Abstract
BACKGROUND:
Although stereotactic body radiation therapy (SBRT) is an established treatment option for early-stage lung cancer, there are no guidelines for reassessing patients for local treatment failure or intrathoracic recurrence after treatment. This study reports the sensitivity, specificity, and positive and negative predictive values for 18F-fluorodeoxyglucose (FDG) PET-CT scanning when used to evaluate patients after SBRT.
METHODS:
Charts were reviewed of all patients who received SBRT and a subsequent FDG PET-CT scan at a university hospital over a 5-year period. Pretreatment and 3-month posttreatment tumor characteristics on PET-CT scan and outcome data (adverse events from SBRT, need for repeat biopsy, rate of local treatment failure and recurrent disease, and all-cause mortality) were recorded.
RESULTS:
Eighty-eight patients were included in the study. Fourteen percent of patients (12 of 88) had positive 3-month PET scans. Of the positive results, 67% (eight of 12) were true positives. Eighty-six percent (76 of 88 patients) had negative 3-month FDG PET-CT scans, with 89% (68 of 76) true negatives. FDG PET-CT scan performed 3 months after SBRT for non-small cell lung cancer (NSCLC) had a sensitivity of 50% (95% CI, 0.26-0.75), a specificity of 94% (95% CI, 0.89-1.0), a positive predictive value of 67% (95% CI, 0.4-0.93), and a negative predictive value of 89% (95% CI, 0.83- 0.96).
CONCLUSIONS:
FDG PET-CT scan 3 months after treatment of NSCLC with SBRT was a specific but insensitive test for the detection of recurrence or treatment failure. Serial CT scans should be used for early surveillance following SBRT, whereas FDG PET-CT scans should be reserved to define suspected metastatic disease or to evaluate new abnormalities on CT scan, or for possible reassessment later in the follow-up period after radiation-related inflammation subsides.
Lung cancer is the third most common cause of cancer and the leading cause of cancer death among men and women in the United States. Unfortunately, lung cancer is detected in an advanced stage approximately 70% of the time, with a 16% 5-year survival rate.1 Surgical resection is the treatment of choice for early-stage non-small cell lung cancer (NSCLC), with a 70% 5-year survival rate and a 55% to 75% recurrence rate. In patients who are medically inoperable or who decline surgery, stereotactic body radiation therapy (SBRT) has emerged as a noninvasive treatment option with a 97.6% 3-year primary tumor control rate.2‐5 Although SBRT is an established treatment, there is a paucity of data on how and when to reassess patients radiographically after treatment to detect local treatment failure or intrathoracic recurrence.
The Radiation Therapy Oncology Group 0236 trial followed patients with CT scans every 3 months during the first 2 years after treatment and then every 6 months for 2 more years. 18F-fluorodeoxyglucose (FDG) PET (FDG PET) scanning was used only if CT scans showed progressive soft tissue abnormalities.6 Despite this protocol, many physicians in clinical practice use FDG PET-CT scanning for interval follow-up. FDG PET-CT scanning is 72% to 94% sensitive and 77% to 92% specific for evaluating malignancy in lung nodules.7 It is superior to CT scan for detecting mediastinal metastasis, with a sensitivity and specificity of 77% and 86%, respectively.8 However, it is unclear whether routine FDG PET-CT scanning improves the detection of recurrence following SBRT for early-stage NSCLC during the period when postradiation-related inflammation increases the maximal standard uptake value (SUVmax) measurements and may degrade the sensitivity of the FDG PET-CT scans.
The aim of this study was to determine the rate of treatment failure and recurrence for 88 consecutive patients who had an FDG PET-CT scan 3 months after SBRT completion. These data were used to determine the sensitivity, specificity, and positive and negative predictive value of FDG PET-CT scanning in this setting.
Materials and Methods
Patients
The Medical University of South Carolina (MUSC) Institutional Review Board (Pro00013650) and the MUSC Hollings Cancer Center Protocol Review Committee approved this study. A retrospective chart review was performed for patients aged ≥ 18 years who underwent SBRT at MUSC for stage I or II NSCLC between July 9, 2008, and January 12, 2012. The diagnosis of cancer was made based on tissue biopsy for all patients except for 10 in whom the risk of morbidity or mortality from a biopsy was too high. In these cases, a presumptive diagnosis was based on a review of clinical and radiographic data by a multidisciplinary thoracic tumor board. Staging was performed using the seventh edition TNM classification and, if any uncertainty arose, via review by a multidisciplinary tumor board.5 Patients with more than one lung nodule were included if each nodule represented a synchronous primary lung cancer. However, only data from the first cancer treated were analyzed. Exclusion criteria included the following: (1) SBRT for malignancies other than early-stage NSCLC, (2) palliative SBRT in the setting of advanced-stage lung cancer, (3) absence of pretreatment FDG PET-CT scan for comparison, (4) failure to return for 3-month follow-up PET-CT scan and (5) FDG PET-CT scan performed at another facility and unavailable for review.
SBRT Treatment Protocol
For treatment simulation, the patient’s upper thorax was positioned in a customizable mold that overlapped a head rest and indexed wing-board. Several helical CT scans were taken through the chest. The first CT scan taken through the involved region was used to triangulate and mark the treatment isocenter on the patient’s skin. This was followed by a four-dimensional (4D) CT scan that extended from the chin inferiorly to include the extent of the lung volume. The 4D CT scan was reconstructed to generate 10 CT image sets, each representing a different respiratory phase. The medical physicist reviewed these image sets under a cine display to inspect for artifacts and adequacy of the images before transferring them via digital imaging and communications in medicine to the treatment planning system (TPS) (PINNACLE v 9.0; Philips Healthcare). The reference CT scan was an average-intensity projection CT image set generated from raw, untagged 4D CT scan projection data. The average-intensity projection and 10 phase-based image sets were registered in the TPS by aligning their common digital imaging and communications in medicine origin. The motion exhibited on the phase-based image sets permitted the radiation oncologist to delineate the internal target volume (ITV) that included all respiratory-induced motion of the gross tumor volume. An intermediate margin to define a clinical target volume was not applied. An isotropic setup margin of 5 mm was added to the ITV to generate the planning target volume (PTV). Normal tissue structures contoured included the left and right lungs, heart, esophagus, trachea, skin, great vessels, and spinal canal. The brachial plexus was contoured in instances in which it was in proximity to the PTV. No block margin was used axially, but a 3-mm margin in addition to the PTV in the craniocaudal direction improved coverage, thereby reducing high-and intermediate-dose spillage. Heterogeneous dose calculations were performed by the collapsed-cone convolution algorithm available in the TPS.
Plan evaluation criteria and normal tissue constraints followed were consistent with currently available protocols, including Radiation Therapy Oncology Group 0618, 0813, and 0915. Prescription doses were either 50 Gy in five fractions or 48 Gy in four fractions. No more than two fractions were delivered per week. Standard practice was to separate fractions by ≥ 2 or 3 days but ≤ 5 days. Treatment plans consisted of eight to 11 static 6-MV photon beams in a mix of noncoplanar and coplanar beam arrangements, although several included dynamic conformal arcs. In rare circumstances in which the PTV abutted a critical structure, an intensity-modulated radiation therapy treatment plan was created allowing only two to three segments per beam and a minimum of 30 MU per segment to reduce the effect of respiratory motion on dose-delivery error.
Before each treatment, each patient was set up by triangulating the treatment isocenter marked during simulation, using the room lasers. This was followed by a cone-beam CT scan to facilitate image-guided patient setup. The visualized ITV in the cone-beam CT images was registered with its position in the reference CT images at the treatment console by overlaying the images and ITV/PTV contours. After the radiation oncologist approved the registration, patient shifts were applied remotely, and the treatment was delivered.
Radiographic follow-up consisted of a 1-month posttreatment CT scan followed by a 3-month posttreatment FDG PET-CT scan. FDG PET-CT imaging was performed on a GE Discovery whole-body PET-CT scanner. Prior to FDG PET-CT scanning, a fasting blood glucose level was taken to ensure reliable results. No patients were injected if blood glucose level was > 230 mg/dL. After 10 mCi FDG was injected, 60 min was allowed for uptake time, except for the case of a solitary pulmonary nodule for which 90 min was allowed. A noncontrasted CT scan was acquired from the base of the skull through the inguinal region, followed by a three-dimensional emission scan of the same area. Images were reviewed in the trans-axial, coronal, and sagittal planes.
After the 3-month FDG PET-CT scan, if findings suggested treatment failure or disease recurrence, the multidisciplinary thoracic tumor board made recommendation for further follow-up FDG PET-CT scan, CT scan, or biopsy. If the 3-month FDG PET-CT scan was not concerning for treatment failure or disease recurrence, CT scans were performed every 6 months for 2 years following SBTR, and then annually to complete a 5-year follow-up period.
Measurements
Data collected included demographics, prior malignancy and treatment, pretreatment and 3-month posttreatment tumor characteristics (size, SUVmax, TNM staging, histology), follow-up imaging results, and outcome data (adverse events, repeat biopsies, rate of local treatment failure and recurrent disease, and all-cause mortality).
The 3-month posttreatment FDG PET-CT scan was considered positive if the reviewing radiation oncologist and dedicated thoracic radiologist agreed that new findings were consistent with recurrence. Disagreements were settled by majority vote among the multidisciplinary tumor board. Criteria for recurrence included an increased tumor size, SUVmax > 3 (in the absence of known infection), and the presence of new intrathoracic abnormalities beyond expected radiation pneumonitis changes. A negative 3-month posttreatment FDG PET-CT scan was defined as a scan lacking the above-mentioned features. A true positive FDG PET-CT scan was defined as biopsy-proven recurrence or radiographic findings so suggestive of recurrence that biopsy was unnecessary (ie, new bone metastases without evidence of a second primary). A true negative test was defined by negative repeat biopsy or long-term CT scan follow-up without evidence of recurrence.
Statistical Analysis
Descriptive statistics including frequencies and percentages were used to describe the demographic and staging characteristics of the data. Sensitivity, specificity, negative predictive value, positive predictive value, and their respective 95% CIs were calculated to evaluate the ability of FDG PET-CT scanning to detect recurrent disease.
Results
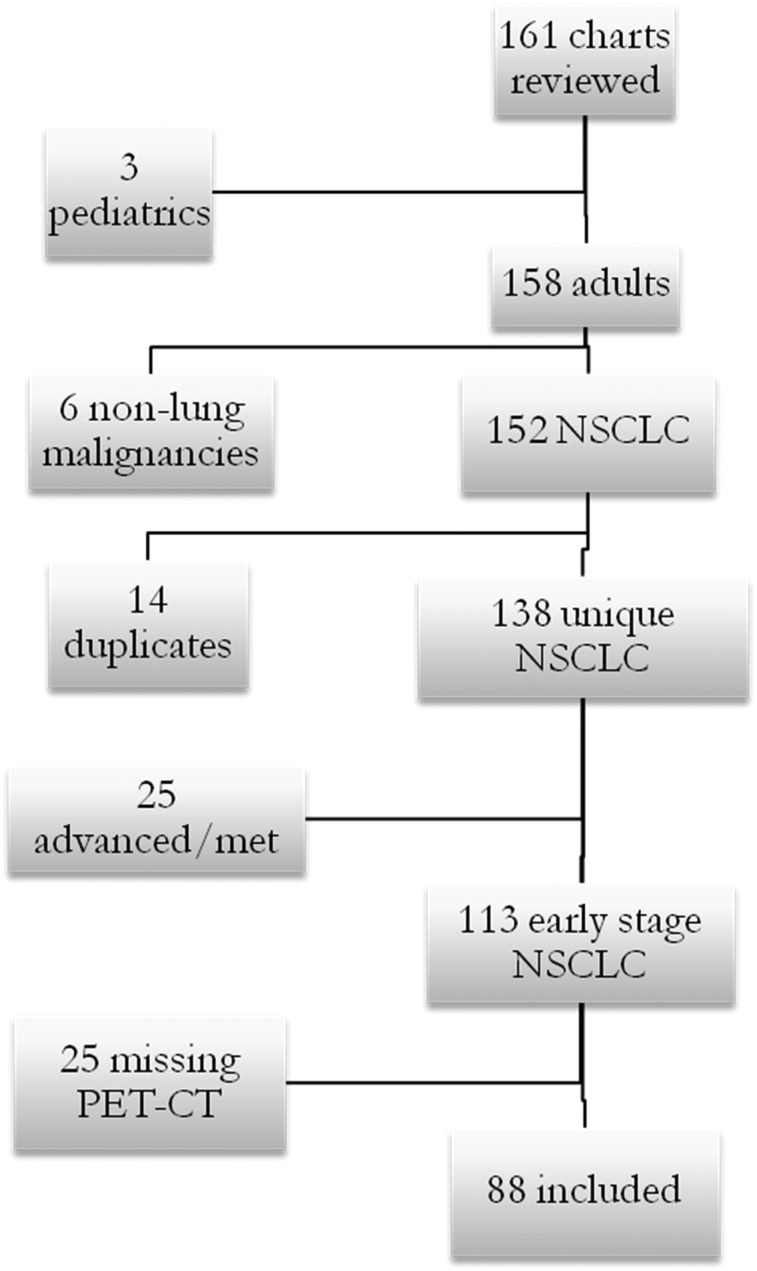
A total of 161 consecutive charts were reviewed for patients receiving SBRT at MUSC between 2008 and 2012. Eighty-eight patients met the inclusion criteria (Fig 1) and were followed radiographically for a mean of 14 months (range, 4-19 months).
Figure 1 .
– Patient inclusion flowchart. The charts of 161 patients were reviewed. Three patients were pediatric patients under the age limit. Six received stereotactic body radiation therapy (SBRT) for nonlung malignancies. Fourteen were duplicate patients who underwent SBRT twice, often for synchronous primaries, for which they were included only once, for the first treatment. Twenty-five received SBRT for advanced disease or as palliation for metastatic disease. Another 25 were missing initial or 3-mo posttreatment PET-CT scan data and, therefore, an adequate comparison could not be made. NSCLC = non-small cell lung cancer.
Demographic characteristics are detailed in Table 1. The study population included twice as many men as women, three-quarters were white, and nearly all (93%) were current or former smokers. More than one-half of the patients had a history of malignancy. Initial lung cancer staging characteristics are included in Table 2. The majority of patients (95%) had stage IA or IB disease. Adenocarcinoma was the most common histologic group.
TABLE 1 .
] Demographic Characteristics of the Study Population
| Variable | No. | % |
| Sex | ||
| Female | 30 | 34 |
| Male | 58 | 66 |
| Ethnicity | ||
| White | 68 | 77 |
| Black | 20 | 23 |
| Smoking status | ||
| Current | 18 | 20 |
| Former | 65 | 74 |
| Never | 5 | 6 |
| History of cancer | ||
| No | 43 | 49 |
| Yes | 45 | 51 |
TABLE 2 .
] Initial Tumor Characteristics of the Study Population
| Variable | No. | % |
| Location | ||
| RUL | 25 | 28 |
| RML | 8 | 9 |
| RLL | 16 | 18 |
| LUL | 24 | 27 |
| Lingula | 0 | 0 |
| LLL | 15 | 17 |
| T stage | ||
| T1a | 42 | 48 |
| T1b | 20 | 23 |
| T2a | 21 | 24 |
| T2b | 4 | 4 |
| T3 | 1 | 1 |
| T4 | 0 | 0 |
| Tx | 0 | 0 |
| N stage | ||
| N0 | 87 | 99 |
| N1 | 0 | 0 |
| N2 | 0 | 0 |
| N3 | 0 | 0 |
| Nx | 1 | 1 |
| M stage | ||
| M0 | 85 | 97 |
| M1a | 0 | 0 |
| M1b | 0 | 0 |
| Mx | 3 | 3 |
| Stage | ||
| IA | 63 | 72 |
| IB | 20 | 23 |
| IIA | 4 | 4 |
| IIB | 1 | 1 |
| IIIA | 0 | 0 |
| IIIB | 0 | 0 |
| IV | 0 | 0 |
| Histology | ||
| Squamous | 28 | 32 |
| Adenocarcinoma | 37 | 42 |
| Large cell | 2 | 2 |
| Carcinoid | 1 | 1 |
| BAC | 5 | 6 |
| Other | 15 | 17 |
BAC = bronchoalveolar cell carcinoma; LLL = left lower lobe; LUL = left upper lobe; RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe.
Three-month posttreatment FDG PET-CT scan results are outlined in Table 3. A minority of patients (12 of 88) had a positive 3-month FDG PET-CT scan. Of these, eight were true positives. Conversely, 76 of 88 patients had negative 3-month PET-CT scans, with 68 true negatives. Sixteen patients had recurrent disease, of which nine were confirmed by tissue diagnosis (three malignant pleural effusions, four mediastinal or hilar lymph nodes, two distant metastases) and seven presumed recurrences based on radiographic and clinical findings. The mean time for documentation of recurrence was 19 months (range, 4-38 months). There were four cases of benign results when sampling tissue to evaluate for recurrence (parapneumonic pleural effusion, two lung biopsy specimens with inflammation only, and benign reactive mediastinal lymphadenopathy).
TABLE 3 .
] Results of 3-Mo Posttreatment PET-CT Scan
| Evidence of Recurrent Disease | |||
| 3-Mo PET (+) | No | Yes | Total |
| No | 68 | 8 | 76 |
| Yes | 4 | 8 | 12 |
| Total | 72 | 16 | 88 |
Areas of postradiation ground glass or consolidation were read in 17 cases. Imaging features suggesting recurrence included new PET scan (+) mediastinal or hilar lymphadenopathy, new nodules, or metastasis to bone and liver. Two patients developed pleural effusions, but each was associated with PET scan (+) mediastinal lymphadenopathy.
Based on these results, FDG PET-CT scanning performed 3 months after SBRT for NSCLC had a sensitivity of 50% (95% CI, 0.26-0.75), a specificity of 94% (95% CI, 0.89-1.0), a positive predictive value of 67% (95% CI, 0.4-0.93), and a negative predictive value of 89% (95% CI, 0.83-0.96).
Discussion
This study is the first, to our knowledge, to define the lack of usefulness of FDG PET-CT scanning in the evaluation of treatment response within the first year after SBRT for NSCLC. It demonstrates that an FDG PET-CT scan performed 3 months after SBRT for NSCLC is an insensitive test for the evaluation of recurrence. However, despite the concern about increased FDG activity in areas of inflammation, the specificity of FDG PET-CT scanning for recurrence was high.
The inability to accurately identify true negative results could be related to postradiation pneumonitis, which manifests as new ground-glass opacities or consolidation on follow-up imaging.9,10 Inflammation can obscure the initial tumor bed, making it difficult to assess residual tumor size and other characteristics. Therefore, without definitive evidence of recurrence, FDG PET-CT scanning may be misinterpreted as showing expected postradiation changes and reported as a negative test, with cancer becoming evident after initial inflammation subsides.
A 3-month follow-up FDG PET-CT scan appears premature, because tumor recurrence becomes radiographically visible later in the follow-up period. Thus, the lack of evidence of recurrence on a 3-month FDG PET-CT scan may not be a fault of the test but simply an issue of timing. FDG PET-CT scanning should be applied later in the follow-up period or guided by abnormalities seen on CT scanning.11
The low sensitivity of FDG PET-CT scanning in this study was surprising, because FDG PET-CT scanning was shown previously to be a very sensitive test with few false-negatives in the initial detection of malignancy.12‐14 This can be explained in that the detection of metabolic activity, which makes FDG PET-CT scanning so useful in the initial evaluation of malignancy, is confounded after treatment by the inflammation caused by radiation. The inflammation involved in radiation pneumonitis can falsely elevate SUVmax within and around the treated tumor bed and, based on such a well-recognized finding, FDG PET-CT scans may have been “underread” rather than “overread.”
This study has some limiting factors. First, with 88 patients, the sample size was relatively small; however, it is as large as, or larger than, many existing studies evaluating radiographic changes following SBRT. In addition, 10 of the included patients underwent SBRT for presumed malignancy but were deemed too high risk to undergo confirmatory biopsy. In each instance, however, the case was discussed by a multidisciplinary tumor board, and consensus was reached that the lesions had an extremely high likelihood of being malignant and treatment was warranted. The final potential limitation is that during the follow-up period, not every patient underwent a repeat biopsy, which some would consider to be the only true gold standard of recurrence. In actual practice, however, this is not a realistic expectation in all cases, and long-term radiographic follow-up without overt signs of recurrence can be an adequate surrogate.
This study adds to the existing body of knowledge on the use of FDG PET-CT scanning as a reassessment tool after lung cancer therapy. With this information on FDG PET-CT scanning, practitioners may become more restrictive in their use of FDG PET-CT scans in the follow-up of patients treated with SBRT for NSCLC. Future studies are needed in this area to delineate if FDG PET-CT scanning has any additional role in the follow-up period besides evaluating new findings on CT scan. As stated previously, a routine FDG PET-CT scan added later in the follow-up course after the acute inflammatory changes have resolved seems to be of more benefit. Takeda et al10 showed that the SUVmaxs of FDG PET-CT scans were superior to those of CT scans in the detection of local recurrence 1 year after SBRT for localized NSCLC. One year appears to be a more reasonable time interval for FDG PET-CT scanning because the chance of recurrence and the resolution of confounding acute inflammation are then more likely. Emerging imaging modalities, such as volumetric tumor measurements made before and after treatment, may also prove to be effective at predicting patients at risk of recurrence and detecting recurrence early, thus allowing for salvage treatment options.15
Conclusions
In conclusion, a FDG PET-CT scan 3 months after treatment of NSCLC with SBRT was a specific but insensitive test, and its routine use in post-SBRT follow-up is not supported. A rationale for these findings includes the following: Postradiation ground-glass opacities can obscure accurate measurements of tumor size, and 3 months after treatment is a short interval that may not allow sufficient time to demonstrate tumor recurrence.
Further studies are needed in this area to fully assess the role and optimal timing of FDG PET-CT scanning in the posttreatment period. Future studies could evaluate the use of volumetric measurements of the tumor before and after treatment to aid in the prediction and detection of recurrence.
Based on the results of this study and until further outcome data are published, we cannot recommend that FDG PET-CT scans be used routinely during the first year after SBRT. A rational approach is to perform serial CT scans at least as frequently as recommended for patients who have undergone curative-intent surgical resection of NSCLC (every 6 months for the first 2 years and every year thereafter)15 for routine surveillance, and to reserve FDG PET-CT scans to help define suspected metastatic disease or to further evaluate new abnormalities present in the tumor bed.
Acknowledgments
Author contributions: N. J. P. is guarantor of the manuscript and takes responsibility for the integrity of the data and the accuracy of the analysis. N. J. P., N. T. T., L. L. G., A. K. S., and G. A. S. contributed to the conception and design of the study; N. J. P. and T. J. G. contributed to the acquisition, analysis, and interpretation of the data, writing of the first drafts of the manuscript, and approval of the final version of the manuscript; N. T. T., A. E. W., L. L. G., A. K. S., and G. A. S. contributed to the analysis and interpretation of the data; and N. T. T., A. E. W., L. L. G., A. K. S., N. C. K., and G. A. S. contributed to the editing and revision of the manuscript, and approval of the final version of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ABBREVIATIONS
- 4D
four-dimensional
- FDG
18F-fluorodeoxyglucose
- ITV
internal target volume
- MUSC
Medical University of South Carolina
- NSCLC
non-small cell lung cancer
- PTV
planning target volume
- SBRT
stereotactic body radiation therapy
- SUVmax
maximal standard uptake value
- TPS
treatment planning system
Footnotes
FUNDING/SUPPORT: This study was supported by the National Center for Research Resources [UL1RR029882 and UL1TR000062].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300 [DOI] [PubMed] [Google Scholar]
- 2.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111(6):1710-1717 [DOI] [PubMed] [Google Scholar]
- 3.Pairolero PC, Williams DE, Bergstralh EJ, Piehler JM, Bernatz PE, Payne WS. Postsurgical stage I bronchogenic carcinoma: morbid implications of recurrent disease. Ann Thorac Surg. 1984;38(4):331-338 [DOI] [PubMed] [Google Scholar]
- 4.Feld R, Rubinstein LV, Weisenberger TH. Sites of recurrence in resected stage I non-small-cell lung cancer: a guide for future studies. J Clin Oncol. 1984;2(12):1352-1358 [DOI] [PubMed] [Google Scholar]
- 5.Goldstraw P, Crowley J, Chansky K, et al. ; International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706-714 [DOI] [PubMed] [Google Scholar]
- 6.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070-1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5_suppl):93S-120S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5_suppl):211S-250S [DOI] [PubMed] [Google Scholar]
- 9.Trovo M, Linda A, El Naqa I, Javidan-Nejad C, Bradley J. Early and late lung radiographic injury following stereotactic body radiation therapy (SBRT). Lung Cancer. 2010;69(1):77-85 [DOI] [PubMed] [Google Scholar]
- 10.Takeda A, Kunieda E, Fujii H, et al. Evaluation for local failure by 18F-FDG PET/CT in comparison with CT findings after stereotactic body radiotherapy (SBRT) for localized non-small-cell lung cancer. Lung Cancer. 2013;79(3):248-253 [DOI] [PubMed] [Google Scholar]
- 11.Cronin P, Dwamena BA, Kelly AM, Carlos RC. Solitary pulmonary nodules: meta-analytic comparison of cross-sectional imaging modalities for diagnosis of malignancy. Radiology. 2008;246(3):772-782 [DOI] [PubMed] [Google Scholar]
- 12.Grgic A, Yüksel Y, Gröschel A, et al. Risk stratification of solitary pulmonary nodules by means of PET using (18) F-fluorodeoxyglucose and SUV quantification. Eur J Nucl Med Mol Imaging. 2010;37(6):1087-1094 [DOI] [PubMed] [Google Scholar]
- 13.Kagna O, Solomonov A, Keidar Z, et al. The value of FDG-PET/CT in assessing single pulmonary nodules in patients at high risk of lung cancer. Eur J Nucl Med Mol Imaging. 2009;36(6):997-1004 [DOI] [PubMed] [Google Scholar]
- 14.Dinkel J, Khalilzadeh O, Hintze C, et al. Inter-observer reproducibility of semi-automatic tumor diameter measurement and volumetric analysis in patients with lung cancer. Lung Cancer. 2013;82(1):76-82 [DOI] [PubMed] [Google Scholar]
- 15.Colt HG, Murgu SD, Korst RJ, Slatore CG, Unger M, Quadrelli S. Follow-up and surveillance of the patient with lung cancer after curative-intent therapy: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5_suppl):e437S-e454S [DOI] [PubMed] [Google Scholar]