = 0.41x + 7.3. Time course of correlations between changes in cross education and changes in the facilitation of motor evoked potentials in the left, untrained FDI by TMS of the right primary motor cortex at an intensity 160% above resting motor threshold combined with concurrent contraction of the right FDI at 80% MVC (right column). The relationships became stronger across session 5: R2 = 0.34, y = 0.07x + 3.8; session 10: R2 = 0.43, y = 0.07x + 12.5; session 15: R2 = 0.43, y = 0.13x + 10.0, and session 20: R2 = 0.78, y = 0.18x + 8.6.
Fig. 5 also shows that the percent changes in cross education were initially unrelated to the percent changes in IHI in session 5 (r = 0.21, p = 0.511) but moderate relationships emerged by session 10 (r = 0.61, p = 0.036) and session 15 (r = 0.66, p = 0.020) that further strengthened by session 20 (r = 0.72, p = 0.008). At no time point was there a significant correlation between the changes in IHI and changes in MEP size measured with RCs at rest (p > 0.05).
Discussion
Repeated effortful contractions of a finger muscle increased maximal voluntary force of the homologous muscle in the unexercised contralateral hand. A reduction in IHI at rest from the trained to the untrained hemisphere and an upregulation of iM1 excitability, especially during muscle contraction mediated this cross education effect. These results provide the first evidence for the evolution and consolidation of interhemispheric plasticity in intact humans.
Behavioral changes
For over 100 years, the majority of studies on cross education have reported an increase in motor function in the contralateral homologous muscle after unilateral practice involving strong voluntary contractions with no skill (3, 9, 29) or involving complex motor skills (24). The present results extend these findings by showing that serial voluntary isometric contractions at submaximal intensity involving minimal skills can also produce functionally and statistically significant inter-limb transfer of maximal voluntary force. Two-thirds of cross education appeared by the 10th session with its gain slowing to about 7% between sessions 10 to 20, suggesting that crossed-adaptations peaked by session 20, after 1000 contractions.
The value of 21.8% cross education observed here agrees with the magnitude (27%, adjusted for the changes in the control group) reported previously for a similarly unfamiliar movement, ulnar deviation (9) and, as expected, exceed the 8% value reported in meta analyses for familiar movements (3, 29). During effortful unilateral muscle contractions, the contralateral homologous muscle can exhibit as high as ∼20% mirror EMG activity and involuntary force (40). Keeping the mirror activity low under these conditions was important because exercise with loads as light as 10% of maximum can increase maximal force (22). Therefore, higher levels of mirror activity could have confounded the observed cross education. However, the average mirror EMG activity in the present study was only 1.3% of maximum EMG activity and did not correlate cross education. Therefore, it is unlikely that mirror EMG activity contributed to the 21.8% cross education. The cross education observed in the present study had a high level of spatial specificity because it was confined to the homologous left FDI without changes in the left ADM although both muscles are innervated by the deep branch of the ulnar nerve. The evolution of cross education was linked to a strong dependence on persistent and large gains (49.9%) in motor output of the exercised, right, FDI because the strength gains in each FDI became more strongly correlated as the study progressed from session 5 (r = 0.01), 10 (r = 0.17), 15 (r = 0.55), and to session 20 (r = 0.81). Because there were no changes in the twitch-evoked forces produced by electrical stimulation in the untrained, left, FDI, cross education, was the result of neural mechanisms and not muscle hypertrophy, as suggested previously (3, 9, 29). The progressively stronger association between the increases in EMG activity recorded during test MVCs of the untrained, left FDI and the increases in cross education suggests that an increase in gross neural drive to the untrained, left, FDI contributed to cross education, strengthening the argument for a neural, possibly supraspinal mechanism mediating cross education (23).
Corticospinal excitability of iM1 contributes to inter-limb force transfer
TMS studies provide evidence that activation of iM1 and the excitability of the corticospinal path targeting the resting hand increase during contralateral unilateral voluntary isometric contractions (16, 26, 27, 30). Especially relevant to the present study are the observations from cross-sectional studies that in hand and wrist muscles iM1 excitability increases with increasing contraction intensity in a task-specific manner (16, 27, 30) and that twitch interpolation after chronic unilateral training revealed increases in neural drive from iM1 to the untrained muscles (23). Together these data point to the existence of a model that forceful unilateral motor practice would repeatedly and bilaterally increase excitability of both M1s that in turn would consolidate the increase in excitability in iM1 and mediate inter-limb force transfer.
The present data provide evidence for this model on multiple levels. First, chronic effortful motor practice made iM1 more excitable during contralateral muscle contraction. In previous studies and at baseline in the present study, conditioning of weak TMS with a weak muscle contraction did not facilitate MEPs in iM1 (27). Although motor practice was done at 80% MVC, adaptations also occurred at weak contractions: MEP size still increased 10.3% (p = 0.001) when weak TMS at 120% rMT was combined with a weak (20%) muscle contraction (Figs. 4 and 4C), suggesting that iM1 became more sensitive to weak input. Second, the capacity of iM1 to respond to TMS also increased because when strong TMS at 160% rMT was conditioned with strong, 80% MVC, MEP size increased 63.9% by session 20 (Figs. 4B and 4D). In previous acute experiments, iM1 facilitation was normally saturated when TMS at 160% rMT was conditioned with strong voluntary contraction (27) but here we found a profound increase in the ipsilateral corticospinal path’s ability to respond to strong TMS conditioned with a strong muscle contraction. Finally, the 6% increase in MEP size at rest also supports the idea of iM1 becoming more sensitive to TMS and undergoing plastic changes with chronic unilateral motor practice, confirming a previous report that found bilateral increase in corticospinal excitability after unimanual ballistic motor practice (4). While this increase in MEP size at rest did not correlate with cross education and probably plays a minimal role in between-limb force transfer (see also (32)), the significant associations that evolved by session 20 between changes in MEP size during contraction and cross education suggest, for the first time, a functional role for these adaptations in the corticospinal paths projecting to the non-exercised, left FDI. These results are compatible with the model that unilateral motor practice can upregulate the excitability of iM1, especially during muscle contraction, and improve motor behavior.
Several studies support the view that GABAa-mediated SICI contributes to M1 plasticity (39). Indeed, maximal voluntary contraction or skill practice by one hand cause reductions in SICI in the other hand (2, 27, 32) and this diminishment in SICI strengthens with increasing unimanual force generation (30). Therefore, it was reasonable to expect that with the evolution and consolidation of cross education SICI would decrease and this would increase corticospinal excitability in iM1. Contrary to this expectation, we observed no changes in SICI and ICF. In previous studies motor skill training acutely, within a single session, reduced SICI in small hand muscles (6) and a leg muscle (31). Although SICI is lower in musicians vs. non-musicians (34), it is unclear if such adaptations are not the result of a selection bias because long-term sensorimotor training produced no changes in SICI (1). One possibility is that we instructed subjects to suppress mirror activity during exercise and this volitional inhibition of mirror activity in the left FDI during contractions of the right FDI negated any training-induced decrease of SICI and increase in ICF. This is because volitional inhibition deepens SICI, lessens ICF, and suppresses corticospinal excitability (37). Thus, had subjects not suppressed mirror activity in the left FDI, SICI could have diminished, ICF could have increased, and cortical excitability measured with the RCs could have also increased more than the observed 6%.
The locus and the cellular mechanisms that contribute to persistent changes in corticospinal excitability in iM1 after unilateral motor practice are not known. The mechanisms could involve changes in membrane properties of corticospinal neurons and in the efficiency of excitatory synaptic inputs onto corticospinal neurons produced by the forceful muscle contractions. Whether the site of these adaptations is in iM1 circuits is unclear because some human and animal experiments suggest that the nature of the motor task has to be complex to produce plasticity in the task and non-task hemisphere (18). However, several studies, using magnetic and electrical stimulation (see references in (15), and imaging (9) show increases in excitability of supraspinal structures after chronic high-intensity motor training using a simple motor task. rTMS studies strongly suggest that motor practice-induced increases in corticospinal excitability were caused by cortical rather than spinal adaptations (28). Although based on imaging and TMS studies the possibility cannot be excluded that remote cortical areas subserving M1 such as supplementary motor areas (32), caudal cingulate, cerebellum (36), and parietal cortices (20) contributed to the increase in iM1 excitability, the most likely scenario involves the modulation of transcallosal paths. Finally, it is still possible that spinal mechanisms played a role in cross education because there were no changes in SICI and ICF and spinal mechanisms can also increase MEPs in the recruitment curves caused by a more synchronized corticospinal conduction producing a more efficient summation of descending volleys at the spinal motoneurons.
Interhemispheric inhibition
To the best of our knowledge, the present data provide the first longitudinal evidence for unimanual motor activity producing a regulatory effect on IHI in intact humans. Repeated effortful contractions of the right FDI increased maximal voluntary force in the left, untrained, FDI 21.8%, decreased IHI from the trained to the untrained hemisphere 30.9%, and these changes in IHI and the transferred force became progressively more strongly and significantly correlated across 20 sessions. The data suggest that interhemispheric plasticity most likely contributed to cross education.
Short-interval IHI is mediated by glutaminergic excitatory transcallosal motor fibers that arise from the M1 that receives the conditioning TMS pulse and project onto local GABAergic inhibitory interneurons located in the opposite M1 that receives the test TMS pulse (11). Under non-pathological conditions IHI from the active to the “non-active” hemisphere increases with increasing intensity of unimanual muscle contraction, presumably to suppress unwanted mirror (co-) activation of the voluntarily “non-active M1” and to minimize mirror EMG activity in the non-task homologous muscle (17). In the present study subjects were successful in volitionally suppressing mirror EMG activity in the left FDI as they strongly contracted the right FDI. While volitional suppression of voluntary command decreases M1 excitability and deepens SICI in the task (37) and non-task hemisphere (25), the specific effects of such suppression are unknown on IHI. In the present study the net effect of repeated forceful muscle contractions on one side and suppression of mirror activity on the other side was that the muscle contractions had a conditioning effect on IHI, which over 20 sessions became progressively and significantly more diminished from the active to the non-active M1. Several lines of evidence support this scenario. One, although in the original experiments IHI deepened with a slight unimanual muscle contraction (11) when the size of the conditioning and test MEPs are properly adjusted across force levels, IHI actually decreases (30). Two, a suppression of mirror EMG activity has little influence on the duration of ipsilateral silent period, a measure of interhemispheric inhibition (13). Three, although the unimanual serial reaction-time task that transferred implicit knowledge and the unimanual pinching task that produced transfer of speed and accuracy are eminently different from each other and still are more complex than the simple task of serial isometric contractions used in the present study, each produced reductions in IHI from the practiced to the non-practiced M1 (2, 32). It thus appears that IHI is amenable to diminishment independent of the nature of the task. Finally, although in cross-sectional studies IHI influenced SICI during unimanual wrist flexions (30), we found no changes in SICI and the changes in IHI and SICI did not correlate at any time point, further supporting the contention that IHI acted as a key moderator of the increase in voluntary force of the untrained, left, FDI. Together, the behavioral outcome in the present study was the transfer of voluntary force from the trained to the non-practiced finger so that the magnitude of transfer (i.e., cross education) became strongly associated with the magnitude of attenuation in IHI. These changes were topographically confined to the homologous, left FDI as there were no changes in IHI and force transfer in the non-homologous, control ADM muscle. Therefore, it seems that IHI contributed to inter-limb transfer of the ability to produce maximal voluntary force by the untrained homologous muscle following a unimanual exercise intervention involving a simple motor skill.
When healthy adults execute a unilateral motor task there can be as much as 20% of maximum mirror EMG activity (13, 40) and large increases in excitability of iM1 (16, 27). There has been a lingering suspicion in previous longitudinal exercise studies that the rarely quantified but undoubtedly present mirror EMG activity in the “inactive” contralateral homologous muscle (14) and the increased excitability of the non-task M1 contributed if not caused the observed cross education effects (3, 5, 9, 29). However, we controlled for mirror EMG activity by repeatedly instructing the subjects in a standardized manner to relax the homologous muscle pair contralateral to unimanual muscle contractions (16). Therefore mirror EMG activity (∼1.3%) very likely did not cause cross education. Yet the present and previous results (2, 32) shed paradoxical light on IHI concerning its role in mirror EMG activity and mediating cross education. IHI’s function is to eliminate unwanted mirror EMG activity and mirror movements in the resting limb when muscles of the other limb execute a unilateral motor task (17). The prediction is that unimanual motor practice, by repeatedly activating IHI-networks, would tend to inhibit the homologous and even non-homologous muscles (25) in the opposite limb and strengthen inter-limb independence. In contrast, the concept of cross education predicts that unilateral exercise would increase performance in the untrained limb and making, perhaps, the two limbs not more but less independent. Indeed, we observed here and others also reported previously (2, 32) that unimanual motor activity decreased IHI and reductions in IHI correlated with transfer of motor function. Such a diminishment would tend to actually increase instead of decrease mirror EMG activity and, more broadly, interference between limbs. Because cortical excitability of iM1 at rest increased little (6%) and mirror EMG activity was virtually absent, the diminishment of IHI seems to be a key contributor to the observed cross education. Although cross-sectional studies link mirror activity to IHI (5, 17), under the current chronic experimental conditions practice of a simple unilateral voluntary force production task reduced IHI with virtually no covariation or progressive increases in mirror activity (∼1.3%), suggesting that the two variables are independent. Again, the current experimental approach do not allow us to exclude the possibility of changes in spinal excitability contributing to cross education and controlling mirror activity.
Clinical relevance
The present results have clinical relevance to neurological and orthopedic conditions. Unilateral exercise of the free limb reduced voluntary strength loss produced by experimental immobilization (10). It is possible that unilateral interventions can modulate excitatory balance between the two hemispheres and yield diagnostic or therapeutic benefits in certain clinical conditions such as multiple sclerosis where IHI can be affected due to demyelination of callosal fibers, cortical myoclonus where deficient IHI can facilitate the spread of myoclonic activity between hemispheres, and subacute stroke where reduced IHI can lead to hyperexcitability of M1 in the unaffected hemisphere.
Acknowledgements
Supported in part by NIH NS049783, NIH NINDS intramural program, and an East Carolina University Research Development Award. We thank Dr. Paul DeVita for their helpful comments and Mr. Patrick Rider and Mr. Jonathan Gomez for preparing the figures. The results of this study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beck S, Taube W, Gruber M, Amtage F, Gollhofer A, Schubert M. Task-specific changes in motor evoked potentials of lower limb muscles after different training interventions. Brain Res. 2007;1179(11):51–60. doi: 10.1016/j.brainres.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 2.Camus M, Ragert P, Vandermeeren Y, Cohen LG. Mechanisms controlling motor output to a transfer hand after learning a sequential pinch force skill with the opposite hand. Clin Neurophysiol. 2009;120(10):1859–1865. doi: 10.1016/j.clinph.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carroll TJ, Herbert RD, Munn J, Lee M, Gandevia SC. Contralateral effects of unilateral strength training: evidence and possible mechanisms. J Appl Physiol. 2006;101(5):1514–1522. doi: 10.1152/japplphysiol.00531.2006. [DOI] [PubMed] [Google Scholar]
- 4.Carroll TJ, Lee M, Hsu M, Sayde J. Unilateral practice of a ballistic movement causes bilateral increases in performance and corticospinal excitability. J Appl Physiol. 2008;104(6):1656–1664. doi: 10.1152/japplphysiol.01351.2007. [DOI] [PubMed] [Google Scholar]
- 5.Carson RG. Neural pathways mediating bilateral interactions between the upper limbs. Brain Res Brain Res Rev. 2005;49(3):641–662. doi: 10.1016/j.brainresrev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Classen J, Liepert J, Hallett M, Cohen L. Plasticity of movement representation in the human motor cortex. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:162–173. [PubMed] [Google Scholar]
- 7.Cramer SC, Finklestein SP, Schaechter JD, Bush G, Rosen BR. Activation of distinct motor cortex regions during ipsilateral and contralateral finger movements. J Neurophysiol. 1999;81(1):383–387. doi: 10.1152/jn.1999.81.1.383. [DOI] [PubMed] [Google Scholar]
- 8.Di Lazzaro V, Oliviero A, Profice P, Meglio M, Cioni B, Tonali P, Rothwell JC. Descending spinal cord volleys evoked by transcranial magnetic and electrical stimulation of the motor cortex leg area in conscious humans. J Physiol. 2001;537(Pt 3):1047–1058. doi: 10.1111/j.1469-7793.2001.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farthing JP. Cross-education of strength depends on limb dominance: implications for theory and application. Exerc Sport Sci Rev. 2009;37(4):179–187. doi: 10.1097/JES.0b013e3181b7e882. [DOI] [PubMed] [Google Scholar]
- 10.Farthing JP, Krentz JR, Magnus CR. Strength training the free limb attenuates strength loss during unilateral immobilization. J Appl Physiol. 2009;106(3):830–836. doi: 10.1152/japplphysiol.91331.2008. [DOI] [PubMed] [Google Scholar]
- 11.Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453(Pt 4):525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143(2):240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- 13.Giovannelli F, Borgheresi A, Balestrieri F, Zaccara G, Viggiano MP, Cincotta M, Ziemann U. Modulation of interhemispheric inhibition by volitional motor activity: an ipsilateral silent period study. J Physiol. 2009;587(Pt 22):5393–5410. doi: 10.1113/jphysiol.2009.175885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hortobágyi T, Hill JP, Lambert NJ. Greater cross education following training with muscle lengthening than shortening. Med. Sci. Sports Exerc. 1997;29(1):107–112. doi: 10.1097/00005768-199701000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Hortobágyi T, Richardson SP, Lomarev M, Shamim E, Meunier S, Russman H, Dang N, Hallett M. Chronic low-frequency rTMS of primary motor cortex diminishes exercise training-induced gains in maximal voluntary force in humans. J Appl Physiol. 2009;106(2):403–411. doi: 10.1152/japplphysiol.90701.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hortobágyi T, Taylor JL, Russell G, Petersen N, Gandevia SC. Changes in segmental and motor cortical output with contralateral muscle contractions and altered sensory inputs in humans. J Neurophysiol. 2003;90(4):2451–2459. doi: 10.1152/jn.01001.2002. [DOI] [PubMed] [Google Scholar]
- 17.Hoy KE, Fitzgerald PB, Bradshaw JL, Armatas CA, Georgiou-Karistianis N. Investigating the cortical origins of motor overflow. Brain Res Brain Res Rev. 2004;46(3):315–327. doi: 10.1016/j.brainresrev.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Jensen JL, Marstrand PC, Nielsen JB. Motor skill training and strength training are associated with different plastic changes in the central nervous system. J Appl Physiol. 2005;99(4):1558–1568. doi: 10.1152/japplphysiol.01408.2004. [DOI] [PubMed] [Google Scholar]
- 19.Kawashima R, Matsumura M, Sadato N, Naito E, Waki A, Nakamura S, Matsunami K, Fukuda H, Yonekura Y. Regional cerebral blood flow changes in human brain related to ipsilateral and contralateral complex hand movements--a PET study. Eur J Neurosci. 1998;10(7):2254–2260. doi: 10.1046/j.1460-9568.1998.00237.x. [DOI] [PubMed] [Google Scholar]
- 20.Koch G, Ruge D, Cheeran B, Fernandez Del Olmo M, Pecchioli C, Marconi B, Versace V, Lo Gerfo E, Torriero S, Oliveri M, Caltagirone C, Rothwell JC. TMS activation of interhemispheric pathways between the posterior parietal cortex and the contralateral motor cortex. J Physiol. 2009;587(Pt 17):4281–4292. doi: 10.1113/jphysiol.2009.174086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993 Nov;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laidlaw DH, Kornatz KW, Keen DA, Suzuki S, Enoka RM. Strength training improves the steadiness of slow lengthening contractions performed by old adults. J Appl Physiol. 1999;87(5):1786–1795. doi: 10.1152/jappl.1999.87.5.1786. [DOI] [PubMed] [Google Scholar]
- 23.Lee M, Gandevia SC, Carroll TJ. Unilateral strength training increases voluntary activation of the opposite untrained limb. Clin Neurophysiol. 2009;120(4):802–808. doi: 10.1016/j.clinph.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Lee M, Hinder MR, Gandevia SC, Carroll TJ. The ipsilateral motor cortex contributes to cross-limb transfer of performance gains after ballistic motor practice. J Physiol. 2010;588(Pt 1):201–212. doi: 10.1113/jphysiol.2009.183855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123(Pt 6):1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- 26.Liepert J, Dettmers C, Terborg C, Weiller C. Inhibition of ipsilateral motor cortex during phasic generation of low force. Clin Neurophysiol. 2001;112(1):114–121. doi: 10.1016/s1388-2457(00)00503-4. [DOI] [PubMed] [Google Scholar]
- 27.Muellbacher W, Facchini S, Boroojerdi B, Hallett M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol. 2000;111(2):344–349. doi: 10.1016/s1388-2457(99)00243-6. [DOI] [PubMed] [Google Scholar]
- 28.Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415(6872):640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- 29.Munn J, Herbert RD, Gandevia SC. Contralateral effects of unilateral resistance training: a meta-analysis. J Appl Physiol. 2004;96(5):1861–1866. doi: 10.1152/japplphysiol.00541.2003. [DOI] [PubMed] [Google Scholar]
- 30.Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci. 2008;28(22):5631–5640. doi: 10.1523/JNEUROSCI.0093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez MA, Lungholt BK, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004;159(2):197–205. doi: 10.1007/s00221-004-1947-5. [DOI] [PubMed] [Google Scholar]
- 32.Perez MA, Tanaka S, Wise SP, Sadato N, Tanabe HC, Willingham DT, Cohen LG. Neural substrates of intermanual transfer of a newly acquired motor skill. Curr Biol. 2007;17(21):1896–1902. doi: 10.1016/j.cub.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 33.Post M, Bakels R, Zijdewind I. Inadvertent contralateral activity during a sustained unilateral contraction reflects the direction of target movement. J Neurosci. 2009;29(19):6353–6357. doi: 10.1523/JNEUROSCI.0631-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenkranz K, Williamon A, Rothwell JC. Motorcortical excitability and synaptic plasticity is enhanced in professional musicians. J Neurosci. 2007;27(19):5200–5206. doi: 10.1523/JNEUROSCI.0836-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salmelin R, Forss N, Knuutila J, Hari R. Bilateral activation of the human somatomotor cortex by distal hand movements. Electroencephalogr Clin Neurophysiol. 1995;95(6):444–452. doi: 10.1016/0013-4694(95)00193-x. [DOI] [PubMed] [Google Scholar]
- 36.Sehm B, Perez MA, Xu B, Hidler J, Cohen LG. Functional neuroanatomy of mirroring during a unimanual force generation task. Cereb Cortex. 2009;20(1):34–45. doi: 10.1093/cercor/bhp075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sohn YH, Wiltz K, Hallett M. Effect of volitional inhibition on cortical inhibitory mechanisms. J Neurophysiol. 2002;88(1):333–338. doi: 10.1152/jn.2002.88.1.333. [DOI] [PubMed] [Google Scholar]
- 38.Urbano A, Babiloni C, Onorati P, Carducci F, Ambrosini A, Fattorini L, Babiloni F. Responses of human primary sensorimotor and supplementary motor areas to internally triggered unilateral and simultaneous bilateral one-digit movements. A high-resolution EEG study. Eur J Neurosci. 1998;10(2):765–770. doi: 10.1046/j.1460-9568.1998.00072.x. [DOI] [PubMed] [Google Scholar]
- 39.Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517(Pt 2):591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zijdewind I, Butler JE, Gandevia SC, Taylor JL. The origin of activity in the biceps brachii muscle during voluntary contractions of the contralateral elbow flexor muscles. Exp Brain Res. 2006;175(3):526–535. doi: 10.1007/s00221-006-0570-z. [DOI] [PubMed] [Google Scholar]