Abstract
Background:
Neuropathology and surveys of retired National Football League (NFL) players suggest that chronic brain damage is a frequent result of a career in football. There is limited information on the neurological statuses of living retired players. This study aimed to fill the gap in knowledge by conducting in-depth neurological examinations of 30- to 60-year-old retired NFL players.
Hypothesis:
In-depth neurological examinations of 30- to 60-year-old retired players are unlikely to detect objective clinical abnormalities in the majority of subjects.
Study Design:
A day-long medical examination was conducted on 45 retired NFL players, including state-of-the-art magnetic resonance imaging (MRI; susceptibility weighted imaging [SWI], diffusion tensor imaging [DTI]), comprehensive neuropsychological and neurological examinations, interviews, blood tests, and APOE (apolipoprotein E) genotyping.
Level of Evidence:
Level 3.
Methods:
Participants’ histories focused on neurological and depression symptoms, exposure to football, and other factors that could affect brain function. The neurological examination included Mini-Mental State Examination (MMSE) evaluation of cognitive function and a comprehensive search for signs of dysarthria, pyramidal system dysfunction, extrapyramidal system dysfunction, and cerebellar dysfunction. The Beck Depression Inventory (BDI) and Patient Health Questionnaire (PHQ) measured depression. Neuropsychological tests included pen-and-paper and ImPACT evaluation of cognitive function. Anatomical examination SWI and DTI MRI searched for brain injuries. The results were statistically analyzed for associations with markers of exposure to football and related factors, such as body mass index (BMI), ethanol use, and APOE4 status.
Results:
The retired players’ ages averaged 45.6 ± 8.9 years (range, 30-60 years), and they had 6.8 ± 3.2 years (maximum, 14 years) of NFL play. They reported 6.9 ± 6.2 concussions (maximum, 25) in the NFL. The majority of retired players had normal clinical mental status and central nervous system (CNS) neurological examinations. Four players (9%) had microbleeds in brain parenchyma identified in SWI, and 3 (7%) had a large cavum septum pellucidum with brain atrophy. The number of concussions/dings was associated with abnormal results in SWI and DTI. Neuropsychological testing revealed isolated impairments in 11 players (24%), but none had dementia. Nine players (20%) endorsed symptoms of moderate or severe depression on the BDI and/or met criteria for depression on PHQ; however, none had dementia, dysarthria, parkinsonism, or cerebellar dysfunction. The number of football-related concussions was associated with isolated abnormalities on the clinical neurological examination, suggesting CNS dysfunction. The APOE4 allele was present in 38% of the players, a larger number than would be expected in the general male population (23%-26%).
Conclusion:
MRI lesions and neuropsychological impairments were found in some players; however, the majority of retired NFL players had no clinical signs of chronic brain damage.
Clinical Relevance:
These results need to be reconciled with the prevailing view that a career in football frequently results in chronic brain damage.
Keywords: concussion, brain injury, neuroradiology, neuropsychology, clinical neurology, chronic traumatic encephalopathy (CTE)
Recent articles have reported an abnormal neuropathology in the brains of deceased football players.4,22,23,49-51,60,61,72,75 Two surveys of retired National Football League (NFL) players have suggested that depression and cognitive problems occur at increased frequency.25,26 However, there has only been 1 report of neurological, neuropsychological, and neuroradiological examinations of living, retired NFL players.31
This stands in contrast to the CTE of boxers. Numerous scientific articles have documented the clinical neurological findings, neuropsychological test results, and neuroradiological findings that characterize CTE in living boxers.11,15,35,45,56,62,70,71 A well-defined neuropathologic pattern of findings for boxers has been reported with correlation to the clinical picture.14
The purpose of this study was to fill in this gap in our knowledge by performing clinical neurological, neuropsychological, and neuroradiological examinations on a group of living, retired NFL players. It complements other work.10,12,13,68,69,80 As originally envisioned, the purpose of the study was to determine whether there was clinical evidence of chronic brain damage related to a career in the NFL. As a result of nonscientific factors, recruitment of study subjects stopped part way through the study, and the authors are reporting on a convenience sample of the 45 retired NFL players who were thoroughly examined.
Materials and Methods
The methodology for this clinical research was modeled after a similar study of 18 retired and active boxers.11 The boxers underwent neurological examination, electroencephalography (EEG), brain computed tomography scans, and neuropsychological testing. Most of the boxers (16/18) had definite signs of brain damage, and all had abnormal results on at least 1 neuropsychological test. The conclusion was that brain damage is a frequent result of a career in professional boxing.
With the assistance of the NFL Players Association, recruitment letters were mailed to more than 5000 retired NFL players whose contact information was on file at the union. The letter explained the purpose and nature of this study. Recipients who were interested in participating in the study or who had questions about the study were asked to call the study coordinator at a confidential, dedicated telephone number. Recipients were also informed that they might be called on the telephone by the study coordinator to ask for their participation. The study coordinator randomly selected names from the list and called them on the telephone to invite their participation. As the study progressed, some of the subjects who went through the study evaluation spontaneously contacted former teammates and other retired NFL or college friends of theirs and suggested that they might also wish to participate. Some of those who were contacted in that manner called the study coordinator and expressed interest in participating. They were accepted into the study if they met the inclusion criteria (see Appendix, Supplement S1; available at http://sph.sagepub.com/content/suppl).
For this study, a more comprehensive magnetic resonance imaging (MRI) evaluation of the brain was used based on state-of-the-art methods under development at Wayne State University.27-30,37-41,53,73,74,77 Emphasis was also placed on using modern neuropsychological tests, clinical examination, and obtaining a detailed neurological and concussion history. The research methods were subjected to institutional review board review and approval at Wayne State University, including informed consent, methodologies, confidentiality, and statistical analysis. Details regarding the recruitment process and exclusion criteria are available in Supplement S1 (see Appendix).
Each subject underwent all study-related testing during 1 day at the medical center. Written informed consent was obtained from each subject by the study coordinator on the day of testing before any evaluations were performed.
Each subject underwent a comprehensive clinical neurological examination performed by the same experienced neurologist. Details of the neurological examination and history-taking procedures can be found in Supplement S2 (see Appendix, available at http://sph.sagepub.com/content/suppl).
Each subject had blood drawn that was sent to an accredited commercial laboratory for the following tests: complete blood count (CBC), routine chemistries, liver function, thyroid-stimulating hormone (TSH), B12, and folate and Lyme antibodies. A portion of each subject’s serum sample was frozen and sent to Duke University Medical Center in North Carolina for APOE (apolipoprotein E) genotyping.
A registered nurse (RN) administered the Patient Health Questionnaire (PHQ) and the Coding Race/Ethnicity in the Columbia University ADRC questionnaire to each subject. The RN also supervised administration of the computerized ImPACT test to each subject.
A board-certified neuropsychologist (PhD) or senior-level neuropsychology PhD candidate (in 42 cases, the neuropsychologist was not affiliated with the NFL or any of its teams; in 3 cases, the neuropsychologist was a team neuropsychologist for an NFL team) administered a battery of pen-and-paper neuropsychological tests to each subject. This test battery was put together by a committee of 5 National Academy of Neuropsychology members, 3 of whom were not affiliated with the NFL or its teams. Details regarding the written neuropsychological test administration can be found in Supplement S3 (see Appendix, available at http://sph.sagepub.com/content/suppl).
Each subject was assigned an integer score for each aspect of the testing results. Details of the scoring system can be found in Supplement S4 and Table S1 (see Appendix, available at http://sph.sagepub.com/content/suppl).
Neuroradiology Imaging Protocol
The MRI protocol consisted of baseline T1, T2, T2* gradient echo, and fluid attenuated inversion recovery (FLAIR) sequences, as well as susceptibility weighted imaging (SWI) and diffusion tensor imaging (DTI) sequences. The imaging parameters are given in Table S2 (see Appendix, available at http://sph.sagepub.com/content/suppl). The total data acquisition time lasted approximately 1 to 1.5 hours. The SWI sequence consists of a strongly susceptibility weighted, low bandwidth (80 Hz/pixel) 3D FLASH sequence (TR [repetition time]/TE [echo time] = 50 ms/40 ms, FA = 15°) with first-order flow compensated in all 3 orthogonal directions.78 The SWI sequence included the majority of the cerebral hemispheres and the posterior fossa, with an acquisition time of approximately 7 minutes and 42 seconds. DTI data were collected with 6 gradient directions uniformly spaced on the surface of a b = 1000 s/mm2 sphere (TR/TE = 6500 ms/100 ms, voxel size = 2 × 2 × 3 mm3, EPI factor = 96, time duration = 7 minutes and 43 seconds).
Postprocessing
Susceptibility weighted imaging data were reviewed by a neuroradiologist and MR scientist, both with more than 30 years’ experience, and suspicious hemorrhagic lesions were confirmed by both. The total number and volume of hemorrhagic lesions detected by SWI were analyzed and quantified with our in-house developed software package (Signal Processing for NMR [SPIN]; MRI Institute for Biomedical Research).
DTI Data Processing
A global white matter (WM) fractional anisotropy (FA) mean analysis was performed by using an approach described in previous work6 that has been shown to be sensitive to mild traumatic brain injury (TBI). In this approach, each subject’s FA map was first spatially normalized to an FA template (mean, 50 normal controls) and then segmented using SPM8 to give gray matter (GM), WM, and cerebrospinal fluid (CSF) masks. A WM-only FA image was then generated to give a global WM FA.
All MRI interpretations were performed by board-certified neuroradiologists. Each subject was then given an integer score for anatomical MRI results and DWI results. The scoring for SWI was as follows: 0 = no microbleeds, 1 = 1 or more microbleeds. Anatomical MRI scoring was as follows: 1 = completely normal; 2 = pituitary microadenoma and/or empty sella; 3 = small cavum septum pellucidum (no cavum vergae, no enlarged ventricles); 4 = large cavum septum pellucidum plus cavum vergae plus enlarged ventricles; 5 = unidentified bright objects (UBOs), cerebral white matter, 3 or fewer; 6 = cortical scar, cerebral; 7 = pituitary macroadenoma; 8 = old small intraparenchymal hemorrhage. Subjects can be assigned more than 1 integer score for anatomical MRI.
Statistics
Descriptive statistics were used to characterize the information and to study correlations and associations among the results. The various demographic and personal history data were correlated to the medical findings using stepwise logistic regression using cumulative, general, or binary logit or linear regression.
Results
The biometric and clinical data on the sample of 45 retired NFL players is given in Tables S3 and S4 (see Appendix, available at http://sph.sagepub.com/content/suppl). The mean age of the retired players was 45.6 ± 8.9 years (range, 30-60 years). The mean number of years in the NFL and NFL training camps was 6.8 ± 3.2 years (range, up to 14 years). The players had 4.2 ± 0.4 years (range, 3.0-5.0 years) of college football, 3.5 ± 0.9 years (range, up to 5 years) of high school, and 2.5 ± 2.3 years (range, up to 9 years) of pre–high school football experience. Their mean height was 75.0 ± 1.8 inches (range, 71-79 inches), and their mean weight was 255 ± 46 lb (range, 178-365 lb). The mean body mass index (BMI) was 31.4 ± 4.8 kg/m2 (range, 22.6-42.1 kg/m2).
The players in the sample reported having 6.9 ± 6.2 concussions (range, up to 25) in the NFL. Thirty-four subjects (75.6%) reported that they had sustained 3 or more concussions during their NFL careers. Overall, they reported experiencing 9.0 ± 6.9 concussions (range, up to 25) in all football play. The primary football positions played in the NFL were: 14 linebackers, 9 offensive linemen, 8 defensive linemen, 8 defensive backs, 2 wide receivers, 2 running backs, 1 tight end, and 1 who played both offensive and defensive line. There were no NFL quarterbacks in the sample. Almost all subjects had played on special teams at some point during their NFL careers.
Symptoms
All subjects were asked 9 specific questions relating to cognition and memory. Twenty-three subjects endorsed between 0 and 2 of these symptoms, 11 endorsed between 3 and 5 of these symptoms, and 11 endorsed between 6 and 9 of these symptoms. Every subject was asked 9 specific questions relating to anxiety and/or depression. Nineteen subjects reported 0 or 1 of these symptoms, 14 reported 2 or 3 of these symptoms, and 12 reported 4 to 8 of these symptoms.
Family History
Ten subjects had a family history of Alzheimer disease, dementia, or “senility.” Eight subjects had a family history of depression, anxiety, and/or suicidality. Nine subjects had a family history of stroke. Four subjects had a family history of other neurological diseases.
Clinical Neurological Examination
The clinical neurological examination results have been broken down into 3 categories in Table S5 (see Appendix, available at http://sph.sagepub.com/content/suppl): mental status, examination of CNS functions excluding mental status, and examination of peripheral nervous system functions.
Bedside Mental Status
Thirty-eight subjects were normal, 3 subjects could only name 10 or fewer “B” words in 1 minute, 3 subjects could only name 3 or fewer US presidents in reverse order, 1 subject was unable to give the correct meaning of a well-known proverb, and 1 subject had bilateral palmomental reflexes. The range of Mini-Mental State Examination (MMSE) scores was between 25 and 30. For more details, see Supplement S5 (see Appendix, available at http://sph.sagepub.com/content/suppl).
Central Nervous System Examination
Thirty-four subjects were normal, 3 subjects had Babinski signs (2 bilateral and 1 unilateral), 2 subjects had abnormal smell sensation, 2 subjects had mild tremors (sustention and/or intention, not resting), 1 subject had minimal horizontal nystagmus on lateral gaze, 1 subject had diminished pin sensation unilaterally on the chin, and 4 subjects had abnormal dynamic visual acuity testing. None of the subjects had any parkinsonian signs.
Peripheral Nervous System Examination
Twenty-seven subjects were normal, and 18 subjects were abnormal. Seven subjects had signs of lumbar radiculopathy, 4 subjects had signs of cervical radiculopathy, 7 subjects had signs of carpal tunnel syndrome, 6 subjects had signs of ulnar nerve dysfunction, and 3 subjects had signs of diabetic polyneuropathy. Some subjects had more than 1 peripheral nervous system (PNS) abnormality.
APOE Genotyping
Seventeen subjects (37.8%) had at least one allele 4. Two of these had 2 copies of allele 4, while 2 were paired with an allele 2 and 13 were paired with an allele 3. Four subjects had 1 copy of allele 2. Two of these were paired with an allele 4, and the other 2 were paired with an allele 3. Twenty-four subjects had 2 copies of allele 3. Three subjects with at least 1 copy of allele 4 had a family history of Alzheimer disease or dementia. Seven of 28 subjects not carrying an allele 4 had a family history of Alzheimer disease, dementia, or senility. One of the allele 4 carriers had a family history of depression, anxiety, or suicidality, compared with 7 of 28 not carrying that allele who had such a family history. Five of the allele 4 carriers were offensive linemen, 5 were linebackers, 4 were defensive backs, 2 were wide receivers, and 1 was a running back. None were defensive linemen.
Depression Testing
On the Beck Depression Inventory (BDI), 30 subjects scored between 0 and 13 (not depressed), 9 subjects scored between 14 and 19 (mildly depressed), 3 subjects scored between 20 and 28 (moderately depressed), and 3 subjects scored 29 or higher (markedly depressed). Nine subjects fulfilled the criteria for either major depression or other depression on the PHQ. Eight of these 9 subjects also scored 14 or higher on the BDI.
Laboratory Results
There were no major abnormalities. For details, see Supplement S6 (see Appendix, available at http://sph.sagepub.com/content/suppl).
Anatomical MRI
Two cases were found with abnormally enlarged ventricles and thin corpus callosum, which suggests brain atrophy. The brain images can be found in Supplement Figures S1 and S2 (see Appendix, available at http://sph.sagepub.com/content/suppl), which show that both cases had significant atrophy of the brain.
Thirty-four subjects had a cavum septum pellucidum (CSP) on their MRIs. Three of these were large and associated with a cavum vergae, and 31 were small. There were no other anatomical MRI findings that occurred with any significant frequency. There were 3 subjects with large CSPs: One (patient 5) played 4 years in the NFL, 4 years in college, 4 years in high school, and 6 years of pre–high school football. He reported 4 total concussions (2 in the NFL) and 10 dings (all in the NFL, see supplement S2 for definition of “ding”; Appendix). The second player (patient 10) played 13 years in the NFL, 4 years in college, 4 years in high school, and 4 years in pre–high school. He reported 25 concussions (all in the NFL) and 30 total dings (25 in the NFL). The third player (patient 36) played 1 year in the NFL (went to 2 NFL training camps, thus would have been considered a “control” subject under the original study criteria), 5 years in college, 2 years in high school, and 1 year before high school. He reported 5 concussions (0 in the NFL) and 6 dings (0 in the NFL).
One subject had a pituitary macroadenoma. Two subjects had developmental venous abnormalities. There were no extra-axial collections and minimal unidentified bright objects.
Neuroradiology/SWI
Susceptibility weighted imaging detected 4 cases with microbleeds and 1 case (patient 30) with abnormal vascular malformation (possible telangiectasia). Table 1 shows the SWI lesion number and total volume (mm3) for the 4 cases in the study.
Table 1.
Detailed data on 4 players
| Case ID |
||||
|---|---|---|---|---|
| 10 | 16 | 22 | 31 | |
| Age, y | 57 | 30 | 32 | 52 |
| Years of NFL Play | 13 | 5 | 8 | 2 |
| No. of Concussions | 20+ | 5 | 8 | 0 |
| Bleed Location | CR | FP GM | CR | BS |
| Volume, mm3 | 21 | 434 | 62 | 57 |
| T1 | Enlarged ventricles | − | − | − |
| CSP/CV | +/+ | +/− | +/− | +/− |
| MMSE | 27 | 28 | 26 | 29 |
| BDI | 4 | 3 | 17 | 8 |
| APOE4 Allele | + | + | − | − |
| Prehistory | None | Severe TBI* | None | None |
| Cardiovascular Record | High cholesterol | Hypertension | None | Hypertension |
APOE, apolipoprotein E; BDI, Beck Depression Inventory; BS, brain stem; CR, corona radiata; CSP/CV, cavum septi pellucidi/cavum vargae; CVI, cavum veli interpositi; FP GM, left frontal gray matter; MMSE, Mini-Mental State Examination; TBI, traumatic brain injury. All central nervous system examinations were normal.
Left parietal skull surgery with thick dura under it.
One subject was 57 years old (patient 10), played 13 years in the NFL, had more than 20 NFL concussions, normal CNS examination, MMSE score of 27, BDI 4, carried the APOE4 allele (E3/E4), and has a history of high cholesterol. Figure 1 shows the microbleed. The second subject was 30 years old (patient 16), played 5 years in the NFL, had 5 NFL concussions, had a normal CNS examination, normal MMSE and BDI, had the APOE4 allele (E3/E4), had a serious head injury with possible skull fracture when he was 9 years old, and has a history of hypertension. Figure S3 in the supplemental information shows the microbleed (see Appendix, available at http://sph.sagepub.com/content/suppl). The third subject (patient 22) was 32 years old, played 8 years in the NFL, had 8 NFL concussions, normal CNS examination, BDI 17, MMSE 26, no history of medical illnesses, and did not carry the APOE4 allele (E3/E3). Figure 2 shows the microbleed. The fourth player (patient 31) was 52 years old, played only 2 years in the NFL as a backup, had 0 concussions in the NFL (or for that matter, at any level of football), completely normal clinical examinations, did not carry the APOE4 allele (E3/E3), and has a history of hypertension and treated CLL. He is one of the subjects designated as having limited NFL exposure in our article. Figure S4 in the supplemental information shows the microbleed (see Appendix, available at http://sph.sagepub.com/content/suppl).
Figure 1.

Microbleed in a 57-year-old player (patient 10). Both SWI magnitude and phase images detect an isolated microbleed at the subcortical gray matter/white matter (GM/WM) junction, while conventional FLAIR and T2-weighted images at the same level fail to visualize the microbleed. Red arrows point to a hemorrhagic lesion, which is not shown on the T2 and FLAIR images. FLAIR, fluid attenuated inversion recovery; SWI, susceptibility weighted imaging.
Figure 2.
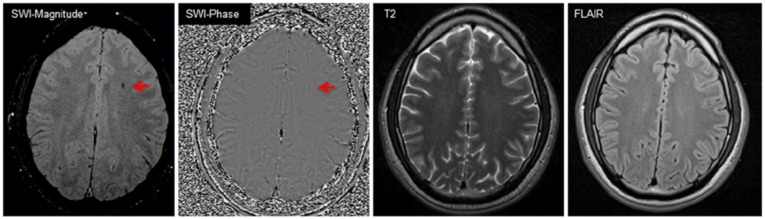
Microbleed in a 32-year-old player (patient 22). Both SWI magnitude and phase images demonstrate a microbleed (arrows) in left superior corona radiata, which conventional MRI (FLAIR and T2-weighted) did not detect. FLAIR, fluid attenuated inversion recovery; MRI, magnetic resonance imaging; SWI, susceptibility weighted imaging.
Diffusion Tensor Imaging Findings
The average DTI FA mean, peak, and mean/peak for the sample’s whole-brain global white matter is given in Table S5 in the supplement (see Appendix).
Neuropsychology
None of the players had dementia (defined as impairments on 2 or more spheres of cognition that interfere with activities of daily living). Twenty-eight players (62%) had no impairments (score 0 or 1). Eleven (24%) players had impairments on 1 or 2 of the subtests with normal Test of Memory Malingering (TOMM) and verbal IQ (score 2 or 3). Six players (13%) had borderline or impaired performance on 1 or more subtests, but the results are confounded by verbal IQ less than 80 or lack of effort as determined by the TOMM results. The overall results of the written neuropsychological test battery are provided in Tables S4 and S5 in the supplement (see Appendix).
Computerized neuropsychological testing was scored by a computer, and the results generated included raw scores and composite scores on various aspects of memory/cognitive function. In the absence of a control group, we used the composite test scores for statistical analysis.
Statistical Analysis
In the absence of a control group, we performed a set of statistical analyses aimed at determining if there were associations between a representative group of “epidemiological” variables (x) that serve as markers of football exposure, head injury exposure, and non-football- or head injury–related variables such as ethanol use, hereditary factors, and BMI and the results of the diagnostic tests (y) performed on the subjects. The football-related variables were chosen as markers of total football exposure, football exposure before college, football exposure at the college level, and football exposure in the NFL. Statistical analyses of the association between these variables and the results of MRI, neuropsychological testing, clinical neurological testing including mental status, and depression testing can help to elucidate the role of these exposures in causing any abnormalities or other findings detected by the clinical tests.
There was no statistical association between the anatomical MRI findings and any football exposure–related variables. There was a correlation between the presence of findings on SWI MRI and family history of neurological disease, employment status, and number of dings in football at all levels (χ2 = 2.75, df = 6, Pr > χ2 = 0.8399 for the Hosmer and Lemeshow goodness of fit). This indicates that the presence of findings on SWI MRI might be because of both genetic and environmental (ie, number of dings) factors. SWI microbleeds were related to the number of dings in football (χ2 = 0.276, df = 4, Pr > χ2 = 0.9913 for the Hosmer and Lemeshow goodness of fit). None of the other “x” variables related to playing in the NFL correlated with the presence of microbleeds on SWI MRI.
Peak FA was negatively associated with presence of the APOE4 allele, participation in other contact sports besides football in high school and/or college, and with ever having been “dinged” in football at any level. Peak FA was positively associated with 1 marker of inappropriate ethanol use, employment status, and number of years of participation in pre–high school football. Mean FA was negatively associated with the number of concussions sustained in the NFL.
Depression scores were not statistically associated with any of the markers of football exposure. There was no statistical association between written neuropsychological test results and any football exposure–related variables. Written neuropsychological test results were statistically associated with BMI and ethanol usage.
In regard to computerized neuropsychological subtest results, there were statistical associations with having played a line position (visual memory subtest), years of pre-NFL football play (verbal memory and motor subtests), the number of “dings” sustained in NFL play (motor subtest), BMI (visual memory), and ethanol usage (multiple subtests). Additional discussion of statistical associations is provided in Supplement S7 and Table S6 (see Appendix, available at http://sph.sagepub.com/content/suppl).
Discussion
This report provides findings of comprehensive neurological, neuropsychological, and neuroradiological evaluations of 45 retired NFL players between the ages of 30 and 60 years. Up until now, there have been 3 mail/telephone surveys of retired NFL players, a number of neuropathological case reports, and 1 clinical evaluation of older retired NFL players in the medical literature.4,22,23,25,26,31,49-51,60,61,75 There are a number of inherent methodological weaknesses in mail/telephone surveys that cast doubt on their validity and reliability. One major limitation of these surveys is the absence of any objectively verified reports of clinical, neuropsychological, or neuroradiological examinations by physicians on any of the survey respondents. The neuropathological cases that have been reported in the scientific literature have not included detailed reports of clinical, neuropsychological, or neuroradiological findings by physicians on the subjects prior to their demise.4,22,23,49-51,60,61,75 The medical community is thus confronted by a neuropathological picture without clinical correlation and a dearth of detailed clinical reports of neurological, neuropathological, or neuroradiological findings in living, retired NFL players. The present report is intended to fill in this gap in our knowledge.
The absence of clinical evidence of dementia, dysarthria, parkinsonism, or cerebellar dysfunction in the retired players stands in stark contrast to boxers, who often showed signs of dysarthria, dementia, parkinsonism, pyramidal tract dysfunction, and/or cerebellar dysfunction.11,13,15,35,45,54,62,70,71 There was a statistical association between the presence of abnormalities on the clinical CNS examination and the total number of football concussions sustained at all levels of football. This suggests that mild clinical abnormalities may be the result of sustaining a relatively greater number of football concussions at all levels of play. Whether this is related to CTE is not demonstrated by these data. For example, Roberts70 specifically excluded the presence of isolated abnormalities, such as an isolated Babinski sign, as evidence of CTE.
Many more subjects had clinical evidence of PNS dysfunction than CNS dysfunction on clinical examination. The signs of diabetic polyneuropathy found in 3 subjects cannot be attributed to the effects of football-related trauma, but the lumbar and cervical radiculopathies and the ulnar and median nerve compressions found in the subjects most likely are of traumatic origin.
The clinical mental status evaluation did not reveal any subjects with dementia. Among college graduates in the general population, MMSE scores of 24 or lower indicate dementia, and the lowest score among the study subjects was 25 (subject 25). If one uses a clinical definition of dementia being characterized by disorientation, confusion, and memory loss, there were no study subjects who met these criteria either. If one defines dementia as impairments in multiple spheres of cognitive and memory functions that adversely affect daily activities, there were no study subjects who met these criteria either. There was a statistical association between the presence of abnormalities on clinical mental status testing and the number of years of college football played. This suggests that pre-NFL football exposure might result in mild mental status abnormalities years later.
Depression
Using the BDI criteria, the 15 subjects (33%) with any severity of depression is higher than the reported prevalence of depression in the general population (15%-20%). The 6 subjects with moderate or severe depression (13.3%) are more in line with the overall population numbers. Nine players (20%) met the PHQ criteria for depression, which is in line with the general population prevalence. The evidence in this study does not support the contention that a career in the NFL is causally related to later-life depression. For further discussion, see Supplement S8 (see Appendix, available at http://sph.sagepub.com/content/suppl).
APOE Genotyping
It has been suggested that people who carry at least 1 copy of the E4 allele are at increased risk of developing Alzheimer disease as a result of head trauma.46,79 It has also been suggested that those carriers have poorer outcomes following head trauma than non–E4 carriers.21,36,76,79 One study found that the E4 allele is a risk factor for chronic brain dysfunction in boxers.34 Another study reported that older professional football players (still active in the sport), who were E4 carriers, performed poorer on a battery of cognitive tests than those who did not carry the E4 allele.42 It is well known that people who carry the E4 allele have an increased risk of developing Alzheimer disease and an earlier age of onset than noncarriers.79 Some studies indicate that the E4 allele enhances brain tau deposition.56,79 All of these factors suggest that there might be a link between the E4 allele and chronic CNS dysfunction in retired NFL players.
In all, 37.7% of the study cohort carried at least 1 copy of the E4 allele. This is higher than the 23.2% to 25.6% of men in the general population who are E4 carriers.17,52,79 This is also higher than the 26.4% of the 53 active NFL players who carried the E4 allele in another study.42 One might hypothesize that the APOE4 allele could be associated with athletic prowess and/or improved physical performance. Some studies have demonstrated an effect of APOE allele status on serum lipid responses to exercise and other physical activities.16,43,59 It is also possible that the APOE gene could be linked to another gene that affects physical performance. These possibilities deserve further investigation.
The absence of a statistical association of the APOE4 genotype with any anatomical MRI, clinical neurologic, depression, or neuropsychological test results in this sample raises doubts about the possibility that APOE genotype has a clinical expression in retired NFL players 60 years and younger. The statistical association of the APOE4 genotype with FA peak on DTI is consistent with a recent report on the effects of APOE on FA in healthy non–football player volunteers.83
Neuroradiology
Most of the effects of mild TBI, including sports concussion, have been reported to be occult to clinical neuroimaging, including computed tomography and conventional MRI. However, a handful of techniques are sensitive to the subtle changes of the brain after concussion,5,41 including SWI41 and DTI.2,6,41,57,85 For further details on neuroradiology techniques, see Supplement S9 (see Appendix, available at http://sph.sagepub.com/content/suppl).
Microbleeds
In this study, none of the 4 subjects with microbleeds belonged to the “control” or limited NFL exposure group (see Table 1). One subject had sustained a significant TBI and skull fracture before playing in the NFL. After brain injury, hemorrhagic lesions may undergo a series of temporal evolutions, and macrophage cells may leave hemosiderin in the bleed site.41 The hemosiderin may stay in the brain for a long time as evidence of previous injuries. Consequently, the microbleed in this case may be attributed to a previous severe brain injury.
The microbleeds in the remaining 3 cases are likely related to head trauma occurring in football at some level. SWI-detected microbleeds can also be related to amyloid angiopathy and/or hypertension,3,27,37 but in subjects younger than 60 years, it is more likely that they are etiologically related to head trauma. In several other studies covering hundreds of patients, including mild cognitive impairment and normal controls, microbleeds were rarely found.3 It is not yet known whether the 9% frequency of microbleeds is higher than what might appear in an age-matched normal population; it is unusual to have more than 1 microbleed in a sample our size.3 Statistical analysis determined an association between total number of dings reported at all levels of football and the presence of microbleeds on SWI, adding further support to the suggestion that head trauma is related to SWI microbleeds in the study subjects.
Magnetic Resonance Diffusion Tensor Imaging
The association between number of years of pre–high school and high school football and FA findings suggests that head injury occurring before the age of 18 years may result in DTI abnormalities that can still be detected many years later. This is consistent with evidence from some other studies suggesting that younger brains are more susceptible to the deleterious effects of head trauma than mature brains.18,44,63-67 On the other hand, it has also been suggested that younger brains may be more tolerant to traumatic biomechanical forces than adult brains.47
The association between number of NFL concussions and the subject’s mean FA suggests that concussions occurring at the NFL level may result in DTI abnormalities that are detectable after NFL retirement. DTI could detect “microstructural” lesions that account for a patient’s neurocognitive symptoms but are invisible on structural MRI.38,57 The fact that the number of NFL concussions is not correlated with neuropsychological test results raises the possibility that NFL concussions may not result in changes in FA that can be related to clinical or neurocognitive abnormalities. Whether these DTI abnormalities correlate with tau deposition in the brain or other pathological indicators of “CTE” remains a question.
The association between measures of excessive/inappropriate ethanol use and FA results indicates that environmental factors other than trauma can affect FA or that brain-injured subjects could be predisposed to excessive ethanol use or more susceptible to the effects of ethanol. The correlation between presence of the APOE4 allele and FA findings suggests that genetic factors contribute to the amount of anisotropy in the brain.83 Recent literature suggests that concussion and APOE4 allele are risk factors for neural behavioral impairment.24 Other studies have demonstrated that axonal injury is a progressive process instead of a single event.8 This suggests that sports concussion makes those individuals with APOE4 allele genotype more susceptible to WM injury.
Anatomical MRI
Three subjects had large CSPs on MRI. Large CSPs have been radiologically and neuropathologically associated with CTE in boxers. Interestingly, there was no correlation between the presence of a large CSP and any of the “x” factors related to exposure to football and/or head trauma. The prevalence of large CSPs in this group of retired NFL players (6.6%) is much lower than the prevalence (20%) in prior CAT scan studies of retired boxers.11,71 This is another important difference between the brains of retired football players and retired boxers. Small CSPs have not been associated with CTE in boxers. The absence of a correlation between a small CSP and any of the “x” factors in this study suggests that football-related head trauma is also not associated with small CSPs. A small CSP in 31 of 42 (74%) MRIs (excluding the 3 MRIs with a large CSP) at first glance seems to be higher than what is seen in MRIs of the general population. However, review of the literature reveals that small CSPs have been seen in up to 76% of healthy subjects on 1.5-T MRIs, as were used in this study.20,48 The radiologists interpreting the MRIs in the present study paid special attention to the septum region because of the known association between septal abnormalities and CTE of boxers.11,14,54,71 It is possible that paying special attention to the septal region on all MRIs (not only those of football players) may result in a higher incidence of small CSPs being reported in MRIs of the general population in clinical practice.
Written Neuropsychological Testing
None of the subjects had dementia using criteria defined as impairments in 2 or more modalities of cognition/memory (verbal and visual memory, executive functions, motor speed, sustained attention/working memory) along with impairments in functions of daily living. Eleven players (24%) had isolated impairments on 1 or 2 subtests not rising to the level of dementia. Three of these 11 had depression (score 2 or 3 on combined depression score). It is known that depression can impair neuropsychological test performance. Nevertheless, the incidence of isolated impairments seems higher than would be expected in the general population younger than 60 years. It is difficult to be certain how this compares with the general male population in the absence of a control group and validated data on the incidence of similar impairments in a general population of similar-aged males.
Statistical analysis suggests that impaired performance on written neuropsychological test results after retirement from the sport and before the age of 60 years is related to non-football factors. The association of impairments on written neuropsychological tests with BMI is not surprising given the evidence in the medical literature linking midlife obesity with cognitive impairment or dementia.1,19,84 The association of impairments on written neuropsychological testing with ethanol use is also not surprising as it is well known that excessive ethanol use can impair cognition.7,81,82 These results should raise a cautionary red flag to those who would ascribe all findings of cognitive and memory dysfunction in retired NFL players to the effects of football-related head trauma.
Computerized Neuropsychological Testing
In the absence of any association with any football-related factors, the associations between visual memory composite score and those who had played line positions and with higher BMI points more toward an effect of midlife obesity on cognition rather than a football-related etiology. The associations between verbal memory and motor composite scores and years of football play in pre–high school and college suggest that exposure to football before the NFL may impair these cognitive functions later in life. The association between motor scores and the number of NFL “dings” suggests that NFL exposure might impair motor functions later in life. In view of the much higher prevalence of signs of PNS than CNS dysfunction in the study cohort, it is unclear what roles PNS and CNS impairments may play in these motor composite test results. In summary, computerized neuropsychological test scores were related to non-football-, non–head injury–related variables, pre-NFL football exposure, and in regard to motor scores only, the number of dings in the NFL.
Playing football at the pre-NFL level was associated with mild abnormalities on some subtest composites on computerized neuropsychological testing and FA findings on DTI MRI. Playing football in the NFL was associated with FA findings on DTI MRI and abnormalities only on the motor subtest composite of the computerized neuropsychological test battery. Length of NFL career was not associated with any abnormal findings on any part of the diagnostic test battery. In fact, none of the variables used as markers of exposure to football and football head injury were associated with abnormal findings on the great majority of the diagnostic tests that were performed. Non-football-related head trauma was associated with DTI MRI FA findings and abnormalities on some subtest composites of the computerized neuropsychological test battery, indicating that the effects of non-football-related head trauma must be considered when evaluating the neurological status of retired NFL players. Non–head trauma–related variables such as BMI, inappropriate/excessive ethanol use, hereditary factors, and social factors were associated with at least as many, and perhaps more, abnormal or poorer performances on various parts of the entire test battery than were head trauma– and football-related factors. Others9 have pointed out the influence of non–head trauma–related factors on neuropsychological test results. They reported poorer test results with obstructive sleep apnea and consequent hypoxemia, which may be a factor in football linemen.
Is There Chronic Brain Damage?
Some have claimed that there is an “epidemic” of chronic brain injury due to the cumulative effects of head impacts in NFL players.4,22,23,49-51,60,61,72,75 They have suggested that chronic brain damage is a frequent occurrence in retired NFL players. The MRI scans in this study revealed probable signs of chronic brain injury in 13% (n = 6) of the players and an association between FA and football exposure. However, FA was also associated with non-football factors such as heredity (APOE status), and 87% (n = 39) of the players did not have MRI findings suggesting chronic brain injury. Eleven players (24.4%) had isolated impairments on written neuropsychological testing, which possibly are related to prior brain injuries, but the presence of these impairments was only statistically associated with non-football head injury factors such as BMI and ethanol overuse and not with any measures of football head injury exposure.
Computerized neuropsychological testing results were statistically associated with numerous factors, including both non-football and football exposures. The prevalence of depression in the cohort is similar to that of the general population.
Comprehensive neurological examinations revealed a few isolated signs of CNS dysfunction (eg, Babinski signs), but no players had dementia, dysarthria, parkinsonism, or cerebellar dysfunction. In his classic book on the subject of brain damage in boxers, Roberts70 excluded using isolated findings such as Babinski signs alone in diagnosing chronic brain damage.
Is the Study Cohort Representative of All NFL Retired Players?
Whether or not the study cohort is representative of the entire group of retired NFL athletes plays a major role in how these findings are interpreted. If the study group is representative, then there is not a clinical epidemic of objective neurological dysfunction in living, retired NFL players. Furthermore, the neuropathological picture that has been reported has few clinical correlates. NFL players have a significant genetic susceptibility to Alzheimer type pathology and tau pathology by dint of their increased frequency of APOE4 genotypes compared with the general population and their high frequency of reported family history of Alzheimer disease, dementia, and “senility.”56,79
If the study group is not representative, then one needs to ask how it may differ from the entire group of retired NFL players. Have the members of the study group been exposed to more or less NFL football or football at other levels than the overall population of retired NFL players? Do the football positions played by the study subjects reflect those played by the entire population of retired players, and, if not, are the positions played by the study group subjects representative of the NFL positions more or less at risk of sustaining concussions? Are the numbers of concussions reported by the study subjects more or less than those reported by the entire group of retired NFL players? Are the members of the study group of similar ages as the entire population of retired NFL players? Did the members of the study cohort report symptoms of cognitive/memory dysfunction and depression/anxiety at similar, higher, or lower rates than the entire group of retired NFL players? Are the medical, social, and family histories reported by the study subjects representative of those of the entire population of retired NFL players?
The evidence suggests that the study cohort is representative of the entire group of retired NFL players in some respects, and when not representative, consists of subjects with an increased exposure to NFL football and an increased incidence of cognitive/memory and depression/anxiety symptoms compared with the entire group of retired NFL players. Additional discussion is provided in Supplement S11 (see Appendix, available at http://sph.sagepub.com/content/suppl).
Limitations
The authors acknowledge many limitations of the study:
Control populations. This study was stopped for nonscientific reasons, limiting the number of available age-matched controls. Ideally, it should have at least 2 groups of controls: 1 group with limited duration of NFL exposure and 1 group of normal and healthy controls. They would be the ideal populations to contrast with the retired NFL players to answer the questions of (1) how an NFL career affects an individual’s neurocognitive and imaging profile and (2) how playing football itself affects an individual’s neurocognitive and imaging profile. This is particularly true for DTI analysis.
DTI analytical approaches. A global histogram approach, which is used in this study, has been reported as being sensitive to brain injury by 2 groups concurrently.6,55 However, the data sets of both studies are more populated with moderate to severe TBI patients instead of patients with mild TBI. Given the subtle nature of possible concussion, a regional or voxel-based instead of global analysis approach could be more sensitive to microstructural changes of the brain after mild TBI.57,58
Timing point of MR scan after injury. Studies reported that metabolic levels might be normalized in the chronic stage after brain injury.32,33 Given the nature of this study design, the subjects are post–playing stage of life, when MRI data may not be sensitive.
Magnetic field strength. The imaging community is migrating to 3-T magnets from the 1.5-Tesla platform used in this study. The doubled signal to noise ratio of 3-T over 1.5-T magnets provides greater potential to detect subtle changes of the brain, including possible microbleeds, white matter injury, or abnormal metabolic levels.
MRI spectroscopy was intended to be a part of the MRI study performed on each subject. Due to technical difficulties, adequate spectroscopy could only be obtained on 10 subjects. Because of this small number, spectroscopy results are not included in this report.
Conclusion
The present study indicates that MRI detects evidence of probable chronic brain injury related to football in up to 13% of the retired players and neuropsychological testing detects evidence of isolated cognitive impairments not rising to the level of dementia and related to multiple factors, not only football/head trauma, in 24.4% of the retired players. There is no clear evidence of chronic brain damage on depression testing or neurological examination. These results need to be reconciled with the prevailing view that a career in football frequently results in chronic brain damage.
A recent report of autopsy results indicates that 34 of 35 retired professional football players’ brains had evidence of “CTE” with a specific pattern of tau pathology, which correlated with a myriad of clinical symptoms ascertained by postmortem interviews with family members and some reviews of medical records.51 There is clearly a large disconnect between that report and the clinical, neuropsychological, and neuroradiological findings in the 45 living, retired NFL players detailed here. For further details, see Supplement S10 (see Appendix, available at http://sph.sagepub.com/content/suppl).
Acknowledgments
The authors were involved with the NFL’s MTBI (Mild Traumatic Brain Injury) Committee during the clinical examinations conducted in this research. The examinations were sponsored by the National Football League and were coordinated with the NFL Players Association. The study was suspended and not brought to its original conclusion. The existing convenience sample was available for analysis and represents an initial view of brain injury and neuropsychological affects in a sample of retired NFL players. We appreciate the neuroradiological assistance of Thomas Naidich, MD (Professor of Radiology, Mt Sinai School of Medicine, New York, NY); Victor Haughton, MD (Professor and Chief Neuroradiology, University of Wisconsin, Madison, WI); and Chi-Sing Zee, MD (Director of Neuroradiology, USC School of Medicine, Professor of Radiology, Los Angeles, CA). We appreciate the radiology staff at ProHEALTH (Kathleen Finzel, MD, and Patricia Roche, DO) and MR Research Center of Wayne State University (Yang Xuan, BS; Ramtilak Gattu, MS; and Randall R. Benson, MD) and the neuropsychological expertise and assistance of Mark R. Lovell, PhD; Paul Mattis, PhD; Kenneth Perrine, PhD; Reuben Echemendia, PhD; and Jennifer Manly, PhD, is appreciated. The APOE sample analysis was conducted at Duke University Medical Center. The statistical analysis of the data was performed under the direction of Dr Daniel R. Jeske, Director of Statistical Consulting Collaboratory, University of California, Riverside. Carl St Martin, MD, reviewed the article. The various sources of data were collated, organized, and archived by Chantal Parenteau, PhD, whose assistance is appreciated. Additional acknowledgments can be found in Supplement S12 (see Appendix, available at http://sph.sagepub.com/content/suppl).
Footnotes
The authors reported no potential conflicts of interest in the development and publication of this manuscript.
References
- 1. Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12:426-437 [DOI] [PubMed] [Google Scholar]
- 2. Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23:794-802 [PMC free article] [PubMed] [Google Scholar]
- 3. Ayaz M, Boikov AS, Haacke EM, Kido DK, Kirsch WM. Imaging cerebral microbleeds using susceptibility weighted imaging: one step toward detecting vascular dementia. J Magn Reson Imaging. 2010;31:142-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baugh CM, Stamm JM, Riley DO, et al. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012;6:244-254 [DOI] [PubMed] [Google Scholar]
- 5. Belanger HG, Vanderploeg RD, Curtiss G, Warden DL. Recent neuroimaging techniques in mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2007;19:5-20 [DOI] [PubMed] [Google Scholar]
- 6. Benson RR, Meda SA, Vasudevan S, et al. Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. J Neurotrauma. 2007;24:446-459 [DOI] [PubMed] [Google Scholar]
- 7. Brust JC. Ethanol and cognition: indirect effects, neurotoxicity and neuroprotection; a review. Int J Environ Res Public Health. 2010;7:1540-1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Büki A, Povlishock JT, All roads lead to disconnection? Traumatic axonal injury revisited. Acta Neurochir (Wien). 2006;148:181-193 [DOI] [PubMed] [Google Scholar]
- 9. Canessa N, Castronovo V, Cappa SF, et al. Neurocognitive impairment in obstructive sleep apnea. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Chest. 2012;141:1601-161022670023 [Google Scholar]
- 10. Casson IR, Pellman EJ, Viano DC. Concussion in the National Football League: an overview for neurologists. Neurol Clin. 2008;26:217-241 [DOI] [PubMed] [Google Scholar]
- 11. Casson IR, Siegel O, Sham R, Campbell EA, Tarlau M, DiDomenico A. Brain damage in modern boxers. JAMA. 1984;251:2663-2667 [PubMed] [Google Scholar]
- 12. Casson IR, Viano DC, Powell JW, Pellman EJ. Twelve years of National Football League concussion data. Sports Health. 2010;2:471-483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Casson IR. Do the ‘facts’ really support an association between NFL players’ concussions, dementia and depression? Neurol Today. 2010;June 3:6-7 [Google Scholar]
- 14. Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. 1973;3:270-303 [DOI] [PubMed] [Google Scholar]
- 15. Critchley M. Medical aspects of boxing, particularly from a neurological standpoint. Br Med J. 1957;1:357-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bray MS, Hagberg JM, Pérusse L, et al. The human gene map for performance and health related fitness phenotypes: the 2006-2007 update. Med Sci Sports Exerc. 2009;41:35-73 [DOI] [PubMed] [Google Scholar]
- 17. Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC. Apolipoprotein E polymorphism and cardiovascular disease: a huge review. Am J Epidemiol. 2002;155:487-495 [DOI] [PubMed] [Google Scholar]
- 18. Field M, Collins MW, Lovell MR, et al. Does age play a role in recovery from sports related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142:546-553 [DOI] [PubMed] [Google Scholar]
- 19. Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009:66:336-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flashman LA, Roth RM, Pixley HS, et al. Cavum septum pellucidum in schizophrenia: clinical and neuropsychological correlates. Psychiatry Res. 2007;154:147-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Friedman G, Froom P, Sazbon L, et al. Apolipoprotein E-e4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology. 1999;52:244-248 [DOI] [PubMed] [Google Scholar]
- 22. Gavett BE, Cantu RC, Shenton M, et al. Clinical appraisal of chronic traumatic encephalopathy: current perspectives and future directions. Curr Opin Neurol. 2011;24:525-531 [DOI] [PubMed] [Google Scholar]
- 23. Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med. 2011;30:179-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250-2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39:903-909 [DOI] [PubMed] [Google Scholar]
- 26. Guskiewicz KM, Marshall SW, Jordan BD, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719-726 [DOI] [PubMed] [Google Scholar]
- 27. Haacke EM, DelProposto ZS, Chaturvedi S, et al. Imaging cerebral amyloid angiopathy with susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2007;28:316-317 [PMC free article] [PubMed] [Google Scholar]
- 28. Haacke EM, Duhaime AC, Gean AD, et al. Common data elements in radiologic imaging of traumatic brain injury. J Magn Reson Imaging. 2010;32:516-543 [DOI] [PubMed] [Google Scholar]
- 29. Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med. 2004;52:612-618 [DOI] [PubMed] [Google Scholar]
- 30. Haacke EM. Susceptibility weighted imaging (SWI). Z Med Phys. 2006;16:237. [DOI] [PubMed] [Google Scholar]
- 31. Hart J, Kraut M, Womack K, et al. Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players. JAMA Neurol. 2013;70:326-335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holshouser BA, Ashwal S, Luh GY, et al. Proton MR spectroscopy after acute central nervous system injury: outcome prediction in neonates, infants, and children. Radiology. 1997;202:487-496 [DOI] [PubMed] [Google Scholar]
- 33. Holshouser BA, Tong KA, Ashwal S, et al. Prospective longitudinal proton magnetic resonance spectroscopy imaging in adult traumatic brain injury. J Magn Reson Imaging. 2006;24:33-40 [DOI] [PubMed] [Google Scholar]
- 34. Jordan BD, Relkin NR, Ravdin LD, Jacobs AR, Bennett A, Grandy S. Apolipoprotein E e4 associated with chronic traumatic brain injury in boxing. J Am Med Soc. 1997;278:136-140 [PubMed] [Google Scholar]
- 35. Jordan BD. Chronic neurologic injuries in boxing. In: Jordan BD, ed. Medical Aspects of Boxing. Boca Raton, FL: CRC Press; 1993:177-185 [Google Scholar]
- 36. Katzman R, Galasko DR, Saitoh T, et al. Apolipoprotein e4 and head trauma: synergistic or additive risks? Neurology. 1996;46:889-892 [PubMed] [Google Scholar]
- 37. Kirsch W, McAuley G, Holshouser B, et al. Serial susceptibility weighted MRI measures brain iron and microbleeds in dementia. J Alzheimers Dis. 2009;17:599-609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kou Z, Benson R, Haacke EM. Magnetic resonance imaging biomarkers of mild traumatic brain injury. In: Dambinova SA, Hayes RL, Wang KKW, eds. Biomarkers for Traumatic Brain Injury. London, UK: Royal Society of Chemistry; 2012:19-44 [Google Scholar]
- 39. Kou Z, Benson RR, Gattu R, Haacke EM, Improving the detection of diffuse axonal injury by a complementary use of advanced MRI. J Head Trauma Rehabil. 2008;23:351-352 [Google Scholar]
- 40. Kou Z, Benson RR, Haacke EM. Susceptibility weighted imaging in traumatic brain injury. In: Gillard J, Waldman A, Barker P, eds. Clinical MR Neuroimaging, 2nd ed. Cambridge, UK: Cambridge University; 2008 [Google Scholar]
- 41. Kou Z, Wu Z, Tong KA. The role of advanced magnetic resonance imaging findings as biomarkers of traumatic brain injury. J Head Trauma Rehabil. 2010;25:267-282 [DOI] [PubMed] [Google Scholar]
- 42. Kutner KC, Erlanger DM, Tsai J, Jordan B, Relkin NR. Lower cognitive performance of older football players possessing Apolipoprotein E e4. Neurosurgery. 2000;47:651-658 [DOI] [PubMed] [Google Scholar]
- 43. Leon AS, Togashi K, Rankinen T, et al. Association of apolipoprotein E polymorphism with blood lipids and maximal oxygen uptake in the sedentary state and after exercise training in the HERITAGE family study. Metabolism. 2004;53:108-116 [DOI] [PubMed] [Google Scholar]
- 44. Lovell MR, Collins MW, Iverson GL, et al. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98:296-301 [DOI] [PubMed] [Google Scholar]
- 45. Martland HS. Punch drunk. JAMA. 1928;91:1103-1107 [Google Scholar]
- 46. Mayeux R, Ottoman R, Maestre G, et al. Synergistic effects of head injury and apolipoprotein e4 in patients with Alzheimer’s disease. Neurology. 1996;45:555-557 [DOI] [PubMed] [Google Scholar]
- 47. McCrory P, Collie A, Ardsson V, Davis G. Can we manage sport related concussion in children the same as in adults. Br J Sports Med. 2004;38:516-519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McCrory P. Cavum septi pellucidii—a reason to ban boxers? Br J Sports Med. 2002;36:157-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709-735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McKee AC, Gavett BE, Stern RA, et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2010;69:918-929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McKee AC, Stein TD, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(pt 1):46-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Menzel HJ, Kladetzky RG, Assmann G. Apolipoprotein E polymorphism and coronary artery disease. Arteriosclerosis. 1983;3:310-315 [DOI] [PubMed] [Google Scholar]
- 53. Morri S. Introduction to Diffusion Tensor Imaging. Amsterdam, Netherlands: Elsevier; 2007 [Google Scholar]
- 54. Morrison R. Medical and public health aspects of boxing. JAMA. 1986;255:2475-2480 [PubMed] [Google Scholar]
- 55. Newcombe VF, Williams GB, Nortje J, et al. Analysis of acute traumatic axonal injury using diffusion tensor imaging. Br J Neurosurg. 2007;21:340-348 [DOI] [PubMed] [Google Scholar]
- 56. Nicoll JAR, Roberts GW, Graham DI. Apolipoprotein E e4 allele is associated with deposition of amyloid beta-protein following head injury. Nat Med. 1995;1:135-137 [DOI] [PubMed] [Google Scholar]
- 57. Niogi SN, Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil. 2010;25:241-255 [DOI] [PubMed] [Google Scholar]
- 58. Niogi SN, Mukherjee P, Ghajar J, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. Am J Neuroradiol. 2008;29:967-973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Obisesan TO, Ferrell RE, Goldberg AP, Phares DA, Ellis TJ, Hagberg JM. APOE genotype affects black-white responses of high density lipoprotein cholesterol subspecies to aerobic exercise training. Metabolism. 2008;57:1669-1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Omalu BI, DeKosky ST, Hamilton RL, et al. Chronic traumatic encephalopathy in a National Football League player: II. Neurosurgery. 2006;58:1086-1092 [DOI] [PubMed] [Google Scholar]
- 61. Omalu BI, DeKosky ST, Minster RL, et al. Chronic traumatic encephalopathy in a National Football League Player: I. Neurosurgery. 2005;57:128-134 [DOI] [PubMed] [Google Scholar]
- 62. Payne E. Brains of boxers. Neurochirurgia (Stuttg). 1968;11:173-188 [DOI] [PubMed] [Google Scholar]
- 63. Pellman EJ, Lovell MR, Viano DC, Casson IR, Tucker A. Concussion in professional football: neuropsychological testing—part 6. Neurosurgery. 2004;55:1290-1305 [DOI] [PubMed] [Google Scholar]
- 64. Pellman EJ, Lovell MR, Viano DC, Casson IR. Concussion in professional football: recovery of NFL and high school athletes assessed by computerized neuropsychological testing—part 12. Neurosurgery. 2006;58:263-274 [DOI] [PubMed] [Google Scholar]
- 65. Pellman EJ, Powell JW, Viano DC, et al. Concussion in professional football: epidemiological features of game injuries and review of the literature—part 3. Neurosurgery. 2004;54:81-97 [DOI] [PubMed] [Google Scholar]
- 66. Pellman EJ, Viano DC, Casson IR, Arfken C, Powell J. Concussion in professional football: injuries involving 7+ days out—part 5. Neurosurgery. 2004;55:1100-1119 [DOI] [PubMed] [Google Scholar]
- 67. Pellman EJ, Viano DC, Casson IR, et al. Concussion in professional football: repeat injuries—part 4. Neurosurgery. 2004;55:860-876 [DOI] [PubMed] [Google Scholar]
- 68. Pellman EJ, Viano DC, Tucker AM, Casson IR, Waeckerle JF. Concussion in professional football: reconstruction of game impacts and injuries. Neurosurgery. 2003;53:799-814 [DOI] [PubMed] [Google Scholar]
- 69. Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM, Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997;204:272–277 [DOI] [PubMed] [Google Scholar]
- 70. Roberts AH. Brain Damage in Boxers. London, UK: Pitman Medical; 1969 [Google Scholar]
- 71. Ross RJ, Cole M, Thompson JS, et al. Boxers: computed tomography, EEG and neurological evaluation. JAMA. 1983;249:211-213 [DOI] [PubMed] [Google Scholar]
- 72. Saulle M, Greenwald BD. Chronic traumatic encephalopathy: a review. Rehabil Res Pract. 2012;2012:816069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sehgal V, Delproposto Z, Haacke EM, et al. Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging. 2005;22:439-450 [DOI] [PubMed] [Google Scholar]
- 74. Sehgal V, Delproposto Z, Haddar D, et al. Susceptibility-weighted imaging to visualize blood products and improve tumor contrast in the study of brain masses. J Magn Reson Imaging. 2006;24:41-51 [DOI] [PubMed] [Google Scholar]
- 75. Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC. Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM R. 2011;3(suppl 2):S460-S467 [DOI] [PubMed] [Google Scholar]
- 76. Teasdale GM, Nicoll JA, Murray G, Fiddes M. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet. 1997;350:1069-1071 [DOI] [PubMed] [Google Scholar]
- 77. Tong KA, Ashwal S, Holshouser BA, et al. Diffuse axonal injury in children: clinical correlation with hemorrhagic lesions. Ann Neurol. 2004;56:36-50 [DOI] [PubMed] [Google Scholar]
- 78. Tong KA, Ashwal S, Holshouser BA, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology. 2003;27:332-339 [DOI] [PubMed] [Google Scholar]
- 79. Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10:241-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Viano DC, Casson IR, Pellman EJ, et al. Concussion in professional football: comparison with boxing head impacts—part 10. Neurosurgery. 2005;57:1154-1173 [DOI] [PubMed] [Google Scholar]
- 81. Victor M. Alcoholic dementia. Can J Neurol Sci. 1994;21:88-99 [DOI] [PubMed] [Google Scholar]
- 82. Victor M. Persistent altered mentation due to ethanol. Neurol Clin. 1993;11:639-661 [PubMed] [Google Scholar]
- 83. Westlye L, Reinvang I, Rootwelt H, Espeseth T. Effects of APOE on brain white matter microstructure in healthy adults. Neurology. 2012;79:1961-1969 [DOI] [PubMed] [Google Scholar]
- 84. Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4:103-109 [DOI] [PubMed] [Google Scholar]
- 85. Wilde EA, McCauley SR, Hunter JV, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948-955 [DOI] [PubMed] [Google Scholar]


