Summary
Background
Gemtuzumab Ozogamicin (GO) was the first example of antibody directed chemotherapy in cancer and developed for Acute Myeloid Leukaemia. Its role has been unclear. Five randomised trials where it was combined with standard induction chemotherapy in adults have produced different results. In an effort to clarify the level of benefit, if any, and in which patients outcomes might be improved, an individual patient data meta-analysis of these 5 trials has been undertaken.
Methods
All randomised trials of GO in adults (age >15), given in conjunction with the first course of intensive induction chemotherapy for AML (excluding APL) were identified. In a collaboration between the groups involved, source data concerning demographics, treatment was requested in May 2013 and collected in 3325 randomised patients (median age 58). All trials were centrally randomised and open-label, with survival as primary endpoint. Analyses are presented by standard techniques, and within standardised risk groups
Results
Remission rates were not increased, but by significantly reducing the risk of relapse overall survival at 5 years was improved irrespective of patient age (30.7% vs 34.6%; HR 0.90 (95% CI 0.82-0.98), p=0.01). The survival benefit was particularly clear in those with favourable cytogenetics (55.2% vs 76.3%; HR0.47 (0.31-0.73), p=0.0005), but also observed in intermediate risk patients (34.1% vs 39.4%; HR 0.84 (0.75-0.95), p=0.007) Patients with adverse karyotype did not benefit overall or within any trial. Dose levels of 3mg/m2 were associated with less toxicity and equal efficacy.
Interpretation
GO can be safely added to conventional induction therapy. For patients who do not have adverse cytogenetics there is a significant survival benefit. These data suggest that the use of GO should be re-evaluated and the license status of GO may need to be reviewed.
Role of funding source
There was no funder for this meta-analysis.
Introduction
The development of treatments for AML to gain regulatory approval has been elusive. One of the few successes was the immuno-conjugate, gemtuzumab ozogamicin (MylotargTM, Pfizer Inc, New York, NY USA) which gained approval in the United States in 2000 based on unrandomised phase 2 data conducted in 142 patients with relapsed disease1,2. The label restricted approval to “older patients with relapse who were not suitable for intensive treatment”. A confirmatory randomised trial was required. Approval in Japan followed for the same indication. Here, the schedule was a single dose on days 1 and15 of 9mg/m2. Combining this dose with chemotherapy was associated with important toxicity3, but a dose-finding study in combination with frequently used chemotherapy combinations in induction and consolidation provided evidence that a single 3mg/m2 dose was safe and apparently effective4. This study was the prelude to a randomised trial where GO would be added to each course of chemotherapy or not. Feasibility was established in combination with courses 1 and 3. This pilot was the precursor of two large trials where GO 3mg/m2 was added to induction in younger and older patients (UK MRC AML155 and UK NCRI AML166). The RCT to support regulatory approval in the US was conducted by the South West Oncology Group (SWOG-01067). Here, GO (6mg/m2) was given on day 4 of a traditional “3+7” (Daunorubicin/Ara-C) induction where the Daunorubicin dose in the GO arm was reduced to 45mg/m2 compared with 60mg/m2 in the control arm. The French GOELAMS Group adopted a similar design to the SWOG group except that GO, given on day 4, was combined with Daunorubicin at a dose of 60mg/m2 (GOELAMS AML2006IR8). In a further development by the French ALFA Group GO administration was fractionated (3mg/m2 on days 1,4,7 of a DA combination with each GO dose limited to a maximum of 5mg9). This was intended to take advantage of CD33 re-expression which occurred after initial exposure10. This proved to be feasible and encouraging in relapsed disease, leading to the frontline trial (ALFA-070111) in which patients received fractionated GO in induction and consolidation.
The overall results of these trials were that remission rates were not improved, although relapse was reduced in 4 of 5 trials with a significant survival benefit in two, AML16 and ALFA-0701. Furthermore, all trials suggested that there was a benefit in well-recognised cytogenetic risk groups with a consistent absence of benefit in adverse risk patients across all trials. Unfortunately the pivotal SWOG-0106 trial was prematurely terminated by the Data Monitoring Committee because of a significant excess of early mortality (17/295 (6%) vs 4/300 (1%)), which was not counterbalanced by a later benefit. It is noticeable that in the control arm early mortality was exceptionally low, and mortality in the GO arm was as expected with conventional treatments. This result was the most influential, because of its registration status, and resulted in GO being withdrawn from the US market in June 2010.
In view of the experience in the other larger trials it has been suggested that the approval status should be reviewed. Ultimately the issue as to whether GO provides overall benefit with acceptable early mortality remains unclear. So too, does the optimal dose and schedule. To gain insight, we performed an individual patient data (IPD) meta-analysis of the five trials in adults which simultaneously combined GO with standard induction chemotherapy.
An IPD meta-analysis has several advantages over one using published data. Up-to-date data can be included (e.g. updating follow-up beyond the point of the original publication). Similar coding systems can be applied (e.g. for cytogenetics), and risk groups can be explored in a standard format. Importantly, the question of which patients may derive benefit, or differences between dosing schedules, can be explored using stratified analyses and interaction tests. It is important that patients are compared within trials, i.e. only within randomised comparison; the analyses for each trial are then combined.
Patients and Methods
All trials with data available as of May 1 2013 were identified. Trials were identified subject to the following eligibility criteria:
An unconfounded comparison of GO in course 1 of induction chemotherapy (i.e. Chemo plus GO vs Chemo), excluding trials where GO was used in place of part of a chemotherapy regimen, before chemotherapy, or only in consolidation.
Patients with newly diagnosed AML (either de Novo or Secondary) or high risk MDS, excluding acute promyelocytic leukaemia.
An intensive induction chemotherapy regimen, designed to induce complete remission in patients. Trials involving less intensive regimens such as low-dose Ara-C were excluded
Patients had to be aged 15 or older.
The group identified and contacted all collaborative groups who had run such a trial, and requested data on baseline characteristics, including age, sex, chemotherapy given, cytogenetics, and FLT-3 ITD and NPM1 mutation status, together with dates of entry, first complete remission, transplant, death and relapse. All five groups responded in the affirmative. Data provided was the most up-to-date information available.
Cytogenetics were initially coded according to each group's individual criteria. As there are some differences between different coding systems, the karyotype was requested for all patients and coding performed according to the revised MRC classification12. A minimum of 20 metaphases were required to ascertain a normal karyotype.
Endpoints were defined according to Revised International Working Group Criteria13, with the exception that peripheral count recovery was not required for complete remission. Data were analysed using standard meta-analytic techniques14 using an assumption free (or fixed-effect) methodology. Comparisons were made within trial, and o-e, V statistics obtained for each trial (or each stratum within each trial). The overall (o-e), V (and hence effect sizes and confidence intervals), were calculated as the sum over all trials. To investigate interactions between baseline parameters and treatment effectiveness, stratified analyses were performed, with interactions assessed by means of standard heterogeneity and trend tests. Survival curves were created using the methodology of the EBCTCG, where (o-e) and V statistics and the number of person years at risk during a given time period are combined to produce a survival curve adjusted for any disparities between arms across trials. Analysis of time-to-event outcomes was by the logrank test, giving rise to Peto odds ratios; for binary outcomes, the Mantel-Haenszel test was used. In all cases, significance was set at p<0.05 and overall results given with 95% confidence intervals. Analyses were performed using SAS Version 9.3.
Role of the Funding Source
There was no funding source for this meta-analysis. The corresponding author had full access to all data and the final responsibility to submit for publication.
Results
A total of five trials comprising 3325 randomised patients were identified (Table 1). The ages of patients ranged from 15 to 84 (median 58); 1842/3325 (55%) were male; 2927/3325 (88%) had de Novo disease, 285 (9%) secondary and 113 (3%) high risk MDS. (Only two trials, MRC AML15 and NCRI AML16 allowed secondary AML patients; only AML16 recruited patients with high risk MDS, defined as >10% marrow blasts at diagnosis). NPM1 mutation data was available on 1370 patients, of whom 398 (29%) had an NPM1 mutation; 354/1802 (20%) patients were FLT3-ITD mutant. Median follow-up was 60.8 months (range 0.5-125.4); in the entire cohort the remission rate was 78% (2589/3324), with median survival of 22.5 months and pooled 5-year survival of 34% (total 2108 deaths). A total of 785 patients underwent transplantation, 90 before remission, 457 in first CR and 238 post 1st CR. In addition to the four published trials, source data were made available for the GOELAMS AML2006IR trial of intermediate risk patients. Details of the trial designs and treatment schedules are given in Table 1. Briefly, a variety of induction schedules were used, although the majority of patients were treated with an anthracycline plus Ara-C combination. GO was given variously at 3mg/m2 on day 1 in 2 trials (UK MRC15, UK NCRI AML16), 6mg/m2 on day 4 in two trials (GOELAMS AML2006IR and SWOG 0106), and at 3mg/m2 (capped at 5mg per dose) on days 1,4,7 in one trial (ALFA-0701). Analyses were therefore stratified by GO schedule, and chemotherapy schedule was included as a stratification variable to see whether the effect of GO varied by induction regimen.
Table 1.
Details of Trials Included in the Meta-Analysis – 14 patients in AML15 aged <15 years are excluded from this meta-analysis. All trials excluded APL, and required no previous treatment.
| Trial | Dates of recruitment |
Number of patients included |
Eligibility criteria |
Median age of included patients (range) |
Cytogenetic grouping (MRC 2009)* |
Chemotherapy given |
Dose/schedule of GO |
Median follow- up for survival |
Last follow- up (publication) |
Last follow- up (meta- analysis) |
|---|---|---|---|---|---|---|---|---|---|---|
| MRC AML15 | 2002-2006 | 1099 | AML, either de Novo or secondary. Usually aged <60 | 50 (15-71) | Fav: 133(15%) Int: 565 (63%) Adv: 196 (22%) Unk: 205 |
DA (3+10, 3+8) or ADE (3+10+5, 3+8+5) or FLAG-Ida | 3mg/m2 on day 1 of chemotherapy | 86.0 months | January 2009 | March 2013 |
| SWOG-0106 | 2004-2009 | 595 | De Novo AML. Aged 18-60 | 47 (18-60) | Fav: 72 (17%) Int: 283 (67%) Adv: 67 (16%) Unk: 173 |
DA (3+7) + G-CSF/GM-CSF | 6mg/m2 on day 4 of chemotherapy | 55.2 months | February 2013 | June 2013 |
| NCRI AML16 | 2006-2010 | 1115 | AML, either de Novo or Secondary or high risk MDS. Usually aged 60+ | 67 (51-84) | Fav: 33(4%) Int: 576 (66%) Adv: 264 (30%) Unk: 242 |
DA (3+10, 3+8) or Daunorubicin (d,1,3, 5)/Clofarabine (d 1-5) | 3mg/m2 on day 1 of chemotherapy | 45.5 months | July 2011 | March 2013 |
| GOELAMS AML2006IR | 2007-2010 | 238 | De Novo AML, aged 18-60 | 50.5 (18-60) | Fav: 0 (0%) Int: 224 (100%) Adv: 0 (0%) Unk: 14 |
DA (3+7) | 6mg/m2 on day 4 of chemotherapy | 39.3 months | unpublished | January 2013 |
| ALFA-0701 | 2008-2010 | 278 | De Novo AML aged 50-70 | 62 (50-70) | Fav: 9 (4%) Int: 179 (73%) Adv: 57 (23%) Unk: 33 |
DA (3+7) | 3mg/m2 d 1,4,7 up to 5mg per dose | 24.1 months | August 2011 | August 2011 |
proportions exclude those with unknown or undetermined cytogenetics. All trials were open-label, centrally randomised and had OS as primary endpoint – thus viewed as at low risk of bias.
Remission and Early Mortality
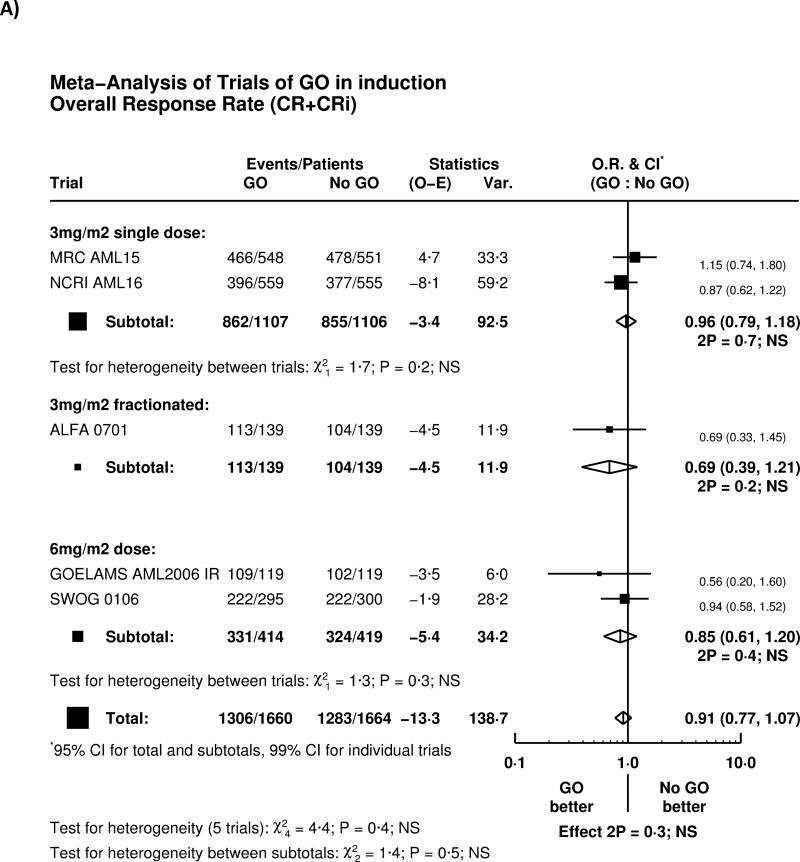
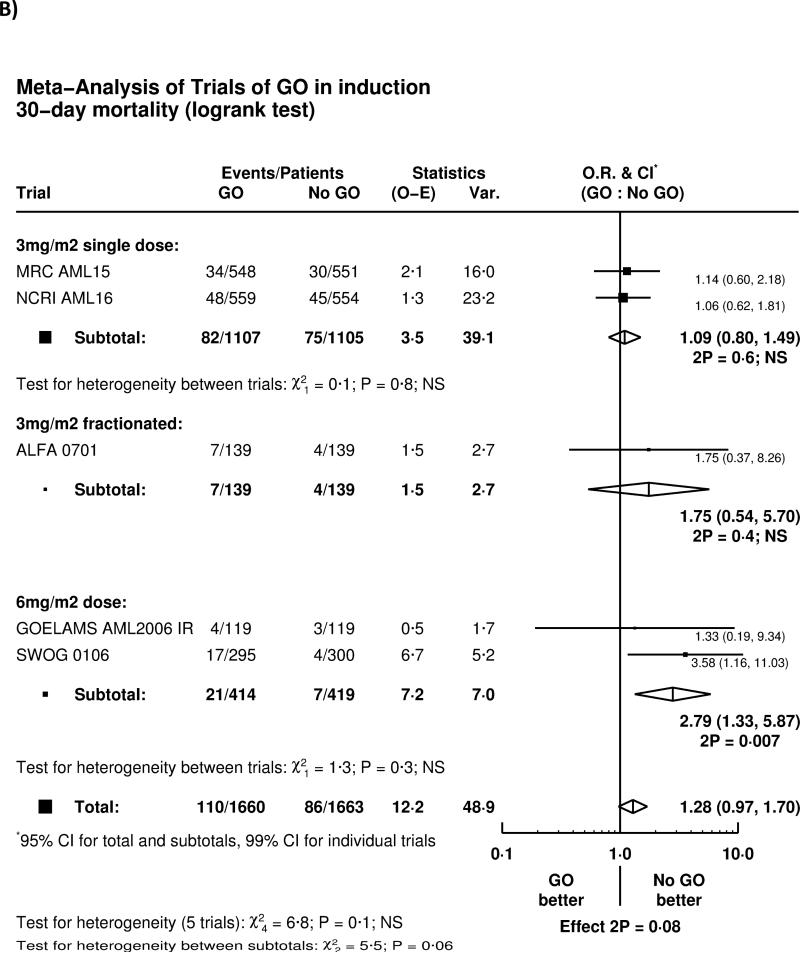
Overall, there was no significant effect of GO on complete remission rate (OR 0.91 (0.77-1.07) p=0.3), with no heterogeneity by either trial or GO administration schedule (Figure 1A). There was a trend for higher 30-day mortality (HR 1.28 (0.97-1.70) p=0.08), with some evidence of heterogeneity between different dosing regimens of GO (Figure 1B). Greater early mortality was seen among patients given GO at 6mg/m2 (p-value for heterogeneity 6mg vs 3mg p=0.03); the result of the SWOG-0106 study differed from all the other randomised trials (p=0.01). When the SWOG-0106 trial was excluded, the hazard ratio for 30-day mortality was 1.13 (0.84-1.53) p=0.4, meaning that there is no evidence of harm in the remaining 2728 patients.
Figure 1.
Effect of GO on A) Remission and B) 30-day mortality
Relapse and Deaths in Remission
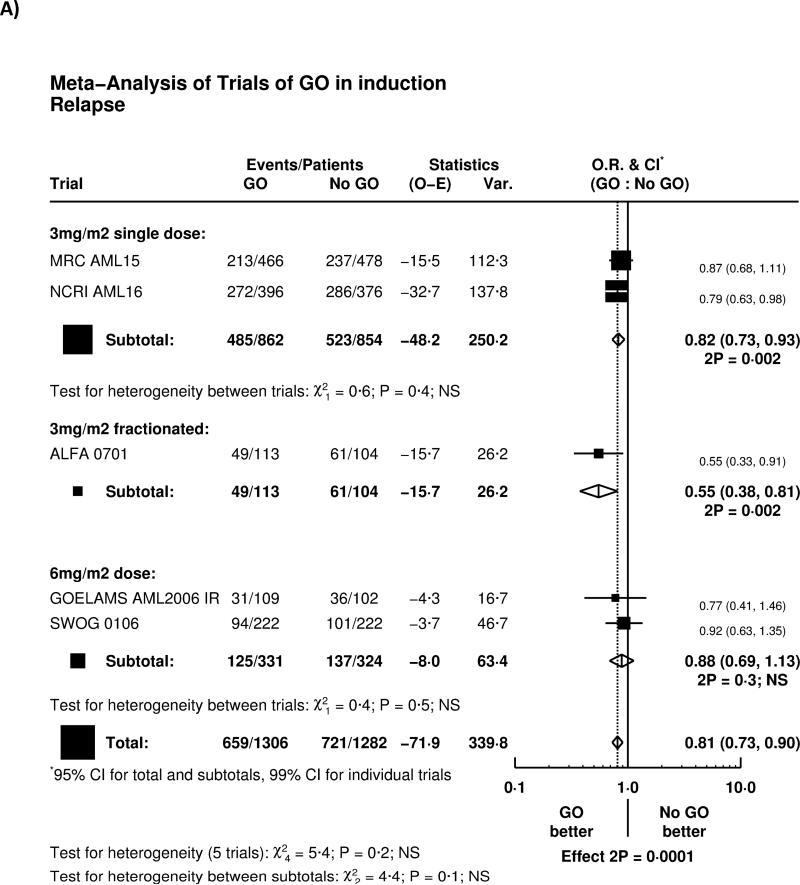
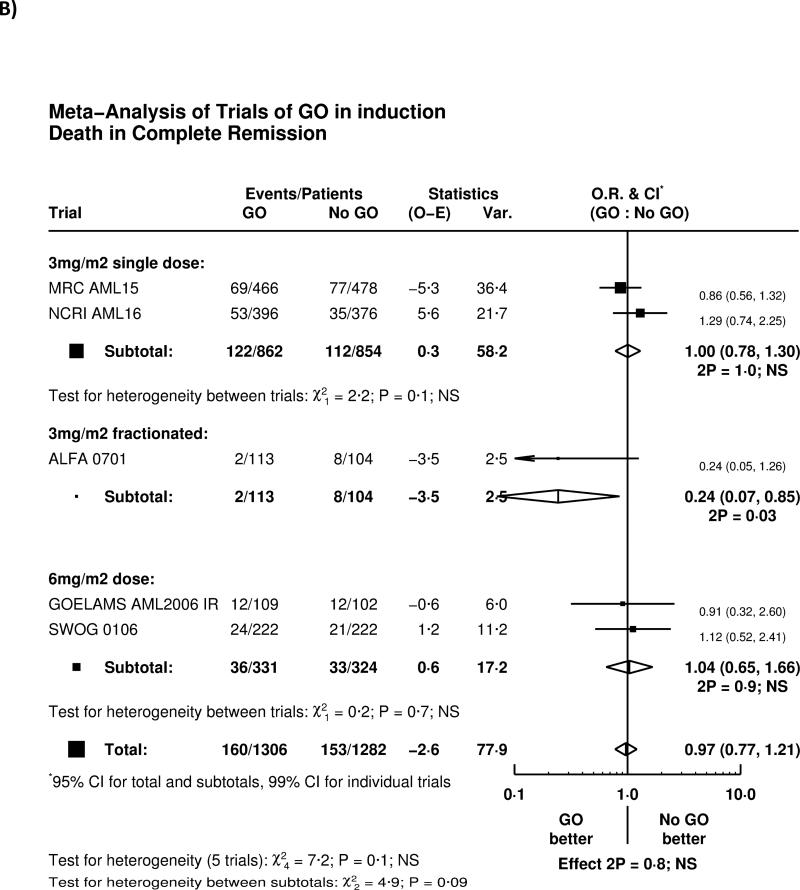
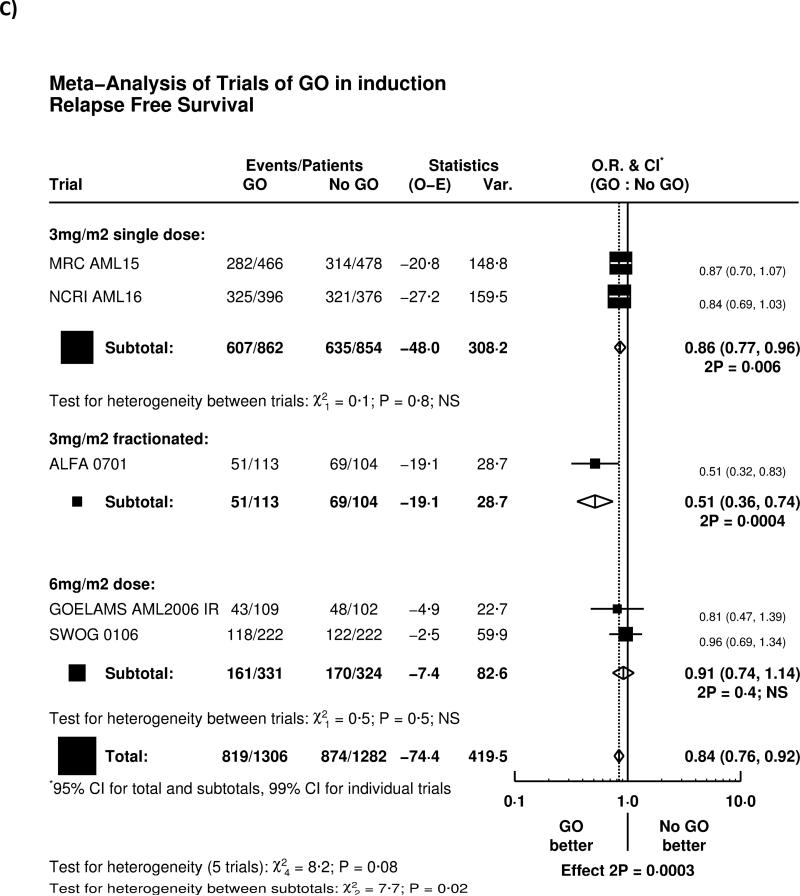
Overall, adding GO to induction chemotherapy significantly reduced relapse (HR 0.81 (0.73-0.90) p=0.0001; Figure 2A). The greatest effect was seen in the French ALFA-0701 study; with evidence of heterogeneity with the other trials (p=0.04), although the remaining four trials when combined together also showed a highly significant reduction in relapse (HR 0.84 (0.75-0.93) p=0.001). There was, overall, no significant difference in deaths in remission (HR 0.97 (0.77-1.21) p=0.8; Figure 2B); while there was a significant benefit in the ALFA-0701 trial, and some heterogeneity (p=0.03), there was no evidence from any trial that deaths in remission were increased among patients receiving GO.
Figure 2.
Effect of GO on A) relapse, B) Death in Complete Remission, C) Relapse Free Survival, D) Survival from Remission
As a result, relapse free survival was significantly improved (HR 0.84 (0.76-0.92) p=0.0003; Figure 2C); with the largest effect in the ALFA-0701 trial, although the other four trials combined also showed a significant improvement in RFS (HR 0.87 (0.79-0.96) p=0.005).
Survival and Predictive Factors
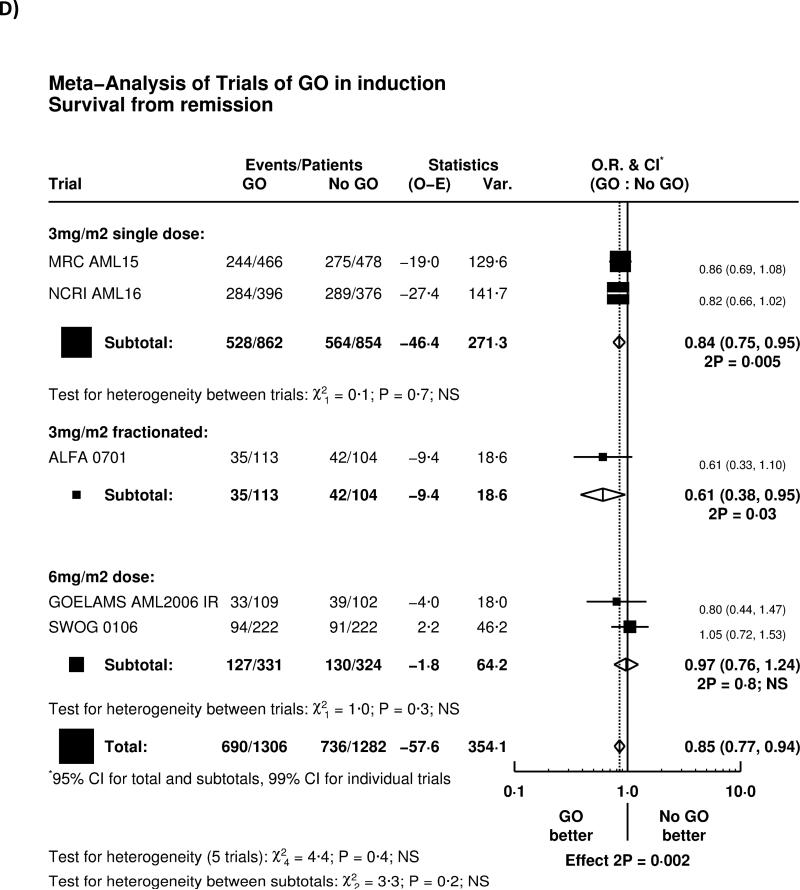
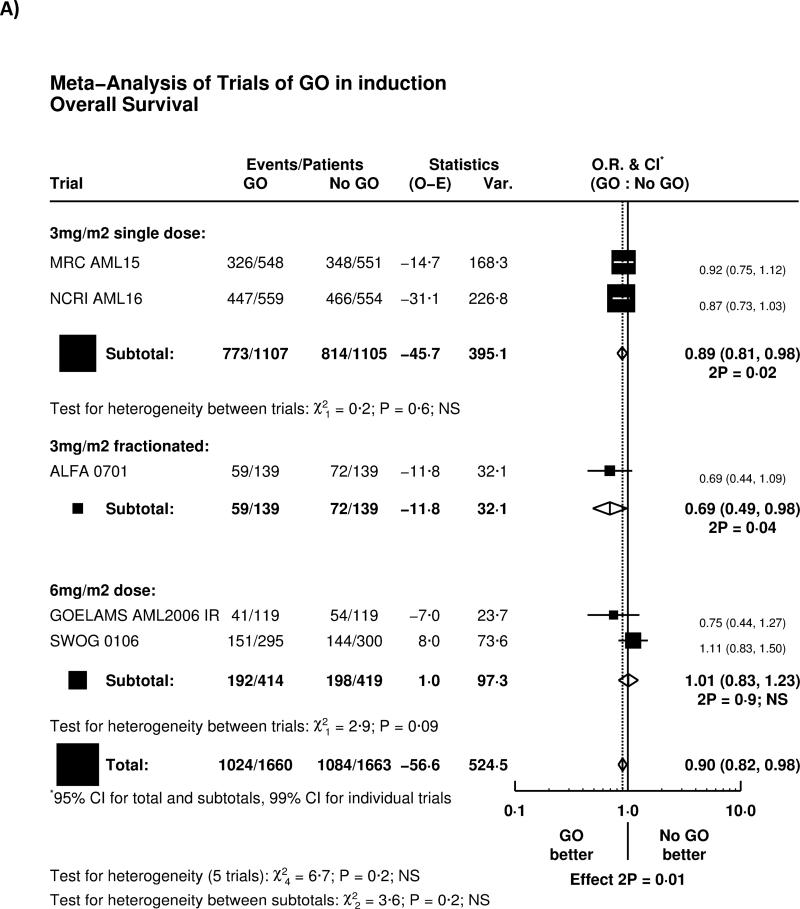
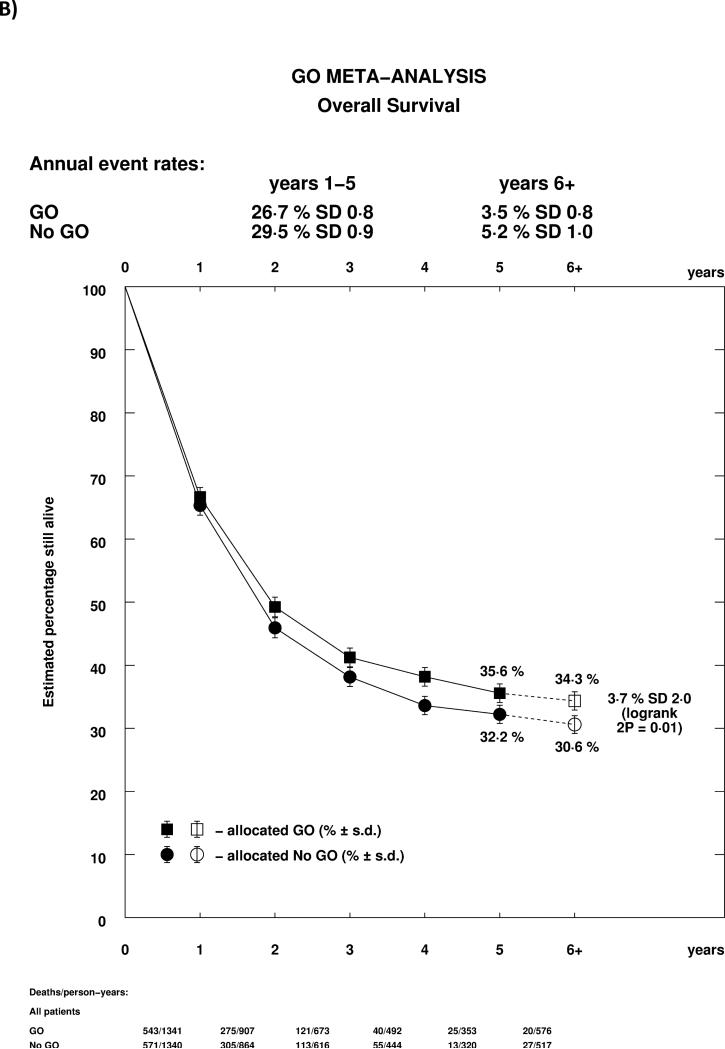
The reduction of relapse led to significantly improved survival from remission (HR 0.85 (0.77-0.94), p=0.002, Figure 2D). Overall, adding GO to induction chemotherapy led to a significant improvement in overall survival (HR 0.90 (0.82-0.98) p=0.01; Figure 3A). There was no significant heterogeneity by dosing regimen or by trial, and the overall absolute improvement was approximately 4% at 5 years (Figure 3B).
Figure 3.
Effect of GO on Overall Survival. A) Overall by Trial, B) Survival Curve. Denominators in Figure 3B represent person-years at risk during the time period in question.
Exploratory stratified analyses were undertaken to identify whether any baseline features predicted a greater or lesser benefit of GO. In analyses stratified by age, sex, diagnosis and induction chemotherapy, there was no evidence of interaction (Supplemental Figure 1). In the ALFA-0701 trial it was suggested that there was greater benefit for patients with a FLT3 mutation, but this was not seen in this overall analysis. Similarly, since NPM1c mutation has been associated with an increase in CD33 expression15, it has been hypothesised that there may be a differential benefit in NPM1c positive disease. However this was not seen.
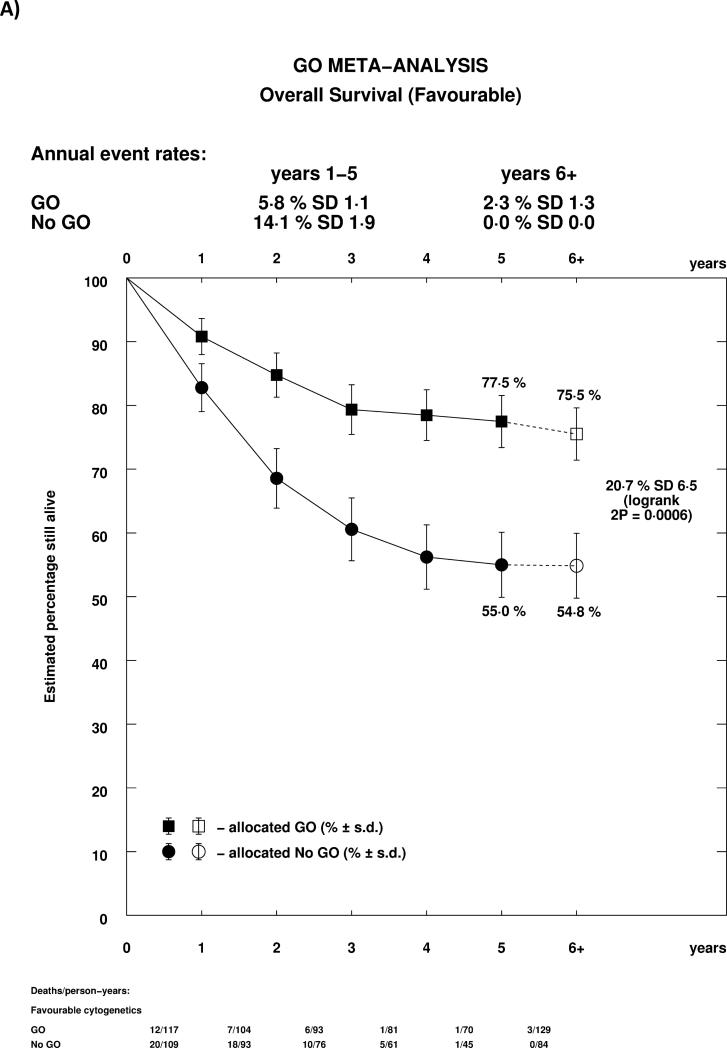
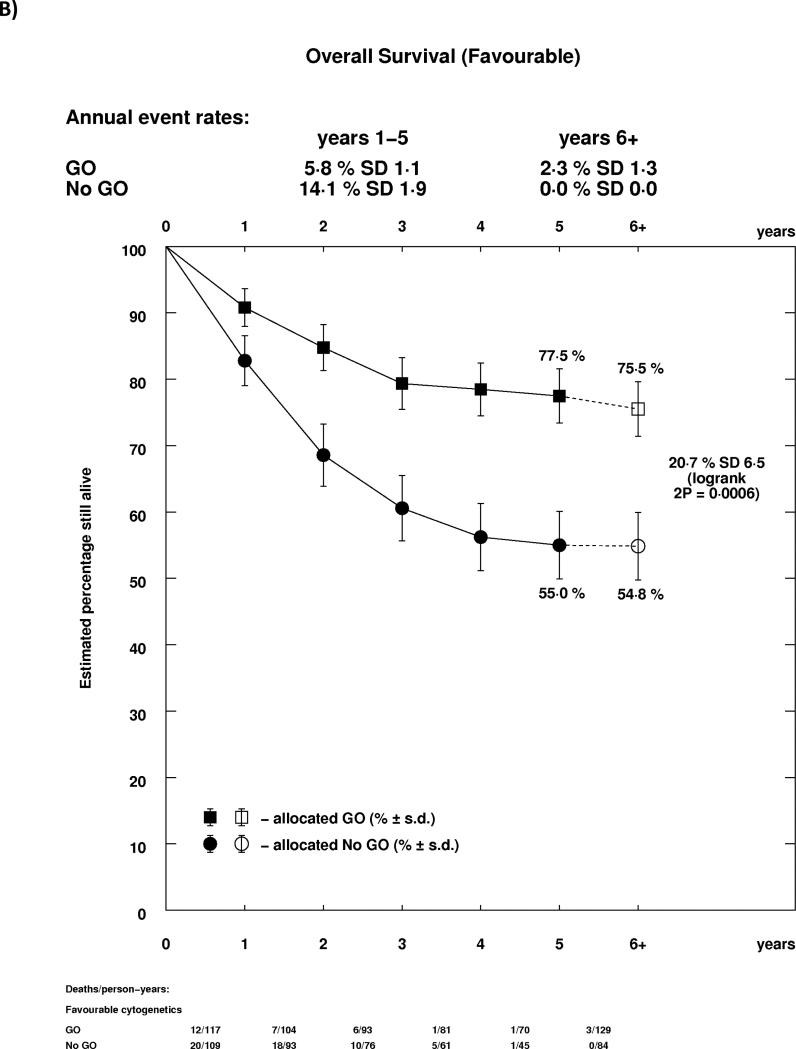
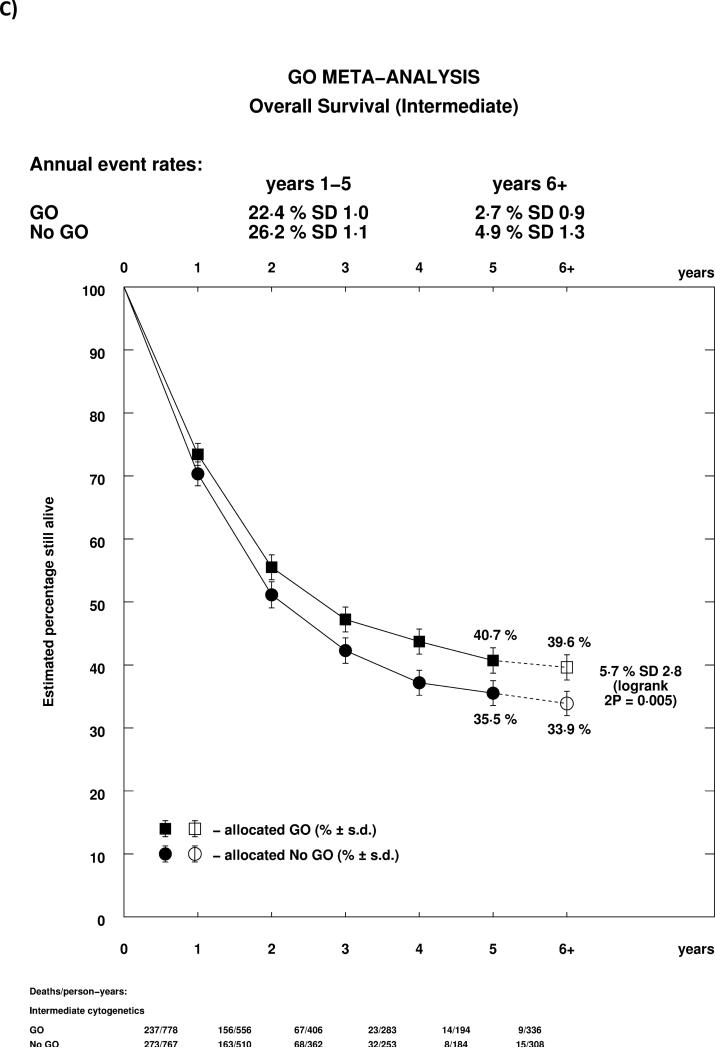
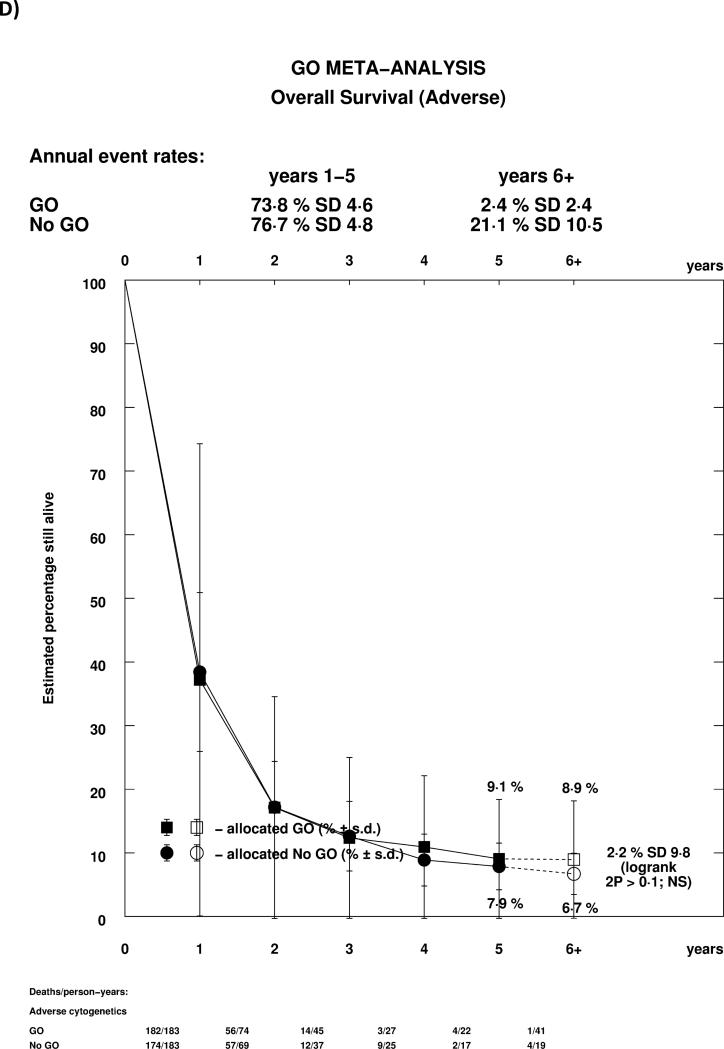
As reported previously in the MRC AML15 trial, and confirmed in the ALFA trial, the effect of GO differed by cytogenetic group, whether each group's own coding was used, or whether analyses were performed using the revised MRC classification (Supplemental Table 1, Figure 4A). In both cases, there was a highly significant test for trend, indicating that the benefit was greatest in patients with favourable risk cytogenetics. Indeed, while for both favourable and intermediate cytogenetics there was a statistically significant survival benefit for GO, there was no evidence of benefit for patients with adverse cytogenetics. Survival curves stratified by cytogenetics illustrate the effect graphically, with meaningful absolute benefits of 20.7% (HR 0.47 (0.31-0.73)) and 5.7% (HR 0.84 (0.75-0.95)) in favourable and intermediate cytogenetic patients, but no evidence of benefit in adverse cytogenetics (Figures 4B-D). Within cytogenetic groups there was no significant between study heterogeneity.
Figure 4.
Analysis of Overall Survival Stratified by Cytogenetics; A: Overall; B: Favourable (MRC 2009); C: Intermediate (MRC 2009); D: Adverse (MRC 2009). Patients with insufficient karyotype data or <20 metaphases are classified as unknown in the MRC 2009 classification.
As noted earlier, 785/3325 (24%) of patients underwent stem cell transplant. A sensitivity analysis was performed, censoring these patients at transplant. The results were in line with those for overall survival (HR 0.88 (0.80-0.97) p=0.01; Supplemental Figure 2). In the 785 patients who were transplanted, there was no overall dis-benefit from transplant among patients given GO: however, while day 100 mortality was improved compared to control in patients given 3mg/m2, there was a non-significant detriment to day 100 mortality in patients allocated 6mg/m2, although numbers are small (Supplemental Figure 3).
In the MRC AML15 trial of younger patients, a prognostic score based on age, cytogenetics and performance status was developed in patients with even trial numbers and identified patients with an absolute 10% survival benefit from GO. In the validation set (odd trial numbers) this was confirmed: overall approximately 75% of patients, including all favourable risk patients, 70% of those with intermediate risk cytogenetics, and no adverse risk patients were predicted to benefit. This score was tested in patients aged under 60 in this meta-analysis, excluding those in which the prognostic score was developed, and 762/1110 (69%) patients were identified as likely to derive benefit by the score. In this group, there was significantly better survival with GO (HR 0.76 (0.63-0.92) p=0.004; Supplemental Figure 4) and an absolute 5-year survival benefit of 10%, with no evidence of benefit from GO among the remaining patients (p-value for heterogeneity p=0.04).
Discussion
This meta-analysis of 3325 adult patients with AML demonstrates that overall survival is improved by the addition of Gemtuzumab Ozogamicin to induction chemotherapy. Importantly this analysis differs from others16,17 which address this issue, firstly because it has the advantages and rigour of individual patient data, enabling, for example, standardisation of risk groups. A further advantage is that it enables data to be updated from the original publication, which happened for all trials except the ALFA trial where follow up data was not available due to contractural arrangements with the supplying company. It also focuses on the specific question of the simultaneous administration of GO with intensive chemotherapy, not pre-induction therapy18, as a replacement for anthracycline19 or combined with low dose therapy20. Importantly, the improvement appears to be achievable without a penalty in terms of early mortality particularly if a dose of 3mg/m2 is used, either as a single or fractionated schedule. At this dose level no excess early mortality was detected in patients post stem-cell transplant. The findings of excess early mortality in the SWOG-0106, which is not seen in the other trials, was seen in the context of an untypically low rate in the control group. The improved survival was clearly a result of reduction in relapse rather than an improved rate of remission, suggesting that the “quality” of remission was improved. It is of interest that in the NCRI AML16 trial where minimal residual disease (MRD) detection in the remission marrow was available, albeit in a minority of patients (n=186)6, this did not show a difference in the “quality” of remission between arms when assessed by flow cytometry at a 104 detection level21. Other data suggest that the level of MRD is reduced by GO in induction22,23.
In identifying patients most likely to benefit from adding GO to induction chemotherapy, only cytogenetics showed a significant interaction with treatment. In particular, the effect of GO was not moderated by age, sex, or choice of induction chemotherapy, with no significant evidence of interaction with FLT-3 or NPM1 mutation status, although numbers were smaller in this comparison. With respect to age, the spectrum of age normally offered intensive induction chemotherapy was well represented. For example the NRCI AML16 trial focused on patients >60, the median age of the ALFA trail was 62, and 154 patients in the MRC AML15 trial were over 60 years. FLT3 was assayed using previously published methods24,25 in reference labs for each collaborative group, where 5% threshold defined positive. Because of limited numbers allelic ratio and various FLT3/NPM1 genotypes were not examined to avoid the dangers inherent in underpowered subgroup analysis. There was significant survival benefit for favourable and intermediate cytogenetic risk groups when assessed separately or together, but a consistent observation was a lack of benefit in patients with adverse risk. The benefit is particularly clear in the favourable risk group, which came primarily from the trials including younger patients (AML15 and SWOG-0106 (Table 1)). Overall 667/3325 (20%) patients did not have cytogenetic data. As an individual group we could not see them benefitting from GO (Supplemental figure 5), however there is no significant interaction and they are included in the total survival analysis. Similarly, there is no interaction between GO and the presence/absence of molecular data. In terms of optimal use, the present data suggests that these groups should be rapidly identified so that GO can be avoided in the case of the adverse risk patients, and given in the case of favourable risk. This may justify routine adoption of rapid diagnostic techniques such as fluorescent in-situ hibridisation (FISH), which would identify between 50% and 80% of patients with adverse risk cytogenetics within the MRC 2009 classification. The validated risk score reported for younger patients in the MRC AML15 trial, was confirmed in this dataset where all 197 favourable, 828/1041 (81%) intermediate and 0/250 adverse risk patients aged >60 are predicted an absolute 10% survival benefit.
While we found no significant interaction between the different schedules of GO, there was nonetheless significant interaction between GO given at 3mg/m2 and 6mg/m2 in terms of 30-day mortality. The increased early mortality observed in the SWOG-0106 study was not replicated in trials which used a lower dose of GO. This would suggest that a dose of 3mg/m2 tends to provide similar survival benefit, while at the same time avoiding excess early mortality. This further implies that future treatment of patients with GO should concentrate upon a dose of 3mg/m2. However, within that dose, the evidence also suggests that the ALFA-0701 fractionated schedule gave a greater reduction in relapse. This schedule capped each dose at a total of 5mg (one vial of drug), so patients with a body surface area >1.67m2 would receive a dose less than 3mg/m2 at each occasion. Future research could therefore focus upon the optimal dosing schedule and whether fractionated dosing provides significant advantages over a single dose given on day 1. In the UK NCRI AML17 trial (ISRCTN55675535) 788 patients have been randomised to 3mg/m2 versus 6mg/m2. Initial (unpublished) results suggest that there is no benefit for the higher dose.
A similarly designed trial in children, where GO was given at a dose of 3mg/m2 on day 6, has recently been presented and shows similar trends26, i.e. no change in remission rate, significant reduction in relapse and a trend for survival benefit. Taken together these data suggest that GO can help patients with AML of all ages who do not have adverse risk disease.
In summary, with respect to the specific question of the addition of GO to induction therapy in adults, CD33 represents a legitimate target in AML. These data provide strong evidence that consideration should be given to revision of its regulatory status with a view to making it available to patients as already suggested27.
Research in Context
Systematic review
This paper reports an IPD meta-analysis of 5 RCTs of Gemtuzumab Ozogamicin (GO) added to intensive induction chemotherapy in adult patients. Trials were identified using a literature search of PubMed using search term “Randomi* and gemtuzumab”, supplemented with contact with individual trialists, and the drug company to identify all studies for which GO had been provided. IPD was collected on all 5 trials, in 3 cases supplementing published data (plus one trial which had not yet been reported in full).
Interpretation
GO significantly improves survival, particularly in patients with favourable and intermediate risk cytogenetics. There is no evidence of benefit in patients with adverse risk cytogenetics. The results indicate that GO has a role in the treatment of AML and that licensing decisions on the drug may need to be revised.
Supplementary Material
Footnotes
Author Contributions:
FRA led a trial and conceived the study, submitted data, reviewed the manuscript. RKH undertook the analysis and wrote the manuscript. SP and MO led a trial, submitted data and reviewed the manuscript, SC, HD and CC led a trial, submitted data and reviewed the manuscript JD, NI, J-Y C & CR led a trial, submitted data and reviewed the manuscript, LC & AVM standardised the cytogenetic data and reviewed the manuscript. EE reviewed the manuscript, AKB led 2 trials, conceived the study, submitted data, and wrote the manuscript. The authors declare no conflict of interest.
References
- 1.Bross PF, Beitz J, Chen G, et al. Approval Summary: Gemtuzumab Ozogamicin in Relapsed Acute Myeloid Leukemia. Clin. Cancer Res. 2001;7:1490–1496. [PubMed] [Google Scholar]
- 2.Larson RA, Boogaerts M, Estey E, et al. Antibody-targeted chemotherapy of older patients with acute myeloid leukemia in first relapse using Mylotarg (gemtuzumab ozogamicin). Leukemia. 2002;16:1627–1636. doi: 10.1038/sj.leu.2402677. [DOI] [PubMed] [Google Scholar]
- 3.Giles FJ, Kantarjian HM, Kornblau SM, et al. Mylotarg (gemtuzumab ozogamicin) therapy is associated with hepatic venoocclusive disease in patients who have not received stem cell transplantation. Cancer. 2001;92:406–413. doi: 10.1002/1097-0142(20010715)92:2<406::aid-cncr1336>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Kell WJ, Burnett AK, Chopra R, et al. A feasibility study of simultaneous administration of gemtuzumab ozogamicin with intensive chemotherapy in induction and consolidation in younger patients with acute myeloid leukemia. Blood. 2003;102:4277–4283. doi: 10.1182/blood-2003-05-1620. [DOI] [PubMed] [Google Scholar]
- 5.Burnett AK, Hills RK, Milligan D, et al. Identification of Patients With Acute Myeloblastic Leukemia Who Benefit From the Addition of Gemtuzumab Ozogamicin: Results of the MRC AML15 Trial. J. Clin. Oncol. 2011;29:369–377. doi: 10.1200/JCO.2010.31.4310. [DOI] [PubMed] [Google Scholar]
- 6.Burnett AK, Russell NH, Hills RK, et al. Addition of Gemtuzumab Ozogamicin to Induction Chemotherapy Improves Survival in Older Patients With Acute Myeloid Leukemia. Journal of Clinical Oncology. 2012;30:3924–3931. doi: 10.1200/JCO.2012.42.2964. [DOI] [PubMed] [Google Scholar]
- 7.Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121:4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delaunay J, Recher C, Pigneux A, et al. Addition of gemtuzumab ozogamycin to chemotherapy improves event-free survival but not overall survival of AML patients with intermediate cytogenetics not eligible for allogeneic transplantation: results of the GOELAMS AML 2006 IR study. Blood. 2011;118(21):37–38. (ASH Annual Meeting Abstracts) [Google Scholar]
- 9.Taksin AL, Legrand O, Raffoux E, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21(1):66–71. doi: 10.1038/sj.leu.2404434. [DOI] [PubMed] [Google Scholar]
- 10.van Der Velden VH, te Marvelde JG, Hoogeveen PG, et al. Targeting of the CD33- calicheamicin conjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalisation by leukemic and normal myeloid cells. Blood. 2001;97:3197–3204. doi: 10.1182/blood.v97.10.3197. [DOI] [PubMed] [Google Scholar]
- 11.Castaigne S, Pautas C, Terre C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised,open-label, phase 3 study. Lancet. 2012;379:1508–16. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 12.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010 Jul 22;116(3):354–65. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Early Breast Cancer Trialists’ Collaborative Group . Treatment of Early Breast Cancer, Volume 1: Worldwide Evidence 1985-1990. Oxford University Press; Oxford, United Kingdom: 1990. [Google Scholar]
- 15.Falini B, Mecucci C, Tiacci E, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 16.Kharfan-Dabaja MA, Hamadani M, Reljic T, et al. Gemtuzumab ozogamicin for treatment of newly diagnosed acute myeloid leukaemia: a systematic review and meta-analysis. Brit J Haematol. 2013;163:315–25. doi: 10.1111/bjh.12528. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Xu SN, Qin DB, et al. Effect of adding gemtuzumab ozogamicin to induction chemotherapy for newly diagnosed acute myeloid leukemia: a meta-analysis of prospective randomized phase III trials. Ann Oncol. 2014;25:455–61. doi: 10.1093/annonc/mdt566. [DOI] [PubMed] [Google Scholar]
- 18.Amadori S, Suciu S, Salih H, et al. The sequential combination of gemtuzumab ozogamicin and intensive chemotherapy does not benefit older patients with untreated AML: results of the EORTC-GIMEMA AML-17 randomized trial. Haematologica. 2012;97(s1):446. (abstract 1091D) [Google Scholar]
- 19.Brunnberg U, Mohr M, Noppeny R, et al. Induction therapy of AML with ara-C plus daunorubicin versus ara-C plus gemtuzumab ozogamicin: a randomized phase II trial in elderly patients. Ann Oncol. 2012;23:990–6. doi: 10.1093/annonc/mdr346. [DOI] [PubMed] [Google Scholar]
- 20.Burnett AK, Hills RK, Hunter AE, et al. The addition of gemtuzumab ozogamicin to low-dose ara-C improves remission rate but does not significantly prolong survival: results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia. 2013;27:75–81. doi: 10.1038/leu.2012.229. [DOI] [PubMed] [Google Scholar]
- 21.Freeman SD, Virgo P, Couzens S, et al. Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J Clin Oncol. 2013;1031(32):4123–31. doi: 10.1200/JCO.2013.49.1753. [DOI] [PubMed] [Google Scholar]
- 22.Borthakur G, Faderl S, Verstovsek S. Molecular response in core binding factor acute myelogenous leukemia with fludarabine, cytarabine, G-CSF and gemtuzumab ozogamicin. Blood. 2008;112:676. (abstract) [Google Scholar]
- 23.Lambert J, Lambert J, Nibourel O, et al. Minimal Residual Disease Assessed by WT1 Expression and NPM1 Mutations Specific RQ-PCR Assays Identifies Patients with Distinct Outcomes in the ALFA 0701 Trial and Is Decreased by Treatment with Gemtuzumab Ozogamicin. Blood. 2012;120:659. (abstract) [Google Scholar]
- 24.Lazenby M, Gilkes AF, Marrin C, et al. The prognostic relevance of flt3 and npm1 mutations on older patients treated intensively or non-intensively: a study of 1312 patients in the UK NCRI AML16 trial. Leukemia. 2014 Feb 27; doi: 10.1038/leu.2014.90. doi: 10.1038/leu.2014.90. [Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Boissel N, Cayuela JM, Preudhomme C, et al. Prognostic significance of FLT3 internal tandem repeat in patients with de novo acute myeloid leukemia treated with reinforced courses of chemotherapy. Leukemia. 2012;16(9):1699–704. doi: 10.1038/sj.leu.2402622. [DOI] [PubMed] [Google Scholar]
- 26.Glamis AS, Aplenc R, Alonzo TA, et al. Gemtuzumab Ozogamicin (GO) In Children With De Novo Acute Myeloid Leukemia (AML) Improves Event-Free Survival (EFS) By Reducing Relapse Risk – Results From The Randomized Phase III Children's Oncology Group (COG) Trial, AAML0531. Blood. 2013;122:355. doi: 10.1200/JCO.2014.55.3628. (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravandi F, Estey EH, Appelbaum FR, et al. Gemtuzumab ozogamicin: time to resurrect? J Clin Oncol. 2012;30:3921–3. doi: 10.1200/JCO.2012.43.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.