Abstract
Background:
The leaves of the Neurolaena lobata (Asteraceae) plant are used to control diabetes and heal wounds and infections.
Aim:
The ethanolic extract of N. lobata leaf was evaluated for its ability to heal inflicted wounds in rats using the excision wound model.
Materials and Methods:
Animals were divided into three groups of six each. Test group animals were treated topically with an ethanolic extract of N. lobata (1:1 with petroleum jelly, 100 mg/kg/day). Standard and control group animals were treated with mupirocin and petroleum jelly, respectively. Treatment was given for 13 days and the wound area was measured on alternate days. Parameters of healing assessed were the rate of wound contraction, period of epithelialization and hydroxyproline content. Antimicrobial activity of the extract was observed against Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli.
Results:
Phytochemical analysis of the extract showed the presence of saponins, tannins, alkaloids and flavanoids. Extract-treated animals exhibited 87% reduction in the wound area over 13 days when compared with the control (78%) and standard (83%) groups (P < 0.05). A significant decrease in the epithelialization period was noticed with the extract-treated test group animals compared with the controls and the standard group animals (P < 0.008). The hydroxyproline content of the extract-treated animals was higher (230.5 ± 42.1) when evaluated against the control and (79.0 ± 32.2) and the standard (115.0 ± 44.5) groups (P < 0.05).
Conclusion:
Increase in the rate of wound contraction and hydroxyproline content with decrease in epithelialization time in extract-treated animals support further evaluation of N. lobata as a pharmacotherapy for wound healing.
Keywords: Antimicrobial activity, epithelialization, hydroxyproline, Neurolaena lobata
INTRODUCTION
Regeneration of dermal and epidermal tissue is the body's natural process of wound healing. The pathological sequence after injury is inflammation; cells below the dermis begin to generate collagen production and, later, epithelial tissue (the outer skin layer) is regenerated.[1] The three stages of wound healing are inflammation, proliferation and remodeling. The proliferative phase is characterized by angiogenesis, collagen deposition, epithelialization and wound contraction. In angiogenesis, new blood vessel growth occurs from the endothelial cells. Subsequently, epithelial cells move slowly across the wound bed to cover it and the wound is contracted by myofibroblasts, which grip the wound edges, and the lesion undergoes contraction.
Zebapique is a popular herb of the Asteraceae family that is used in Trinidad and Tobago and the Caribbean for a number of aliments, most commonly the flu. It is also called “Jackass Bitters” (or by its scientific name “Neurolaena lobata”). In Trinidad, the leaves of the plant are used to make a tea that is very bitter. The leaves of the Zebapique contain a potent anti-parasitic agent called sesquiterpene dialdehyde that is effective against intestinal parasites, Candida and fungal infections. It is also used to control diabetes and heal wounds and infections.[2]
However, there is no scientifically proven data to our knowledge demonstrating the wound-healing activity of N. lobata. We undertook the present study to explore the effects of N. lobata leaf extract on excision wound.
MATERIALS AND METHODS
Plant material and extract preparation
The leaves of N. lobata were collected locally in March 2012, identified by the plant taxonomist and curator, National Herbarium of Trinidad and Tobago, the University of the West Indies, St. Augustine, Trinidad and a voucher specimen (specimen number: 36519) was deposited at the above-mentioned herbarium. Fresh leaves were shade dried and powdered using an electric blender. The fine powder (250 g) was suspended in 500 mL of ethanol for 20 h at room temperature. The mixture was filtered using a fine muslin cloth followed by filter paper (Whatman No. 1) and the filtrate was made into residue in a fume food with an exhaust fan. The clear residue (50 g) was used to evaluate the wound healing and antimicrobial activity and the phytochemical analysis.
Animals
The study was approved by the Ethics Committee for animal experimentation (AHC06/07/1), The Faculty of Medical Sciences, the University of the West Indies, St. Augustine.
An acute toxicity study was conducted to select the dose. The (n = 4) rats were treated orally by the staircase method with increasing (in geometrical progression) (100, 200, 400 and 800 mg/kg body weight) doses of extract dissolved in water for 14 days. With the 400 mg/kg body weight dose, no toxicity or mortality was observed and all the animals were physically active. On the basis of this observation, we selected the dose of 100 mg/kg body weight for the study.
Healthy inbred male Sprague Dawley rats weighing 200-220 g were used for the study. The animals were divided into three groups of six each. They were individually housed and maintained on normal food and water ad libitum. Animals were periodically weighed before and after the experiment. The rats were anesthetized prior to and during infliction of the experimental wounds. Ketamine at a dose of 80mg/kg body weight was used to anesthetize the animals and all the surgical interventions were carried out under sterile conditions. Animals were closely observed for any infection and excluded from the study and replaced if they showed signs of infection.
Wound-healing activity
An excision wound model was used to evaluate the wound-healing activity of N. lobata.
Excision wound model
Surgical intervention was carried out under aseptic conditions. Animals were anesthetized to inflict excision wounds as described by Morton and Malone.[3] The dorsal fur was shaved with an electric clipper and the anticipated area of the wound to be created was outlined on the back of the animals with methylene blue using a circular plastic cup. A full-thickness of the skin was excised to obtain a wound of circular area of approximately 150 mm2 diameter and 2 mm depth along the markings using a tooth forceps, surgical blade and pointed scissors. Wounds were blotted with cotton swabs soaked in normal saline. The test group animals received topical application of the ethanolic extract of N. lobata (1:1 ratio mixed with petroleum jelly, 100 mg/kg/day). The standard and control groups received treatment with mupirocin and petroleum jelly, respectively (100 mg/kg/day). Dosing was carried out for 13 days and the wound area was measured on alternate days. Parameters of efficacy studied were percentage wound contraction, time to epithelialization and collagen estimation.
The wound closure rate was assessed by tracing the wound on alternate days (1, 3, 5, 7, 9, 11 and 13) using transparency paper and a permanent marker. Subsequently, the recorded wound areas were measured using a graph paper. The point at which the eschar fell off without any residual raw wound was considered as the period of epithelialization.
On Day 13, the animals were sacrificed with an overdose of ketamine. The wounds were excised, leaving a 5 mm margin of normal skin around the edges of the wound. The wet weight of the granulation tissue was noted. The granulation tissue obtained was dried at 60°C for 12 h and weighed, and the dry weight was recorded. Five milliliters of 6N HCl was added to the dried tissue, which was then kept at 110°C for 24 h. The neutralized acid hydrolysate of the dry tissue was used for determination of hydroxyproline.[4] An additional piece of wet granulation tissue was preserved in 10% formalin for histological studies. For the better appreciation of collagen deposition, Mason Trichrome stain was used that stains the fibers blue green.
Phytochemical screening methods
Saponins
The extract (2 g) was boiled with 20 mL of water for about 4 min. This was mixed vigorously and left for a few minutes. The formation of frothing indicated the presence of saponins.[5]
Tannins
To an aliquot of the extract (dissolved in water), 2 mL of 1% ferric chloride was added. Color development from red-brown to blue-black indicated the presence of tannins.[5]
Triterpenes
The extract (1 g) was mixed with 10 mL of chloroform and warmed at 55°C for 30 min. Few drops (1-2 mL) of concentrated sulfuric acid were added and mixed well. The appearance of a reddish brown color indicated the presence of triterpenes.[6]
Alkaloids
The extract (1 g) was boiled with 50 mL methanol for 20 min in a water bath and the cooled filtrate was tested separately with Mayer's, Wagner's, Hager's and ammonium reineckate reagents. Cloudy precipitate of the alcoholic layer indicated the presence of alkaloids.[6]
Flavonoids
About 1 g of extract was boiled with 10 mL of ethyl acetate over a steam bath for 3 min. The filtrate of about 4 mL was mixed with 1 mL of dilute ammonia solution and a yellow precipitate indicated the presence of flavonoids.[5]
Antimicrobial Activity
The antibacterial effect of N. lobata was evaluated using laboratory strains of Pseudomonas aeruginosa (ATCC 27853), Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 25922) using the agar well diffusion method of Lovian.[7] Sensitivity testing was performed using Muller Hinton agar plates with a known volume of bacterial suspension in microplate wells. Ten microliters of the ethanol extract of N. lobata was added to the microplate wells and incubated at 35-37°C for 18-20 h. Results were determined by visual inspection of zones of growth inhibition.
Histological Study
The granulation tissues obtained on Day 13 from all the three groups were fixed in 10% buffered formal saline and processed for routine histological evaluation. Sections of 7.0 micron were cut from the tissues and stained with Masson's trichrome stain.
Statistical Analysis
The means of the wound area measurements between groups at different time intervals were compared using one-way ANOVA, followed by Tukey's post hoc tests. Data were analyzed using SPSS (Version 12.0, Chicago, IL, USA) and the P value was set at <0.05 for all analyses.
RESULTS
The phytochemical analysis of the leaf extract by the qualitative study showed the presence saponins, tannins, alkaloids and flavonoids and the absence of volatile oils.
Table 1 shows the effects of the N. lobata leaf extract administered topically on wound-healing activity in rats inflicted with excision wound. On Day 13, animals treated with the extract exhibited 87% reduction in the wound area compared with the controls (78%) and the standard (83%) group (P < 0.05) [Figure 1]. After treatment with the extract of N. lobata, animals showed a significant decrease in the epithelialization period (9.0 ± 0.7) compared with the control (12.1 ± 0.30) and the standard groups (10.0 ± 0.68) (P < 0.008). The hydroxyproline content of the extract-treated animals was higher (230.5 ± 42.1) in comparison with the control (79.0 ± 32.2) and the standard group animals (115.0 ± 44.5) (P < 0.05). A histological study of the granulation tissue obtained on Day 13 from the experimental animals showed marked, well-organized bands of collagen, moderate fibroblasts, few inflammatory cells and blood vessels [Figure 2]. The mupirocin-treated animals showed moderate collagen and marked number of fibroblasts [Figure 3]. The granulation tissue obtained from petroleum jelly-treated animals showed minimal collagen fibers, moderate inflammation and fibroblasts [Figure 4]. Pseudomonas aeruginosa (ATCC 27853), Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 25922) were sensitive to the leaf extract of N. lobata, determined by zones of growth inhibition.
Table 1.
Wound healing effect of N. lobata in the excision wound model
Figure 1.
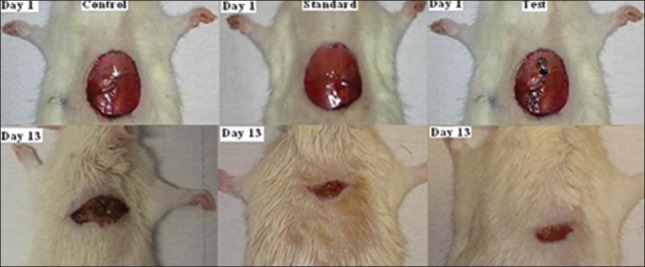
Wound area contraction of the control, standard and experimental animals on Days 1 and 13
Figure 2.
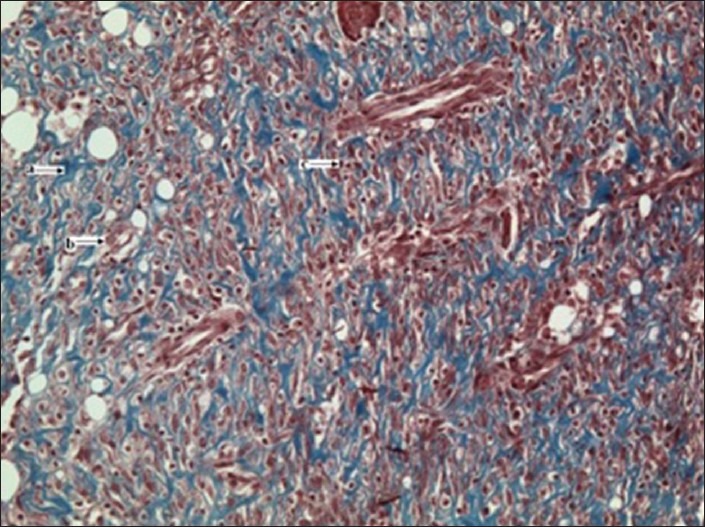
(x20 magnification): Experimental group granulation tissue from the excision wound showing abundant collagen deposits (Masson's trichrome stain); a = collagen fibers, b = fibroblast, c = inflammatory cells
Figure 3.
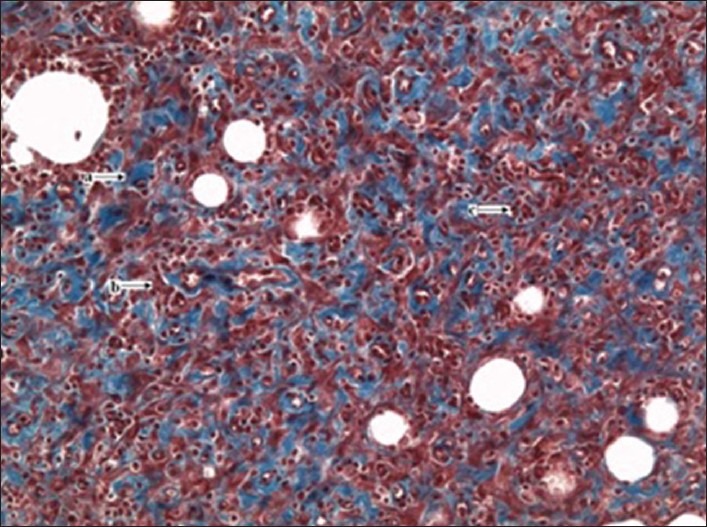
(x20 magnification): Standard group granulation tissue from the excision wound showing moderate collagen deposits (Masson's trichrome stain); a = collagen fibers, b = fibroblast, c = inflammatory cells
Figure 4.
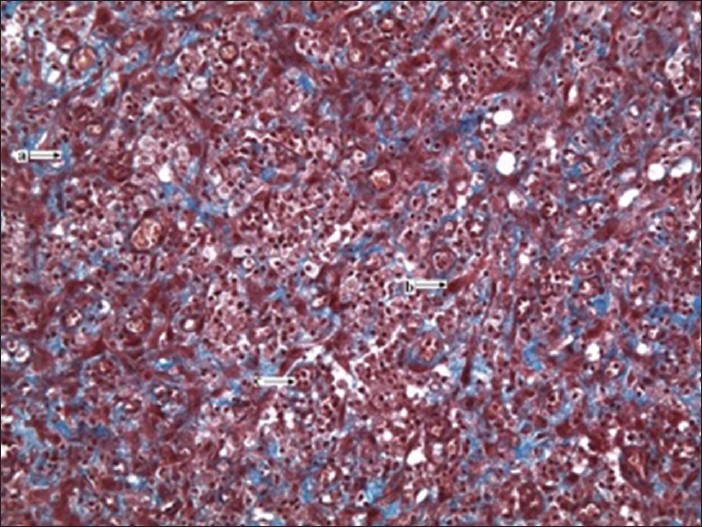
(x20 magnification): Control group granulation tissue from the excision wound model showing minimal collagen deposits (Masson's trichrome stain); a = collagen fibers, b = fibroblast, c = inflammatory cells
DISCUSSION
Our observation of increased rate of wound contraction and hydroxyproline content and decrease in epithelialization time of the extract of N. lobata in the excision wound model-treated animals support its healing activity in cutaneous wounds. Successful wound healing is accomplished by early healing, with minimal pain and closure by scar tissue that has high tensile strength.
The ethanol extract of N. lobata increased the rate of wound contraction and the rate of epithelialization in the excision wound. Epithelialization after injury involves the proliferation and migration of epithelial cells toward the center of the wound while wound contraction is largely due to the action of myofibroblasts.[8] This suggests that N. lobata may enhance the migration and proliferation of epithelial cells with little or no effect on myofibroblasts principally responsible for contraction. The increased hydroxyproline content of the granulation tissue is an index of increased collagen turnover.[9] Collagen, the major component of granulation tissue, is the foremost constituent that provides strength and support to the extracellular tissue. It is composed of the amino acid, hydroxyproline, which is used as a biochemical marker for tissue collagen.[10,11]
The crude extract demonstrated the antimicrobial activity against Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli. Lentz et al. also reported an inhibitory activity of N. lobata on bacterial cultures.[12] The antimicrobial effect of the extract might have inhibited the growth of microorganisms that infect and delay the healing process. Antimicrobial activity may also contribute to the enhanced wound healing activity of the extract in terms of reduced wound area in this excision wound model, a feature reported elsewhere.[13,14] It is thus likely that the antibacterial activity of N. lobata may contribute to its wound-healing potential.
The phytochemical analysis of the leaf extract by the qualitative method showed the presence of saponins, tannins, alkaloids and flavanoids, all of which promote the process of wound healing.[15,16] Tannins act as scavengers of free radicals, saponins have antimicrobial and antioxidant effects that contribute to increased epithelialization and thereby wound contraction, and flavonoids are also antioxidant and free radical scavengers.[17] The abundance of tannins, which has been implicated in the hemostatic activity of plants where they arrest bleeding from damaged or injured vessels by precipitating proteins to form vascular plugs, may partly contribute to the hemostatic activity of N. lobata. In the process of healing, the neutrophil migration in cutaneous wounds causes lipid peroxidation and generation of free oxygen radicals. The improved antioxidant profile of these phytochemicals would enhance collagen deposition and strength.
Throughout the course of the experimentation, the extract was well tolerated by the animals. There was no abnormal behavior such as restlessness, aggressiveness or irritation at the wound site. Our data demonstrate that the topical administration of the ethanol extract of N. lobata facilitates wound healing in the excision wound model in rats. Promotion of wound healing could be attributed to the phytochemical constituents in the plant, which, by their individual or collective activity, enhance antioxidant and antimicrobial properties. Further studies with the purified extract may elucidate the mechanism of wound healing of N. lobata.
CONCLUSIONS
The results of our study showed that the extract of N. lobata has potential for use in wound care due to the ability of its constituents to arrest bleeding from fresh wounds, inhibit the growth of bacteria and accelerate wound healing by enhancing epithelialization and stimulating tissue proliferation.
ACKNOWLEDGMENT
The authors sincerely thank Mr. Terry Ramnanan Singh and Miss Sabana Meyers for their technical assistance.
Footnotes
Source of Support: Nill.
Conflict of Interest: None declared.
REFERENCES
- 1.Mahabir D, Gulliford MC. Use of medicinal plants for diabetes in Trinidad and Tobago. Rev Panam Salud Publica. 1997;1:174–9. doi: 10.1590/s1020-49891997000300002. [DOI] [PubMed] [Google Scholar]
- 2.Iba Y, Shibata A, Kato M, Masukawa T. Possible involvement of mast cells in collagen remodeling in the late phase of cutaneous wound healing in mice. Int Immunopharmacol. 2004;4:1873–80. doi: 10.1016/j.intimp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Morton JJ, Malone MH. Evaluation of vulnerary activity by an open wound procedure in rats. Arch Int Pharmacodyn. 1972;196:117–26. [PubMed] [Google Scholar]
- 4.Neuman RE, Logan MA. The determination of hydroxyproline. J Biol Chem. 1950;184:299. [PubMed] [Google Scholar]
- 5.Kapoor LD, Singh A, Kapoort SL, Strivastava SN. Survey of Indian Medicinal Plants for Saponins. Alkaloids and Flavonoids. Lloydia. 1969;32:297–302. [PubMed] [Google Scholar]
- 6.Harborne JB. Photochemical Methods: A Guide to Modern Techniques of Plant Analysis. London: Chapman A and Hall; 1973. p. 279. [Google Scholar]
- 7.Lovian V. Antibiotics in Laboratory Medicine. Baltimore, London: Williams and Williams; 1980. [Google Scholar]
- 8.Cotran RS, Kumar V, Robbins SL, Schoen FJ. Inflammation and repair. 5th ed. Pennsylvania: W.B. Saunders Company; 1994. Robbins Pathologic Basis of Disease; pp. 51–92. [Google Scholar]
- 9.Sasidharan S, Logeswaran S, Latha LY. Wound healing activity of Elaeis guineensis leaf extract ointment. Int J Mol Sci. 2012;13:336–47. doi: 10.3390/ijms13010336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar R, Katoch SS, Sharma S. β-Adrenoceptor agonist treatment reverses denervation atrophy with augmentation of collagen proliferation in denervated mice gastrocnemius muscle. Indian J Exp Biol. 2006;44:371–6. [PubMed] [Google Scholar]
- 11.Pattanayak SP, Sunita P. Wound healing, anti-microbial and antioxidant potential of Dendrophthoe falcata (L.F) Ettingsh. J Ethnopharmacol. 2008;120:241–7. doi: 10.1016/j.jep.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Lentz DL, Clark AM, Hufford CD, Meurer-Grimes B, Passreiter CM, Cordero J, et al. Antimicrobial properties of Honduran medicinal plants. J Ethnopharmacol. 1998;63:253–63. doi: 10.1016/s0378-8741(98)00100-7. [DOI] [PubMed] [Google Scholar]
- 13.Okoli CO, Akah PA, Okoli AS. Potentials of leaves of Aspilia africana (Compositae) in wound care: An experimental evaluation. BMC Complement Altern Med. 2007;7:24–9. doi: 10.1186/1472-6882-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carson CF, Riley TV, Cookson BD. Efficacy and safety of tea tree oil as a topical antimicrobial agent. J Hosp Infect. 1998;40:175–8. doi: 10.1016/s0195-6701(98)90135-9. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhari M, Mengi S. Evaluation of phytoconstituents of Terminalia arjuna for wound healing activity in rats. Phytother Res. 2006;20:799–805. doi: 10.1002/ptr.1857. [DOI] [PubMed] [Google Scholar]
- 16.Sumitra M, Manikandan P, Gayathri VS, Mahendran P, Suguna L. Emblica officinalis exerts wound healing action through up-regulation of collagen and extracellular signal-regulated kinases (ERK1/2) Wound Repair Regen. 2009;17:99–107. doi: 10.1111/j.1524-475X.2008.00446.x. [DOI] [PubMed] [Google Scholar]
- 17.Thakur R, Jain N, Pathak R, Sandhu SS. Practices in wound healing studies of plants. Evidence Based Complement Alternat Med. 2011;2011:438056. doi: 10.1155/2011/438056. [DOI] [PMC free article] [PubMed] [Google Scholar]


