Abstract
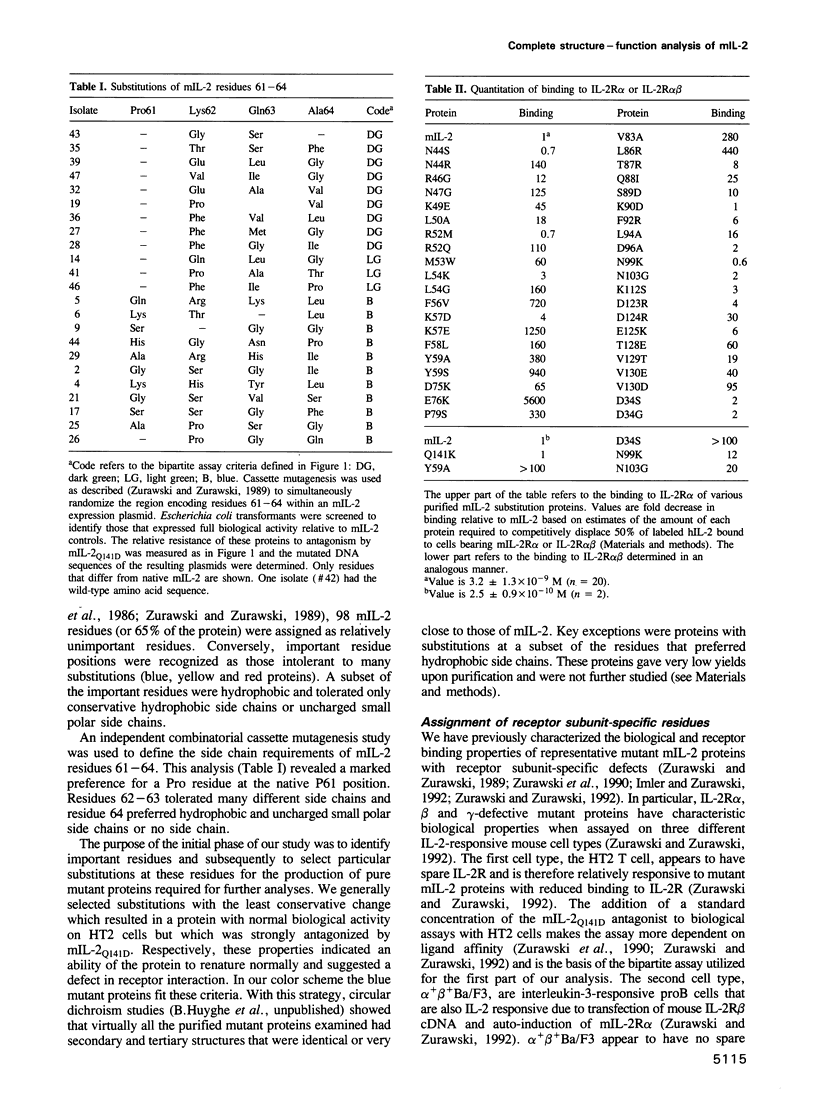
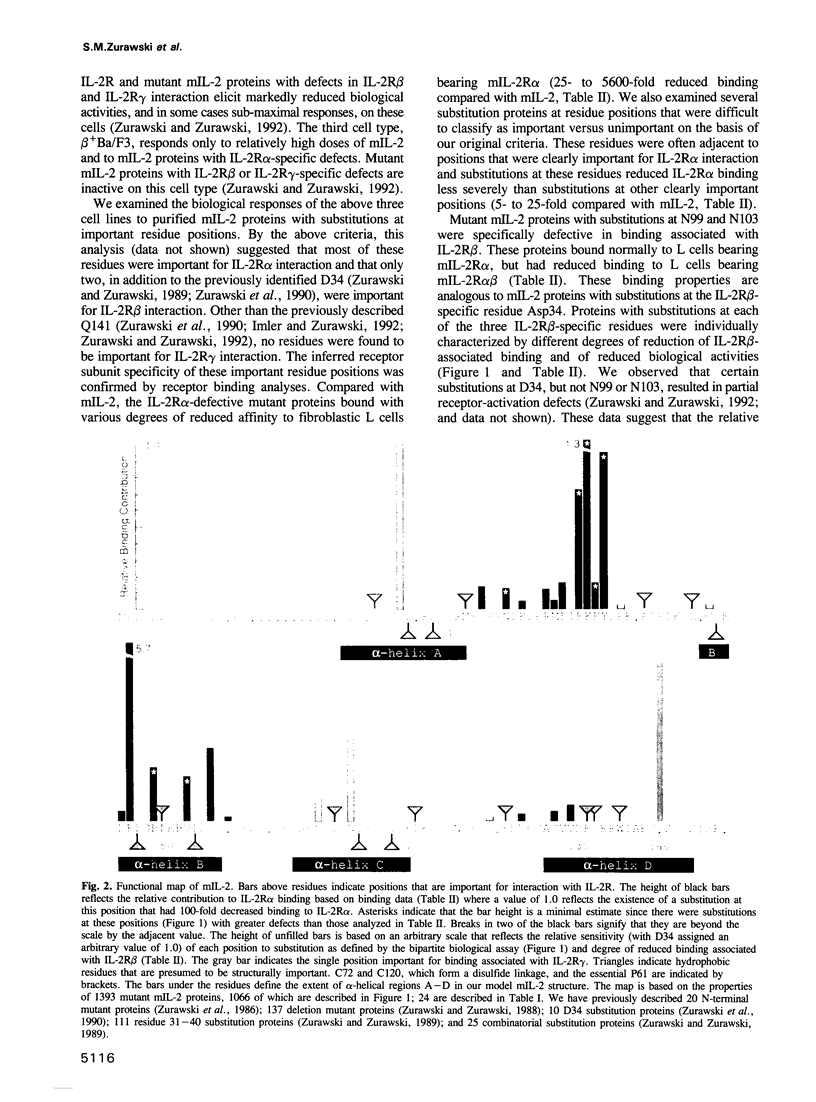
The high affinity receptor for interleukin-2 (IL-2) contains three subunits called IL-2R alpha, beta and gamma. A biological and receptor binding analysis based on 1393 different mutant mouse IL-2 (mIL-2) proteins was used to define the function of each of the 149 residues. By this genetic analysis, 44 residues were assigned important functions, 21 of which were structural. The remaining 23 residues consisted of 19 residues, from three separate regions, that were important for IL-2R alpha interaction; three residues, from two separate regions, that were important for IL-2R beta interaction; and a single residue important for IL-2R gamma interaction. We built a model mIL-2 structure based on the homologous human IL-2 (hIL-2) crystal structure. The roles of the 21 residues presumed to be important for structure were consistent with the model. Despite discontinuity in the primary sequence, the residues specific for each IL-2R subunit interaction were clustered and located to three disparate regions of the tertiary mIL-2 structure. The relative spatial locations of these three surfaces are different from the two receptor binding sites known for the structurally related human growth hormone and the significance of this observation is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F. Unraveling the structure of IL-2. Science. 1992 Jul 17;257(5068):410–413. doi: 10.1126/science.1631562. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Collins L., Tsien W. H., Seals C., Hakimi J., Weber D., Bailon P., Hoskings J., Greene W. C., Toome V., Ju G. Identification of specific residues of human interleukin 2 that affect binding to the 70-kDa subunit (p70) of the interleukin 2 receptor. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7709–7713. doi: 10.1073/pnas.85.20.7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Ultsch M., De Vos A. M., Mulkerrin M. G., Clauser K. R., Wells J. A. Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science. 1991 Nov 8;254(5033):821–825. doi: 10.1126/science.1948064. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Wells J. A. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989 Jun 2;244(4908):1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Mori H., Doi T., Taniguchi T. A restricted cytoplasmic region of IL-2 receptor beta chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989 Dec 1;59(5):837–845. doi: 10.1016/0092-8674(89)90607-7. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Imler J. L., Miyajima A., Zurawski G. Identification of three adjacent amino acids of interleukin-2 receptor beta chain which control the affinity and the specificity of the interaction with interleukin-2. EMBO J. 1992 Jun;11(6):2047–2053. doi: 10.1002/j.1460-2075.1992.tb05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imler J. L., Zurawski G. Receptor binding and internalization of mouse interleukin-2 derivatives that are partial agonists. J Biol Chem. 1992 Jul 5;267(19):13185–13190. [PubMed] [Google Scholar]
- Ju G., Collins L., Kaffka K. L., Tsien W. H., Chizzonite R., Crowl R., Bhatt R., Kilian P. L. Structure-function analysis of human interleukin-2. Identification of amino acid residues required for biological activity. J Biol Chem. 1987 Apr 25;262(12):5723–5731. [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Crabtree G. R., Rudikoff S., Pumphrey J., Robb R. J., Krönke M., Svetlik P. B., Peffer N. J., Waldmann T. A. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- McKay D. B. Response. Science. 1992 Jul 17;257(5068):412–413. doi: 10.1126/science.257.5068.412. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nikaido T., Shimizu A., Ishida N., Sabe H., Teshigawara K., Maeda M., Uchiyama T., Yodoi J., Honjo T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):631–635. doi: 10.1038/311631a0. [DOI] [PubMed] [Google Scholar]
- O'Garra A. Interleukins and the immune system 1. Lancet. 1989 Apr 29;1(8644):943–947. [PubMed] [Google Scholar]
- O'Garra A. Interleukins and the immune system 2. Lancet. 1989 May 6;1(8645):1003–1005. doi: 10.1016/s0140-6736(89)92640-8. [DOI] [PubMed] [Google Scholar]
- Patthy L. Homology of a domain of the growth hormone/prolactin receptor family with type III modules of fibronectin. Cell. 1990 Apr 6;61(1):13–14. doi: 10.1016/0092-8674(90)90208-v. [DOI] [PubMed] [Google Scholar]
- Ringheim G. E., Freimark B. D., Robb R. J. Quantitative characterization of the intrinsic ligand-binding affinity of the interleukin 2 receptor beta chain and its modulation by the alpha chain and a second affinity-modulating element. Lymphokine Cytokine Res. 1991 Jun;10(3):219–224. [PubMed] [Google Scholar]
- Sauvé K., Nachman M., Spence C., Bailon P., Campbell E., Tsien W. H., Kondas J. A., Hakimi J., Ju G. Localization in human interleukin 2 of the binding site to the alpha chain (p55) of the interleukin 2 receptor. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4636–4640. doi: 10.1073/pnas.88.11.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita T., Asao H., Ohtani K., Ishii N., Kumaki S., Tanaka N., Munakata H., Nakamura M., Sugamura K. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992 Jul 17;257(5068):379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- Wang A., Lu S. D., Mark D. F. Site-specific mutagenesis of the human interleukin-2 gene: structure-function analysis of the cysteine residues. Science. 1984 Jun 29;224(4656):1431–1433. doi: 10.1126/science.6427925. [DOI] [PubMed] [Google Scholar]
- Weigel U., Meyer M., Sebald W. Mutant proteins of human interleukin 2. Renaturation yield, proliferative activity and receptor binding. Eur J Biochem. 1989 Mar 15;180(2):295–300. doi: 10.1111/j.1432-1033.1989.tb14647.x. [DOI] [PubMed] [Google Scholar]
- Weir M. P., Chaplin M. A., Wallace D. M., Dykes C. W., Hobden A. N. Structure-activity relationships of recombinant human interleukin 2. Biochemistry. 1988 Sep 6;27(18):6883–6892. doi: 10.1021/bi00418a034. [DOI] [PubMed] [Google Scholar]
- Zurawski S. M., Imler J. L., Zurawski G. Partial agonist/antagonist mouse interleukin-2 proteins indicate that a third component of the receptor complex functions in signal transduction. EMBO J. 1990 Dec;9(12):3899–3905. doi: 10.1002/j.1460-2075.1990.tb07610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski S. M., Mosmann T. R., Benedik M., Zurawski G. Alterations in the amino-terminal third of mouse interleukin 2: effects on biological activity and immunoreactivity. J Immunol. 1986 Nov 15;137(10):3354–3360. [PubMed] [Google Scholar]
- Zurawski S. M., Zurawski G. Identification of three critical regions within mouse interleukin 2 by fine structural deletion analysis. EMBO J. 1988 Apr;7(4):1061–1069. doi: 10.1002/j.1460-2075.1988.tb02914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski S. M., Zurawski G. Mouse interleukin-2 structure-function studies: substitutions in the first alpha-helix can specifically inactivate p70 receptor binding and mutations in the fifth alpha-helix can specifically inactivate p55 receptor binding. EMBO J. 1989 Sep;8(9):2583–2590. doi: 10.1002/j.1460-2075.1989.tb08397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski S. M., Zurawski G. Receptor antagonist and selective agonist derivatives of mouse interleukin-2. EMBO J. 1992 Nov;11(11):3905–3910. doi: 10.1002/j.1460-2075.1992.tb05483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M., Kossiakoff A. A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992 Jan 17;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]