Abstract
Background:
The fixed dose combinations (FDCs) of muscle relaxants, non-steroidal anti-inflammatory drugs and paracetamol are commonly prescribed in the treatment of acute lower backache.
Aim:
The present study was undertaken with the aim of comparing the efficacy and safety of FDCs of thiocolchicoside and aceclofenac versus chlorzoxazone, aceclofenac and paracetamol in patients with acute lower backache associated with muscle spasm.
Materials and Methods:
A total of 100 patients between ages range from 18 and 55 years having low back pain of ≤7 days duration were randomly divided into two groups. Group A was prescribed thiocolchicoside (4 mg) + aceclofenac (100 mg) while Group B was prescribed chlorzoxazone (500 mg) + aceclofenac (100 mg) + paracetamol (325 mg) orally twice daily for 7 days. Severity of pain at rest and on movement was recorded using visual analogue scale. Muscle spasm was evaluated by hand-to-floor distance and Lasegue's maneuver. Readings were noted on day 1 (baseline), day 3 and day 7.
Results:
There was statistically significant reduction in severity of pain and muscle spasm on day 3 and day 7 in both groups. There was no statistically significant difference in pain relief and muscle spasm among the treatment groups but clinically showed better improvement in the Group A. The adverse drug reactions occurring during study showed a statistically significant better safety profile in the Group A than Group B.
Conclusion:
These findings confirm that FDC of thiocolchicoside and aceclofenac is a preferred option for patients with lower backache pain associated with muscle spasm.
Keywords: Fixed dose combinations, low backache, muscle relaxants, non-steroidal inflammatory drugs, visual analog scale
INTRODUCTION
Low back pain (LBP), a very common complaint among middle aged population affecting 90% of all adults at least once in a lifetime and is usually associated with “muscle spasm” that is responsible for persistence pain.[1] Higher incidence of LBP is reported in Indian population in younger patients due to occupational exposure and lack of exercise.
It is a major health and socio-economic problem[2] and is associated with high costs of health care, work absenteeism and disablement.[3] In general, LBP is managed with the short-term use of non-steroidal anti-inflammatory drugs (NSAIDs) and centrally acting skeletal muscle relaxants.[4] Unfortunately NSAIDs have gastric intolerance, whereas most of the centrally acting muscle relaxants have central nervous system depressant side-effects such as sedation, dizziness, impairment of co-ordination, mental confusion, weakness etc.[5] Hence, these limiting factors demands a need for an ideal fixed dose combination (FDC) which is devoid of effects on psychomotor performance, free of sedation and higher tolerability.
Chlorzoxazone, a centrally muscle relaxant acts primarily at the level of the spinal cord and subcortical areas of the brain, where it inhibits multisynaptic reflex arcs involved in producing and maintaining skeletal muscle spasm. The exact mode of action is not clear, may be related to the sedative properties of the drug. The side-effect profile is similar to that of most of other muscle relaxants, except for a limited number of reported cases of significant hepatotoxicity particularly by chlorzoxazone.[6]
Thiocolchicoside is a semi-synthetic derivative of colchicines, a natural glycoside originated from flower seeds of superba gloriosa.[7] It has an affinity for the inhibitory glycine and gamma-aminobutyric acid (GABA)-A receptors i.e., have glycomimetic and gaba mimetic activity, therefore shows muscle relaxant action. As it has GABA-mediated action, so it shows both myorelaxant as well as analgesic activity. It has demonstrated its clinical efficacy and safety in many clinical trials.[5,7,8,9,10,11,12] It has also been reported that thiocolchicoside produces muscle relaxation without any subjective or objective sedative side-effects.[8]
There is no published clinical study which compared two FDCs of thiocolchicoside + aceclofenac versus chlorzoxazone + aceclofenac + paracetamol in patients suffering from LBP in Indian population. Hence the present study was undertaken to compare the efficacy and safety of FDCs commonly available in the market and prescribed by orthopedicians, i.e., thiocolchicoside and aceclofenac versus chlorzoxazone, aceclofenac and paracetamol in patients with acute lower backache associated muscle spasm.
MATERIALS AND METHODS
Study design
This preliminary, prospective, open labeled, randomized, comparative drug study was undertaken in the out-patient Department of Orthopedics, in North Indian Government Medical Institute. The study protocol was approved by the institutional review board. Patients attending the outpatient department were screened and assessed according to the specified inclusion and exclusion criteria. A total of 100 eligible patients of both sexes having acute LBP of moderate to severe intensity with muscle spasm with history of trauma, sprain or sustained injury with no finding of fracture and severe spinal diseases such as spondylitis, cancer and severe arthritis were taken and willing to take medications as directed and come for the follow-up were enrolled in the study. The written consent of patients was taken on inform consent form in the local language.
Inclusion criteria
Patients of either sex in the age range of 18-55 years with a history of LBP and muscle spasm of ≤7 days were included in the study.
Exclusion criteria
Patients with back pain due to malignancy, infection, abnormal metabolism, osteoarthritis of hip or any other disease, back pain referred from other organs, patients with a history of presence of peptic ulceration or gastrointestinal bleeding or severe dyspepsia, patients allergic to NSAIDs and skeletal muscle relaxants or suffering from asthma or other allergic disorders, patients treated with NSAIDs or skeletal muscle relaxants for 3 days prior to study, patients with severe concurrent systemic disease including bleedings diathesis, patients on anticoagulation therapy, patients suffering from hepatic or renal impairment and pregnant or lactating women were excluded from the study.
Treatment procedure
Demographic data and relevant medical history was obtained from all patients prior to initiation of therapy. Patients were randomly divided into two groups: Group A and Group B, 50 each. Group A received a FDC of thiocolchicoside (4 mg) and aceclofenac (100 mg) orally twice a day for 7 days. Group B received FDC of chlorzoxazone (500 mg), aceclofenac (100 mg) and paracetamol (325 mg) orally twice a day for 7 days. Commercial available preparations were used. Patients were evaluated on day 3 and day 7 for severity of pain and muscle spasm. At each visit patients were asked to report any if adverse effect present.
Criteria for evaluation
Primary efficacy measures
Pain assessment scale
Assessment of intensity of pain at rest and pain on movement was carried out on day 0 (visit 1, baseline), day 3 (visit 2) and day 7 (visit 3) by means of a 10 cm visual analogue scale (VAS)[13] as reported by a patient between 0 (no pain) and 10 (unbearable pain). The patients were asked to score by ticking off the scale between 0 (no pain) and 10 (unbearable pain).
Muscle spasm assessment
Finger-to floor distance
It was measured by flexion at the hip joint in a standing position. The patients were told to bend down as far as possible without bending the knees and try to touch the floor with their fingers. The remaining distance between the floor and fingertips was measured by the ruler in centimeters.[14]
Lasegue's maneuver
In this test, articular excursion of the hip in degrees on performing Lasegue's manoeuvre before inducing pain in the supine position which involved gradually raising of lower extremity by flexing the hip with the knee in extension passively. The angle between the raised limb and tabletop was measured.[14]
Secondary efficacy measure
Global efficacy evaluation was evaluated based on the global assessment based on a 4-point scale marked as excellent/good/average/poor.
Safety measures
Side-effects such as tiredness, drowsiness, dizziness and alertness were noted based on history, observations of adverse reactions. Furthermore, global assessment of tolerability to therapy was assessed on a four-point scale of excellent/good/average/poor.
Statistical analysis
Comparison was done both inter- and intra-group between baselines, day 3 and day 7 values and expressed in mean ± standard deviation. Then the data was pooled and analyzed. One-way analysis of variance (ANOVA) was applied in the intra-group analysis for pain at rest, pain on movement and FFD. Post-hoc analysis was also done using Tukey's test. Independent Student's t-test is applied for intergroup analysis. Pearson's Chi-square test was used for overall assessment of tolerability. All tests were carried out using SPSS 16 software by SPSS Inc. Released 2007. SPSS for Windows, Version 16.0. Chicago, SPSS Inc.
RESULTS
A total of 100 patients were enrolled in the study. During the study period, three patients from both groups did not come for follow-up, so data of these six patients were not included in the statistical analysis. The effects of the FDCs on pain on rest were shown in Figure 1 and pain on movement was shown in Table 1. In Group A, severity of pain on rest was reduced from 100% to 62.5% on day 3 and 6.9% on 7th day whereas in Group B, it was reduced from 100% to 73.9% on day 3 and 20.3% on day 7 respectively [Figure 1]. There was statistically significant decline in the pain intensity on day 3 and at the end of the treatment (day 7) in both groups, i.e., A and B while using one-way ANOVA (P < 0.001 in both groups). There was clinically significant difference was observed, but no statistically significant difference was observed between the two groups of treatment while reporting pain on the rest while using independent Student's t-test (P > 0.05). Similar results were obtained in the evaluation of pain on movement, i.e., in Group A, severity of pain on movement was reduced from 100% to 71.7% on day 3 and 15.2% on 7th day whereas in Group B, it was reduced from 100% to 77.3% on day 3 and 30.7% on day 7 [Table 1]. There was statistically significant decline in the pain intensity on day 3 and at the end of the treatment (day 7) in both groups, i.e. A and B while using one-way ANOVA (P < 0.01 in both groups). There was clinically significant difference was observed, but no statistically significant difference was observed between the two groups of treatment after using independent Student's t-test (P > 0.05) while reporting pain on movement.
Figure 1.
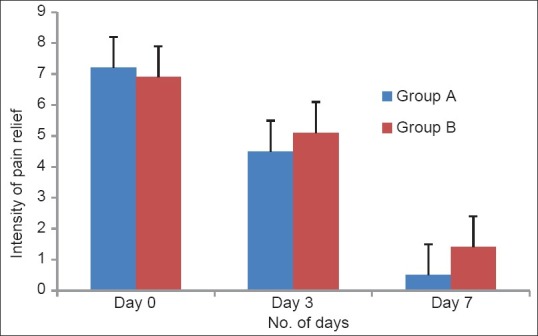
Intensity of pain relief on rest as per visual analogue scale (VAS) effects of a 7 day treatment with fixed dose combinations of thiocolchicoside (4 mg) and aceclofenac (100 mg) versus chlorzoxazone (500 mg), aceclofenac (100 mg) and paracetamol (325 mg) on pain at rest in patients with acute low back pain; pain was evaluated by means of 10-cm VAS. No statistically significant difference was observed between the two groups of patients at any time
Table 1.
Intensity of pain relief on movement as per VAS

The distance decreased from 46.3 to 25.8 cm on day 3 (–44.2%) and 15.2 cm (–67.2%) on day 7 in Group A and from 46.7 to 32.5 cm (–30.4%) on day 3 and 20.9 cm (–55.2%) on day 7 in Group B [Figure 2]. There was a decrease in muscle spasm in both the groups which is statistically significant while using one-way ANOVA (P < 0.001) in both groups). Although the results achieved in the Group A were slightly better than those in Group B, no statistically significant difference was observed between the two groups at any time (P > 0.05).
Figure 2.
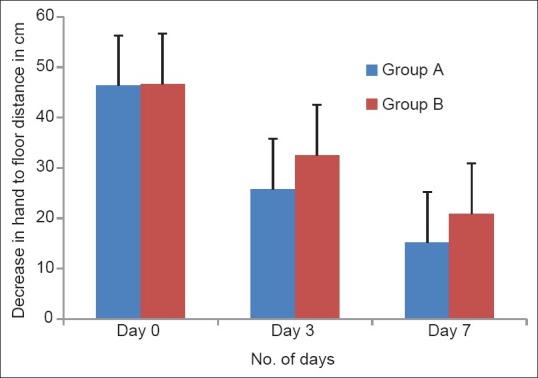
Hand-to-floor distance effects of a 7 day treatment with fixed dose combinations of thiocolchicoside (4 mg) and aceclofenac (100 mg) versus chlorzoxazone (500 mg), aceclofenac (100 mg) and paracetamol (325 mg) on the “hand-to-floor” distance (cm); no statistically significant difference was observed between the two groups of patients at anytime
The articular excursion performed before inducing pain was on average 70.7° at baseline and increased to 78.5° on day 7 (P < 0.01 vs. basal) in the Group A, while in the Group B patients, the excursion increased from 74.5° at baseline to 80.1° at the end of the treatment (P < 0.01 vs. basal) [Figure 3].
Figure 3.
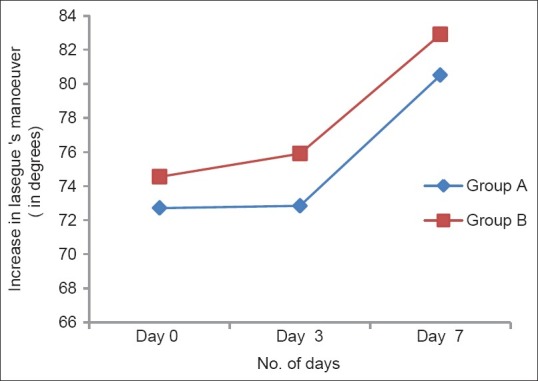
Increase in excursion by Lasegue's manoeuvre effects of a 7 day treatment with fixed dose combinations of thiocolchicoside (4 mg) and aceclofenac (100 mg) versus chlorzoxazone (500 mg), aceclofenac (100 mg) and paracetamol (325 mg) on the Lasegue’ maneuver (expressed as degrees); no statistically significant difference was observed between the two groups of patients at anytime
The adverse drug reactions occurring during study showed a statistically significant (P < 0.0001) better safety profile in the Group A than Group B. In fact, none of patient reported with sedation, drowsiness or dizziness and only two patients out 47 (4.2%) manifested gastrointestinal side-effects during the study treated in the Group A, while in Group B, 34 (72.3%) patients reported with sedation, drowsiness or dizziness and 20 patients out 47 (42.5%) manifested gastrointestinal side-effects. None of the patient presented with hepatic dysfunction.
As per investigators’ assessment about efficacy, 65% of patients reported excellent, 30% good, 3% average and 2% reported poor efficacy in the Group A respectively. Nearly 20% of patients reported excellent, 50% good, 24% average and 6% reported poor efficacy in Group B.
As per investigators’ opinion about tolerability, 65% of patients reported excellent, 30% good, 4% average and 1% reported poor in the Group A. In Group B, 20% of patients reported excellent, 20% good, 50% and 10% reported poor.
DISCUSSION
NSAIDs and skeletal muscle relaxants are effective in acute LBP for short-term pain relief. FDCs of these drugs provide relief from acute muscle spasm by blocking the spasm-pain-spasm cycle associated with LBP. Unfortunately, most of central muscle relaxants are central nervous system depressants and are associated with sedation as major side-effect. Chlorzoxazone is associated with usually reversible and mild hepatotoxicity.[15] Efficacy of thiocolchicoside in the treatment of acute LBP has been demonstrated by several randomized, double-blind, placebo-controlled trials. Pain.[5,8,10,12,13] Lahoti[11] concluded that FDC of aceclofenac + thiocolchicoside + paracetamol significantly reduced intensity of pain and improve the mobility of the patients.
In the present comparison was done to compare the efficacy and safety of FDCs commonly available in the market and prescribed by orthopedicians, i.e. thiocolchicoside and aceclofenac versus chlorzoxazone, aceclofenac and paracetamol in patients with acute lower backache associated muscle spasm. Severity of pain at rest was assessed using VAS between two groups. The scores decreased significantly as compared to baseline score on day 3 and day 7 within the two groups of patients. However, the decrease in severity of pain at rest was more pronounced within Group A as compared to Group B patients, though the difference between the two groups was not found to be statistically significant. This result is in accordance with the result reported by Lahoti[11] which states that combination of aceclofenac with thiocolchicoside show significantly improvement in pain at rest and during movement.
Present study confirms the efficacy of both FDCs in the treatment of painful muscle spasm. The study reported a statistically significant improvement in the hand-to-floor distance on the 3rd (P < 0.001) and 7th day (P < 0.001) as compared to baseline on both the groups. However, the decrease hand-to-floor distance was more pronounced within Group A as compared to Group B, though the difference between the two groups was not found to be statistically significant.
Most of the FDCs containing muscle relaxants have a major limiting side-effect as sedation. In our study, none of the patient reported sedation in Group A compared to Group B. In various other studies also neither thiocolchicoside alone or in combination with aceclofenac reported any sedative effect.[10,11,12,13] Few patients reported mild incidence of nausea and vomiting.
CONCLUSION
The incidence of acute backache is increasing in the present scenario due to modernization, lack of exercise and postural problems. Although currently used FDCs of central muscle relaxants and NSAIDs provide relief from back pain associated spasm have central nervous system side-effects such as sedation, tiredness, weakness etc., Today, patients look for a speedy recovery and returning back to daily activities without any compromise on quality of life.
In this randomized trial, FDC of thiocolchicoside and aceclofenac in patients with acute lower backache associated muscle spasm was found to be slightly more effective and without any sedation as compared to FDC of chlorzoxazone, aceclofenac and paracetamol. In addition, FDC of thiocolchicoside and aceclofenac showed better pain control with no effect on psychomotor parameters in comparison to FDC of chlorzoxazone, aceclofenac and paracetamol. This favorable therapeutic profile in terms efficacy, better quality of life and speedy recovery makes FDC of thiocolchicoside and aceclofenac over chlorzoxazone, aceclofenac and paracetamol combination as a preferred option of clinicians in the treatment of painful muscle spasm. In conclusion, Combination of aceclofenac + thiocolchicoside for 7 days in the treatment of LBP significantly reduces the intensity of pain and improves the mobility without causing side-effects like sedation.
Footnotes
Source of Support: Nill.
Conflict of Interest: None declared.
REFERENCES
- 1.Gautschi OP, Hildebrandt G, Cadosch D. Acute low back pain - Assessment and management. Praxis (Bern 1994) 2008;97:58–68. doi: 10.1024/1661-8157.97.2.58. [DOI] [PubMed] [Google Scholar]
- 2.Andersson GB. The epidemiology of spinal disorders. In: Frymoyer JW, editor. The Adult Spine: Principles and Practice. 2nd ed. New York: Raven Press; 1997. pp. 93–143. [Google Scholar]
- 3.van Tulder MW, Koes BW, Bouter LM. A cost-of-illness study of back pain in The Netherlands. Pain. 1995;62:233–40. doi: 10.1016/0304-3959(94)00272-G. [DOI] [PubMed] [Google Scholar]
- 4.Chou R, Huffman LH. American Pain Society, American College of Physicians. Medications for acute and chronic low back pain: A review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:505–14. doi: 10.7326/0003-4819-147-7-200710020-00008. [DOI] [PubMed] [Google Scholar]
- 5.Ketenci A, Ozcan E, Karamursel S. Assessment of efficacy and psychomotor performances of thiocolchicoside and tizanidine in patients with acute low back pain. Int J Clin Pract. 2005;59:764–70. doi: 10.1111/j.1742-1241.2004.00454.x. [DOI] [PubMed] [Google Scholar]
- 6.Powers BJ, Cattau EL, Jr, Zimmerman HJ. Chlorzoxazone hepatotoxic reactions. An analysis of 21 identified or presumed cases. Arch Intern Med. 1986;146:1183–6. doi: 10.1001/archinte.146.6.1183. [DOI] [PubMed] [Google Scholar]
- 7.Umarkar AR, Bavaskar SR, Yewale PN. Thiocolchicoside as muscle relaxant: a review. Int J Pharm Biol Sci. 2011;1:364–71. [Google Scholar]
- 8.Patat A, Klein MJ, Surjus A, Renault M, Rezvani Y, Granier J. Effects of acute and repeated doses of two muscle relaxants chlormezanone and thiocolchicoside, on vigilance and psychomotor performance of healthy volunteers. Hum Psychopharmacol. 1991;6:285–92. [Google Scholar]
- 9.Tüzün F, Unalan H, Oner N, Ozgüzel H, Kirazli Y, Içağasioğlu A, et al. Multicenter, randomized, double-blinded, placebo-controlled trial of thiocolchicoside in acute low back pain. Joint Bone Spine. 2003;70:356–61. doi: 10.1016/s1297-319x(03)00075-7. [DOI] [PubMed] [Google Scholar]
- 10.Marcel C, Rezvani Y, Revel M. Evaluation of thiocolchicoside as monotherapy in low back pain. Results of a randomized study versus placebo. Presse Med. 1990;19:1133–6. [PubMed] [Google Scholar]
- 11.Lahoti G. To evaluate efficacy and safety of fixed dose combination of aceclofenac+paracetamol+thiocolchicoside (acenac-MR) in the treatment of acute low back pain. J Indian Med Assoc. 2012;110:56–8. [PubMed] [Google Scholar]
- 12.Soonawalla DF, Joshi N. Efficacy of thiocolchicoside in Indian patients suffering from low back pain associated with muscle spasm. J Indian Med Assoc. 2008;106:331–5. [PubMed] [Google Scholar]
- 13.Desai AA, Sachdeva PD, Arora BD. A comparative study of combined use of aceclofenac along with thiocolchicoside and aceclofenac alone in patients diagnosed of low back pain. Int J Pharm Sci. 2011;2:141–50. [Google Scholar]
- 14.Cabitza P, Randelli P. Efficacy and safety of eperisone in patients with low back pain: A double blind randomized study. Eur Rev Med Pharmacol Sci. 2008;12:229–35. [PubMed] [Google Scholar]
- 15.Chou R, Peterson K, Helfand M. Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: A systematic review. J Pain Symptom Manage. 2004;28:140–75. doi: 10.1016/j.jpainsymman.2004.05.002. [DOI] [PubMed] [Google Scholar]


