Abstract
Cardiovascular health effects of near-roadway pollution appear more substantial than other sources of air pollution. The underlying cause of this phenomenon may simply be concentration-related, but the possibility remains that gases and particulate matter (PM) may physically interact and further enhance systemic vascular toxicity. To test this, we utilized a common hypercholesterolemic mouse model (Apolipoprotein E-null) exposed to mixed vehicular emissions (MVE; combined gasoline and diesel exhausts) for 6 h/d × 50 days, with additional permutations of removing PM by filtration and also removing gaseous species from PM by denudation. Several vascular bioassays, including matrix metalloproteinase 9 (MMP9) protein, 3-nitrotyrosine, and plasma-induced vasodilatory impairments, highlighted that the whole emissions, containing both particulate and gaseous components, was collectively more potent than MVE-derived PM or gas mixtures, alone. Thus, we conclude that inhalation of fresh whole emissions induce greater systemic vascular toxicity than either the particulate or gas phase alone. These findings lend credence to the hypothesis that the near-roadway environment may have a more focused public health impact due to gas-particle interactions.
Keywords: Air pollution, Particulate matter, Volatile Organic compounds, Engine Emissions, Cardiovascular toxicity
INTRODUCTION
The adverse cardiovascular health outcomes of air pollutants are well reported(Brook et al., 2010, Pope et al., 2004, Pope, 2007) but mechanistic underpinnings of systemic effects arising from inhaled toxicants remain unclear (Brook and Rajagopalan, 2012, Campen et al., 2012). This lack of insight hampers efforts to protect specific at-risk populations, as well as identify and regulate specific components of the pollutant mixture. In recent years, various epidemiological studies have found that traffic-derived pollution may drive greater cardiovascular health impacts compared to specific components, such as particulate matter (PM) (Sarnat et al., 2008, Hoffmann et al., 2007). These findings suggest that complex mixtures of pollutants may have additive toxic effects, either by acting through similar toxic pathways or by chemical interactions that enhance the toxicity of specific components.
Recent work in our laboratory revealed that the gas phase of both gasoline engine exhaust (GEE) and diesel engine exhaust (DEE) drive an independent systemic vascular toxicity in hyperlipidemic mice (Campen et al., 2010, Lund et al., 2011, Lund et al., 2009, Lund et al., 2007). High-efficiency filtration of the particulate matter (PM) phase failed to reduce the vascular toxicity of GEE (Lund et al., 2007) and had a modest, non-significant attenuation of DEE toxicity (Campen et al., 2010). However, the GEE was notable for a very low concentration of PM, thus it was not surprising that filtration of this fraction had minimal biological benefit. Subsequent investigation of a mixed vehicle emission (MVE) that combined GEE and DEE revealed compelling evidence of an interaction in terms of enhancing systemic vascular lipid peroxidation (Lund et al., 2011). Interestingly, in this instance, removal of MVE PM by filtration led to substantial reductions in toxicity and, in further permutations, we observed that combining MVE gases (without MVE PM) with non-vehicular PM, such as road dust or generated sulfate or nitrate PM, led to increases in toxicity (Vedal et al., 2013). These findings were suggestive of gas-particle interactions, but the overall study design was sub-optimal for clearly delineating such effects. An essential permutation was missing, that of exposure to fresh engine emissions PM in the absence of copollutant gases. The present study, therefore, sought to further explore the vascular impact of mixed vehicular emissions, directly comparing the independent roles of the gas and particle phases.
MATERIALS AND METHODS
Animals and inhalation exposure protocol
8-week-old male ApoE−/− mice (Taconic, Hudson, NY) on a high fat diet (Harlan Teklad 88137) were randomized into one of four exposure groups: (1) mixed vehicular emissions (MVE), 300 µg PM/m3 (50 µg PM/m3 derived from gasoline engine combined with 250 µg PM/m3 derived from a diesel engine); (2) MVE at the 300 µg PM/m3 with PM filtered (MVE–PM), using a high efficiency particulate air (HEPA) filter; (3) MVE at the 300 µg PM/m3 with the gases removed (MVE–G), using a HARVARD parallel plate denuder to remove gases (Ruiz et al., 2006); and (4) filtered air (FA, controls). Each exposure group included 6 mice and all mice survived the 50d exposure, but certain assays may only include 5 subjects due to loss of samples or failed assays. Naïve (non-exposed) C57BL/6 mice (male, 8–10 weeks old) were used as tissue donors for aortic ring preparations (see bleow). All groups of mice were exposed sub-chronically by whole body inhalation for 6 h per day, 7 days per week for a period of 50 days.
Engines used to generate emissions have been previously characterized in detail, and included a Yanmar diesel engine generator operated under constant load combined with emissions from a gasoline powered 4.3 L engine operated on a transient engine cycle (Lund et al., 2007, Lund et al., 2009, McDonald et al., 2004). General characteristics of the exposure atmospheres are shown in Table 1, including particulate matter (PM), nitrogen oxides (NOx), and carbon monoxide (CO). Characterization of PM was conducted gravimetrically using an aluminum in-line filter holder with quartz filters. NOx and CO were determined using chemiluminescence- and infrared spectroscopic-based detectors, respectively. A more thorough characterization fo the base MVE atmosphere is provided by Vedal and colleagues (2013). These relative proportions were selected based on the maximum achievable PM that could be obtained from the gasoline engine exhaust without adding excess heat and humidity. The gasoline engine exhaust was diluted approximately 10:1 from the tailpipe. The diesel exhaust was selected to account for the remaining PM to achieve the target of 300 µg/m3. The ratio of GEE to DEE based on PM was 1:5. However, GEE accounted for the majority of CO and non-methane volatile organic compounds (NMVOC).
Table 1.
Target proportions of gasoline and diesel engine exhaust in motor vehicle exhaust (MVE) atmospheres.
| Exposure Atmosphere Targets | |||
|---|---|---|---|
| Test Atmosphere | PM (µg/m3) |
NOx (ppm) |
CO (ppm) |
| MVE | |||
| Gasoline Engine Exhaust | 50 | 25 | 97 |
| Diesel Engine Exhaust | 250 | 5 | 2.2 |
All procedures were approved by both the University of New Mexico and the Lovelace Respiratory Research Institute animal care and use committees. For myograph experiments, male C57BL/6 mice (Harlan Laboratories) were used. Mice were euthanized with an overdose of anesthesia (isoflurane; concentration 5%) or by exsanguination via cardiac puncture while under anesthesia (isoflurane; concentration 1.5–2%).
Collection of blood plasma and bronchoalveolar lavage (BALF)
Blood samples were collected via cardiac puncture immediately before euthanasia into ice-cooled tubes containing ethylenediaminetetraacetic acid (EDTA). Blood samples were centrifuged at 1500 g for 5 min, and plasma aliquots stored at −80°C until further analysis. BALF was collected and assayed for lactate dehydrogenase (LDH) and total protein, as previously described (Robertson et al., 2013).
Effect of ApoE−/− plasma samples on ACh responses of aortas from naïve WT mice
Rings from the thoracic aorta were isolated from naïve WT mice, and cleaned of connective tissue. Segments of aorta (2–3 mm length) were mounted in a 4-chamber myograph system (610 M; Danish Myo Technology A/S, Aarhus, Denmark). Vessels were submerged in physiological salt solution (PSS with the following composition (mM): 119.0 NaCl, 25.0 NaHCO2, 5.5 glucose, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 0.03 ethylenediaminetetraacetic acid, 2.5 CaCl2) bubbled at 37°C with 21%O2–5%CO2-balance N2, and left to equilibrate at 2 mN of tension for approximately 30 min. Tension was gradually applied over 10 min to an optimal passive tension of 10 mN. Preliminary experiments showed that this tension produced optimal contraction and relaxation responses. Data from force transducers were processed by a MacLab/4e convertor displayed through LabChart™ software (AD Instrument, USA).
Vessel viability was confirmed by a contractile response on addition of high potassium PSS (KPSS, in mM: 64.9 NaCl, 25.0 NaHCO3, 5.5 glucose, 58.9 KCl, 1.2 MgSO4, 1.2 KH2PO4, 0.03 ethylenediaminetetraacetic acid, 2.5 CaCl2), repeated twice. After a 30 min equilibration period, vessels were incubated with 2.5% plasma from 1 of the 4 exposure groups. Plasma alone caused little or no constriction, the degree of which was not altered by treatment group. On attainment of a stable plateau, rings were pre-constricted with a predetermined submaximal concentration of U-46619 (5.6×10−9M; Sigma-Aldrich), tested in a separate set of aortas from naïve WT mice (Supplementary Figure I). Once responses stabilized, cumulative concentration-response curves were generated with the endothelium-dependent vasorelaxant acetylcholine (ACh; 10−9–10−5M; Sigma-Aldrich).
Immunohistochemistry – Monocyte/Macrophage (MOMA) staining
After exsanguination, hearts were removed, immediately embedded and frozen in Tissue Freezing Media (Fisher Scientific, Pittsburgh, PA), and the ascending aorta/aortic root was sectioned at 7µm thickness. Immunohistochemistry of MOMA-2 expression on the sections was completed as previously described (8). Analysis of imaging was done using Image J (NIH) software of 2 slides, with 2 consecutive sections each per animal, with an n=6 per group.
Immunofluorescence of Vascular Endpoints
Aorta sections were prepared for either 3-nitrotyrosine (3-NT), matrix metalloproteinase-9 (MMP-9), cluster of differentiation 36 (CD-36), or lectin-like receptor of oxidized low density lipoprotein cholesterol (LOX-1) immunofluorescence staining, as described above. The slides were thawed at RT (45 min), and then placed into ice-cold acetone for fixation (15 min), and rinsed with PBS (3×). Aorta sections were then blocked (3% BSA, 0.1% Tween, 10% goat serum into 5 ml volume of PBS) for 1 hr at RT in a moisture chamber. Blocked slides were incubated with the primary antibody, either 3-NT (1:500; Invitrogen, Grand Island, NY), MMP-9 (1:1000; Abcam, Cambridge, MA), CD36 (1:1000; Abcam), or LOX-1 (1:1000; Abcam) for 1 hr at RT in a moisture chamber. Slides were then rinsed, followed by incubation with the appropriate secondary Ab labeled with Alexa Fluor 594 (1:500; Invitrogen) for 1 hr at RT in a moisture chamber, rinsed, and then stained with Hoechst (1:100 in blocking solution) for nuclear visualization. Slides were then cover-slipped with Fluoro-Gel mounting medium (EMS, Hatfeld, PA), visualized, and imaged by microscopy, and analyzed for fluorescence as previously described (8). A minimum of 2 slides, with 2 consecutive sections each per animal, with an n=6 per group were used for analysis.
Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM). Vascular contractile responses were expressed as either absolute values in tension (in millinewton, mN) or as a percentage of the maximal contraction induced by KPSS (58.9 nM KCl). The negative logarithm concentration of U-46619 eliciting 50% of the maximum response to KPSS (EC50) was calculated with a nonlinear regression analysis. Relaxation responses are expressed as the percentage reduction to the initial constriction to 5.6×10−9M U-46619, with 100% representing basal tension. Statistical comparisons were performed by two-way analysis of variance (ANOVA) using the Bonferroni post-hoc test, unless otherwise stated. For immunohistochemical studies an ANOVA was used to compare groups with Dunnett’s post-hoc comparison. Student’s T-tests were used in limited fashion to provide sub-group comparisons on a limited basis. Statistical analyses were performed using GraphPad Prism software (V5.0; GraphPad Software Inc, USA). P < 0.05 was considered to be statistically significant.
RESULTS
Atmosphere Characterization
Table 2 provides a summary of the test atmosphere concentrations of PM, NOx and CO for each of the test atmospheres. A more detailed characterization of PM and gases from this mixed emissions atmosphere has been previously described (Vedal et al., 2013). The high efficiency filter used to remove PM from the MVE test atmosphere provided a 99% removal based on the particle mass measurement. The removal was also high for total particle number, which was reduced from 1.85×105 particles/cm3 to <0.020×105 particles/cm3, or 99%. The denuder was also effective in removing the gases from the test atmosphere, with a removal rate of approximately 96% for both CO and NOx. Based on the design of the denuder, other gases were also removed but not measured in the present study. CO and NOx were selected as surrogate measures for gas removal efficiency as they were present in the highest abundance. The particle number in the denuded atmosphere was 1.7×105 particles/cm3, suggesting a particle loss of approximately 10% while transiting the denuder. The particle size in the atmosphere showed a median diameter of 0.13 µm by particle mass and 0.08 µm by particle number. It was noted by Ruiz et al (Ruiz et al., 2006) that some particle loss can occur when the size approaches 0.05 µm.
Table 2.
Test atmosphere concentrations of nitrogen oxides (NOx), particulate matter (PM), and carbon monoxide (CO).
| Exposure Atmosphere Composition |
Units | MVE | MVE-PM | MVE-Gases | Control |
|---|---|---|---|---|---|
| Nitrogen Oxides (NOx) | ppm | 27 ± 6 | 28 ± 9 | 1 ± 1 | 0 ±0.05 |
| Carbon Monoxide (CO) | ppm | 99 ± 9 | 99 ± 10 | 4 ± 3 | 0.5 ± 0.1 |
| Particle Mass | µg/m3 | 295 ± 51 | 12 ± 9 | 295 ± 55 | 8 ± 5 |
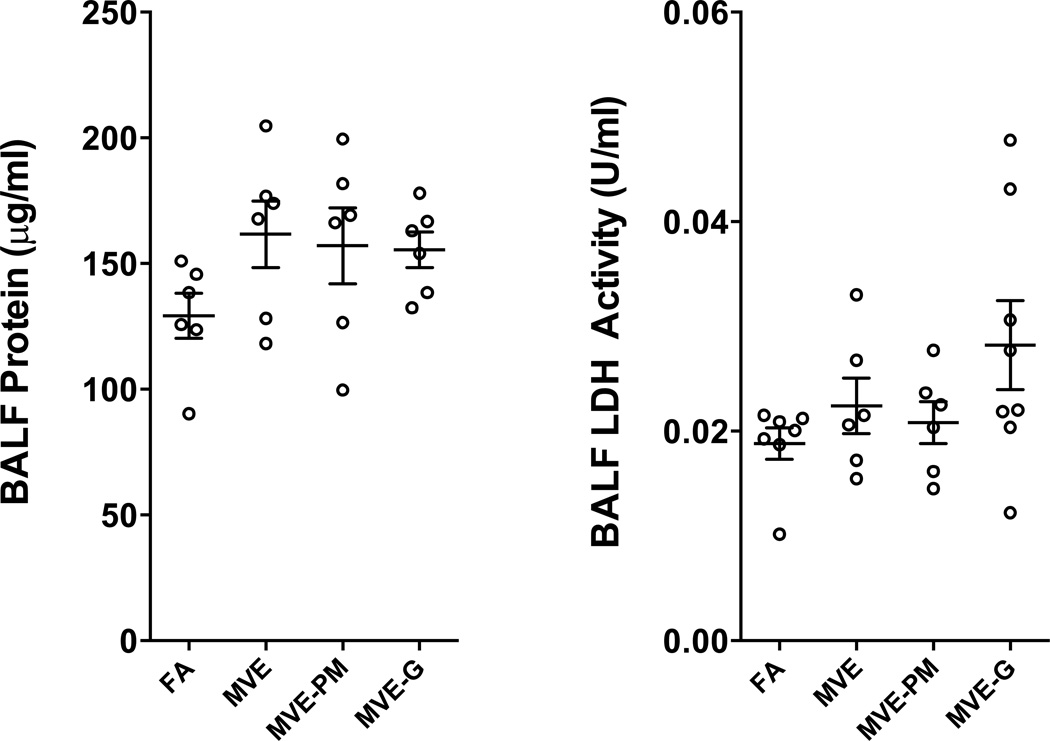
MVE and derivative atmospheres did not induce BALF Protein or LDH Activity
The 50 days exposure did not induce significant increases in lavage markers of inflammation. Lavage fluid was assayed for total protein and LDH activity (Figure 1). However, while no exposure induced a significant increase in either marker compared to filtered air controls, the distribution of data showed a greater variability in values from exposed mice, which may suggest some earlier response with variable adaptation and/or recovery. Thus, conclusions related to a lack of pulmonary inflammation and injury must be tempered by the lack of temporal information derived in this study.
Figure 1.
Lung injury, assessed by BALF protein (A) and LDH activity (B), were not significantly elevated after 50 d of exposure to any atmosphere. Though an increasing tendency is noted, ANOVA testing rendered p-values of 0.2176 and 0.1463 for protein and LDH, respectively.
MVE-induced plasma circulating factors impair vasorelaxation to ACh
Thoracic aorta from naïve WT (C57BL/6) mice demonstrated impaired vasorelaxation to ACh when incubated with 2.5% plasma samples obtained from ApoE−/− mice exposed to MVE compared to plasma from ApoE−/− littermate control mice exposed to FA (Figure 2). Plasma from ApoE−/− mice exposed to MVE-PM (i.e., the gaseous portion, only) resulted in no observable effect on relaxant responses to ACh in aortic rings, relative to plasma from FA controls (Figure 3), strongly suggesting that the PM fraction is vital in driving the systemic vascular effects of vehicular emissions. However, MVE-G atmosphere did not appear to induce as severe an impairment of vasorelaxation to ACh as did the whole MVE atmosphere (Figure 2). Such observations suggest the importance of both the PM and gaseous components to the systemic vascular effects, although the PM components are likely the primary mediators in our 50-d MVE exposures. Additionally, these differences in vasorelaxation among the groups cannot be explained by an altered contractile sensitivity of the aortic rings to the thromboxane mimetic, U-46619, which was used for preconstricting vessels. Diluted plasma samples obtained from the four groups of ApoE−/− mice did not modify the increase in force (mN) to 5.6×10−9M U-46619 in thoracic aortas from naïve WT (7.6 ± 1.5 for FA; 7.9 ± 2.2 for MVE; 6.8 ± 2.4 for MVE-PM filtered; and 6.8 ± 2.2 for MVE-gases filtered; n=5–6).
Figure 2.
Relaxation in response to acetylcholine (ACh) of thoracic aortas from naïve WT mice incubated with 2.5% serum obtained from ApoE−/− mice exposed filtered air (FA; open circles), mixed vehicular emissions (ME; 30 µg particulate matter (PM)/m3 gasoline emissions and 270 µg PM/m3 diesel exhaust emissions; solid squares); ME with PM filtered (solid triangle); and ME with gases filtered (up-side down solid triangle). Data are expressed as mean ± SEM (n=5–6). *P<0.05 versus ApoE−/− plasma FA-exposed; two-way ANOVA followed by Bonferroni post-hoc test.
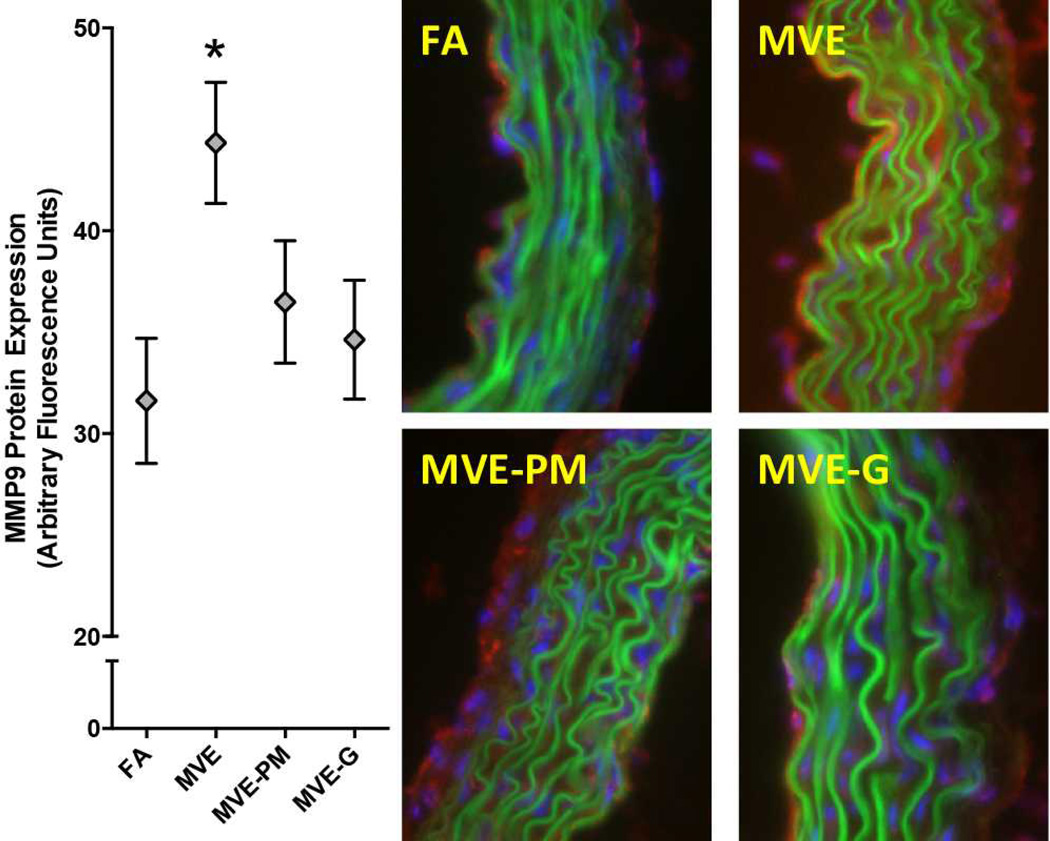
Figure 3.
MMP9 protein levels (Red fluorescence) in aortic wall from ApoE−/− mice exposed inhalationally to FA, MVE, MVE-PM, or MVE-G for 50 days. Green fluorescence reflects lamellar autofluorescence and blue fluorescence indicates nuclei. Removal of PM or Gases led to a significant reduction in vascular MMP9 staining (N=5/group; P<0.05).
Immunohistochemistry
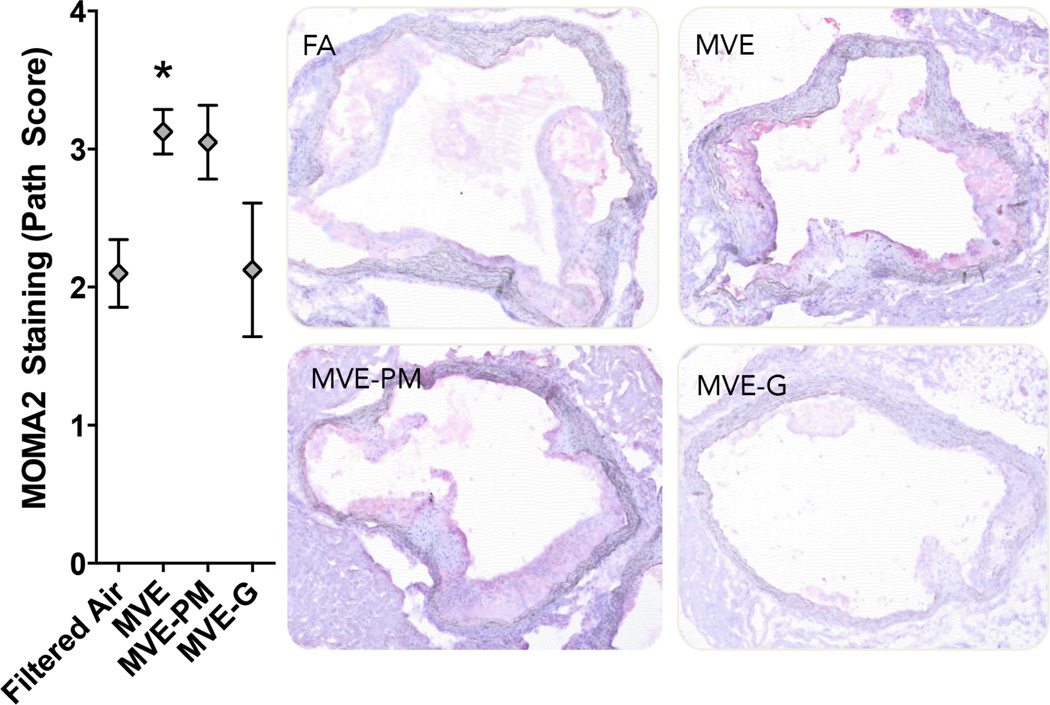
Several vascular responses were characterized by immunohistochemistry, revealing varied contributions from 50-d exposures to the PM and gaseous phases of whole emissions. MVE exposure induced a significant increase in vascular MMP9 protein levels throughout the aorta cross-section (Figure 3), but notably elevated in the intimal region, consistent with previous studies showing elevated vascular gelatinase activity (Lund et al., 2011) (Vedal et al., 2013). Exposures to PM-filtered and gas-denuded emissions MVE did not induce significant effects on vascular MMP9 levels, although these groups were not significantly different from MVE by ANOVA, either. Sub-group analysis by t-test did reveal a significant difference between MVE and MVE-gases (p<0.05), providing some level of confidence as to the contribution of the gaseous portion of whole emissions in contributing to MMP9 upregulation. A MVE-related effect was also seen for vascular inflammation, as measured by MOMA2 staining in the valve leaflets of the aortic outflow tract (Figure 4). Clear increases in staining density were seen with the MVE atmosphere relative to control, while mixed effects were noted when gases or, to a lesser extent, PM were removed from the atmosphere. Assessment of vascular CD36 and LOX-1 expression was also conducted, but no significant effects were observed for any exposure atmosphere (Supplementary Figures 2).
Figure 4.
Macrophage/monocyte (MOMA2, Red) staining levels in aortic outflow tract from ApoE−/− mice exposed inhalationally to FA, MVE, MVE-PM, or MVE-G for 50 days. Removal of Gases led to a significant reduction in vascular MOMA2 staining (N=5/group; P<0.05).
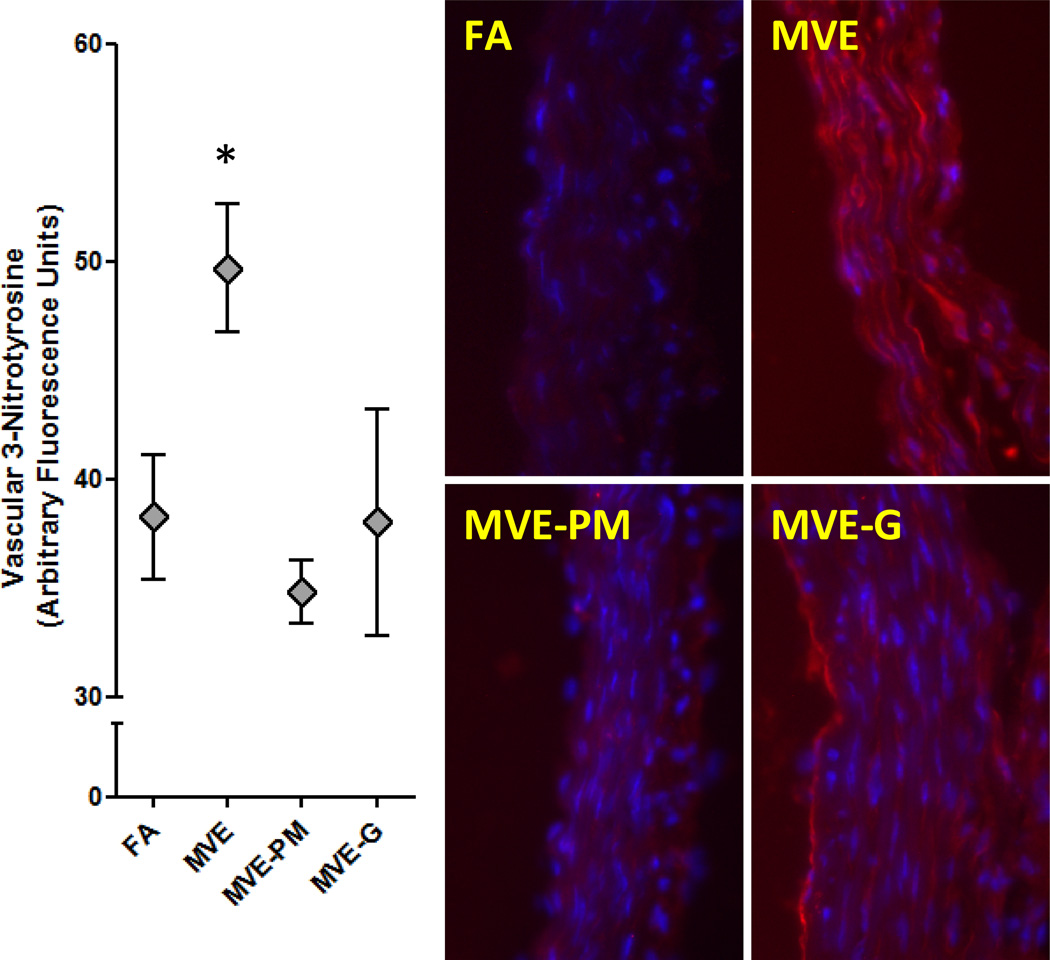
Lastly, vascular 3-NT staining suggested enhanced presence of nitrosative stress thoughout the aortic cross-section following inhalation of MVE (Figure 5). PM filtration completely abolished this response, returning 3-NT fluorescence levels to that seen in control mice. Denudation of gaseous co-pollutants led to varying responses in the overall group. In vessels from MVE-gas exposed mice with high 3-NT staining, the predominance of the effect appeared relatively localized to the intimal region, compared with intimal and medial staining seen in MVE-exposed mice.
Figure 5.
3-Nitrotyrosine (3-NT; Red) staining in aortic wall from ApoE−/− mice exposed inhalationally to FA, MVE, MVE-PM, or MVE-G for 50 days. Removal of PM or gases abrogated the generation of 3-NT. Blue fluorescence indicates nuclei.
DISCUSSION
Removal of gaseous components from MVE PM by thermal denudation or filtration of PM abrogated much of the vascular toxicity observed from the whole emissions. These observations highlight the interactive potency conferred by the combination of gases and PM. However, vasodilatory impairments induced by circulating components in the plasma were largely abrogated by removal of PM, moreso than the gases, consistent with numerous studies of PM-induced endothelial dysfunction. (Brook et al., 2002) (LeBlanc et al., 2010) (Langrish et al., 2013) (Mills et al., 2011) Epidemiological findings of traffic-derived pollution or proximity to roadways as more focused drivers of cardiovascular disease outcomes (Hoffmann et al., 2006) (Sarnat et al., 2008) may relate to the present findings, which show that the interaction of fresh gases and PM enhances systemic vascular toxicity compared to either component, alone.
Plasma from ApoE−/− mice exposed to MVE for 50 days, at a dilution of 1:40, caused a diminution of the vasorelaxant response to ACh and the removal of PM largely restored normal responses. Thermal denudation of the PM had an intermediate effect (i.e., statistically undifferentiated from MVE and FA controls), suggesting that that particulate fraction remains a principal driver of systemic vascular dysfunction. We have previously noted vascular dilatory impairments in aortas from ApoE−/− mice exposed to MVE, but not MVE with PM filtered(Vedal et al., 2013). Thus, the 50-day exposure to MVE PM appears to be the major factor from the whole emissions in causing endothelial dysfunction, and the present study provides plausible data that this effect is mediated by interactions with plasma-borne factors. What those factors are remains uncertain.
Recent work with ozone found that acutely this gas modifies endogenous components in the serum leading to interaction with vascular CD36, a multi-ligand scavenger receptor (Robertson et al., 2013). The present study observed no changes in vascular CD36 levels, but the presence of the receptor in the intimal regions of vessels (Supplementary Figure 2) is consistent with a potential role for this or other scavenger receptors, such as LOX-1 or TLR4 (Kampfrath et al., 2011) (Lund et al., 2011), in mediating endothelial cell responses to circulating factors. With acute ozone exposure, the generation of the altered serum components appeared independent of lung inflammation, which is again consistent with the present findings, wherein serum induced clear vascular effects despite MVE inducing no significant elevation in BALF protein or LDH activity. Furthermore, the outcomes caused by plasma/serum from exposed mice are consistent with recent work in humans exposed to either whole diesel exhaust or a principal gaseous component thereof, nitrogen dioxide (Channell et al., 2012). In that study, the dilute plasma obtained post-exposure induced inflammatory activation of primary human coronary artery endothelial cells in culture, characterized by increases in adhesion molecules and interleukin-8 release. While lung inflammation was not assessed in that cohort, Frampton and colleagues have previously conducted lavage cell differentials on comparable exposures in healthy subjects, with no significant findings except at much higher concentration × time levels of NO2. Diesel emissions inhalation caused a more consistent and prolonged increase in serum inflammatory potential, while the effect of NO2 was immediate but subsided by 24 h post-exposure. In the present study, the absence of effects of the gas phase may relate to the collection of blood 24h after the final exposure, while the particulate phase has a longer lasting impact, possibly due to prolonged retention relative to the reactive gas phase. A more detailed time course of toxicity from these phases of whole emissions remains to be elaborated.
Several other human studies provide hints that combined gases and PM may be necessary to cause adverse vascular effects. Inhaled diesel emissions led to reduced brachial artery vasodilation in humans (Mills et al., 2005) and removal of PM via a continuously-regenerating particle trap has been shown to eliminate vascular response to diesel (Lucking et al., 2011). However, several subsequent studies of NO2 and concentrated ambient PM have led to null findings (Langrish et al., 2010) (Barath et al., 2013). Exposure to a pure carbon nanoparticulate (diesel is also enriched for elemental carbon) or PM-filtered diesel emissions could not recapitulate effects of whole emissions (Mills et al., 2011). The recombination of carbon nanoparticulate and PM-filtered diesel emissions was not included in that study design, nor was a permutation with thermal denudation of the diesel PM conducted, thus the interaction question remains unanswered. Certainly, it is clear from many of these acute human studies that PM remains a principal driver of systemic effects, but the modifying effect of copollutant gases remains an understudied phenomenon and an important consideration for assessment of cumulative public health impacts of combustion source pollution.
Other endpoints assessed in the present study are consistent with previous observations for whole gasoline, diesel, or MVE (Lund et al., 2007, Lund et al., 2011, Lund et al., 2009). Gasoline emissions at slightly higher (~20%) concentrations induced MMP activity and nitrotyrosine formation in the aortas. MMP-9 levels are predicted to contribute to vascular remodeling and potentially destabilization of advanced plaques (Lund et al., 2007). We have previously seen this protein and its activity elevated in vessels from exposed mice (gasoline and diesel separately and together) and recently found it to be unregulated in human plasma post-diesel emissions (Lund et al., 2007) (Lund et al., 2011). Other labs have confirmed the upregulation of nitrotyrosine following diesel exhaust exposure (Bai et al., 2011). We propose that this is formed due to interaction of superoxide radicals with nitric oxide generated by nitric oxide synthase, leading to the generation of peroxynitrite and resultant protein adduction. While we have not shown this at the molecular level, previous studies confirm that endothelial nitric oxide synthase is uncoupled and becomes a vascular source of oxidative stress (Cherng et al., 2011).
In previous work, we filtered the whole emissions from gasoline and diesel engine exposures to remove PM, but arrived at different conclusions based on the findings. Gasoline engine emissions have a remarkably low level of PM relative to the copollutant gases, thus the filtration of PM did not have a substantial impact on the health outcomes, which were driven by the independent effects of the gas phase. Similarly, removal of PM from diesel emissions led to a roughly 30% reduction in several endpoints related to vascular oxidative stress and remodeling, but the gases had an independent toxic effect. It was only when combining the two exhausts, with gasoline emissions high in VOCs and diesel emissions high in PM, that the gas-PM interactions were optimally observed. Initial work with this combination of engine emissions indicated a potential synergy in driving vascular lipid peroxidation (Lund et al., 2011), but the present study is the first to denude the MVE PM in an attempt to delineate the importance of adhered volatile/semivolatile species in modifying the potency of inhaled PM.
One major question that remains unanswered is how inhaled agents induced systemic vascular effects. While other routes have been postulated, such as direct translocation of pollutants or neural activation (Brook et al., 2010, Hazari et al., 2011), recent studies demonstrate that serum/plasma obtained after controlled exposures has an altered bioactivity, presumably due to compositional changes. In humans exposed to nitrogen dioxide (NO2) or diesel emissions, it was noted that plasma obtained after exposure had a greater potential for upregulating adhesion molecules and chemokines in cultured human primary coronary artery endothelial cells (Channell et al., 2012). More recently, we observed that serum obtained from O3-exposed mice impaired vasodilation in a manner that was dependent on vascular CD36 receptors, which suggests that circulating ligands are elicited post-exposure. While compositional changes of the serum have not been characterized, it was noted that the dependence of effects on the multi-ligand CD36 receptor, known to affect an inflammatory response, implies that oxidized lipids, peptides and peptide fragments, as well as adducted proteins may have a role (Robertson et al., 2013). Several labs have demonstrated compositional and functional changes in circulating components caused by inhalation of a variety of compounds. For instance, functional high-density lipoprotein alterations and formation of oxidized lipids were noted following exposure to ambient PM (Li et al., 2013) and acrolein inhalation was shown to increase circulating acrolein-protein adducts (Wheat et al., 2011).
Caveats regarding the present model must be noted, including the relatively high concentration of emissions and the hypercholesterolemic transgenic mouse model. Naturally, if the objective to derive health impact estimates from the present work, some consideration of the 300 μg PM/m3, along with high levels of CO, NOx, and hydrocarbons would be necessary before extrapolation to possible human effects. However, we would note that the present study is designed to principally address the gas-PM interaction question. As such, the level of emissions used is quite modest from our perspective, as it yielded no observable changes in airway protein or LDH and no changes in a number of other vascular markers. Similarly, the ApoE−/− model is notorious for its sensitivity to air pollution, and the precise mechanism underlying this vulnerability is unclear. As we are interested in studying the impact of inhaled pollutants on vascular lesions that develop in the ApoE−/− model, the rationale for utilizing the wildtype (C57BL/6J) mouse that does not develop such lesions for comparisons is lacking. While we are assured that we could not recapitulate the vascular histopathological effects of MVE in a wildtype (C57BL/6) mouse, serum obtained from C57BL/6 mice exposed to ozone does confer vasorelaxation impairments in naïve aortas (Robertson et al., 2013).
In summary, removal of the gaseous phase components from MVE PM significantly diminished markers of systemic vascular inflammation and injury incurred by subchronic inhalation exposure. We conclude that combined gas and PM phases in whole emissions are more injurious than either component alone. We speculate that the mechanism by which the combined PM and gas portions lead to potentiated effects relates to more complex and reactive PM surface chemistry, and the delivery or volatile and semivolatile gases deeper in the lungs, as carried by the PM. However, our current means of characterizing the PM physicochemistry does not permit this level of insight. The findings suggest that novel metrics of PM physicochemistry, including surface area and adhered VOCs, may be valuable aids in public health risk assessment. Further inquiry into which specific adhered species or classes of S/VOCs will also be of value in understanding the principal drivers of epidemiologically-observed cardiovascular health effects of near-roadway exposures.
Supplementary Material
Acknowledgments
SOURCE OF FUNDING: This work is supported by the National Institutes of Health (NIH ES014639 to M.J.C. and ES016586 to A.K.L.) and by the Environmental Protection Agency, Assistance Agreement RD-83479601-0 (Clean Air Research Centers). The views expressed in this document are solely those of the authors and the U.S. EPA does not endorse any products or commercial services mentioned in this publication.
ABBREVIATIONS
- ACh
Acetylcholine
- EDTA
Ethylenediaminetetraacetic Acid
- MVE
Mixed Vehicular Emissions
- MVE-G
Mixed Vehicular Emissions with Gases Removed
- MVE-PM
Mixed Vehicular Emissions with Particulate Matter Removed
- NO2
Nitrogen Dioxide
- PM
Particulate Matter
- PSS
Physiological Saline Solution
- KPSS
High Potassium Physiological Saline Solution
- SEM
Standard Error of the Mean.
Footnotes
DISCLOSURES
The authors declare no conflicts of interest with the research presented in this manuscript.
REFERENCES
- Bai N, Kido T, Suzuki H, Yang G, Kavanagh TJ, Kaufman JD, Rosenfeld ME, Van Breemen C, Eeden SF. Changes in atherosclerotic plaques induced by inhalation of diesel exhaust. Atherosclerosis. 2011;216:299–306. doi: 10.1016/j.atherosclerosis.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barath S, Langrish JP, Lundback M, Bosson JA, Goudie C, Newby DE, Sandstrom T, Mills NL, Blomberg A. Short-term exposure to ozone does not impair vascular function or affect heart rate variability in healthy young men. Toxicol Sci. 2013;135:292–299. doi: 10.1093/toxsci/kft157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S. Chronic air pollution exposure and endothelial dysfunction: what you can't see--can harm you. J Am Coll Cardiol. 2012;60:2167–2169. doi: 10.1016/j.jacc.2012.08.974. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, kaufman JD American Heart Association Council on, E., Prevention, C. O. T. K. I. C. D., Council on Nutrition, P. A. & Metabolism. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Campen MJ, Lund A, Rosenfeld M. Mechanisms linking traffic-related air pollution and atherosclerosis. Curr Opin Pulm Med. 2012;18:155–160. doi: 10.1097/MCP.0b013e32834f210a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen MJ, Lund AK, Knuckles TL, Conklin DJ, Bishop B, Young D, Seilkop S, Seagrave J, Reed MD, Mcdonald JD. Inhaled diesel emissions alter atherosclerotic plaque composition in ApoE(−/−) mice. Toxicol Appl Pharmacol. 2010;242:310–317. doi: 10.1016/j.taap.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channell MM, Paffett ML, Devlin RB, Madden MC, Campen MJ. Circulating factors induce coronary endothelial cell activation following exposure to inhaled diesel exhaust and nitrogen dioxide in humans: evidence from a novel translational in vitro model. Toxicol Sci. 2012;127:179–186. doi: 10.1093/toxsci/kfs084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherng TW, Paffett ML, Jackson-Weaver O, Campen MJ, Walker BR, Kanagy NL. Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles. Environ Health Perspect. 2011;119:98–103. doi: 10.1289/ehp.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, Farraj AK. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect. 2011;119:951–957. doi: 10.1289/ehp.1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B, Moebus S, Mohlenkamp S, Stang A, Lehmann N, Dragano N, Schmermund A, Memmesheimer M, Mann K, Erbel R, Jockel KH Heinz Nixdorf Recall Study Investigative, G. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. 2007;116:489–496. doi: 10.1161/CIRCULATIONAHA.107.693622. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Moebus S, Stang A, Beck EM, Dragano N, Mohlenkamp S, Schmermund A, Memmesheimer M, Mann K, Erbel R, Jockel KH Heinz Nixdorf, R. S. I. G. Residence close to high traffic and prevalence of coronary heart disease. Eur Heart J. 2006;27:2696–2702. doi: 10.1093/eurheartj/ehl278. [DOI] [PubMed] [Google Scholar]
- Kampfrath T, Maiseyeu A, Ying Z, Shah Z, Deiuliis JA, Xu X, Kherada N, Brook RD, Reddy KM, Padture NP, Parthasarathy S, Chen LC, Moffatt-Bruce S, Sun Q, Morawietz H, Rajagopalan S. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res. 2011;108:716–726. doi: 10.1161/CIRCRESAHA.110.237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish JP, Lundback M, Barath S, Soderberg S, Mills NL, Newby DE, Sandstrom T, Blomberg A. Exposure to nitrogen dioxide is not associated with vascular dysfunction in man. Inhal Toxicol. 2010;22:192–198. doi: 10.3109/08958370903144105. [DOI] [PubMed] [Google Scholar]
- Langrish JP, Unosson J, Bosson J, Barath S, Muala A, Blackwell S, Soderberg S, Pourazar J, Megson IL, Treweeke A, Sandstrom T, Newby DE, Blomberg A, Mills NL. Altered nitric oxide bioavailability contributes to diesel exhaust inhalation-induced cardiovascular dysfunction in man. J Am Heart Assoc. 2013;2:e004309. doi: 10.1161/JAHA.112.004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc AJ, Moseley AM, Chen BT, Frazer D, Castranova V, Nurkiewicz TR. Nanoparticle inhalation impairs coronary microvascular reactivity via a local reactive oxygen species-dependent mechanism. Cardiovasc Toxicol. 2010;10:27–36. doi: 10.1007/s12012-009-9060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Navab M, Pakbin P, Ning Z, Navab K, Hough G, Morgan TE, Finch CE, Araujo JA, Fogelman AM, Sioutas C, Hsiai T. Ambient ultrafine particles alter lipid metabolism and HDL anti-oxidant capacity in LDLR-null mice. J Lipid Res. 2013;54:1608–1615. doi: 10.1194/jlr.M035014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucking AJ, Lundback M, Barath SL, Mills NL, Sidhu MK, Langrish JP, Boon NA, Pourazar J, Badimon JJ, Gerlofs-Nijland ME, Cassee FR, Boman C, Donaldson K, Sandstrom T, Newby DE, Blomberg A. Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation. 2011;123:1721–1728. doi: 10.1161/CIRCULATIONAHA.110.987263. [DOI] [PubMed] [Google Scholar]
- Lund AK, Knuckles TL, Obot Akata C, Shohet R, Mcdonald JD, Gigliotti A, Seagrave JC, Campen MJ. Gasoline exhaust emissions induce vascular remodeling pathways involved in atherosclerosis. Toxicol Sci. 2007;95:485–494. doi: 10.1093/toxsci/kfl145. [DOI] [PubMed] [Google Scholar]
- Lund AK, Lucero J, Harman M, Madden MC, Mcdonald JD, Seagrave JC, Campen MJ. The oxidized low-density lipoprotein receptor mediates vascular effects of inhaled vehicle emissions. Am J Respir Crit Care Med. 2011;184:82–91. doi: 10.1164/rccm.201012-1967OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund AK, Lucero J, Lucas S, Madden MC, Mcdonald JD, Seagrave JC, Knuckles TL, Campen MJ. Vehicular emissions induce vascular MMP-9 expression and activity associated with endothelin-1-mediated pathways. Arterioscler Thromb Vasc Biol. 2009;29:511–517. doi: 10.1161/ATVBAHA.108.176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald JD, Barr EB, White RK, Chow JC, Schauer JJ, Zielinska B, Grosjean E. Generation and characterization of four dilutions of diesel engine exhaust for a subchronic inhalation study. Environ Sci Technol. 2004;38:2513–2522. doi: 10.1021/es035024v. [DOI] [PubMed] [Google Scholar]
- Mills NL, Miller MR, Lucking AJ, Beveridge J, Flint L, Boere AJ, Fokkens PH, Boon NA, Sandstrom T, Blomberg A, Duffin R, Donaldson K, Hadoke PW, Cassee FR, Newby DE. Combustion-derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation. Eur Heart J. 2011;32:2660–2671. doi: 10.1093/eurheartj/ehr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, Macnee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- Pope CA., 3rd Mortality effects of longer term exposures to fine particulate air pollution: review of recent epidemiological evidence. Inhal Toxicol. 2007;19(Suppl 1):33–38. doi: 10.1080/08958370701492961. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Robertson S, Colombo ES, Lucas SN, Hall PR, Febbraio M, Paffett ML, Campen MJ. CD36 Mediates Endothelial Dysfunction Downstream of Circulating Factors Induced by O3 Exposure. Toxicol Sci. 2013 doi: 10.1093/toxsci/kft107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz PA, Lawrence JE, Ferguson ST, Wolfson JM, Koutrakis P. A counter-current parallel-plate membrane denuder for the non-specific removal of trace gases. Environ Sci Technol. 2006;40:5058–5063. doi: 10.1021/es060563w. [DOI] [PubMed] [Google Scholar]
- Sarnat JA, Marmur A, Klein M, Kim E, Russell AG, Sarnat SE, Mulholland JA, Hopke PK, Tolbert PE. Fine particle sources and cardiorespiratory morbidity: an application of chemical mass balance and factor analytical source-apportionment methods. Environ Health Perspect. 2008;116:459–466. doi: 10.1289/ehp.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedal S, Campen MJ, Mcdonald JD, Kaufman JD, Larson TV, Sampson PD, Sheppard L, Simpson CD, Szpiro AA. National Particle Component Toxicity (NPACT) Initiative Report on Cardiovascular Effects. Res Rep Health Eff Inst. 2013;178:238. [PubMed] [Google Scholar]
- Wheat LA, Haberzettl P, Hellmann J, Baba SP, Bertke M, Lee J, Mccracken J, O'Toole TE, Bhatnagar A, Conklin DJ. Acrolein inhalation prevents vascular endothelial growth factor-induced mobilization of Flk-1+/Sca-1+ cells in mice. Arterioscler Thromb Vasc Biol. 2011;31:1598–1606. doi: 10.1161/ATVBAHA.111.227124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.