Abstract
Context:
Sympathoadrenal response to laryngoscopy and tracheal intubation manifests as transient, but distinct tachycardia and hypertension.
Aims:
The objective of this study is to compare the clinical effects of dexmedetomidine with esmolol and control in attenuating the presser response during laryngoscopy.
Settings and Design:
A randomized, prospective, double-blind, controlled study.
Subjects and Methods:
We studied consented, 90 adult, American Society of Anesthesiologists physical status I and II patients of either sex, scheduled for non-cardiac surgery requiring intubation. The patients were randomly divided into three groups (n = 30). Group C received placebo, Group E received 2.0 mg/kg of esmolol and Group D received 1.0 μg/kg of dexmedetomidine, intravenously over 10 min and 3 min before induction of general anesthesia. All patients were uniformly pre-medicated, induced and intubated using thiopentone and succinylcholine as per standard protocol. Heart rate (HR), systemic arterial pressures were recorded at baseline, after study drug infusion, after induction, immediately and 3, 5, 7, 10 min after intubation.
Statistical Analysis:
Analysis of variance and t-test as appropriate.
Results:
The mean arterial pressure was significantly increased in patients receiving placebo (P < 0.0001) and esmolol (P < 0.0001) after laryngoscopy and intubation compared with baseline value and Group D (P = 0.6294). The rise in HR (P = 0.08481) and rate pressure product (P = 0.0666) at the time of intubation were minimal and was statistically significant up to 15 min in Group D.
Conclusions:
Both the drugs attenuated the pressure response. Of the two drugs administered, dexmedetomidine 1.0 μg/kg provides a consistent, reliable and effective attenuation of pressure responses when compared to esmolol 2.0 mg/kg.
Keywords: Dexmedetomidine, esmolol, hemodynamic response, intubation, laryngoscopy
INTRODUCTION
Laryngoscopy and tracheal intubation are noxious stimuli that evoke a transient but marked sympathetic response manifesting as increase in heart rate (HR), blood pressure these changes are maximum immediately after intubation and lasts for 5-10 min, which may be well-tolerated by normal fit American Society of Anesthesiologists (ASA) 1 patients. In patients with cardiovascular disease the hemodynamic changes may lead to life threatening complications including myocardial ischemia, acute heart failure and cerebrovascular accidents.[1] Treatment modalities include topical lignocaine sprays, deeper planes of anesthesia by inhalational/intravenous (IV) agents or narcotics, calcium channel blockers, vasodilators such as sodium-nitroprusside; nitroglycerine etc.[2] Although there are several methods, research is still going on for attenuation of pressor response to laryngoscopy and intubation.[3] Esmolol is an ultra-short-acting, beta-adrenergic receptor antagonist;[4] with proven efficacy to provide hemodynamic stability during laryngoscopy and tracheal intubation without severe side-effects.[5] In contrast to this, there have been a very few reports on the effects of dexmedetomidine.
Dexmedetomidine is an imidazole derivative and highly selective alpha (α)-2-adrenergic receptor agonist.[6] α2 -agonists produce hyperpolarization of noradrenergic neurons and suppression of neuronal firing in the locus cerelous leads to decreased systemic noradrenalin release results in attenuation of sympathoadrenal responses and hemodynamic stability during laryngoscopy and tracheal intubation.[7] The topic of study was selected because tachycardia and hypertension may get further enhanced in response, to laryngoscope and tracheal intubation, proven dangerous to cardiovascular disease patients.
In a randomized, prospective, double-blind, controlled study, we compare the safety and efficacy of single bolus IV dose of dexmedetomidine with single bolus IV dose of esmolol and placebo in attenuating hemodynamic response to laryngoscopy and tracheal intubation.
SUBJECTS AND METHODS
After approval of the study protocol by the Institutional Ethical Committee, written informed consent was obtained from each patient. 90 normotensive, ASA physical status I and II patients of either sex, aged 40-60 years, who were scheduled for elective non-cardiac surgery under general anesthesia (GA) requiring endotracheal (ET) intubation, were included in this study. All patients were thoroughly examined and routine investigations were carried out. The patients who refuse to consent, patients whose physical characteristics suggested difficulties in intubation (Mallampati grades III and IV), who had hypertension or cardiovascular, respiratory, neurological, psychological, hepatic, endocrinal, renal disease and taking any medication (e.g. opioids or sedatives in the week prior to surgery), having history of alcohol abuse or drug allergies, pregnant and lactating patients were excluded from the study.
Baseline (average of three readings) vital parameters of patients’ including HR, systolic arterial pressure (SAP), diastolic arterial pressure (DAP); mean arterial pressure (MAP) and oxygen saturation were recorded in the pre-operative ward. After 1 h patients were taken to the operation theatre. In the operating room an IV line was secured with 18-G venous cannula and Ringer's lactate infusion (6 ml/kg) was started. Routine standard monitors such as pulse oxymetry, electrocardiography (ECG) and non-invasive blood pressure were applied and monitoring started. All the patients were uniformly pre-medicated with IV ondansetron 0.08 mg/kg and glycopyrrolate 0.004 mg/kg, 10 min before induction. The study drugs were premixed to a volume of 10 ml and were presented as coded syringes by an anesthesiologist who is not involved in the study. The patients were blinded to the treatment group and all recordings were performed by an anesthesiologist blinded to the group allocation, thus the study was made double-blinded.
The patients were randomly divided into three equal groups of 30 each (Balanced randomization using a computer program, block size 10). The patients in control Group C received 10 ml of physiologic saline, Group E received esmolol 2.0 mg/kg and Group D received dexmedetomidine 1.0 μg/kg as slow IV infusion over a period of 10 min. The patient's HR and blood pressure were stabilized and pre-oxygenated for 3 min after study drug infusion. Then the induction of anesthesia was performed with thiopentone 5.0 mg/kg and then succinylcholine 2.0 mg/kg was administered IV as per standard protocol. The patient's lungs were ventilated manually with 100% oxygen. Laryngoscopy was attempted 90 s after the administration of succinylcholine with Macintosh curved blade number 4 by an anesthesiologist having more than 9 years of experience. The trachea was intubated with appropriate size-cuffed disposable ET tube. Laryngoscopy and intubation was limited to 15-20 s in all patients, failure to intubate within this period was excluded from this study. After confirming the position and fixing the ET tube with adhesive plaster anesthesia was maintained with, 66% N2O in 33% oxygen and 1% sevoflurane in 6 l of fresh gas flow. Bolus IV dose of 0.08 mg/kg followed by intermittent dose of 0.02 mg/kg vecuronium was used for muscle relaxation. At the end of the surgery all patients were reversed with neostigmine 0.05 mg/kg and glycopyrrolate 0.008 mg/kg IV. Patients were extubated after adequate recovery and then shifted to anesthesia recovery room and monitored for complications such as pain, respiratory depression; hypertension, hypotension, bradycardia, drowsiness, rigidity, nausea or vomiting and attended appropriately, rescue treatment was also noted during anesthesia and recovery.
Vital parameters such as HR, SAP, DAP and MAP were recorded, at baseline, after study drug infusion, after induction, immediately and 3, 5, 7 and 10 min after intubation and every 5 min there after using multipara monitor. No surgical intervention was allowed throughout the study period of 10 min. The hemodynamic alterations like a decrease in MAP greater than 20% below the baseline value or SAP less than 90 mm of Hg was treated with primarily by increasing the IV fluid infusion rate and then reducing sevoflurane concentration or incremental doses of ephedrine 4 mg bolus IV. Decrease in HR (<50 beats/min) was treated with atropine 0.5 mg IV.
Statistical analysis
After the initial pilot observations, it was decided that a 20% of difference should be the minimum detectable difference of means in all groups. The standard deviation (SD) of residual was also kept same (20% of average difference between the groups). The α value was 0.05 and the power (1-a) of the study was 0.80. Thus, the calculated sample size for each group was 23 patients. Preserving the designing effect it was decided to include 30 patients in each group. Groups were compared for demographic data (age, weight) and hemodynamic parameters (HR, blood pressure) by one way analysis of variance and paired t-test was used for comparison among the groups, while for comparison within the groups unpaired t-test was used. Probability was considered to be significant if less than 0.05. Data are represented as mean and SD.
RESULTS
All Cases were selected from general surgery only; all the 90 patients completed the study. The demographic profile of the patients in terms of age, body weight, male:female ratio, ASA status, Mallampati Class were comparable and there were no significant differences among the three groups (P > 0.05) [Table 1].
Table 1.
Patient's characteristics

The increase in mean HR after intubation was seen in all the three groups. But the mean increase was minimal 5.83% in Group D (4 beats, P = 0.0848), when compared with Group E 14% (9.81beats; P = 0.0152) and Group C 30% (24.9 beats; P < 0.0001), which was highly significant (P < 0.0001). Also, only in the Group D, there was no significant rise of HR at any time interval [Figure 1].
Figure 1.

Mean heart rate of patients in Groups C-D
The mean SAP levels in Group D were significantly lower than Groups C and E immediately after intubation (P < 0.001, P > 0.001 respectively) and until the end of surgery. Esmolol does not prevented the raise in SAP following intubation, but the raise was significantly less (P = 0.0269) when compared with the patients who does not received any drug [Table 2].
Table 2.
Comparison of SAP (mm of Hg) in the three groups

The DAP levels in Group D were significantly lower than Groups C and E at all times after intubation. In esmolol group, there is a transient raise 21.4% (16.63 mm Hg) in DAP following intubation (P < 0.0001) at other times it remained below the baseline level [Table 3].
Table 3.
Comparison of diastolic arterial pressure (mm of Hg) in the three groups

The MAP was comparable in all the three groups at baseline level. The MAP decreased following induction, which was significant in Group C (P = 0.024) but not significant in Group E (P = 0.088) and Group D (P = 0.3145). The MAP rose by 30% (28.04 mm Hg) in Group C, 26% (24.00 mm Hg) in Group E and only 2% (1.67 mm Hg) in Group D at intubation. The rise in MAP was highly significant after intubation in Group C (P < 0.0001) and significant in Group E (P < 0.05) which was not significant in Group D (P > 0.05) [Table 4].
Table 4.
Comparison mean arterial pressure (mm Hg) level in three groups

The rate pressure product (RPP) was calculated as the product of HR and SAP (RPP = HR × SAP). In our study the RPP during intubation revealed a highly significant increase in Group C (76.5%, P < 0.0001) and significant increase in Group E (49%, P < 0.001), whereas the increase was insignificant in Group D (16%, P > 0.0666). These changes were highly significant up to 15 min post-intubation. Although comparing Group E to Group D the increase in RPP in Group E at the time of intubation (P < 0.001) was statistically significant. The rise in mean RPP was least in Group D and highest in Group C [Figure 2].
Figure 2.
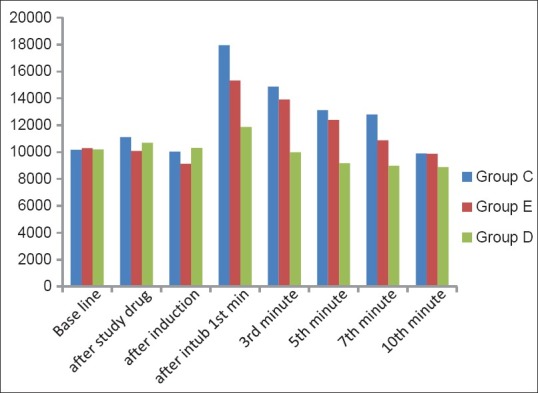
The comprehensive changes in rate pressure product of patients in Groups C-D
DISCUSSION
In this study infusion of dexmedetomidine 1.0 μg/kg prior to induction of anesthesia suppressed the hemodynamic response to tracheal intubation in normotensive patients. This suppression in cardiovascular responses was found to be greater with dexmedetomidine than that resulted from infusion of esmolol 2.0 mg/kg.
Tachycardia and hypertension are more common following laryngoscope and ET intubation. Prophylaxis include topical lignocaine sprays, deeper planes of anesthesia by inhalational agents; narcotics, calcium channel blockers, vasodilators such as sodium-nitroprusside; nitroglycerine etc.,[2] but they have got side-effects such as sedation, respiratory depression, hypotension and bradycardia. Dexmedetomidine has sedative, anxiolytic, analgesic and sympatholytic, effects may blunt the cardiovascular responses in the peri-operative period without causing significant respiratory depression.
Among the β-adrenergic blocking drugs, esmolol seems to be an appropriate selection for attenuating the hemodynamic response to laryngoscopy and tracheal intubation, because of its cardioselectivity, rapid onset of action and short elimination half-life.[8] There have been several reports discussing the effects of esmolol on both HR and arterial blood pressure during laryngoscopy and ET intubation compared with placebo. Miller et al.[9] in their study have reported that 100 mg of single bolus dose of esmolol was effective for controlling the hemodynamic response to tracheal intubation in a Canadian multicenter trial. Liu et al. who used esmolol infusion to control hemodynamic responses associated with intubation, found significant decreases in HR and SAP prior to induction and post-intubation, the increase was 50% less in the esmolol-treated patients compared to the placebo group.[10]
Esmolol decreases the force of contraction and HR by blocking beta-adrenergic receptors of the sympathetic nervous system which are found in the heart, blood vessels and other organs of the body. Esmolol prevents the action of two naturally occurring neurotransmitters epinephrine and nor-epinephrine, there by attenuates the tachycardia and hypertensive responses to laryngoscopy and tracheal intubation.
Although esmolol is considered to have significant effect on both tachycardia and hypertensive response following ET intubation, Oxorn et al.[11] concluded that esmolol in bolus doses of 100 mg and 200 mg affects solely the chronotropic response in a significant manner. Kindler et al. found that esmolol administration before laryngoscopy was sufficient to control HR after intubation but it did not affect SAP.[12] Similarly, in this study, esmolol was not as effective on attenuating the hypertensive response as it was on attenuating the chronotropic response to tracheal intubation. In fact, a significant increase in SAP and a transient raise in DAP was observed after intubation compared to the baseline values and when compared with dexmedetomidine the increase in SAP was greater and more significant in this study.
Direct acting α2 -adrenoceptor agonists represent clinically significant effects on the anesthetic requirements and on the sympathoadrenal and hemodynamic responses induced by anesthesia including tracheal intubation and surgery. Scheinin et al.[13] reported that 0.6 μg/kg dexmedetomidine decreased, but not totally suppressed, the hemodynamic response to tracheal intubation in healthy individuals. Keniya et al. stated that the pre-treatment with dexmedetomidine 1.0 μg/kg attenuated, but not totally obtunded the cardiovascular response to tracheal intubation after induction of anesthesia.[14] In this study, we did not observed any significant differences in HR and arterial blood pressure values between the baseline and post-intubation values in the dexmedetomidine group. Similar to the two studies mentioned above, the mean percentage variation analysis at the stated moments revealed an absence of any increase in HR, SAP and DAP in dexmedetomidine group suggesting dexmedetomidine as an effective agent for blunting the hemodynamic response to laryngoscopy and tracheal intubation. Bradycardia and hypotension have been reported in studies pertaining to the effect of dexmedetomidine administration on peri-operative hemodynamics.[15,16,17] In contrast to the previously mentioned studies,[16,18,19] we did not detected any excessive reduction in HR or systemic blood pressure values in the dexmedetomidine group compared with other groups. Moreover, in this study neither bradycardia nor hypotension was observed in the patients. Dexmedetomidine has been used IV in doses ranging from 0.1 to 10 μg/kg/h but higher doses have been associated with a significant increase in incidence of bradycardia and hypotension. Rapid administration of dexmedetomidine might produce tachycardia, bradycardia and hypertension followed by hypotension. We administered dexmedetomidine, 1.0 μg/kg slowly, over 10 min in our study hence no bradycardia or hypotension was found in our study.
The α-adrenoceptors are involved in regulating the autonomic nervous system and cardiovascular systems. α2 -adrenoceptors are located on blood vessels, where they mediate vasoconstriction and on sympathetic presynaptic terminals where they inhibit epinephrine and nor-epinephrine release.[20] α2 -adrenoceptors are also located within the central nervous system and their activation leads to sedation, a reduction of tonic levels of sympathetic outflow and an augmentation of Vagal activity. This can result in a decrease in HR and cardiac output. The use of α2 -agonists in the peri-operative period has been associated with reduced anesthetic requirements and attenuated HR and blood pressure responses to stressful events.[21,22]
Monitoring of HR and ECG has shown no evidence of myocardial insult in any of the patients in any group in our study. It is advisable and safe to use dexmedetomidine in patients to attenuate the hemodynamic responses of cardiovascular system during laryngoscopy and ET intubation.
CONCLUSION
Evaluation of baseline and immediately after intubation values, revealed a greater percentage variation in MAP in the esmolol and control groups as compared to the dexmedetomidine group. Therefore, within the constraints of this study we demonstrated that administration of a single dose of dexmedetomidine before GA induction was an effective method for attenuating the hemodynamic response to tracheal intubation.
Footnotes
Source of Support: Nill.
Conflict of Interest: None declared.
REFERENCES
- 1.Shribman AJ, Smith G, Achola KJ. Cardiovascular and catecholamine responses to laryngoscopy with and without tracheal intubation. Br J Anaesth. 1987;59:295–9. doi: 10.1093/bja/59.3.295. [DOI] [PubMed] [Google Scholar]
- 2.Kovac AL. Controlling the hemodynamic response to laryngoscopy and endotracheal intubation. J Clin Anesth. 1996;8:63–79. doi: 10.1016/0952-8180(95)00147-6. [DOI] [PubMed] [Google Scholar]
- 3.da Silva Neto WV, Azevedo GS, Coelho FO, Netto EM, Ladeia AM. Evaluation of hemodynamic variations during anesthetic induction in treated hypertensive patients. Rev Bras Anestesiol. 2008;58:330–41. doi: 10.1590/s0034-70942008000400002. [DOI] [PubMed] [Google Scholar]
- 4.Ghause MS, Singh V, Kumar A, Wahal R, Bhatia VK, Agarwal J. A study of cardiovascular response during laryngoscopy and intubation and their attenuation by ultra short acting b-blocker esmolol. Indian J Anaesth. 2002;46:104–6. [Google Scholar]
- 5.Louizos AA, Hadzilia SJ, Davilis DI, Samanta EG, Georgiou LG. Administration of esmolol in microlaryngeal surgery for blunting the hemodynamic response during laryngoscopy and tracheal intubation in cigarette smokers. Ann Otol Rhinol Laryngol. 2007;116:107–11. doi: 10.1177/000348940711600205. [DOI] [PubMed] [Google Scholar]
- 6.Khan ZP, Ferguson CN, Jones RM. Alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–65. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- 7.Grewal A. Dexmedetomidine: New avenues. J Anaesthesiol Clin Pharmacol. 2011;27:297–302. doi: 10.4103/0970-9185.83670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sum CY, Yacobi A, Kartzinel R, Stampfli H, Davis CS, Lai CM. Kinetics of esmolol, an ultra-short-acting beta blocker, and of its major metabolite. Clin Pharmacol Ther. 1983;34:427–34. doi: 10.1038/clpt.1983.193. [DOI] [PubMed] [Google Scholar]
- 9.Miller DR, Martineau RJ, Wynands JE, Hill J. Bolus administration of esmolol for controlling the haemodynamic response to tracheal intubation: The Canadian Multicentre Trial. Can J Anaesth. 1991;38:849–58. doi: 10.1007/BF03036959. [DOI] [PubMed] [Google Scholar]
- 10.Liu PL, Gatt S, Gugino LD, Mallampati SR, Covino BG. Esmolol for control of increases in heart rate and blood pressure during tracheal intubation after thiopentone and succinylcholine. Can Anaesth Soc J. 1986;33:556–62. doi: 10.1007/BF03014260. [DOI] [PubMed] [Google Scholar]
- 11.Oxorn D, Knox JW, Hill J. Bolus doses of esmolol for the prevention of perioperative hypertension and tachycardia. Can J Anaesth. 1990;37:206–9. doi: 10.1007/BF03005471. [DOI] [PubMed] [Google Scholar]
- 12.Kindler CH, Schumacher PG, Schneider MC, Urwyler A. Effects of intravenous lidocaine and/or esmolol on hemodynamic responses to laryngoscopy and intubation: A double-blind, controlled clinical trial. J Clin Anesth. 1996;8:491–6. doi: 10.1016/0952-8180(96)00109-2. [DOI] [PubMed] [Google Scholar]
- 13.Scheinin B, Lindgren L, Randell T, Scheinin H, Scheinin M. Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need for thiopentone and peroperative fentanyl. Br J Anaesth. 1992;68:126–31. doi: 10.1093/bja/68.2.126. [DOI] [PubMed] [Google Scholar]
- 14.Keniya VM, Ladi S, Naphade R. Dexmedetomidine attenuates sympathoadrenal response to tracheal intubation and reduces perioperative anaesthetic requirement. Indian J Anaesth. 2011;55:352–7. doi: 10.4103/0019-5049.84846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Abraham R, Ogorek D, Weinbroum AA. Dexmedetomidine: A promising agent for anesthesia and perioperative care. Isr Med Assoc J. 2000;2:793–6. [PubMed] [Google Scholar]
- 16.Lawrence CJ, De Lange S. Effects of a single pre-operative dexmedetomidine dose on isoflurane requirements and peri-operative haemodynamic stability. Anaesthesia. 1997;52:736–44. doi: 10.1111/j.1365-2044.1997.169-az0303.x. [DOI] [PubMed] [Google Scholar]
- 17.Erkola O, Korttila K, Aho M, Haasio J, Aantaa R, Kallio A. Comparison of intramuscular dexmedetomidine and midazolam premedication for elective abdominal hysterectomy. Anesth Analg. 1994;79:646–53. doi: 10.1213/00000539-199410000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Scheinin H, Jaakola ML, Sjövall S, Ali-Melkkilä T, Kaukinen S, Turunen J, et al. Intramuscular dexmedetomidine as premedication for general anesthesia. A comparative multicenter study. Anesthesiology. 1993;78:1065–75. doi: 10.1097/00000542-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Aantaa RE, Kanto JH, Scheinin M, Kallio AM, Scheinin H. Dexmedetomidine premedication for minor gynecologic surgery. Anesth Analg. 1990;70:407–13. doi: 10.1213/00000539-199004000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Yildiz M, Tavlan A, Tuncer S, Reisli R, Yosunkaya A, Otelcioglu S. Effect of dexmedetomidine on haemodynamic responses to laryngoscopy and intubation: Perioperative haemodynamics and anaesthetic requirements. Drugs R D. 2006;7:43–52. doi: 10.2165/00126839-200607010-00004. [DOI] [PubMed] [Google Scholar]
- 21.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–42. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–94. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]


