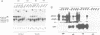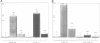Abstract
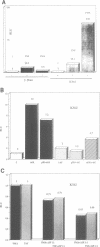
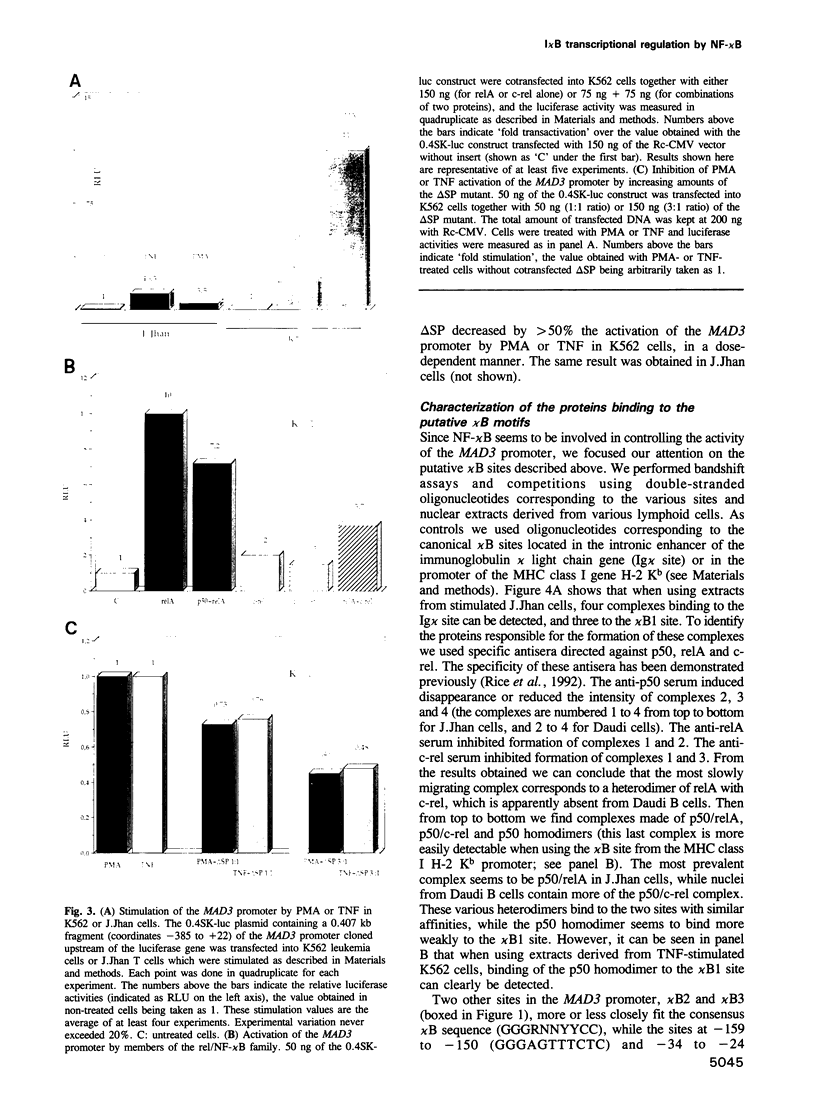
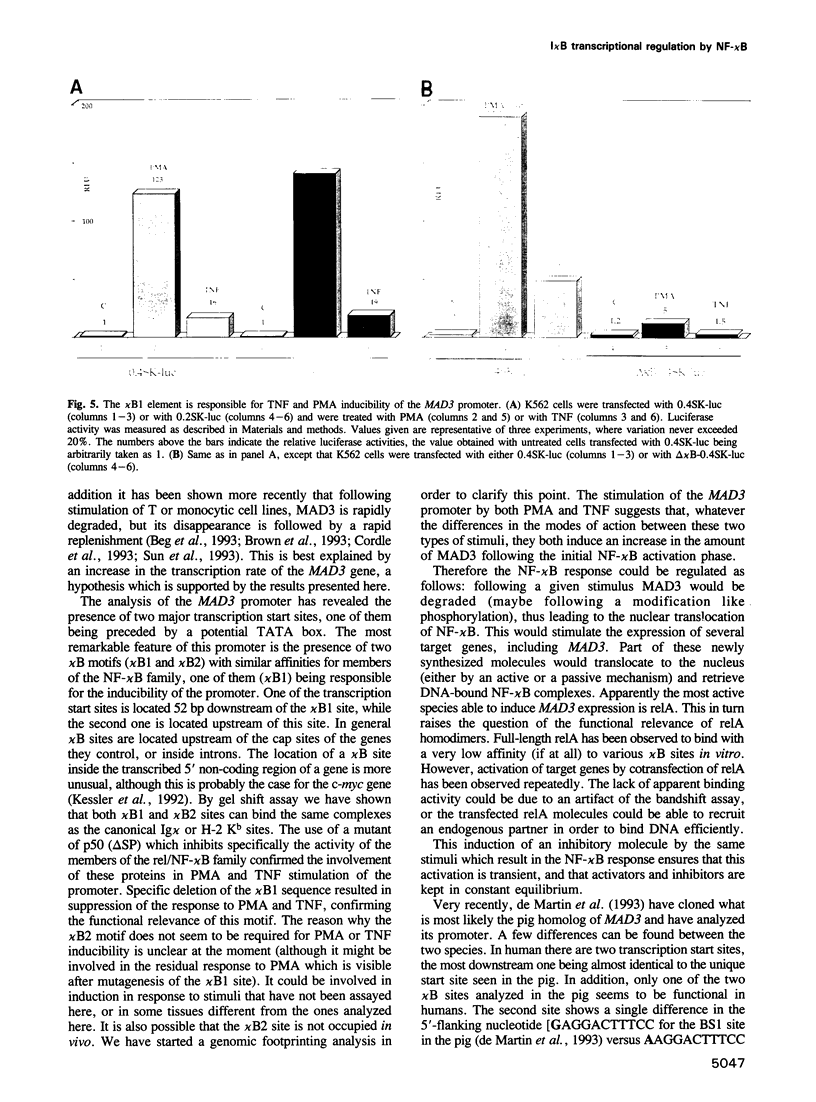
In order to characterize the regulation of the gene encoding the I kappa B-alpha/MAD3 inhibitor of the transcription factor NF-kappa B, we have isolated a human genomic clone and sequenced the promoter of this gene. The MAD3 promoter exhibits a potential TATA element upstream of one of the two major transcription sites, and contains several potential NF-kappa B binding sequences, suggesting that the gene is positively regulated by members of this family. Transfection experiments demonstrate that the MAD3 promoter can be activated by various combinations of members of the rel/NF-kappa B family, as well as by phorbol esters and tumor necrosis factor. Specific deletion of one of the kappa B motifs, located 37 bp upstream of the TATA box, abolishes responses to PMA and TNF. This kappa B motif binds NF-kappa B (p50/relA), p50/c-rel and relA/c-rel heterodimers as well as KBF1 (p50 homodimer). These results help to explain the previously observed transient nature of the NF-kappa B response: following NF-kappa B activation, the expression of the inhibitor is increased, therefore the extent of nuclear translocation of the active complex is reduced, resulting in a decreased activation of its target genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988 Apr 22;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Lenardo M., Pierce J. W., Baltimore D. Phorbol-ester-induced activation of the NF-kappa B transcription factor involves dissociation of an apparently cytoplasmic NF-kappa B/inhibitor complex. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):789–798. doi: 10.1101/sqb.1988.053.01.089. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Finco T. S., Nantermet P. V., Baldwin A. S., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993 Jun;13(6):3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg A. A., Ruben S. M., Scheinman R. I., Haskill S., Rosen C. A., Baldwin A. S., Jr I kappa B interacts with the nuclear localization sequences of the subunits of NF-kappa B: a mechanism for cytoplasmic retention. Genes Dev. 1992 Oct;6(10):1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- Blank V., Kourilsky P., Israël A. NF-kappa B and related proteins: Rel/dorsal homologies meet ankyrin-like repeats. Trends Biochem Sci. 1992 Apr;17(4):135–140. doi: 10.1016/0968-0004(92)90321-y. [DOI] [PubMed] [Google Scholar]
- Brown K., Park S., Kanno T., Franzoso G., Siebenlist U. Mutual regulation of the transcriptional activator NF-kappa B and its inhibitor, I kappa B-alpha. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2532–2536. doi: 10.1073/pnas.90.6.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capobianco A. J., Gilmore T. D. Repression of the chicken c-rel promoter by vRel in chicken embryo fibroblasts is not mediated through a consensus NF-kappa B binding site. Oncogene. 1991 Dec;6(12):2203–2210. [PubMed] [Google Scholar]
- Cordle S. R., Donald R., Read M. A., Hawiger J. Lipopolysaccharide induces phosphorylation of MAD3 and activation of c-Rel and related NF-kappa B proteins in human monocytic THP-1 cells. J Biol Chem. 1993 Jun 5;268(16):11803–11810. [PubMed] [Google Scholar]
- Davis N., Bargmann W., Lim M. Y., Bose H., Jr Avian reticuloendotheliosis virus-transformed lymphoid cells contain multiple pp59v-rel complexes. J Virol. 1990 Feb;64(2):584–591. doi: 10.1128/jvi.64.2.584-591.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N., Ghosh S., Simmons D. L., Tempst P., Liou H. C., Baltimore D., Bose H. R., Jr Rel-associated pp40: an inhibitor of the rel family of transcription factors. Science. 1991 Sep 13;253(5025):1268–1271. doi: 10.1126/science.1891714. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Grilli M., Chiu J. J., Lenardo M. J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Grumont R. J., Gerondakis S. Murine c-rel transcription is rapidly induced in T-cells and fibroblasts by mitogenic agents and the phorbol ester 12-O-tetradecanoylphorbol-13-acetate. Cell Growth Differ. 1990 Aug;1(8):345–350. [PubMed] [Google Scholar]
- Haskill S., Beg A. A., Tompkins S. M., Morris J. S., Yurochko A. D., Sampson-Johannes A., Mondal K., Ralph P., Baldwin A. S., Jr Characterization of an immediate-early gene induced in adherent monocytes that encodes I kappa B-like activity. Cell. 1991 Jun 28;65(7):1281–1289. doi: 10.1016/0092-8674(91)90022-q. [DOI] [PubMed] [Google Scholar]
- Hohmann H. P., Remy R., Scheidereit C., van Loon A. P. Maintenance of NF-kappa B activity is dependent on protein synthesis and the continuous presence of external stimuli. Mol Cell Biol. 1991 Jan;11(1):259–266. doi: 10.1128/mcb.11.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. D., Inoue J., Davis N., Link E., Baeuerle P. A., Bose H. R., Jr, Verma I. M. The rel-associated pp40 protein prevents DNA binding of Rel and NF-kappa B: relationship with I kappa B beta and regulation by phosphorylation. Genes Dev. 1991 Aug;5(8):1464–1476. doi: 10.1101/gad.5.8.1464. [DOI] [PubMed] [Google Scholar]
- Kessler D. J., Duyao M. P., Spicer D. B., Sonenshein G. E. NF-kappa B-like factors mediate interleukin 1 induction of c-myc gene transcription in fibroblasts. J Exp Med. 1992 Sep 1;176(3):787–792. doi: 10.1084/jem.176.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Link E., Kerr L. D., Schreck R., Zabel U., Verma I., Baeuerle P. A. Purified I kappa B-beta is inactivated upon dephosphorylation. J Biol Chem. 1992 Jan 5;267(1):239–246. [PubMed] [Google Scholar]
- Logeat F., Israël N., Ten R., Blank V., Le Bail O., Kourilsky P., Israël A. Inhibition of transcription factors belonging to the rel/NF-kappa B family by a transdominant negative mutant. EMBO J. 1991 Jul;10(7):1827–1832. doi: 10.1002/j.1460-2075.1991.tb07708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R., Hatada E. N., Hohmann H. P., Haiker M., Bartsch C., Röthlisberger U., Lahm H. W., Schlaeger E. J., van Loon A. P., Scheidereit C. Cloning of the DNA-binding subunit of human nuclear factor kappa B: the level of its mRNA is strongly regulated by phorbol ester or tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):966–970. doi: 10.1073/pnas.88.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin P. J., Gilmore T. D. The C terminus of the NF-kappa B p50 precursor and an I kappa B isoform contain transcription activation domains. Nucleic Acids Res. 1992 May 25;20(10):2453–2458. doi: 10.1093/nar/20.10.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., MacKichan M. L., Israël A. The precursor of NF-kappa B p50 has I kappa B-like functions. Cell. 1992 Oct 16;71(2):243–253. doi: 10.1016/0092-8674(92)90353-e. [DOI] [PubMed] [Google Scholar]
- Ruben S. M., Dillon P. J., Schreck R., Henkel T., Chen C. H., Maher M., Baeuerle P. A., Rosen C. A. Isolation of a rel-related human cDNA that potentially encodes the 65-kD subunit of NF-kappa B. Science. 1991 Mar 22;251(5000):1490–1493. doi: 10.1126/science.2006423. [DOI] [PubMed] [Google Scholar]
- Schmitz M. L., Henkel T., Baeuerle P. A. Proteins controlling the nuclear uptake of NF-kappa B, Rel and dorsal. Trends Cell Biol. 1991 Nov;1(5):130–137. doi: 10.1016/0962-8924(91)90118-s. [DOI] [PubMed] [Google Scholar]
- Schwartz O., Virelizier J. L., Montagnier L., Hazan U. A microtransfection method using the luciferase-encoding reporter gene for the assay of human immunodeficiency virus LTR promoter activity. Gene. 1990 Apr 16;88(2):197–205. doi: 10.1016/0378-1119(90)90032-m. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Sun S. C., Ganchi P. A., Ballard D. W., Greene W. C. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993 Mar 26;259(5103):1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- Ten R. M., Paya C. V., Israël N., Le Bail O., Mattei M. G., Virelizier J. L., Kourilsky P., Israël A. The characterization of the promoter of the gene encoding the p50 subunit of NF-kappa B indicates that it participates in its own regulation. EMBO J. 1992 Jan;11(1):195–203. doi: 10.1002/j.1460-2075.1992.tb05042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari M., Dobrzanski P., Mohn K. L., Cressman D. E., Hsu J. C., Bravo R., Taub R. Rapid induction in regenerating liver of RL/IF-1 (an I kappa B that inhibits NF-kappa B, RelB-p50, and c-Rel-p50) and PHF, a novel kappa B site-binding complex. Mol Cell Biol. 1992 Jun;12(6):2898–2908. doi: 10.1128/mcb.12.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari M., Mohn K. L., Yue F. E., Taub R. Sequence of rat RL/IF-1 encoding an IkappaB, and comparison with related proteins containing notch-like repeats [corrected]. Nucleic Acids Res. 1992 Feb 11;20(3):607–607. doi: 10.1093/nar/20.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano O., Kanellopoulos J., Kieran M., Le Bail O., Israël A., Kourilsky P. Purification of KBF1, a common factor binding to both H-2 and beta 2-microglobulin enhancers. EMBO J. 1987 Nov;6(11):3317–3324. doi: 10.1002/j.1460-2075.1987.tb02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel U., Baeuerle P. A. Purified human I kappa B can rapidly dissociate the complex of the NF-kappa B transcription factor with its cognate DNA. Cell. 1990 Apr 20;61(2):255–265. doi: 10.1016/0092-8674(90)90806-p. [DOI] [PubMed] [Google Scholar]
- Zabel U., Henkel T., Silva M. S., Baeuerle P. A. Nuclear uptake control of NF-kappa B by MAD-3, an I kappa B protein present in the nucleus. EMBO J. 1993 Jan;12(1):201–211. doi: 10.1002/j.1460-2075.1993.tb05646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martin R., Vanhove B., Cheng Q., Hofer E., Csizmadia V., Winkler H., Bach F. H. Cytokine-inducible expression in endothelial cells of an I kappa B alpha-like gene is regulated by NF kappa B. EMBO J. 1993 Jul;12(7):2773–2779. doi: 10.1002/j.1460-2075.1993.tb05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]