Abstract
Objective
Mild decrease in core temperature (therapeutic hypothermia; TH) provides lasting neuroprotection following cardiac arrest or cerebral ischemia. However, current methods for producing TH trigger a cold-defense response which must be countered by sedatives, muscle paralytics and mechanical ventilation. We aimed to determine methods for producing hypothermia in the conscious mouse by targeting two transient receptor potential (TRP) channels involved in thermoregulation, TRPV1 and TRPM8.
Design
Controlled prospective animal study.
Subjects
Conscious unrestrained young and aged male mice.
Setting
Research laboratory at academic medical center.
Interventions
Mice were treated with the TRPV1 agonist dihydrocapsaicin (DHC), a TRPM8 inhibitor (“compound 5”) or their combination and the effects on core temperature (Tcore) were measured by implanted thermocouples and wireless transponders.
Measurements and Main Results
TRPV1 agonist DHC produced a dose-dependent (2–4 mg/kg, s.c.) drop in Tcore. A loading dose followed by continuous infusion of DHC produced a rapid and prolonged (>6 hrs) drop of Tcore within the therapeutic range (32–34 °C). The hypothermic effect of DHC was augmented in aged mice and was not desensitized with repeated administration. TRPM8 inhibitor “compound 5” (20 mg/kg s.c.) augmented the drop in core temperature during cold exposure (8 °C). When “compound 5” (30 mg/kg) was combined with DHC (1.25–2.5 mg/kg), the drop in Tcore was amplified and prolonged.
Conclusions
Activating warm receptors (TRPV1) produced rapid and lasting hypothermia in young and old mice. Furthermore, hypothermia induced by TRPV1 agonists was potentiated and prolonged by simultaneous inhibition of TRPM8.
Keywords: transient receptor potential, pharmacological hypothermia, thermoregulation, TRPV1, TRPM8, dihydrocapsaicin
Introduction
Mild-to-moderate hypothermia (32–34 °C) has demonstrated neuroprotection in adult patients following global ischemia due to cardiac arrest (1–3) and is currently being investigated in the treatment of other brain injuries, such as stroke. Traditional therapeutic hypothermia (TH) protocols consist of mild lowering of core body temperature by physical methods (4) that take place over several hours and must be closely monitored to ensure the achievement of target temperature. However, a decrease in both ambient and core temperature trigger profound shivering and increased oxygen consumption (5). In order for TH to be effective and to avoid clinical complications that accompany prolonged shivering, this response must be counteracted by sedating narcotics and/or muscle paralytics. These agents often lead to respiratory depression, thus necessitating mechanical ventilation. The combination of difficult implementation, slow achievement of target temperature and induction of the shiver response during forced cooling limits the applicability and potential effectiveness of traditional TH (6).
In the current study, we investigated pharmacological hypothermia (PH) through targeting two of the thermosensitive transient receptor potential (TRP) ion channels that play a role in thermoregulation. TRPV1 channels are activated by heat and low pH as well as by exogenous (e.g., capsaicin) and endogenous (e.g., anandamide) chemicals. These channels are expressed in warm-sensing afferent nerve fibers and in hypothalamus (7), and it is known that activators of TRPV1 channels, such as capsaicin, produce hypothermia in rodents and larger mammals (8, 9). Systemically administered TRPV1 agonists most likely produce their hypothermic effect by acting on peripheral warm-sensing fibers and/or higher-order hypothalamic sensory neurons within the neural pathway for skin warming (7). Therefore, by activating TRPV1 channels pharmacologically, a “false” signal that the skin has been warmed is provided to the central thermoregulatory neurons in the hypothalamus – thus initiating heat loss. Conceptually, this strategy pharmacologically mimics skin counter-warming techniques, which were shown to substantially, but not clinically sufficiently decrease shivering and heat generation in human patients (10). TRPM8 channels are activated by cooling and are expressed in peripheral and visceral cold-sensing nerve fibers (11–14). Inhibition of TRPM8 channels significantly reduces perception of cold (12, 15) and inhibits non-shivering thermogenesis in response to decreased core temperature (16).
There were two primary goals in this study. The first was to establish therapeutically relevant methods of producing hypothermia in a mouse model. Thermoregulation in mice can be quite peculiar and is affected by a plethora of factors (17). Given the significant potential for transgenic and knockout mouse models, we sought to provide a description of how to promote rapid and sustained hypothermia through TRPV1 channel activation. Because stroke patients are typically conscious at the time of treatment, it was essential that we demonstrate effective methods of hypothermia that are compatible with the awake subject. Further, because stroke patients are typically elderly, it was additionally important to demonstrate that these methods were effective in an aging model. The second goal was to test the hypothesis that TRPM8 channel inhibition facilitates hypothermia as either an independent means or as an adjuvant to TRPV1 channel activation.
Materials and Methods
All studies were performed with male mice from colonies maintained at the Baylor College of Medicine (BCM) animal facility. All experiments were approved by the Institutional Animal Care and Use Committee at BCM. CD-1 mice (8–10 weeks, 30–45 g) were used for all experiments except for Fig 4, in which C57BL/6 mice were used. The C57BL/6 mice (the background of the aged mice) were used at 9–10 weeks (Adult, 26–30 g) or 24 months (Aged, 35–42g).
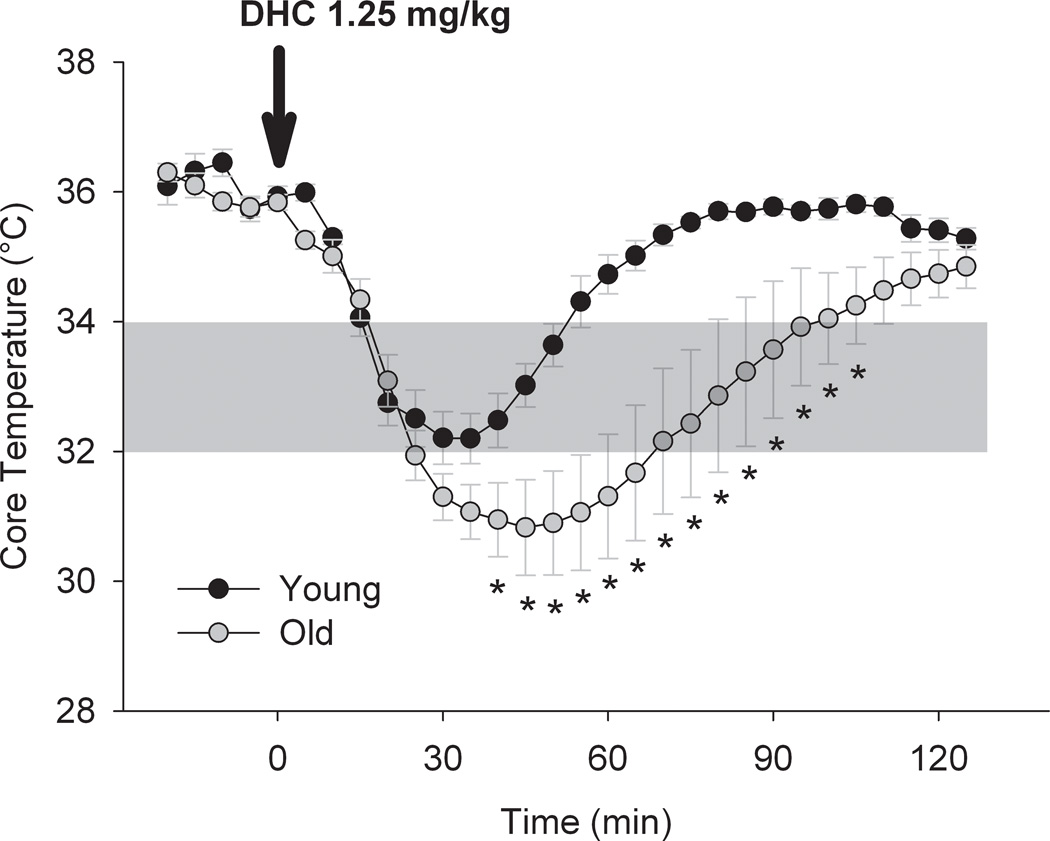
Figure 4. Dihydrocapsaicin -mediated hypothermia is not attenuated in old mice.
Summary of core temperature in response to dihydrocapsaicin (DHC; 1.25 mg/kg s.c.) in young (9–10 weeks) and old mice (24 months). Dihydrocapsaicin was injected as a bolus dose at time 0. * P<0.05 with two-way RM-ANOVA followed by Holm-Sidak test for multiple pairwise compairsons; n = 5, each.
Surgical Procedures
Mice were anesthetized with isofluorane (5% induction, 2% maintenance) for all surgical procedures. Core temperature (rectal) during surgery was maintained at 37 °C by a temperature controller (YSI Incorporated, Yellow Springs, OH) connected to a heating pad under the animal. The mice were transferred to a warming cage until they could maintain core temperature above 36 °C without heat support.
Placement of drug delivery lines and thermocouple
A small opening was made in the back skin around the base of the neck and one or two PE-10 lines (Instech Solomon Inc., San Antonio, TX USA) were inserted. The lines were tunneled under the skin caudally and terminated above the abdominal region (Figure 1A). A small thermocouple (40 G wire; Omega Engineering Inc., Stamford, CT USA) was similarly introduced and directed to a lateral region of the abdomen. The abdominal wall was perforated with a 20G needle to place the thermocouple into the abdominal cavity as a measure of core temperature. The lines and thermocouple were secured with sutures to prevent them from pulling loose in the conscious animal. The lines emanating from the neck were bundled and passed through a hole in the top of the holding cage. The PE-10 lines were connected to independent syringe pumps for delivery of compounds without disturbing the animals with a needle injection. The implanted thermocouple and a free thermocouple to measure ambient temperature were connected to a digital thermometer with data logging capability (HH806W, Omega Engineering Inc., Stamford, CT USA).
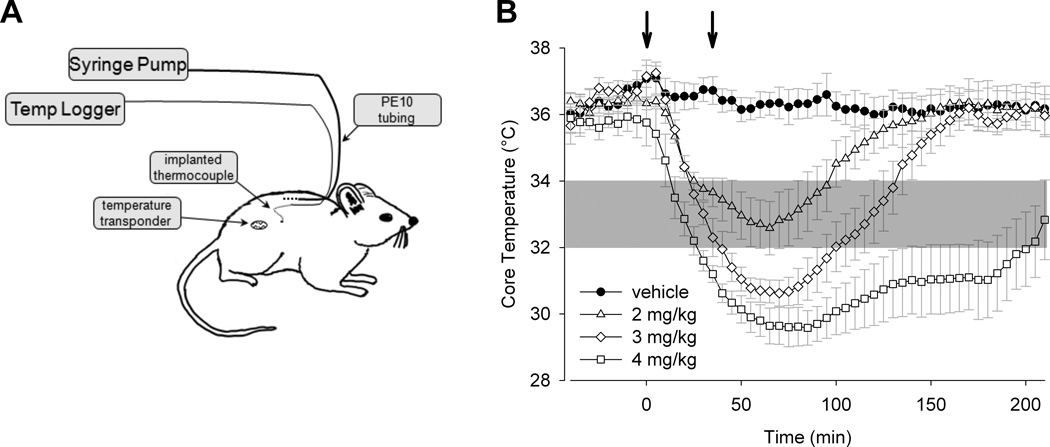
Figure 1. TRPV1 agonist dihydrocapsaicin injection leads to a dose-dependent drop in core temperature.
A) Drawing depicting a conscious mouse with implanted drug delivery lines and temperature probes. Drugs were delivered by one or more syringe pumps and core temperature was measured by abdominal thermocouple or by implanted wireless temperature telemeter. B) Dose-temperature response to a selective TRPV1 agonist (dihydrocapsaicin; DHC) in mouse (n = 4 to 5). Each dose was administered in two bolus injections (indicated by the arrows) 30 minutes apart. The dose indicated reflects the final cumulative dose delivered. In this and subsequent figures, the area shaded in gray shows the temperature range of therapeutic hypothermia (32–34 °C).
Placement of wireless temperature telemeter
In some mice, core temperature was monitored by a miniature wireless Implantable Programmable Temperature Transponder (IPTT-300, BioMedic DataSystems) placed under the skin on the back of the animal. Preliminary studies were performed with mice in which the IPTT-300 and an abdominal thermocouple were placed. There was very good agreement between both methods of measurement both at rest and when core temperature was lowered following dihydrocapsaicin (DHC) infusion (see Drugs section).
Drugs
Dihydrocapsaicin (DHC, Cayman Chemical Company) was prepared in DMSO as a stock solution (1 mg/mL) and then diluted five-fold in sterile saline prior to injection. “Compound 5” (3-[7-Trifluoromethyl-5-(2-trifluoromethyl-phenyl)-1H-benzimid-azol-2-yl]-1-oxa-2-azaspiro[4.5]dec-2-ene Hydrochloride), a selective TRPM8 antagonist kindly provided by Janssen Research & Development (18), was prepared in 5% 1-methyl-2-pyrrolidinone (NMP; Sigma) and 10% Solutol HS-15 (Sigma). “Compound 5” demonstrates high potency in blocking calcium influx through canine, rat and human TRPM8 channels heterologously expressed in HEK cells (with respective IC50 values of 0.8, 4.0, and 3.0 nM) and also inhibits cold-induced currents through human TRPM8 in the same expression system (IC50 = ca. 1 nM). In addition, “compound 5” has minimal off-target selectivity, as evidenced by less than 50% inhibition of reference compound binding to any of the targets in the panel of 50 GPRC, transporter, and ion channel assays (Cerep) and 190 kinase assays (18).
“Wet-dog shakes” (WDS) assay
To determine if “compound 5” effectively inhibits TRPM8 activity in live mice, a WDS assay was performed. In this assay, a potent TRPM8 agonist (icilin 3 mg/kg s.c.) was injected in mice treated with “compound 5” or vehicle. Icilin injections in mice produce vivid and quantifiable shaking behaviors (“wet-dog shakes”), which are TRPM8-dependent (18–22)(2, 3). Accordingly, effective inhibition of TRPM8 is predicted to result in decreased number of icilin-induced behavioral events. “Compound 5” or vehicle was administered 60 minutes prior to icilin. The number of “wet-dog shakes” was counted over a 5-minute period starting 10 minutes after icilin injection.Experimental protocols (summarized in Table 1): In protocol 1, used to establish a method for long-lasting and sustained hypothermia through TRPV1 activation, DHC (2–4 mg/kg) or vehicle (20% DMSO in saline) was infused subcutaneously through the PE-10 line in a conscious mouse. The full dose was delivered in two bolus injections 30 minutes apart (Figure 1B) or as continuous infusion with or without an initial bolus injection (Figure 2). Core temperature was recorded by the thermocouple implanted in the abdomen; a second thermocouple recorded cage ambient temperature (22–24 °C). Temperature was measured at a frequency of 1 sample/min for up to 8 hours after the first infusion.
Table 1.
Summary of experimental protocols.
| Protocol # | Objective | Drug and dose | Delivery route | Tcore measurement |
Figure |
|---|---|---|---|---|---|
| Protocol 1 | Stable hypothermia by TRPV1 agonist | DHC 2–4 mg/kg | s.c. infusion through catheter | Implanted thermocouple | Figures 1–2 |
| Protocol 2 | Repeated TRPV1 activation | DHC 1.25 mg/kg | s.c. bolus | Telemetry | Figure 3 |
| Protocol 3 | Young vs aged mice | DHC 1.25 mg/kg | s.c. bolus | Telemetry | Figure 4 |
| Protocol 4 | TRPM8 inhibition at cold (8°C) | Compound 5 20 mg/kg | s.c. bolus | Telemetry | Figure 5 |
| Protocol 5 | Combined TRPV1 activation and TRPM8 inhibition | Compound 5 20 mg/kg + DHC 0.6–2.5 mg/kg | s.c. bolus | Telemetry | Figure 6 |
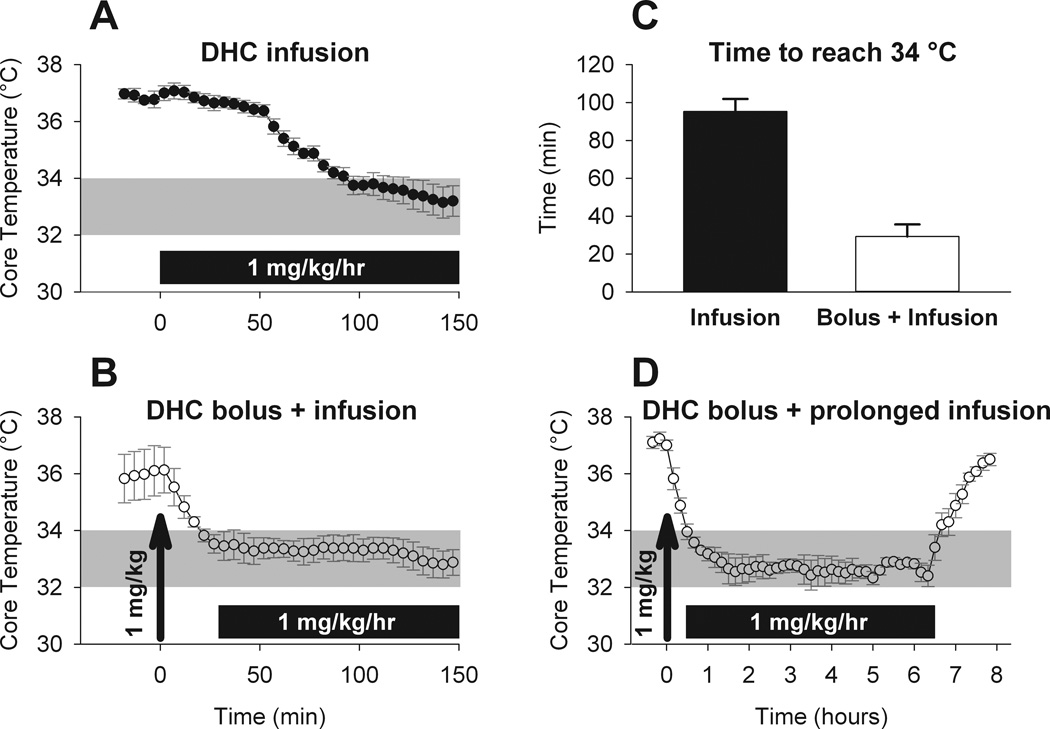
Figure 2. Dihydrocapsaicin bolus injection followed by continuous infusion leads to a rapid drop in core temperature that is stable over the duration of infusion.
A) Time course of core temperature in response to continuous infusion of dihydrocapsaicin (DHC; n = 4) or B) bolus injection (arrow) followed by continuous infusion (B) (n = 4). The bolus dose was 1 mg/kg and the continuous infusion dose was 1 mg/kg/hr. C) Summary of the time to reach the upper therapeutic range (34 °C) for each delivery method. D) The core temperature response to a 6 hour continuous infusion of dihydrocapsaicin (1 mg/kg/hr) following initial bolus dose (1 mg/kg; arrow) (n = 4). * P<0.001, t-test.
In protocol 2 we determined the desensitization of the hypothermic effect of TRPV1 agonists. Individual mice were injected with a single bolus of DHC (1.25 mg/kg s.c.) on four consecutive days (Figure 3). Responses from five mice were averaged to produce a group response from each day. Temperature was measured by telemeter in 10 minute intervals.
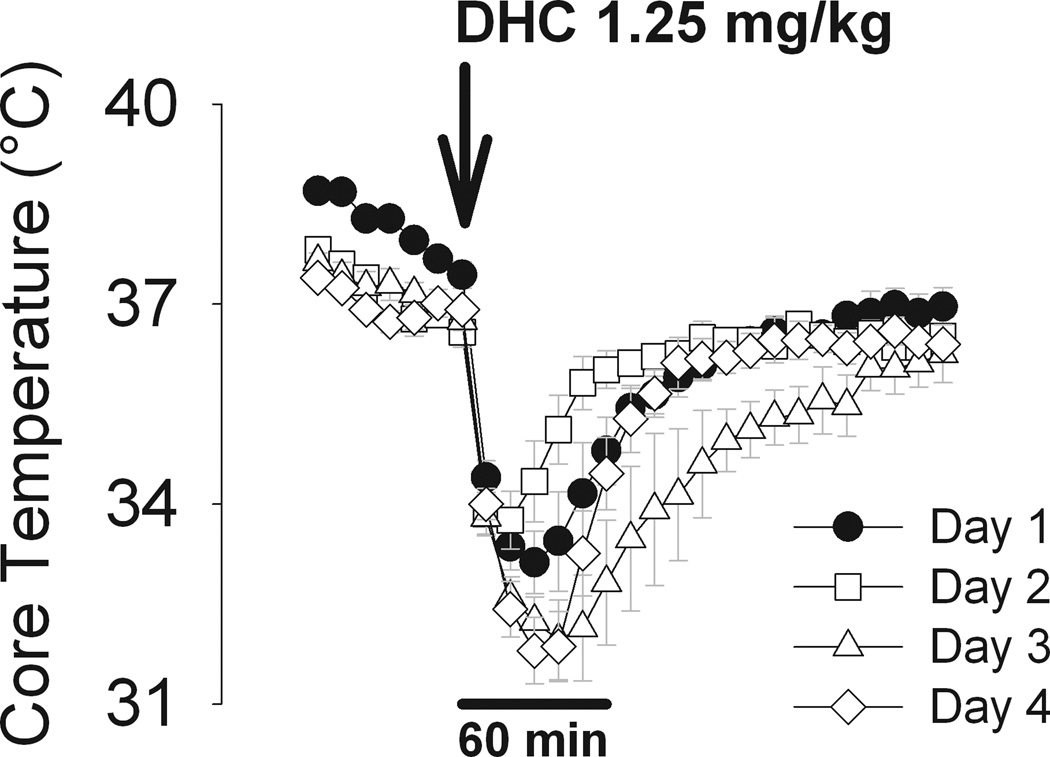
Figure 3. Dihydrocapsaicin-mediated hypothermia does not desensitize over multiple treatments.
Core temperature in response to dihydrocapsaicin (DHC; 1.25 mg/kg i.p.) delivered to the same mice (n=5) on 4 successive days. Mice were at ambient temperature of 24 °C during each experiment.
Protocol 3 was used to determine the effectiveness of TRPV1 agonists in aged subjects. Young (9–10 weeks) or aged (24 months) mice were injected with a single bolus of DHC (1.25 mg/kg s.c.). Temperature was measured by telemeter for 2 hours after injection (Figure 4).
In protocol 4, used to determine the hypothermic effect of TRPM8 inhibition in mildly subneutral and cold environments, “compound 5” (20 mg/kg s.c.) or vehicle was administered to telemeter implanted mice (Figure 5). “Compound 5” is a selective TRPM8 inhibitor (see Drugs section). Core temperature was recorded for 30 minutes starting 30 minutes after the injection, at which point the mice were transferred to a cold room for 2 hours and measured at 20 minute intervals. The individual cages were fitted with a plastic mesh top to allow heat exchange with the atmosphere. Temperature in the cage averaged 8 °C.
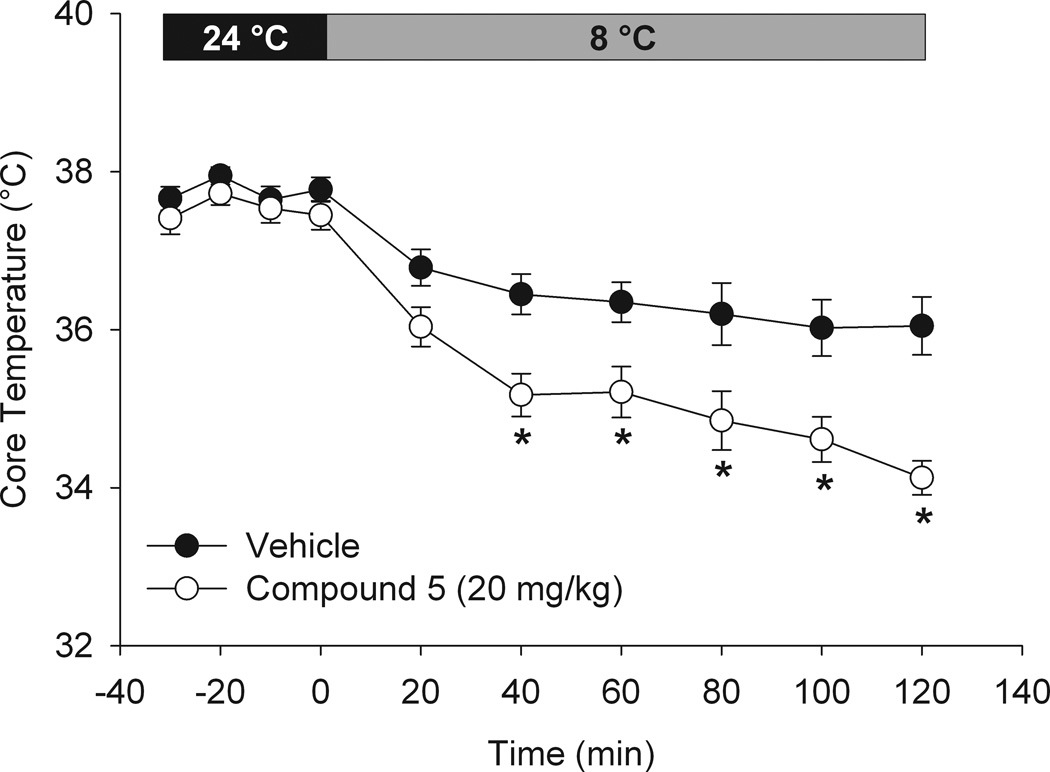
Figure 5. Treatment with TRPM8 inhibitor “compound 5” attenuates the cold-defense response.
Core temperature in mice exposed to cold environment (8 °C) for 2 hours with or without TRPM8 inhibitor “compound 5” (20 mg/kg s.c.). Mice were treated with comp 5 (open circles) or vehicle (filled circles) 60 minutes prior to transfer to cold environment (n = 8, each). * P<0.05, two-way RM-ANOVA with Holm-Sidak test for for multiple pairwise comparisons.
Protocol 5 was used to determine if TRPM8 inhibition potentiates the hypothermic effect of TRPV1 agonists. TRPM8 antagonist “compound 5” (30 mg/kg i.p.) or vehicle was injected in mice with implanted telemeter (Figure 6). After 60 minutes, DHC (0.6, 1.25, or 2.5 mg/kg i.p.) was injected in both groups. Temperature was recorded for 5.5 hours (330 minutes) from the time of “compound 5” or vehicle injection, with a measurement taken every 10 minutes. The depth of hypothermia was determined by calculating the minimum value (nadir) of Tcore with each treatment. The duration of hypothermia was determined by measuring the time from DHC injection until recovery of Tcore to ≥34 °C to the nearest 10 minutes. The 0.6 mg/kg DHC dose failed to reliably drop Tcore below 34 °C during the experiment and was therefore excluded from Tcore recovery analysis. In the two groups treated with higher doses of DHC and pretreated with “compound 5”, the Tcore occasionally failed to return to 34 °C within the 330 minute measurement period. In these cases (6 out of 16 mice), the maximum time value (330 min) was assigned for the recovery time. All mice, however, fully recovered Tcore by the next morning. In addition, in a single mouse in the vehicle group treated with 2.5 mg/kg DHC, Tcore did not drop below 34 °C. This mouse was assigned the minimum time value (0 minutes) for the recovery time.
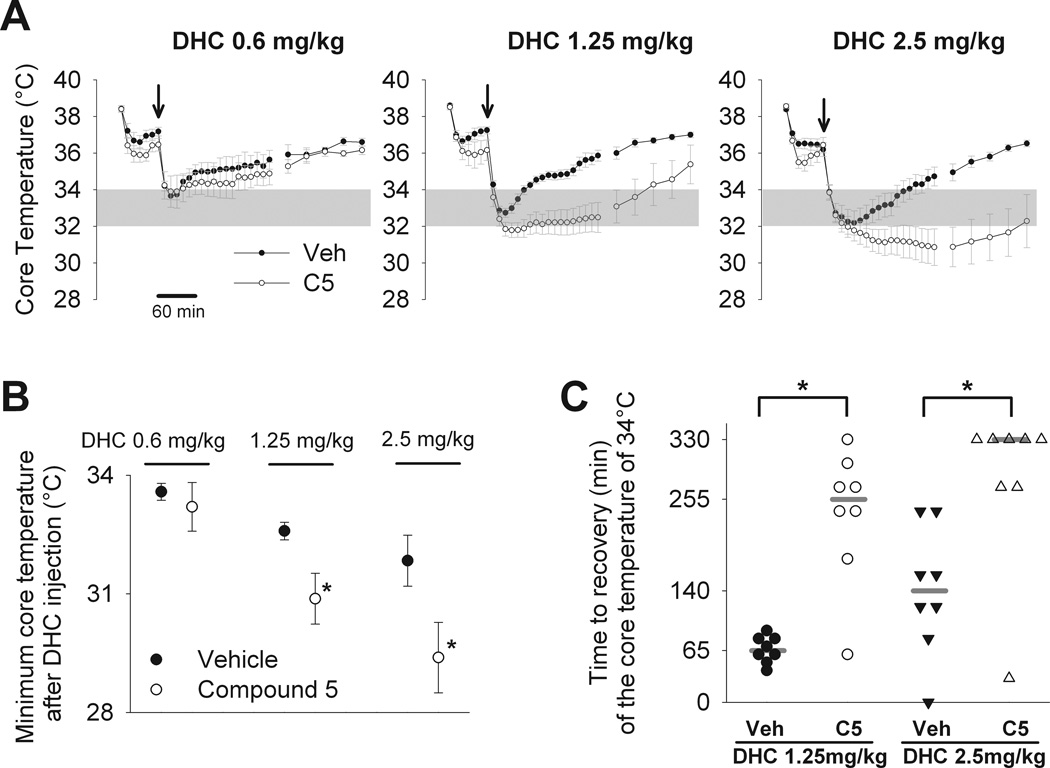
Figure 6. Inhibition of TRPM8 with “compound 5” potentiates and prolongs the hypothermic effect of TRPV1 activation by dihydrocapsaicin.
A) Mice were pretreated with TRPM8 inhibitor “compound 5”, 30 mg/kg i.p. (C5) or vehicle (Veh) 60 minutes prior to dihydrocapsaicin (DHC) injection. At time 0, mice were injected with dihydrocapsaicin 0.6, 1.25, and 2.5 mg/kg i.p. (arrow). Core temperature was measured for 5.5 hours (330 minutes) after dihydrocapsaicin injection. N=8 mice in each group. B) The minimum core temperature reached within the measurement period in each of the groups from experiment shown in A. * P<0.05 for vehicle versus ”compound 5” for the same dose of dihydrocapsaicin, Mann-Whitney rank sum test (for 1.25 mg/kg dihydrocapsaicin) or un-paired t-test (for 2.5 mg/kg dihydrocapsaicin). C) Time from the dihydrocapsaicin injection until core temperature recovered to 34 °C. Time was measured to the nearest 10 minutes. The graph shows individual values for each subject (symbols) and median values for each group (grey horizontal bar). Some mice did not recover the temperature of 34 °C within the measurement period (330 minutes) and were assigned the maximum possible value for the recovery time (330 minutes). A single mouse did not reach the hypothermic temperature of 34 °C and was assigned the minimum possible value for the recovery time (0 minutes). * P<0.01 for vehicle versus “compound 5” for the same dose of DHC, Mann-Whitney rank sum test.
Statistical analysis
Data are expressed as mean±SEM. A t-test was used for single comparisons between two groups with normal distribution. For non-normally distributed data sets, a Mann-Whitney Rank Sum test was applied. The two-way RM-ANOVA was used for comparing groups with repeated measures, using the Holm-Sidak test for individual comparisons. Differences were considered significant at p<0.05.
Results
Our initial experiments focused on achieving rapid and stable hypothermia in conscious mice by selective TRPV1 channel activation with dihydrocapsaicin (DHC) (Protocol 1) (8). Figure 1B shows the resulting core temperature (Tcore) in response to subcutaneously delivered DHC (2–4 mg/kg). To examine the possibility of using bolus injections of DHC for prolonged hypothermic effect in this experiment, each dose was delivered one-half at 0 minutes and one-half at 30 minutes (indicated by the arrows). Ambient temperature was maintained at 24 °C throughout the experiment. DHC delivered at 2 mg/kg cumulative dose resulted in a Tcore within the target therapeutic range (32–34 °C), whereas 3 or 4 mg/kg produced an overshoot of the optimal range. Although the 2 mg/kg dose of DHC achieved the therapeutic target range, it only maintained temperature within that range for 70 minutes.
We employed a continuous infusion protocol to produce a more stable Tcore that could be maintained in the therapeutic range (Figure 2A). DHC was infused at 1 mg/kg/hr based on the dose required to produce a similar drop in Tcore in rats (8). Continuous infusion of DHC indeed produced a more stable drop in Tcore; however, it took 95±7 minutes to reach the upper limit of the therapeutic range (Figure 2C). In an effort to reach the therapeutic range more rapidly, we employed a protocol wherein a bolus dose (1 mg/kg) was delivered at 0 minutes, followed by continuous infusion (1 mg/kg/hr) beginning at 30 minutes (Figure 2B). Through this method, we were able to attain in 29±6 minutes the target temperature (Figure 2C) that was stably maintained in the desired range without overshoot.
In the course of these experiments, DHC infusion did not result in any outward signs of distress or discomfort, such as hunched posture, ruffled fur, vocalization or restlessness. The primary behavioral effect was reduced activity when core temperature dropped below approximately 34 °C. In these studies, the methods of DHC infusion did not induce any perceivable nocifensive behaviors.
In successful hypothermic protocols, low temperature was maintained over 12–24 hours and then gradually increased to normal levels (2, 3). Therefore, next we sought to determine whether we could achieve rapidly attained, long-lasting and reversible hypothermia in mice using pharmacological TRPV1 activation. Figure 2D shows the result of a 6-hour infusion of DHC, as in the protocol above. We achieved rapid drop to 34 °C and then maintained temperature within the 32 to 34 °C range for the duration of the six-hour continuous infusion (Figure 2D). Once infusion was stopped, Tcore gradually increased toward the initial baseline value. The time to increase from 34 to 36 °C was 45 ± 2 minutes (0.045 ± 0.002 °C/min), which was notably slower than the initial drop in temperature.
The hypothermic response to TRPV1 agonists has been shown to desensitize in response to high doses of agonists such as capsaicin and DHC (9, 23). We therefore examined the potential for DHC infusion at a dose of 1.25 mg/kg to produce hypothermia on four consecutive days (Protocol 2, Figure 3). We found no attenuation of the effect of DHC from the initial response on day 1.
In light of the greatest need for neuroprotective strategies in the elderly population (such as for stroke), an ideal therapy should be effective in a model of advanced age. We therefore sought to determine if DHC injection (1.25 mg/kg s.c. in bolus) was still effective in aged mice (24 mo) compared to young (9–10 wk) controls (Protocol 3, Figure 4). We found that the maximum drop in Tcore, as well as the duration of hypothermia, was actually greater in the aged group.
TRPM8 channels are one of the primary molecular sensory transducers of innocuous cold (12, 15). These channels become activated upon cooling (<28 °C) and are shown to contribute to the triggering of certain manifestations of the cold-defense response. We hypothesized that TRPM8 is activated during DHC-induced hypothermia and contributes to the recovery of the core temperature after cessation of the DHC action due to clearance. To test this hypothesis, we used a recently developed TRPM8 channel antagonist, “compound 5”, which was shown to effectively inhibit TRPM8-dependent responses in vivo in rats with peroral delivery and in mice with intraperitoneal delivery (18, 22). Because the effectiveness of this compound has not previously been reported with subcutaneous delivery in mouse, we performed a “wet-dog shakes” (WDS) assay for TRPM8 activity to determine the inhibition of TRPM8 by “compound 5”. The number of TRPM8-dependent WDS events in response to TRPM8 agonist icilin (3mg/kg; s.c) was determined in mice pre-treated with “compound 5” (20 mg/kg; s.c.) or vehicle 60 minutes prior to icilin. Pre-treatment with “compound 5” reduced the number of events from 10.3±2.4 to 0.3±0.3 (p=0.015, data not shown), which demonstrated effective in vivo inhibition of the TRPM8 channels in our mouse model.
Having established an effective dose of “compound 5”, we next examined the potential for pharmacological TRPM8 inhibition to facilitate lowering of Tcore in a cold ambient temperature (Protocol 4). In this experiment, mice were injected with “compound 5” (20 mg/kg, s.c.) or vehicle, after 60 min were transferred to a cold environment (8 °C) and maintained for 2 hours (Figure 5). Vehicle treated mice experienced a slight drop in Tcore but still maintained a core temperature above 36 °C. “Compound 5” treated mice had a significantly lower Tcore compared with vehicle treated mice by 40 minutes of cold exposure, which ultimately dropped to ~34 °C by the end of 2 hours. This result demonstrates that TRPM8 inhibition augments the drop in core temperature during external cooling by physical methods.
TRPM8 inhibitor “compound 5” was then evaluated for the potential to augment and/or prolong the drop in Tcore elicited by a bolus injection of DHC in mice housed at a mildly subneutral ambient temperature of 24 °C (Protocol 5). DHC was administered at three doses (0.6, 1.25, and 2.5 mg/kg i.p.) at time 0 to mice pretreated with either “compound 5” (30 mg/kg i.p.) or vehicle (Figure 6A). Note that “compound 5” treatment alone produced a slightly lower Tcore than vehicle (36.1 vs. 36.9 °C; n=21 each; Mann-Whitney Rank Sum Test, P=0.039), as measured 10 minutes prior to DHC infusion (data not shown), consistent with previous reports on TRPM8 antagonists (17, 33). Figure 6B summarizes the Tcore nadir for each dose of DHC following “compound 5” or vehicle pretreatment. Figure 6C presents individual and group median time from DHC injection until recovery of Tcore to ≥34 °C.
The hypothermic response following treatment with the lowest dose of DHC (0.6 mg/kg) was unaffected by “compound 5” pretreatment (the nadir of the hypothermic response: 33.6 ± 0.2°C in vehicle group versus 33.2 ± 0.6°C in “compound 5” group), whereas the two higher doses (1.25 and 2.5 mg/kg) demonstrated a significant potentiation of both maximum Tcore drop (the nadir of the hypothermic response with 1.25 mg/kg DHC: 32.6 ± 0.2°C in vehicle group versus 30.9 ± 0.6°C in “compound 5” group; with 2.5 mg/kg DHC: 31.8 ± 0.6°C in vehicle group versus 29.4 ± 0.9°C in “compound 5” group) and duration of Tcore in the therapeutic range (median values for the time to recovery of Tcore to ≥ 34°C with 1.25 mg/kg DHC: 65 minutes in vehicle group versus 255 minutes in “compound 5” group; with 2.5 mg/kg DHC: 330 minutes in vehicle group versus 140 minutes in “compound 5” group).
Discussion
Mild-to-moderate hypothermia (32–34 °C) has been shown to be effective in the treatment of cardiac arrest and management of intracranial hypertension following severe head injury in adults, as well as cerebral ischemia in newborns (1–3, 24, 25). In addition, hypothermia has shown promise in a number of animal models of neurological injury, including ischemic stroke (24). Despite this significant promise in animal models, large-scale controlled clinical trials have yet to be performed to evaluate the effectiveness of hypothermia for treatment of stroke in humans. Significant impediments to these trials may include unfavorable balance between potential benefit and the difficulty in implementation, clinical complications and the high cost currently associated with traditional cooling protocols (6, 26).
The most effective hypothermia protocol should be able to achieve target temperature quickly and maintain that temperature for at least 2–8 hours and up to 24–36 hours (27). In addition, because many brain injury patients, such as those with ischemic stroke, often arrive at the hospital conscious, it should ideally be compatible with the conscious spontaneously breathing patient. Most current hypothermia protocols, however, require strong sedatives and/or paralytics in order to combat the cold-defense response that is triggered in mammals when core temperature is lowered even slightly. This response involves shivering and non-shivering thermogenesis as well as heat conservation through cutaneous vasoconstriction (7, 28). These thermogenic processes result in such undesirable effects as increased ATP and O2 consumption, elevated catecholamine release, hyperglycemia, electrolyte and blood gas imbalances and severe pain. In the present study, we have demonstrated the potential for thermo-sensitive TRP channel modulators to produce rapid and sustained hypothermia in mice within the mild hypothermic range. Importantly, these methods do not require sedation or paralytics to blunt the cold-defense response and appear to be well tolerated in conscious mice. Small animals, such as mice, have a relatively low mass-to-surface area relationship and are therefore more susceptible to rapid heat loss and hypothermia. However, cooling of larger animals, such as monkey and calf, by TRPV1 channel agonists has recently been performed (8), demonstrating the potential for this method in larger mammals such as humans.
In our experiments, subcutaneous and intraperitoneal DHC appeared to be effective and well tolerated in mice at mild hypothermia-inducing doses, with reduced activity being the main observable change in behavior. In a separate study, intravenously-delivered DHC, at a similar effective dose in rat (0.75 mg/kg/hr), was also shown to be well tolerated (8). It should be noted that at higher doses (2.2–3 mg/kg/hr), intravenous DHC produced transient episodes (<2 minutes) of hypotension, bradycardia and ECG alterations. Although the doses that produced cardiovascular alterations were at least three times greater than the dose required for mild hypothermia (8), it will still be important to further examine exposure limits and delivery routes of TRPV1 agonists in order to avoid undesirable cardiovascular responses.
Because capsaicin and other TRPV1 agonists are known to produce desensitization with chronic or repeated high dose delivery (9, 23, 29, 30), we were concerned that the hypothermic response may significantly diminish over time or after successive administrations. However, the response to DHC did not show appreciable diminution over a six-hour continuous infusion period, in contrast to several other TRPV1 agonists that have shown substantially decreased effectiveness after 2 or 3 hours of continuous infusion (8). We also demonstrated that the hypothermic effect of DHC was not lost after repeated exposures on successive days in the same mice (5 mg/kg cumulative dose).
Given that a number of neurological pathologies are associated with advanced age, it is critical that methods of pharmacologically induced hypothermia be compatible with an aged population. Aged mice have reduced TRPV1 expression in the dorsal root ganglion and peripheral nerves (31) and have greater nonshivering thermogenesis capacity compared with younger adult animals (32). Surprisingly, when we evaluated DHC-mediated hypothermia in the aged mice, we found that they actually had a greater response to DHC, with significant prolongation of the hypothermic period. This may be explained by reduced capacity for heat production through shivering (33), reduced metabolic rate in aging (34) or age-dependent differences in drug metabolism or clearance. Regardless of the cause, the above findings indicate that DHC-mediated hypothermia can be used repeatedly without significant loss of efficacy and is very effective in both young and aged mice.
One hypothetical strategy to reduce the therapeutically effective dose of TRPV1 agonists is to combine these agonists with TRPM8 antagonists. We therefore examined the potential of TRPM8 channel inhibition to either produce hypothermia by itself or to augment hypothermia produced by a TRPV1 agonist. TRPM8 is a cold-sensitive (<28°C) ion channel, found in cold-sensitive nerve fibers located in the skin and viscera (11). TRPM8 channels are involved in the perception of cold (12, 15) and portions of the autonomic cold-defense response, including brown adipose tissue activation and cutaneous vasoconstriction (16). Because ambient temperature during normal housing conditions is below the thermoneutral zone (30–34 °C) in mice (35), it is likely that a fraction of the TRPM8 channels located in superficial regions, such as the skin, are active and contribute to thermoregulatory balance at room temperature. We thus predicted that selective inhibition of TRPM8 channels would result in reduced activity along the cold-defense response pathways and thereby promote a decrease in Tcore. In our study, TRPM8 inhibitor “compound 5” resulted in a very slight drop in Tcore (0.8 °C) at a dose of 30 mg/kg for mice maintained at room temperature (24 °C), which is consistent with the hypothermic effect of other recently developed TRPM8 blockers (19, 36). TRPM8 inhibition in a cold environment (8 °C) still only produced a ~2 °C drop in Tcore after a 2-hour exposure. Taken together, these findings support the recently proposed idea that that although TRPM8 inhibition results in a mild drop in core temperature, it still leaves a substantial capacity to maintain Tcore in mild to severe cold environment through shivering thermogenesis (22). Therefore, the potential for TRPM8 inhibition alone to produce hypothermia of therapeutic value (even in combination with external cooling) appears to be quite low.
In light of the limited potential of TRPM8 antagonists to promote clinically relevant hypothermia as a single agent, we sought to determine whether “compound 5” could be used as an adjuvant in DHC-mediated hypothermia. We reasoned that TRPM8 channels, located in skin nerve fibers and/or deeper structures such as spinal cord, could become activated as a result of DHC-mediated hypothermia, thereby triggering some portion of the cold-defense response and attenuating or abbreviating the drop in Tcore. If this possibility is valid, blocking the TRPM8 channels should permit a greater or longer-lasting drop in Tcore. To test this hypothesis, we administered bolus doses of DHC with or without pretreatment with “compound 5” and examined the depth and duration of hypothermia. With TRPM8 inhibition, the hypothermic response to DHC was significantly deeper and more prolonged. These data suggest a potentiating effect of TRPM8 channel inhibition on TRPV1-agonist-mediated drop of Tcore. Indeed, in the highest DHC dose group, pre-treatment with “compound 5” significantly extended the time from DHC injection until recovery of Tcore to ≥34 °C to >5.5 hours from a single bolus administration of DHC. Therefore, whereas pharmacological TRPM8 inhibition may not be a useful method to produce hypothermia by itself, it does appear to be an effective method to potentiate and prolong the effect of TRPV1 activation thereby reducing the required dose of TRPV1 agonist. As mentioned above, we propose the following mechanism for the potentiating effect of TRPV1 activation and TRPM8 inhibition: as the core temperature decreases following DHC administration, so does the temperature of deep (spinal cord) and superficial structures (skin) containing TRPM8 channels; TRPM8 channels are thus activated by this lowered temperature and recruit some level of compensatory thermogenesis (most likely in brown adipose tissue), which reduces the amplitude and duration of the DHC-induced hypothermia. However, the direct confirmation of this mechanism requires further study.
Conclusions
In summary, we present methods for producing pharmacological hypothermia in mice via TRPV1 channel activation to promote a rapid and sustainable drop in Tcore. These methods do not require heavy sedation or muscle paralytics to prevent the cold-defense response and are therefore compatible with the conscious, spontaneously breathing subject. Furthermore, these methods were effective with application on successive occasions and in a model of aging. Lastly, we demonstrate that TRPM8 inhibition alone was insufficient to produce hypothermia of therapeutic value. However, TRPM8 inhibition potentiated the TRPV1 agonist-mediated response to promote a deeper and more sustained hypothermia. Therefore, we propose that combined use of TRPM8 inhibitors and TRPV1 agonists may promote more effective hypothermia in conscious subjects with greatly reduced physiological stress associated with traditional physical cooling methods.
Acknowledgements
We would like to thank Dr. Sloan Youngblood for critically reading the manuscript.
Drs. Marrelli, Cao, and Balasubramanian received support for article research from NIH (R01 HL088435, R21 NS077413, AHA #13PRE16900006 (for support of June Cao only). Their institutions received grant support (R01 HL088435, R21 NS077413, AHA #13PRE16900006 (for support of June Cao only). Dr. Feketa disclosed receiving the pharmacological TRPM8 blocker ("compound 5") at no charge from Janssen Research and Development and received support for article research from NIH. His institution received grant support from NIH funding (the research in laboratory is funded by grants from NIH (NS077413 and HL088435).
Footnotes
Copyright Form Disclosures:
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Nolan JP, Morley PT, Vanden Hoek TL, et al. Therapeutic hypothermia after cardiac arrest: An advisory statement by the advanced life support task force of the international liaison committee on resuscitation. Circulation. 2003;108:118–121. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 2.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 3.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 4.Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med. 2010;363:1256–1264. doi: 10.1056/NEJMct1002402. [DOI] [PubMed] [Google Scholar]
- 5.Lay C, Badjatia N. Therapeutic hypothermia after cardiac arrest. Curr Atheroscler Rep. 2010;12:336–342. doi: 10.1007/s11883-010-0119-2. [DOI] [PubMed] [Google Scholar]
- 6.Seif D, Henderson SO. Use of therapeutic hypothermia in postcardiac arrest patients by emergency departments. Therapeutic Hypothermia and Temperature Management. 2011;1:23. doi: 10.1089/ther.2010.0004. [DOI] [PubMed] [Google Scholar]
- 7.Romanovsky AA, Almeida MC, Garami A, et al. The transient receptor potential vanilloid-1 channel in thermoregulation: A thermosensor it is not. 2009 doi: 10.1124/pr.109.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fosgerau K, Weber UJ, Gotfredsen JW, et al. Drug-induced mild therapeutic hypothermia obtained by administration of a transient receptor potential vanilloid type 1 agonist. 2010 doi: 10.1186/1471-2261-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MS, Brendel K, Buck SH, et al. Dihydrocapsaicin-induced hypothermia and substance P depletion. 1982 doi: 10.1016/0014-2999(82)90263-1. [DOI] [PubMed] [Google Scholar]
- 10.Badjatia N, Strongilis E, Prescutti M, et al. Metabolic benefits of surface counter warming during therapeutic temperature modulation. Crit Care Med. 2009;37:1893–1897. doi: 10.1097/CCM.0b013e31819fffd3. [DOI] [PubMed] [Google Scholar]
- 11.Takashima Y, Daniels RL, Knowlton W, et al. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. 2007 doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. 2002 doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 13.Voets T, Droogmans G, Wissenbach U, et al. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. 2004 doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 14.Madrid R, Donovan-Rodriguez T, Meseguer V, et al. Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. 2006 doi: 10.1523/JNEUROSCI.3752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bautista DM, Siemens J, Glazer JM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. 2007 doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 16.Tajino K, Hosokawa H, Maegawa S, et al. Cooling-sensitive TRPM8 is thermostat of skin temperature against cooling. PLoS One. 2011;6:e17504. doi: 10.1371/journal.pone.0017504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon CJ. Thermal physiology of laboratory mice: Defining thermoneutrality. J Thermal Biology. 2012;37:654–685. [Google Scholar]
- 18.Parks DJ, Parsons WH, Colburn RW, et al. Design and optimization of benzimidazole-containing transient receptor potential melastatin 8 (TRPM8) antagonists. 2011 doi: 10.1021/jm101075v. [DOI] [PubMed] [Google Scholar]
- 19.Knowlton WM, Daniels RL, Palkar R, et al. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS One. 2011;6:e25894. doi: 10.1371/journal.pone.0025894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colburn RW, Lubin ML, Stone DJ, Jr, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Dhaka A, Murray AN, Mathur J, et al. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Feketa VV, Balasubramanian A, Flores CM, et al. Shivering and tachycardic responses to external cooling in mice are substantially suppressed by TRPV1 activation but not by TRPM8 inhibition. Am J Physiol Regul Integr Comp Physiol. 2013 doi: 10.1152/ajpregu.00296.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jancso-Gabor A, Szolcsanyi J, Jancso N. Irreversible impairment of thermoregulation induced by capsaicin and similar pungent substances in rats and guinea-pigs. J Physiol. 1970;206:495–507. doi: 10.1113/jphysiol.1970.sp009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs S, Hunt R, Tarnow-Mordi W, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2007;(4) doi: 10.1002/14651858.CD003311.pub2. CD003311. [DOI] [PubMed] [Google Scholar]
- 25.Polderman KH, Tjong Tjin Joe R, Peerdeman SM, et al. Effects of therapeutic hypothermia on intracranial pressure and outcome in patients with severe head injury. Intensive Care Med. 2002;28:1563–1573. doi: 10.1007/s00134-002-1511-3. [DOI] [PubMed] [Google Scholar]
- 26.Zgavc T, Ceulemans AG, Sarre S, et al. Experimental and clinical use of therapeutic hypothermia for ischemic stroke: Opportunities and limitations. Stroke Res Treat. 2011;2011:689290. doi: 10.4061/2011/689290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Worp HB, Sena ES, Donnan GA, et al. Hypothermia in animal models of acute ischaemic stroke: A systematic review and meta-analysis. Brain. 2007;130:3063–3074. doi: 10.1093/brain/awm083. [DOI] [PubMed] [Google Scholar]
- 28.Morrison SF. 2010 carl ludwig distinguished lectureship of the APS neural control and autonomic regulation section: Central neural pathways for thermoregulatory cold defense. J Appl Physiol. 2011;110:1137–1149. doi: 10.1152/japplphysiol.01227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hori T, Tsuzuki S. Thermoregulation in adult rats which have been treated with capsaicin as neonates. Pflugers Arch. 1981;390:219–223. doi: 10.1007/BF00658265. [DOI] [PubMed] [Google Scholar]
- 30.Szallasi A, Joo F, Blumberg PM. Duration of desensitization and ultrastructural changes in dorsal root ganglia in rats treated with resiniferatoxin, an ultrapotent capsaicin analog. Brain Res. 1989;503:68–72. doi: 10.1016/0006-8993(89)91705-8. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Davis BM, Zwick M, et al. Reduced thermal sensitivity and Nav1.8 and TRPV1 channel expression in sensory neurons of aged mice. Neurobiol Aging. 2006;27:895–903. doi: 10.1016/j.neurobiolaging.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talan MI, Kirov SA, Kosheleva NA. Nonshivering thermogenesis in adult and aged C57BL/6J mice housed at 22 degrees C and at 29 degrees C. Exp Gerontol. 1996;31:687–698. doi: 10.1016/s0531-5565(96)00095-2. [DOI] [PubMed] [Google Scholar]
- 33.Lee TF, Wang LC. Improving cold tolerance in elderly rats by aminophylline. Life Sci. 1985;36:2025–2032. doi: 10.1016/0024-3205(85)90452-7. [DOI] [PubMed] [Google Scholar]
- 34.Marcinek DJ, Schenkman KA, Ciesielski WA, et al. Reduced mitochondrial coupling in vivo alters cellular energetics in aged mouse skeletal muscle. J Physiol. 2005;569:467–473. doi: 10.1113/jphysiol.2005.097782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garami A, Pakai E, Oliveira DL, et al. Thermoregulatory phenotype of the Trpv1 knockout mouse: Thermoeffector dysbalance with hyperkinesis. J Neurosci. 2011;31:1721–1733. doi: 10.1523/JNEUROSCI.4671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Almeida MC, Hew-Butler T, Soriano RN, et al. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci. 2012;32:2086–2099. doi: 10.1523/JNEUROSCI.5606-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]