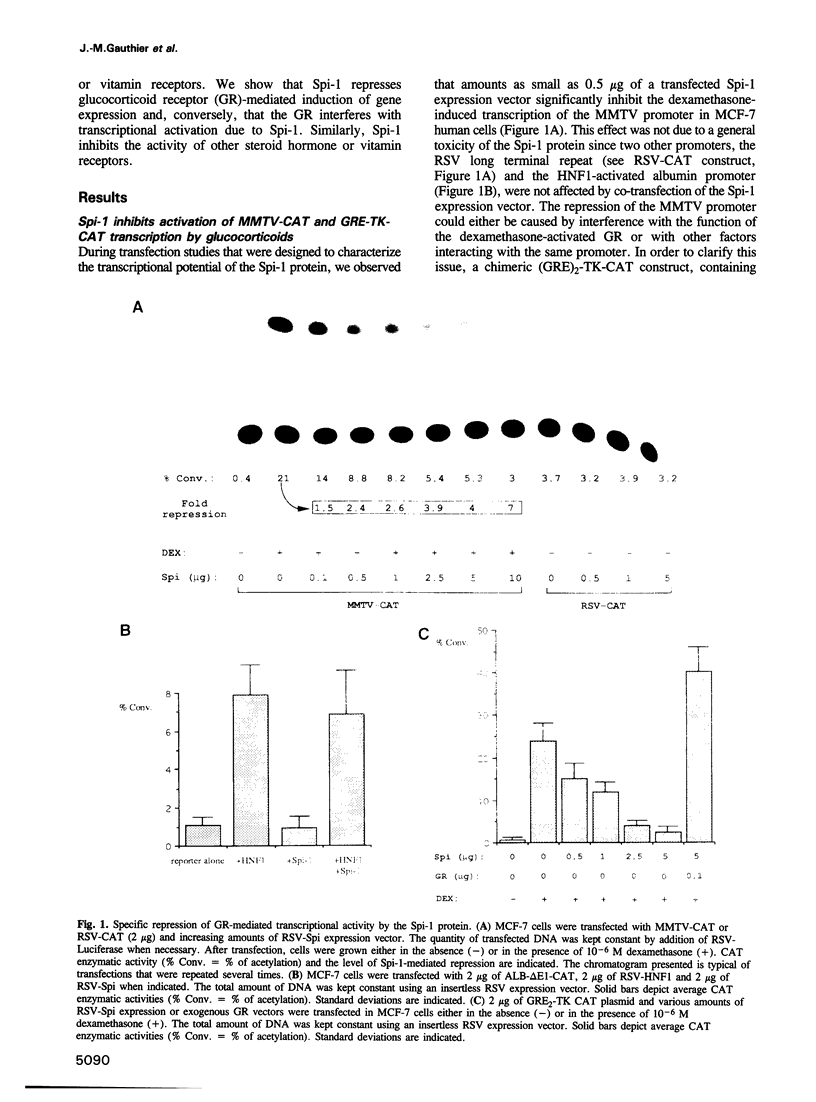
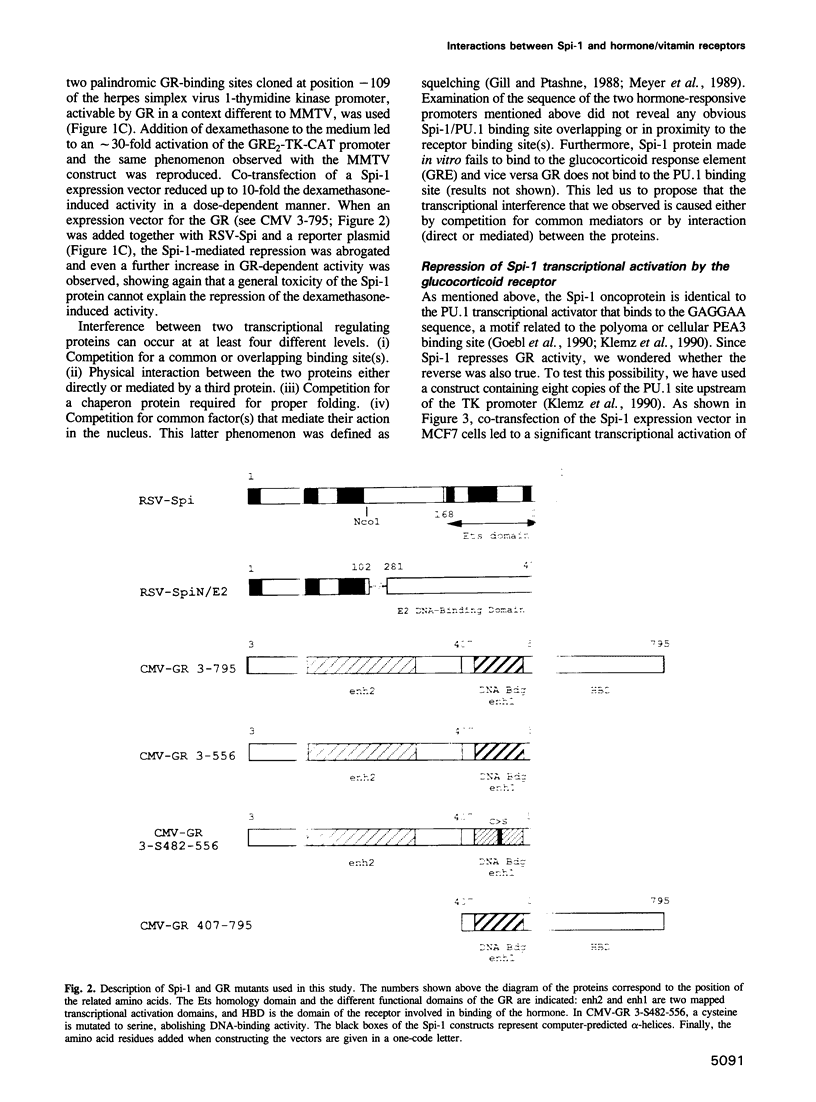
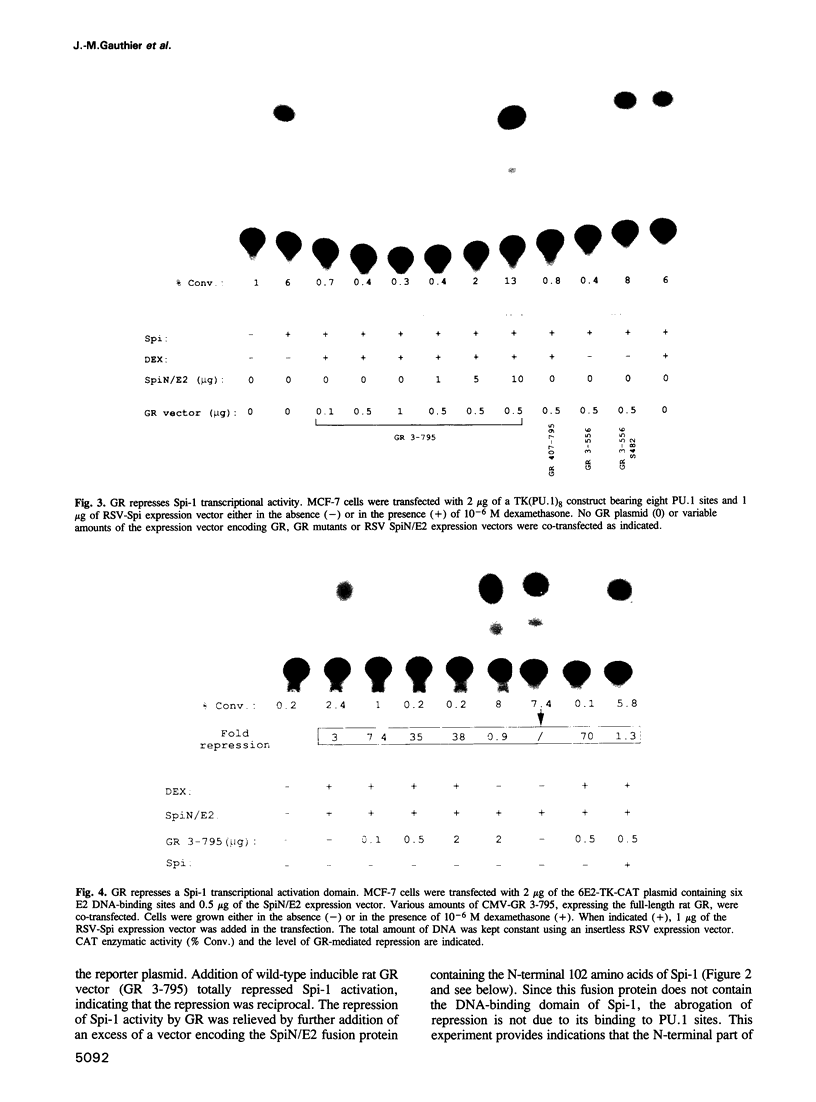
Abstract
The Spi-1/PU.1 protein is an Ets-related transcription factor, whose overexpression is a consequence of SFFV integration in Friend erythroleukemic cells. We present evidence that Spi-1/PU.1 can specifically repress the glucocorticoid-induced activation of promoters carrying a glucocorticoid response element (GRE). Conversely, the glucocorticoid receptor (GR) represses Spi-1/PU.1-mediated transcriptional activation in the presence of hormone. Spi-1/PU.1 also antagonized activation by other nuclear receptors, such as the thyroid hormone or the retinoic acid receptors, in several cell lines, including K562 erythroleukemic cells. These observations suggest that accumulation of the Spi-1/PU.1 protein may interfere with the action of hormones in the erythrocyte differentiation pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-David Y., Bernstein A. Friend virus-induced erythroleukemia and the multistage nature of cancer. Cell. 1991 Sep 6;66(5):831–834. doi: 10.1016/0092-8674(91)90428-2. [DOI] [PubMed] [Google Scholar]
- Ben-David Y., Giddens E. B., Letwin K., Bernstein A. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Dev. 1991 Jun;5(6):908–918. doi: 10.1101/gad.5.6.908. [DOI] [PubMed] [Google Scholar]
- Chouard T., Blumenfeld M., Bach I., Vandekerckhove J., Cereghini S., Yaniv M. A distal dimerization domain is essential for DNA-binding by the atypical HNF1 homeodomain. Nucleic Acids Res. 1990 Oct 11;18(19):5853–5863. doi: 10.1093/nar/18.19.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbois C., Aubert D., Legrand C., Pain B., Samarut J. A novel mechanism of action for v-ErbA: abrogation of the inactivation of transcription factor AP-1 by retinoic acid and thyroid hormone receptors. Cell. 1991 Nov 15;67(4):731–740. doi: 10.1016/0092-8674(91)90068-a. [DOI] [PubMed] [Google Scholar]
- Dostatni N., Thierry F., Yaniv M. A dimer of BPV-1 E2 containing a protease resistant core interacts with its DNA target. EMBO J. 1988 Dec 1;7(12):3807–3816. doi: 10.1002/j.1460-2075.1988.tb03265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucas V., Spyrou G., Yaniv M. Unregulated expression of c-Jun or c-Fos proteins but not Jun D inhibits oestrogen receptor activity in human breast cancer derived cells. EMBO J. 1991 Aug;10(8):2237–2245. doi: 10.1002/j.1460-2075.1991.tb07760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frykberg L., Palmieri S., Beug H., Graf T., Hayman M. J., Vennström B. Transforming capacities of avian erythroblastosis virus mutants deleted in the erbA or erbB oncogenes. Cell. 1983 Jan;32(1):227–238. doi: 10.1016/0092-8674(83)90513-5. [DOI] [PubMed] [Google Scholar]
- Gandrillon O., Jurdic P., Pain B., Desbois C., Madjar J. J., Moscovici M. G., Moscovici C., Samarut J. Expression of the v-erbA product, an altered nuclear hormone receptor, is sufficient to transform erythrocytic cells in vitro. Cell. 1989 Jul 14;58(1):115–121. doi: 10.1016/0092-8674(89)90408-x. [DOI] [PubMed] [Google Scholar]
- Gauthier J. M., Dillner J., Yaniv M. Structural analysis of the human papillomavirus type 16-E2 transactivator with antipeptide antibodies reveals a high mobility region linking the transactivation and the DNA-binding domains. Nucleic Acids Res. 1991 Dec;19(25):7073–7079. doi: 10.1093/nar/19.25.7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier J. M., Dostatni N., Lusky M., Yaniv M. Two DNA-bound E2 dimers are required for strong transcriptional activation and for cooperation with cellular factors in most cells. New Biol. 1991 May;3(5):498–509. [PubMed] [Google Scholar]
- Gill G., Ptashne M. Negative effect of the transcriptional activator GAL4. Nature. 1988 Aug 25;334(6184):721–724. doi: 10.1038/334721a0. [DOI] [PubMed] [Google Scholar]
- Goebl M. K. The PU.1 transcription factor is the product of the putative oncogene Spi-1. Cell. 1990 Jun 29;61(7):1165–1166. doi: 10.1016/0092-8674(90)90676-6. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman A., Wasylyk B. Nuclear targets for transcription regulation by oncogenes. Trends Genet. 1991 Feb;7(2):49–54. doi: 10.1016/0168-9525(91)90231-E. [DOI] [PubMed] [Google Scholar]
- Hagemeier C., Bannister A. J., Cook A., Kouzarides T. The activation domain of transcription factor PU.1 binds the retinoblastoma (RB) protein and the transcription factor TFIID in vitro: RB shows sequence similarity to TFIID and TFIIB. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1580–1584. doi: 10.1073/pnas.90.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen T. H., Turek L. P., Mercurio F. M., Cripe T. P., Olson B. J., Anderson R. D., Seidl D., Karin M., Schiller J. Sequence-specific and general transcriptional activation by the bovine papillomavirus-1 E2 trans-activator require an N-terminal amphipathic helix-containing E2 domain. EMBO J. 1988 Dec 20;7(13):4245–4253. doi: 10.1002/j.1460-2075.1988.tb03322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard J. M., Herbomel P., Ott M. O., Mottura-Rollier A., Weiss M., Yaniv M. Determinants of rat albumin promoter tissue specificity analyzed by an improved transient expression system. Mol Cell Biol. 1987 Jul;7(7):2425–2434. doi: 10.1128/mcb.7.7.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Karim F. D., Urness L. D., Thummel C. S., Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A., Gunther C. V., Nye J. A. The ETS-domain: a new DNA-binding motif that recognizes a purine-rich core DNA sequence. Genes Dev. 1990 Sep;4(9):1451–1453. doi: 10.1101/gad.4.9.1451. [DOI] [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- König H., Ponta H., Rahmsdorf H. J., Herrlich P. Interference between pathway-specific transcription factors: glucocorticoids antagonize phorbol ester-induced AP-1 activity without altering AP-1 site occupation in vivo. EMBO J. 1992 Jun;11(6):2241–2246. doi: 10.1002/j.1460-2075.1992.tb05283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. F., Dostatni N., McBride A. A., Yaniv M., Howley P. M., Arcangioli B. Functional analysis of the papilloma virus E2 trans-activator in Saccharomyces cerevisiae. Genes Dev. 1989 Jan;3(1):38–48. doi: 10.1101/gad.3.1.38. [DOI] [PubMed] [Google Scholar]
- Lavigueur A., Bernstein A. p53 transgenic mice: accelerated erythroleukemia induction by Friend virus. Oncogene. 1991 Dec;6(12):2197–2201. [PubMed] [Google Scholar]
- Lewin B. Oncogenic conversion by regulatory changes in transcription factors. Cell. 1991 Jan 25;64(2):303–312. doi: 10.1016/0092-8674(91)90640-k. [DOI] [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Lucibello F. C., Slater E. P., Jooss K. U., Beato M., Müller R. Mutual transrepression of Fos and the glucocorticoid receptor: involvement of a functional domain in Fos which is absent in FosB. EMBO J. 1990 Sep;9(9):2827–2834. doi: 10.1002/j.1460-2075.1990.tb07471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride A. A., Byrne J. C., Howley P. M. E2 polypeptides encoded by bovine papillomavirus type 1 form dimers through the common carboxyl-terminal domain: transactivation is mediated by the conserved amino-terminal domain. Proc Natl Acad Sci U S A. 1989 Jan;86(2):510–514. doi: 10.1073/pnas.86.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Gronemeyer H., Turcotte B., Bocquel M. T., Tasset D., Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989 May 5;57(3):433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- Moreau-Gachelin F., Ray D., Mattei M. G., Tambourin P., Tavitian A. The putative oncogene Spi-1: murine chromosomal localization and transcriptional activation in murine acute erythroleukemias. Oncogene. 1989 Dec;4(12):1449–1456. [PubMed] [Google Scholar]
- Moreau-Gachelin F., Tavitian A., Tambourin P. Spi-1 is a putative oncogene in virally induced murine erythroleukaemias. Nature. 1988 Jan 21;331(6153):277–280. doi: 10.1038/331277a0. [DOI] [PubMed] [Google Scholar]
- Muller M., Renkawitz R. The glucocorticoid receptor. Biochim Biophys Acta. 1991 Feb 16;1088(2):171–182. doi: 10.1016/0167-4781(91)90052-n. [DOI] [PubMed] [Google Scholar]
- Munroe D. G., Peacock J. W., Benchimol S. Inactivation of the cellular p53 gene is a common feature of Friend virus-induced erythroleukemia: relationship of inactivation to dominant transforming alleles. Mol Cell Biol. 1990 Jul;10(7):3307–3313. doi: 10.1128/mcb.10.7.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn M. F., Hunter T. The ets sequence is required for induction of erythroblastosis in chickens by avian retrovirus E26. J Virol. 1989 Jan;63(1):398–402. doi: 10.1128/jvi.63.1.398-402.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- När A. M., Boutin J. M., Lipkin S. M., Yu V. C., Holloway J. M., Glass C. K., Rosenfeld M. G. The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell. 1991 Jun 28;65(7):1267–1279. doi: 10.1016/0092-8674(91)90021-p. [DOI] [PubMed] [Google Scholar]
- Paul R., Schuetze S., Kozak S. L., Kabat D. A common site for immortalizing proviral integrations in Friend erythroleukemia: molecular cloning and characterization. J Virol. 1989 Nov;63(11):4958–4961. doi: 10.1128/jvi.63.11.4958-4961.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R., Schuetze S., Kozak S. L., Kozak C. A., Kabat D. The Sfpi-1 proviral integration site of Friend erythroleukemia encodes the ets-related transcription factor Pu.1. J Virol. 1991 Jan;65(1):464–467. doi: 10.1128/jvi.65.1.464-467.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D., Bosselut R., Ghysdael J., Mattei M. G., Tavitian A., Moreau-Gachelin F. Characterization of Spi-B, a transcription factor related to the putative oncoprotein Spi-1/PU.1. Mol Cell Biol. 1992 Oct;12(10):4297–4304. doi: 10.1128/mcb.12.10.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyers C. L., Denny C. T., Witte O. N. Leukemia and the disruption of normal hematopoiesis. Cell. 1991 Jan 25;64(2):337–350. doi: 10.1016/0092-8674(91)90643-d. [DOI] [PubMed] [Google Scholar]
- Schroeder C., Gibson L., Zenke M., Beug H. Modulation of normal erythroid differentiation by the endogenous thyroid hormone and retinoic acid receptors: a possible target for v-erbA oncogene action. Oncogene. 1992 Feb;7(2):217–227. [PubMed] [Google Scholar]
- Schuetze S., Paul R., Gliniak B. C., Kabat D. Role of the PU.1 transcription factor in controlling differentiation of Friend erythroleukemia cells. Mol Cell Biol. 1992 Jul;12(7):2967–2975. doi: 10.1128/mcb.12.7.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Severne Y., Wieland S., Schaffner W., Rusconi S. Metal binding 'finger' structures in the glucocorticoid receptor defined by site-directed mutagenesis. EMBO J. 1988 Aug;7(8):2503–2508. doi: 10.1002/j.1460-2075.1988.tb03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemshedini L., Knauthe R., Sassone-Corsi P., Pornon A., Gronemeyer H. Cell-specific inhibitory and stimulatory effects of Fos and Jun on transcription activation by nuclear receptors. EMBO J. 1991 Dec;10(12):3839–3849. doi: 10.1002/j.1460-2075.1991.tb04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry F., Dostatni N., Arnos F., Yaniv M. Cooperative activation of transcription by bovine papillomavirus type 1 E2 can occur over a large distance. Mol Cell Biol. 1990 Aug;10(8):4431–4437. doi: 10.1128/mcb.10.8.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling F., Moreau-Gachelin F., Tambourin P. Emergence of tumorigenic cells during the course of Friend virus leukemias. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3614–3618. doi: 10.1073/pnas.78.6.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S., Döbbeling U., Rusconi S. Interference and synergism of glucocorticoid receptor and octamer factors. EMBO J. 1991 Sep;10(9):2513–2521. doi: 10.1002/j.1460-2075.1991.tb07791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Xin J. H., Cowie A., Lachance P., Hassell J. A. Molecular cloning and characterization of PEA3, a new member of the Ets oncogene family that is differentially expressed in mouse embryonic cells. Genes Dev. 1992 Mar;6(3):481–496. doi: 10.1101/gad.6.3.481. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Gamble C. L., Clark S. P., Joyner A., Shibuya T., MacDonald M. E., Mager D., Bernstein A., Mak T. W. Clonal analysis of early and late stages of erythroleukemia induced by molecular clones of integrated spleen focus-forming virus. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6893–6897. doi: 10.1073/pnas.78.11.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Zhang X. K., Graupner G., Tzukerman M., Sakamoto B., Karin M., Pfahl M. Antagonism between retinoic acid receptors and AP-1: implications for tumor promotion and inflammation. New Biol. 1991 Dec;3(12):1206–1219. [PubMed] [Google Scholar]
- Zenke M., Muñoz A., Sap J., Vennström B., Beug H. v-erbA oncogene activation entails the loss of hormone-dependent regulator activity of c-erbA. Cell. 1990 Jun 15;61(6):1035–1049. doi: 10.1016/0092-8674(90)90068-p. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Wills K. N., Husmann M., Hermann T., Pfahl M. Novel pathway for thyroid hormone receptor action through interaction with jun and fos oncogene activities. Mol Cell Biol. 1991 Dec;11(12):6016–6025. doi: 10.1128/mcb.11.12.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]