Abstract
T-helper 1 and 17 (Th1/Th17) responses are important in inflammatory bowel disease (IBD), and research indicates that Toll-like receptor 6 (TLR6) stimulation leads to Th17 cell development within the lung. The gastrointestinal tract, like the lung, is a mucosal surface that is exposed to bacterially derived TLR6 ligands. Thus, we looked at the effects of TLR6 stimulation on the expression of Th17-, Th1-, and regulatory T-cell-associated transcription factors; RORγt, T-bet, and Foxp3, respectively; in CD4+ T cells within gut-associated lymphoid tissue (GALT) in vitro and in vivo. Cells from GALT and spleen were stimulated with anti-CD3 and TLR ligands for TLR1/2 and TLR2/6 (Pam3CSK4 and FSL-1, respectively). FSL-1 was more effective than Pam3CSK4 at inducing Th1 and Th17 responses in the GALT while Pam3CSK4 rivaled FSL-1 in the spleen. TLR6 was further explored in vivo using experimental colitis. Tlr6−/− mice were resistant to colitis, and oral FSL-1 led to more severe colitis in wild-type mice. Similar pro-inflammatory reactions were seen in human peripheral blood mononuclear cells, and TLR6 expression was directly correlated with RORC mRNA levels in inflamed intestines of IBD patients. These results demonstrate that TLR6 supports Th1- and Th17-skewed responses in the GALT and might be an important target for the development of new medical interventions in IBD.
Introduction
Toll-like receptor 6 (TLR6) is a member of the TLR family of pattern recognition receptors.1 Like TLR1, it forms a heterodimer with TLR2.2 The heterodimer TLR2/6 recognizes diacylated lipopeptides whereas TLR1/2 recognizes triacylated lipopeptides.3, 4 Lipopeptides are cell wall components of Gram-positive bacteria, yeasts, and mycoplasma.
Initial studies on TLR6 have suggested that TLR2/6 stimulation on dendritic cells leads to tolerogenic dendritic cell formation and regulatory T-cell (Treg) development.5, 6 Tregs have the capacity to suppress the pro-inflammatory activities of other immune cells, and they can differentiate into two main groups based on their expression of the transcription factor, forkhead box p3 (Foxp3).7 Foxp3− Treg, often known as regulatory type 1 T cells, are induced in the periphery and produce high amounts of interleukin (IL)-10. Foxp3+ Treg, on the other hand, may be induced or thymus-derived and use additional suppression mechanisms.
Recently, contradictory results were published that demonstrated that TLR6 could support the development of T-helper 17 (Th17) cells in lung, which were protective against fungal infection.8 Th17 cell responses are especially important for the control of extracellular bacterial and fungal pathogens and are particularly well known for their ability to stimulate the accumulation of neutrophils.9 Th17 cells are characterized by the master transcription factor, retinoic acid-related orphan receptor γt (RORγt) and the production of IL-17A, IL-17F, IL-22, IL-26, tumor necrosis factor α, and granulocyte macrophage colony-stimulating factor. Under pro-inflammatory conditions, with high amounts of IL-23 and IL-1β, they also produce interferon γ (IFNγ).10
Genome-wide association scan data indicate a role of Th17 cells in inflammatory bowel disease (IBD),11 and Th17 cells are increased in patients with a magnified activation state.12, 13, 14 This is in addition to the classical T-cell subset associations of the two main forms of IBD: Th1 with Crohn's disease and Th2 with ulcerative colitis.15 Crohn's disease, in particular, appears to be mediated by Th1/Th17 responses,15 including highly inflammatory Th17 cells that produce IFNγ.12, 16
As TLR6 is involved in Th17 induction in the lung,8 we considered that TLR6 might be involved in modulating Th17 responses in IBD. We found that TLR6 stimulation of murine gut-associated lymphoid tissue (GALT) cells was more effective than Pam3CSK4 (TLR1/2 ligand) at inducing Th1/Th17 responses in vitro. Moreover, Tlr6−/− mice were protected against colitis while the addition of TLR6 ligands during colitis led to worsened disease and increased Th17 responses after disease resolution. TLR6 stimulation may be important in controlling Th1/Th17-mediated inflammation in IBD patients.
Results
TLR6 is expressed in the intestines of IBD patients and mice with experimental colitis
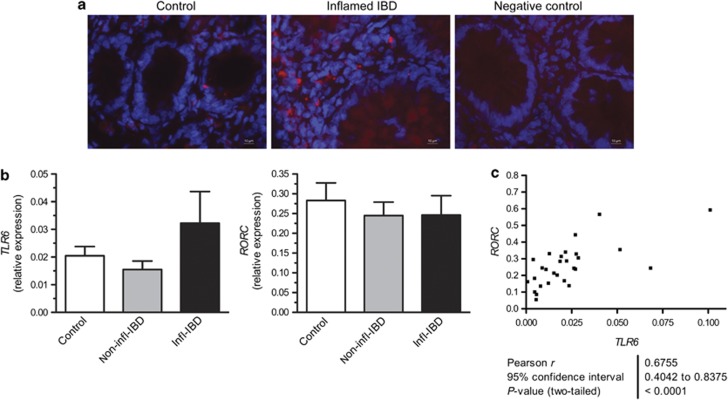
Researchers have reported a role for TLR6 in the development of Th17 cells at the lung mucosa.8 Therefore, we investigated the expression of TLR6 mRNA and protein in inflamed intestinal tissues. Immunofluorescence staining showed that TLR6 was expressed in the intestine by both crypt epithelial and non-epithelial cells, and TLR6+ cells were present in IBD intestinal biopsies (Figure 1a). TLR6 and RORC mRNA were also detected in both patient and control intestines (Figure 1b). Although the mRNA levels were not significantly changed in IBD patients, the levels of the two genes were significantly correlated (Figure 1c), suggesting increased TLR6 expression has a relationship with the Th17-associated master transcription factor RORC in the intestine.
Figure 1.
Toll-like receptor 6 (TLR6) is expressed during inflammatory bowel disease (IBD). (a) Immunofluorescent staining of TLR6 in intestinal tissues obtained from a control (Control) and an IBD patient (Inflamed IBD). Negative control shows background stain without the primary antibody. Representative stains are shown for n=3 controls and IBD patients. Red staining shows TLR6+ cells, and blue shows nucleic acids (cell nuclei). Photomicrographs were taken at × 400 magnification. (b) Relative expression of RORC and TLR6 was determined for RNA obtained from colon carcinoma patients (control, n=10), non-inflamed regions of IBD patient intestinal tissue (non-infl-IBD, n=10) and matched inflamed regions of IBD patient intestines (infl-IBD). Data are presented as mean+s.e.m. The measurement was performed twice independently. (c) A significant correlation was found between the mRNA levels of RORC and TLR6 in colon samples obtained from both control and IBD patient material (n=28).
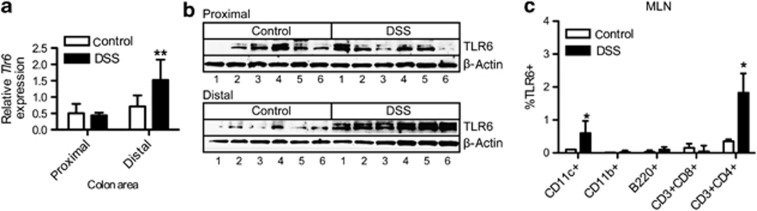
To determine Tlr6 mRNA expression in the murine colon, we isolated mRNA from colon at both the distal (rectum) and proximal (cecum) ends during intestinal inflammation (day 7) in the dextran sodium sulfate (DSS)-induced colitis model. In DSS colitis, the disease manifests more strongly at the distal end. Although both control and DSS-treated mice expressed Tlr6 in the colon, during colitis Tlr6 mRNA was significantly increased in the inflamed distal region of the colon (Figure 2a). The increased expression of TLR6 protein was confirmed by immunoblot (Figure 2b). As expected, we observed a double band of ∼100 kD, demonstrating that TLR6 is upregulated in the colon during colitis. To determine which immune cells express TLR6, mesenteric lymph node (MLN) cells were isolated from control and DSS-treated mice and were examined for TLR6 expression using flow cytometry. TLR6 expression was low in immune cells taken from control mice (Figure 2c). In DSS-treated mice, TLR6 expression was significantly upregulated in CD11c+ and CD3+CD4+ cells.
Figure 2.
Toll-like receptor 6 (TLR6) expression is increased during experimental colitis. (a) Distal and proximal regions of colon tissues isolated from control and dextran sodium sulfate (DSS)-treated mice were analyzed for Tlr6 (n=6). Values are presented as relative to the household gene Rps13. (b) Immunoblot for TLR6 protein content in distal and proximal colon tissues of control and DSS-treated mice (n=6). The double bands are in accordance with the staining pattern shown by the manufacturer of the TLR6 antibody. Beta-actin staining was used as a loading control. (c) mesenteric lymph node (MLN) cells from control and DSS-treated mice were stained (n=4) with either an antibody for TLR6 or a matched isotype control. The percentage of TLR6 staining was determined for various immune cells. Matched isotype values were first subtracted. Data are presented as mean+s.e.m. P-values considered as significant are indicated as *<0.05, **<0.01.
TLR6 stimulation increases RORγt expression in the GALT
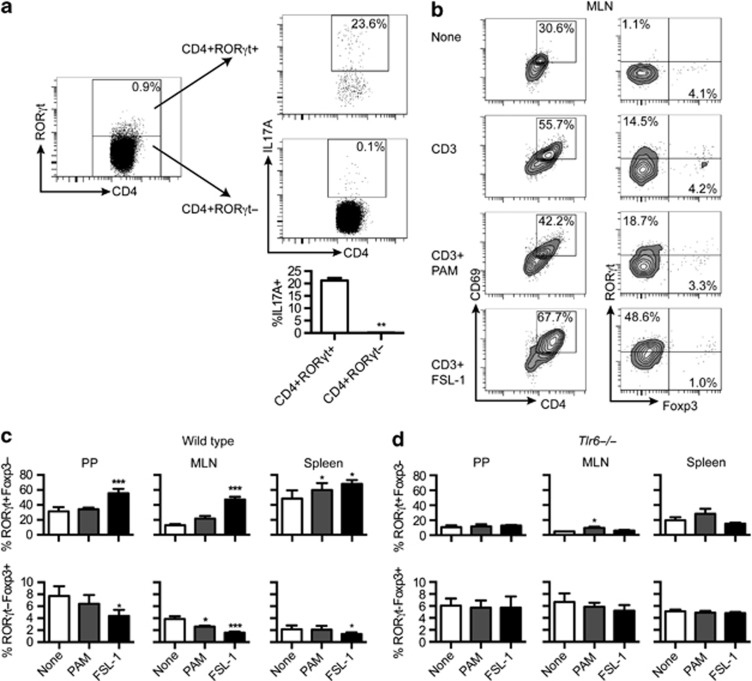
We explored TLR1/2 and TLR2/6 stimulation in the context of murine GALT by looking at the effect of TLR ligands (TLRLs) on CD4+ T-cell responses in Peyer's patches (PPs) and MLN. Spleen was also included as a control tissue. Th17-, Th1-, and Treg-associated responses were measured by master transcription factor expression (RORγt, T-bet, and Foxp3, respectively). CD4+RORγt+ T-cell expression of IL-17A was first confirmed in MLN cells (Figure 3a). Although not all CD4+RORγt+ T cells expressed IL-17A (∼20%), the IL-17A expression was constrained to the CD4+RORγt+ T-cell population.
Figure 3.
FSL-1 stimulation of murine gut-associated lymphoid tissue (GALT) cells increases RORγt expression and lowers Foxp3 expression. (a) Cells were isolated from mesenteric lymph node (MLN) and stimulated with PMA/ionomycin (n=6) and the numbers of IL-17A+ cells in the CD4+RORγt+ and CD4+RORγt- T cells population determined. Representative plots are shown for a single mouse. (b) Left column: percentages CD4+CD69+ T cells from total MLN cells after the indicated stimulation. Right column: RORγt and Foxp3 staining of the CD4+CD69+ cells in the left column. Plots are representative of the data presented in part c. (c) Cells isolated from the indicated lymphoid tissues from wild-type mice were stimulated with anti-CD3 and TLRLs. Percentages of RORγt+Foxp3− and RORγt-Foxp3+ T cells were determined from the gated CD4+CD69+ T-cell population (n=4, pooled from two independent experiments). (d) Cells isolated from lymphoid tissues of Tlr6−/− mice were stimulated and analyzed as in part C (n=3). Data are presented as mean+s.e.m. P-values considered as significant are indicated as *<0.05, **<0.01 and ***<0.001.
The expression of RORγt and Foxp3 in activated CD4+CD69+ T cells was measured in the GALT (MLN and PP) and spleen after stimulation with anti-CD3 and TLRLs. Stimulation with FSL-1 in GALT cells led to a significant increase in RORγt+Foxp3− T cells within the CD4+CD69+ population that was not observed in the other conditions (Figure 3b and c). Parallel reductions in the percentage of RORγt-Foxp3+ T cells in the FSL-1-treated GALT cells were also noted. The effect of FSL-1 was also observed in spleen cells (Figure 3c) and human peripheral blood mononuclear cells (PBMCs) (Supplementary Figure 1A online). Pam3CSK4 had little effect on GALT cells except lowering RORγt-Foxp3+ T cells in the MLN. However, Pam3CSK4 increased the percentage of RORγt+Foxp3− T cells in the spleen (Figure 3c).
Loss of TLR6 expression eliminated all FSL-1-mediated transcription factor changes (Figure 3d). Its effect on the response caused by PAM3CSK4, in contrast, was limited.
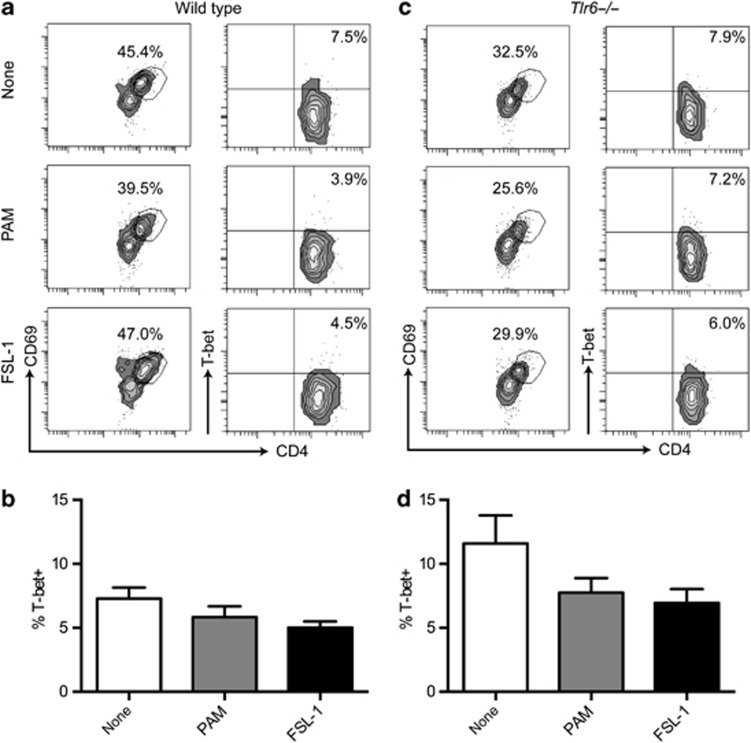
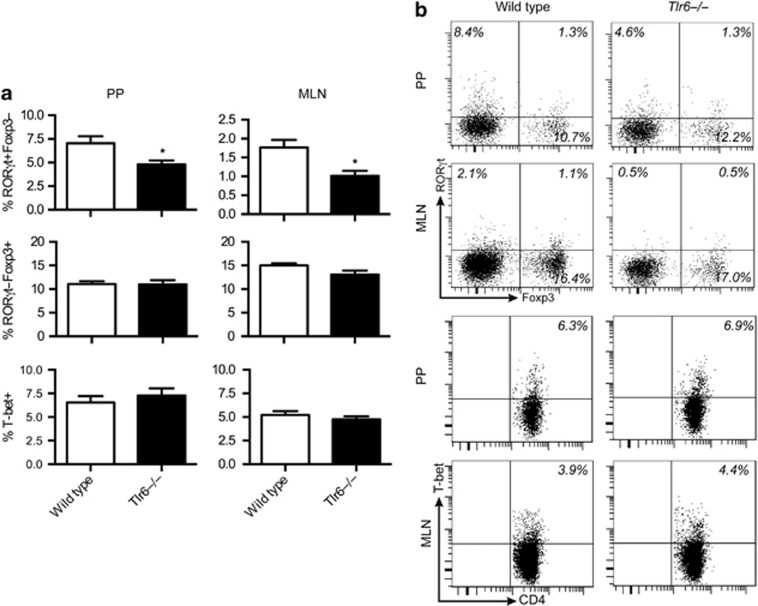
Unlike RORγt, expression of the Th1 transcription factor, T-bet, was not stimulated by the addition of TLRLs during the stimulation of MLN cells with anti-CD3. It also remained unchanged in CD4+ T cells in Tlr6−/− mice (Figure 4a–d).
Figure 4.
TLRL stimulation of murine mesenteric lymph node (MLN) cells does not affect T-bet expression. Wild-type (a and b) and Tlr6−/− (b and c) MLNs were stimulated with TLRLs and anti-CD3. T-bet was measured after 48 h of stimulation. None, anti-CD3 alone; PAM, Pam3CSK4 and anti-CD3; and FSL-1, FSL-1 and anti-CD3. (a and c) Left column: percentages CD4+CD69+ T cells from total MLN cells after the indicated stimulation. Right column: T-bet and CD4 staining of the CD4+CD69+ cells in the left column. T-bet plots are representative of the data (n=5) presented in parts b and d. Data are presented as mean+s.e.m.
T-cell stimulation combined with FSL-1 increases both IFNγ and IL-17A secretion in the GALT
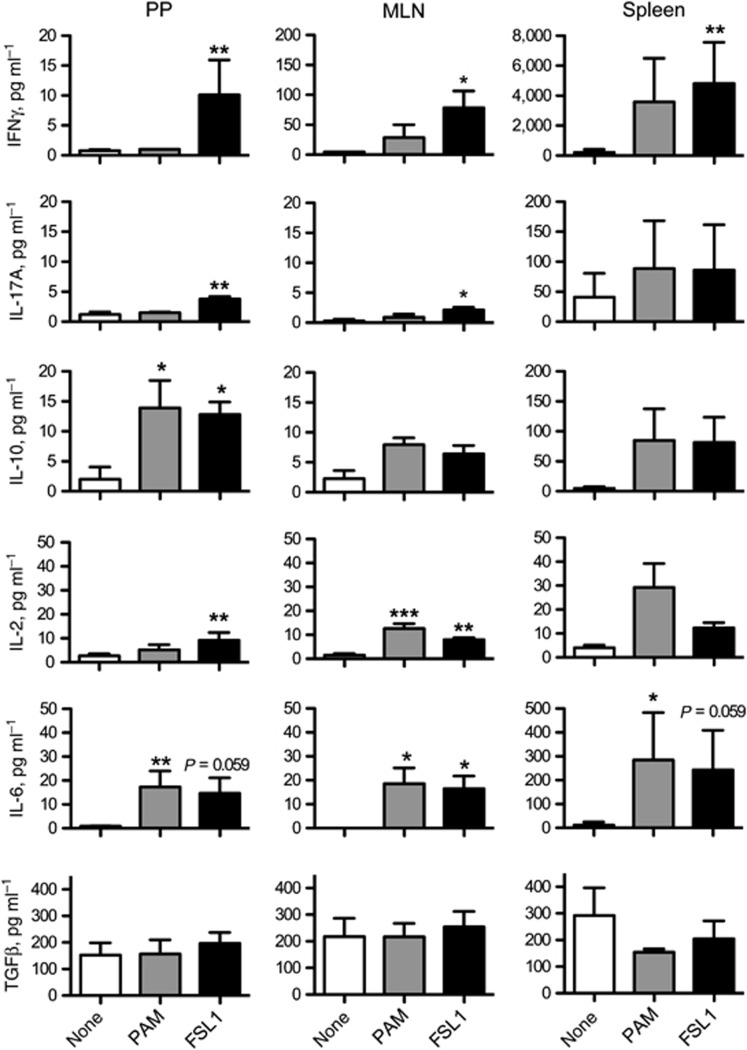
To further investigate influences on T-cell polarization during stimulation with anti-CD3 and TLRLs, we measured IFNγ, IL-17A, IL-10, IL-4, IL-2, IL-6, and transforming growth factor β (TGFβ) in the supernatants after 48 h of culture. IL-4 was hardly detectable in all of the cultures (data not shown). In GALT cultures, the addition of FSL-1 during anti-CD3 stimulation led to significant increases in the production of IFNγ and IL-17A that were not seen after stimulation with Pam3CSK4 (Figure 5). In PP cells, Pam3CSK4 and FSL-1 both induced a significant increase in IL-10, a trend also seen in MLN. FSL-1 significantly increased the levels of IL-2 in the MLN and PP, while Pam3CSK4 had only the same response in the MLN. Interleukin-6 was increased by both TLRLs in the GALT, while TGFβ levels remained the same in all conditions.
Figure 5.
T-cell stimulation with anti-CD3 and FSL-1 leads to increased IFNγ, IL-17A secretion in gut-associated lymphoid tissue (GALT). Cells isolated from murine Peyer's patches (PPs), mesenteric lymph nodes (MLNs), and spleens were stimulated with anti-CD3 and TLRLs (n=4). After 48 h, supernatants were isolated and analyzed for cytokine content. The cytokines measured and the units are given in the y-axis. Data are presented as the mean+s.e.m. P-values considered as significant are indicated as *<0.05, **<0.01, and ***<0.001.
The spleen cells, in general, produced far higher amounts of cytokines than the GALT cells when stimulated with anti-CD3 and TLRLs. All cytokines, except TGFβ, appeared to increase. Significant changes were measured for IFNγ after FSL-1 treatment and IL-6 after Pam3CSK4 (Figure 5). Within the spleen and human PBMCs (Supplementary Figure 1B), FSL-1 and Pam3CSK4 performed almost identically with both TLRLs causing significant and not quite significant increases in the cytokines tested.
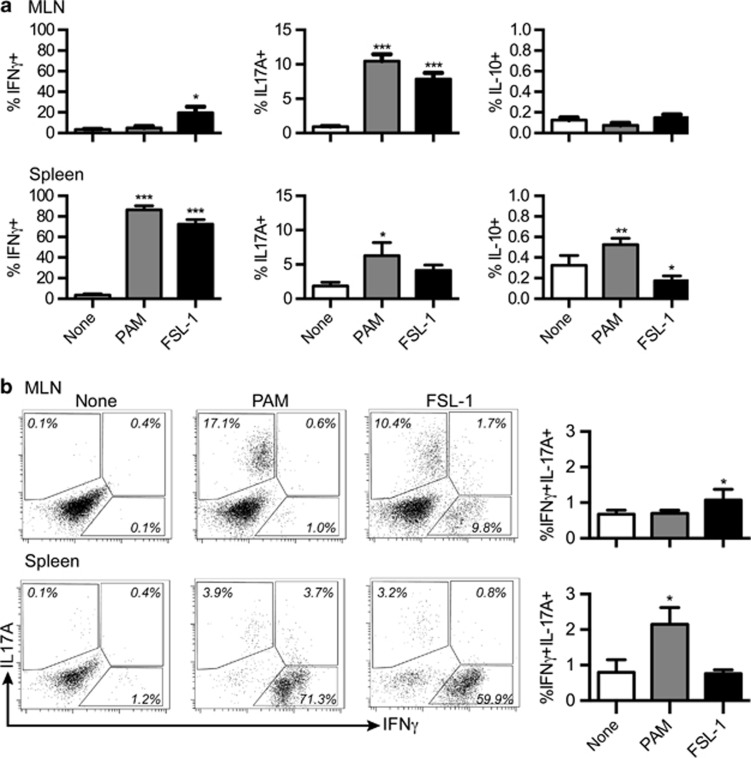
FSL-1 leads to increased Th1 and Th17 cells in GALT long-term cultures
The previous results were after short 48-h stimulations. Early changes in transcription factor expression and cytokines may not be representative of fully differentiated and expanded effector T cells. Therefore, MLN and spleen cells were stimulated with anti-CD3 in combination with the TLRLs and expanded for 7 days in culture. Restimulation with PMA/ionomycin revealed that both TLRLs led to significantly increased amounts of CD4+IL-17A+ T cells (Th17) within MLN cell suspensions (Figure 6a). However, FSL-1 uniquely led to magnified percentages of CD4+IFNγ+ T cells (Th1) in MLN cell cultures. Neither TLRL induced CD4+IL-10+ cells. In the spleen cell cultures, both TLRLs caused similar increases in CD4+IFNγ+ T cells with ∼80% of the CD4+ T cells expressing IFNγ. Though the percentage of Th17 cells appeared to increase in FSL-1-treated spleen cultures, only Pam3CSK4 treatment led to significant increases. Pam3CSK4 treatment also increased the percentage of CD4+IL-10+ T cells, while FSL-1 decreased them.
Figure 6.
FSL-1 leads to increased Th1 and Th17 cells in extended mesenteric lymph node (MLN) cultures. Cells isolated from the MLNs or spleens (n=4) were stimulated with anti-CD3 and TLRLs for 7 days followed by PMA/ionomycin stimulation. (a) Percentages of cytokine-positive CD4+ cells are indicated. (b) Percentages of CD4+IFNγ+IL-17A+ cells are shown in the column figure on the right. Representative FACS plots for a single mouse are also shown. Data in column graphs are presented as the mean+s.e.m. P-values considered as significant are indicated as *<0.05, **<0.01, and ***<0.001.
It has been reported that CD4+ T cells double positive for both IFNγ and IL-17A are particularly potent autoimmune Th17 cells that arise when Th17 are exposed to high IL-23 and IL-1β.10 Therefore, we examined if the populations of CD4+IFNγ+IL-17A+ T cells were also changed in the cultures. These rare cells increased significantly in the FSL-1-treated MLN cultures and were not affected by Pam3CSK4 treatment (Figure 6b). In expanded spleen cultures, Pam3CSK4, but not FSL-1 treatment, significantly increased the CD4+IFNγ+IL-17A+ T-cell population.
Tlr6−/− mice have lowered Th1 and Th17 responses in the GALT and are protected against colitis
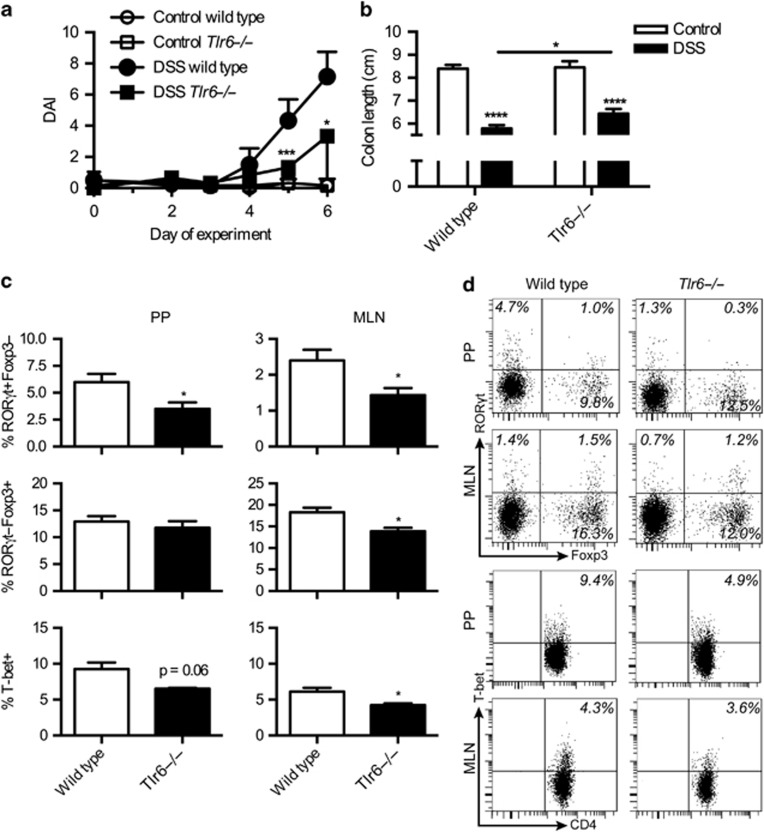
Tlr6−/− mice have less Th17 cells in the lung.8 The possibility existed that lowered Th17 numbers would be found in the GALT as well. To determine if this was the case, T-cell subsets were examined in the MLN and PPs of Tlr6−/− mice. In both tissues, significant reductions of CD4+RORγt+Foxp3− T cells were observed as compared with wild-type controls while the rest remained unchanged (Figure 7a and b). Owing to the changes in the Th17 populations, we speculated that Tlr6−/− mice could be protected against DSS-induced colitis. Tlr6−/− mice were less susceptible to colitis than wild types, as judged by the disease activity index (Figure 8a). This difference was also observed in the colon length (Figure 8b). Both Tlr6−/− mice and wild-type mice had shortened colons after DSS exposure; however, the colons of the DSS-treated wild type mice were significantly shorter than the colons of the DSS-treated Tlr6−/− mice.
Figure 7.
Tlr6−/− mice have lower amounts of CD4+RORγt+ cells. (a) Gut-associated lymphoid tissue (GALT) cells were isolated from both Tlr6−/− and wild-type mice (n=6). RORγt, Foxp3, and T-bet expression were analyzed within the CD4+ T-cell population. (b) Representative plots of a single mouse are shown for the transcription factor staining. Data are given as mean+s.e.m. P-values considered as significant are indicated as *<0.05. PPs, Peyer's patches.
Figure 8.
Tlr6−/− mice have reduced Th1 and Th17 responses and are protected from colitis. (a) Disease activity index (DAI) is shown for healthy controls and dextran sodium sulfate (DSS)-treated wild-type and Tlr6−/− mice (n=6). (b) In the same mice, colon lengths were measured at sacrifice (day 7 after start of DSS). (c) Transcription factor staining is shown for CD4+ T cells from the gut-associated lymphoid tissue (GALT) for the mice in the above experiment treated with DSS. (d) Representative FACS plots are shown for the data presented in c. Data are given as mean+s.e.m. P-values considered as significant are indicated as *<0.05, **<0.01, ***<0.001, and ****<0.0001. MLN, mesenteric lymph node; PPs, Peyer's patches.
An investigation of the transcription factor expression and cytokine production in GALT CD4+ cells revealed that during colitis, CD4+RORγt+Foxp3- T cells remained significantly reduced in Tlr6−/− mice compared with wild types (Figure 8c and d). Significant and almost significant reductions of CD4+T-bet+ cells were also observed in the MLN and PP of Tlr6−/− mice. Lowered CD4+RORγt-Foxp3+ populations were also observed in the MLN, but not in the PP.
Oral FSL-1 increases experimental colitis severity and residual Th17-associated responses
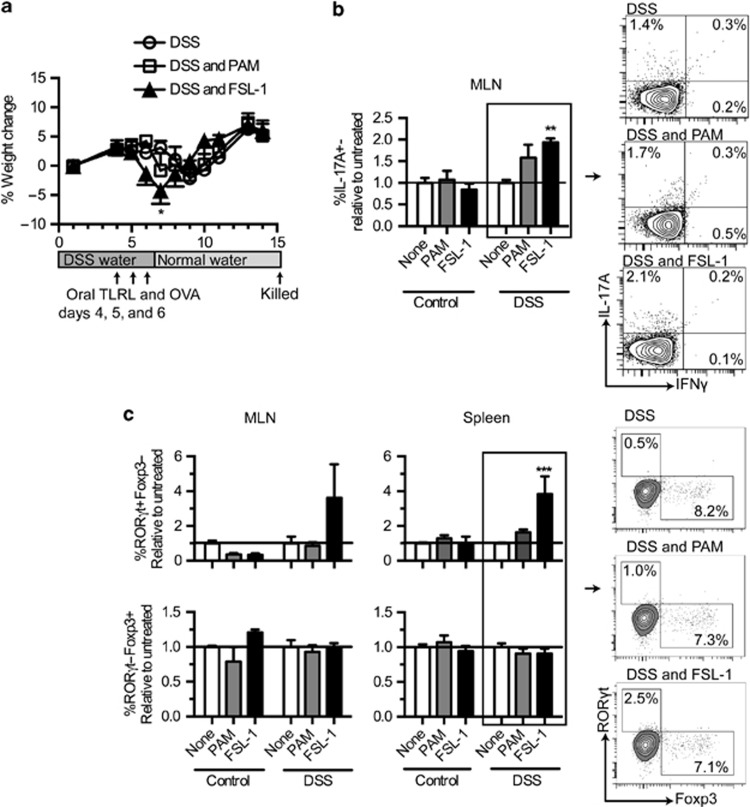
Because TLR6 expression was increased in the colon during DSS colitis, we speculated that in vivo treatment with the TLR6 ligand, FSL-1, during colitis would lead to changes in the disease progression and associated Th1 and Th17 responses. FSL-1, Pam3CSK4, or saline in combination with ovalbumin (OVA) were orally administered on day 4, 5, and 6 after the start of DSS water to both healthy and DSS-treated mice (Figure 9a). Due to the risk that oral TLRL treatment might lead to extremely severe disease, a mild form of DSS colitis was induced. Analysis of the weight scores showed that only FSL-1-treated mice experienced a significantly sharper decrease in weight on day 7 as compared with the DSS controls at these conditions (Figure 9a).
Figure 9.
Oral FSL-1 worsens colitis and increases the Th17 response. Mice were administered regular water or dextran sodium sulfate (DSS) water for 6 days. On day 4, 5, and 6, mice were given oral TLRLs combined with the tracking antigen ovalbumin (OVA). One week after stopping DSS, the mice were sacrificed (n=5). (a) Percent weight gain of the mice over time relative to starting weight. Data are shown as the mean of each day +/−s.e.m. The experimental design is shown under the weight graph. (b) Intracellular cytokine staining on CD4-gated mesenteric lymph node (MLN) cells isolated from the in vivo experiment. Data are presented as relative values of the mean percentage staining found in the untreated group (the group not receiving TLRL treatment). FACS plots on the right show representative staining of the DSS samples. (c) MLN and spleen cell cultures were analyzed for percentages of RORγt+Foxp3− (Th17) and RORγt-Foxp3+ (Treg) T-cell populations in CD4+ T cells after 48 h of anti-CD3 stimulation. Data are presented in the same manner as b. Representative FACS plots on the right show examples of spleen data. Data are shown as the mean+s.e.m. P-values considered as significant are indicated as *<0.05, **<0.01, ***<0.001, and ****<0.0001.
To observe changes in the T-cell compartments after inflammation, mice were sacrificed on day 14, and cytokine expression in CD4+ cells was examined in the MLN and spleen. FSL-1- and DSS-treated mice had significantly increased amounts of CD4+IL-17A+ cells in the MLN relative to the mice treated with just DSS (Figure 9b). Expression of IFNγ was low in the MLN, and no appreciable numbers of CD4+IL-17A-IFNγ+ and CD4+IL-17A+IFNγ+ were noted, preventing analysis (data not shown). Although cytokines were measured in the spleen, there were no differences measured between the groups (data not shown).
Further analysis of the T-cell response using anti-CD3 stimulation and transcription factor staining revealed that FSL-1- and DSS-treated animals had approximately four times as many potential Th17 cells within the total CD4+ T cells as compared with the untreated DSS controls in the spleen while Treg populations remained unchanged (Figure 9c). In the MLNs, similar changes were observed, however, they were not quite significant. Similar responses were also observed for antigen-specific CD4+ T-cell responses directed against the tracking antigen OVA, which was administered simultaneously with the TLRLs (Supplementary Figure 2).
Discussion
The role of TLR6 at the mucosal interface is not well understood. Studies by DePaolo et al.5 demonstrate that TLR2/6 stimulation of dendritic cells, both in vitro and in vivo, is associated with the formation of suppressive immune responses. Recently, the same group also found lowered regulatory responses and enhanced Th1 and Th17 responses in TLR6−/− mice6 during Yersinia enterocolitica infection, supporting their previous findings. In contrast, research on the lung mucosa found conflicting data. Protective Th17 responses were impaired in TLR6−/− mice during Saccharopolyspora rectivirgula and Aspergillus fumigatus infections of the lung.8, 17 Moreover, the administration of macrophage-activating lipopeptide-2 (another TLR2/6 ligand) to rat lung caused the influx of immune cells.18 There are also conflicting reports about the effects of TLR2/6 stimulation on human Treg. Some studies show that TLR2/6 ligands suppress Treg function, like TLR1/2 ligands, while others show that it is ineffective.19, 20
Our results show that there is a relationship between TLR6 stimulation in the GALT, and the induction of Th1/Th17 responses, which is not shared by TLR1/2. Even though Pam3CSK4 stimulation leads to responses in the GALT, they are relatively milder than those induced by FSL-1 and do not support Th1. In contrast, Pam3CSK4 appears superior to FSL-1 at inducing Th1/Th17 in the spleen. Deeper investigation of TLR6 in vivo further supported the in vitro findings for TLR6; TLR6 stimulation worsens colitis development and is involved with Th1/Th17 induction. Furthermore, TLR6 expression is increased in the distal colon during colitis, where inflammation is the highest,21 and TLR6 is expressed in the inflamed intestines of IBD patients where it also correlates with RORC expression. Taken together, this indicates that TLR6 is an interesting player in intestinal inflammation.
Performing re-stimulations with anti-CD3 and TLR2/6 or TLR1/2 ligands showed that FSL-1, but not Pam3CSK4, induced RORγt and reduced Foxp3 in GALT cell suspensions after 48 h of culture. As CD4+ T cells do express TLR6, this effect could be mediated by direct signaling, or it could be mediated by cytokine release from other immune cells. Our results suggest that the latter could be a possibility. Th17 cells require expression of the transcription factor, RORγt.22 Th17 differentiating conditions (anti-CD3 with low IL-2, IL-4, IFNγ, and high IL-6 and TGFβ) increase Rorc mRNA after 25 h.23 In our FSL-1-treated cultures, IL-4 and IL-2 were both at low concentrations; TGFβ was present, and both IFNγ and IL-6 were increased. IL-6 induces STAT3 (signal transducer and activator of transcription 3), which is needed for Rorc transcription.24 STAT3 also induces the loss of FOXP3 in human natural Tregs,25 which was also observed. Despite high concentrations of IFNγ (which is not necessarily detrimental to Th17 induction26), the environment was, at a cytokine level, conducive to Th17 development and detrimental to Treg.
Despite the lack of changes in T-bet expression in GALT cells at 48 h of culture, large amounts of IFNγ were produced as well as other pro-inflammatory cytokines. As 48 h is too short of a period for naïve T cells to be stimulated, differentiate, and produce their own cytokines; the source must be other cells found in our cell suspensions. Existing memory Th1 cells are likely candidates, and it is known that they produce IFNγ and IL-10 when costimulated with TLR2 ligands.27 Other options are memory CD8+ T cells,28 innate lymphoid cells,29 dendritic cells,30 and macrophages.31
To further determine the long-term effects on T-cell differentiation, extended cultures were performed with the TLRLs. In MLNs, Pam3CSK4 induced CD4+IL-17A+ cells just as well as FSL-1 but, unlike FSL-1, did not induce CD4+IFNγ+ cells. Looking at the cytokines produced during the first 48 h shows that MLN stimulated with anti-CD3 and Pam3CSK4 produced significantly higher levels of IL-6. As IL-6 combined with TGFβ is needed to differentiate Th17 cells, the development of Th17 is to be expected. The response is not, however, entirely the same as that with FSL-1, which also induced IFNγ/IL-17A double-positive cells. These cells are indicative of a highly pro-inflammatory cytokine milieu and promote intestinal inflammation found in IBD.12, 16 The main difference between the two stimulations was the presence of IFNγ in the FSL-1 cultures. The presence of IFNγ supports Th1 development32 and also promotes Th17 responses.26 This could be the main reason that FSL-1 leads to Th1/Th17 responses in the GALT.
We observed clear differences between Pam3CSK4 and FSL-1 in the GALT. This does not exclude an important role for TLR1/2 in intestinal inflammation, but does suggest that it is different than that of TLR2/6. Our results and studies by DePaolo et al.6 indicate that TLR1/2 is responsible for intestinal Th17 responses. Still, TLR1/2 stimulation is more potent in the spleen than GALT, inducing both Th1 and Th17. This tissue specific response is an important consideration for future work on TLRLs and may offer some explanation to conflicting data in previous publications.
To investigate TLR6 in intestinal inflammation, DSS colitis was induced in Tlr6−/− mice. Our experiments showed that these mice were partially protected from the disease. Interestingly, Tlr6−/− mice have reduced numbers of Th17 cells as compared with the wild-type mice, and they remain low even during colitis. During colitis, these mice also had fewer CD4+T-bet+ cells and reduced Treg. Although the loss of Treg is puzzling, the reductions in Th1/Th17 may have influenced the severity of disease.
To learn more about the fate of CD4+ T cells primed during DSS-induced colitis, FSL-1 or Pam3CSK4 were orally administered along with the tracking antigen OVA during the induction of colitis in wild-type animals. Significantly increased Th17 responses in the FSL-1- and DSS-treated mice were found in the MLNs and spleen using several different assays. This is particularly striking as these responses were measured 7 days after DSS treatment was stopped. In contrast, residual Th1 responses were not measured. DSS colitis-associated T-cell responses within the spleen after disease resolution are not unusual. It has been observed that after DSS colitis heals, there is a striking increase of CD4+ T cells in the spleen, while the percentage of T cells within the MLN normalizes,33 which may also explain why the percentages of cytokine-expressing T cells was so low in our intracellular cytokine staining assay.
Our results point to TLR6 being an important driver of Th1 and Th17 responses in the GALT. This is supported by both in vitro and in vivo evidence. Moreover, the fact that RORC and TLR6 mRNA levels are correlated in human intestine and that stimulations of human PBMCs mimic the murine results, suggests a similar relationship between TLR6 and pro-inflammatory T-cell polarization in humans. As the reduction of Th1/Th17 responses in IBD is a prime goal for therapy, TLR6 could be a potential target for future IBD treatments.
methods
Animals. Female C57BL/6 mice for the oral TLRL study and for the in vitro experiments were purchased from Charles River Laboratories (Maastricht, The Netherlands). The mice were used at 8–12 weeks of age and were housed under standard conditions in the animal facilities at Utrecht University. Breeding pairs of Tlr6−/− mice were obtained from Shizuo Akira (Osaka University). Male and female Tlr6−/− mice used both in vivo and in vitro were bred and kept in standard conditions at the Central Animal Facility of the Radboud University Nijmegen Medical Centre. Sex and aged-matched C57BL/6 controls used in the Tlr6−/− in vivo experiment were bred in the same locale as the Tlr6−/− mice.
Experimental colitis. Experimental colitis was induced in all mice by adding 1.5% (w/v) DSS (MP Biomedicals LLC, Illkirch, France) to the drinking water of the mice for 6 days, starting at day 1 of the experiment. Mice were sacrificed on either day 7 or 14 after starting DSS, depending on the experiment. Individual mice were considered a single experimental unit.
Oral gavages for TLRLs and OVA administration were performed on day 4, 5, and 6 to coincide with the developing intestinal inflammation. Each gavage contained 400 μg endotoxin-free OVA (Hyglos GmBH, Bernried am Starnberger See, Germany) and 80 μg of either Pam3CSK4 or FSL-1 (both from EMC microcollections, Tuebingen, Germany) in 500 μl saline solution. Both FSL-1 and Pam3CSK4 are synthetic lipopeptides. Control animals were administered saline only.
The disease activity index of colitis was determined by combining the scores collected from the weight measurement, rectal condition, feces condition, and the detectable presence of blood in the feces. The disease activity index was between 0 and 11 for each mouse. The loss of weight was scored as follows relative to starting weight: 0, 0–2.5% weight loss; 1, 2.5–5% weight loss; 2, 5–10% weight loss; 3, 10–15% weight loss; and 4, >15% weight loss. The feces condition score was scored as follows: 0, normal; 1, soft with normal form; 2, loss of form/diarrhea; and 3, no feces produced. The rectal condition score was determined as follows: 0, normal; 1, irritation present; 2, mucus and/or swelling; and 3, visible blood. Fecal blood was tested with a Colo-rectal test kit (Axon Lab AG, Stuttgart, Germany). Fecal blood was scored as follows: 0, no blood; 1, blood.
Messenger RNA expression analysis. The human intestinal tissue samples in this study were obtained from surgical resection specimens and include pairs of macroscopically inflamed and normal-appearing (non-inflamed) mucosa from patients with Crohn's disease (n=5) and ulcerative colitis (n=5), both clinically and histologically confirmed, with normal tissue from patients with a colorectal carcinoma, at least 10 cm from the tumor, as controls. Details on the tissue specimens and patient characteristics are described in a previous study as well as the methods of total RNA isolation.34
For the murine colon samples, the colons were divided into two equal sections: proximal and distal. The total RNA was isolated using the RNAeasy kit (Qiagen, Germantown, MD) and, subsequently, reverse transcribed into cDNA using the iScript cDNA synthesis kit (BioRad, Hercules, CA).
Real-time PCR was performed using iQ SYBR Green super mix kit (BioRad) with the CFX 96 Real-time system (BioRad). Messenger RNA for ribosomal protein S13 (RPS13) was used as the internal control in mice, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA was used as the internal control in the human samples. The primers used for the human real-time PCR were obtained from SA Biosciences (Frederick, MD). The primer sequences used for the mouse studies were as follows: Tlr6 fwd: 5′ GACTCTCCCACAACAGGATACG 3′, Tlr6 rev: 5′ TCAGGTTGCCAAATTCCTTACAC 3′, Rps13 fwd: 5′ GTCCGAAAGCACCTTGAGAG 3′, Rps13 rev: 5′ AGCAGAGGCTGTGGATGACT 3′. The final data for the target samples were normalized against the internal control GAPDH in the human samples and Rps13 in the mouse samples. The relative mRNA expression values were calculated using Bio-Rad CFX manager V1.6.
Immunofluorescence. Cryostat sections (5 μm) of the intestinal tissues of CD patients (n=5), UC patients (n=5) and normal tissue from patients with a colorectal carcinoma (n=5) were cut, fixed in ice-cold acetone for 10 min, blocked with 5% goat serum in 1% BSA/PBS (bovine serum albumin/phosphate-buffered saline) and afterwards incubated with rabbit-anti-TLR6 (1:125, Novus Biologicals, Cambridge, UK, NBP1-54336) in 1% BSA/PBS overnight at 4 °C. After subsequent washings with PBS, the antibodies were visualized by incubation with goat-anti-rabbit-Alexa568 (1:400, Invitrogen, Breda, The Netherlands) in 1% BSA/PBS for 1 h at room temperature. Counterstaining was done with Hoechst 33,342 (Sigma Aldrich Chemie BV, Zwijndrecht, The Netherlands) for 5 min at room temperature and slides were sealed with Prolong gold anti-fade reagent (Invitrogen). Photomicrographs were taken at room temperature with an Olympus BX-60 microscope with a × 40/0.75na objective lens equipped with a Leica DFC425 C camera and controlled with LAS software 4.0.
Ex vivo T-cell stimulation and PBMC culture. PPs, MLN, and splenocytes were isolated from mice. Cells were re-stimulated with either 25 μg ml−l endotoxin-free OVA (Hyglos GmBH) or 2 μg ml−l anti-CD3 (eBioscience, San Diego, CA) with or without Pam3CSK4 or FSL-1 (4 μg ml−l; EMC microcollections) and cultured with RPMI medium (Roswell Park Memorial Institute medium, Life Technologies, Paisley, Scotland) supplemented with 1 unit per ml penicillin, 1 μg ml−l streptomycin, 50 μM β-mercaptoethanol, and 5% FCS in 96-well round-bottom plates at a concentration of 105 cells per well. During the long stimulation, cells were incubated for 7 days before restimulation with PMA/ionomycin (Sigma). Otherwise, cells were incubated for 48 h, after which, supernatants were collected; cells were harvested and stained with fluorescently labeled antibodies.
Human PBMCs were cultured in RPMI medium supplemented with 1 unit per ml penicillin, 1 μg ml−l streptomycin, 1 mM pyruvate, 50 μg ml−l gentamicin, and 2.5% FCS in 96-well round-bottom plates at a concentration of 105 cells per well. Similarly to the murine experiments, the cells were incubated for 48 h with anti-CD3 (1 μg ml−l; eBioscience) with or without TLRLs (Pam3CSK4 or FSL-1, 4 μg ml−l; EMC microcollections). Upon completion of the incubation, the supernatants were collected and the cells harvested for FACS staining.
Flow cytometry. Intracellular cytokine staining was performed on either fresh or cultured cells after 5 h stimulation with PMA/Ionomycin (Sigma) and Brefeldin A (eBioscience). Cells were then stained with antibodies for CD4, IFNγ, IL-17A, IL-10, and IL-4 (all antibodies from eBioscience) using the Foxp3 intracellular staining kit (eBioscience). To prevent background staining, cells were first incubated with unlabeled anti-CD16/32 (eBioscience) for 15 min on ice as suggested in the manufacturer's protocol. Fixed samples were kept at 4 °C until reading with the flow cytometer.
Transcription factor expression was measured in murine cells or PBMCs by performing a surface stain with anti-CD4 and anti-CD69 and then staining intracellularly for Foxp3, T-bet, and RORγt. All antibodies and the Foxp3 intracellular staining reagents were obtained from eBioscience. Before the surface stain and again during the intracellular stain, samples were blocked with unlabeled anti-human/mouse CD16/32 (eBioscience) for 15 min on ice. Fixed samples were kept at 4 °C until reading with the flow cytometer. All samples were read on a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA) and analysis of the flow cytometry data was performed using BD FACSDiva software (BD Biosciences).
Cytokine measurements. Isolated supernatants were analyzed for cytokine production by using either the human or mouse BD Cytometric Bead Array Th1/Th2/Th17 kits (BD Biosciences) and the mouse/human TGFβ Ready-Set-Go! ELISA from eBioscience. Kits were used according to manufacturer's protocol. The bead array samples were read using a FACSCanto II flow cytometer (BD Biosciences). Data analysis was performed using the FCAP Array Analysis software and FCS Filter Software (Soft Flow Hungary Ltd., Pecs, Hungary).
Immunoblot. Intestinal tissues were weighed (100 mg ml−l) and homogenized in radioimmunoprecipitation assay buffer containing protease inhibitors and EDTA (Thermo Fisher Scientific, Rockford, IL) using a Precellys 24 tissue homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France) for four times 10 s at 6,000 rpm with a minimum 5 min cooling period on ice in between. Afterwards, samples were centrifuged for 15 min at 14,000 rpm and the supernatant was transferred to a clean tube. Homogenates were separated on 4–20% (w/v) SDS gels (BioRad) and blotted to nitrocellulose membranes (Millipore, Billerica, MA). Membranes were blocked for 2 h with 5% milk proteins in PBS/0.1% Tween-20 and subsequently incubated with rabbit-anti-mouse TLR6 (Sigma, SAB1300203) in PBS/2% milk/0.1% Tween-20 overnight at 4 °C. After incubation, membranes were washed three times with PBS/2% milk/0.1% Tween-20, incubated with goat-anti-rabbit-horse radish peroxidase (Dako, Heverlee, The Netherlands) in PBS/2% milk/0.1% Tween-20, treated with commercial ECL reagents (Amersham Biosciences, Roosendaal, The Netherlands) and finally exposed to photographic film. Afterwards, blots were stripped with stripping buffer (Thermo Fisher Scientific) and reprobed with rabbit-anti-β-actin (Cell Signaling Technology, Danvers, MA). The TLR6 staining appears as double bands and this is in accordance with the staining pattern depicted by the manufacturer.
Statistical analysis. Means with s.e.m. are represented in each graph. Statistical analysis was performed using GraphPad Prism version 6.0 for windows (GraphPad Software, San Diego, CA). Correlation significance was determined using the Pearson product–moment correlation coefficient. For comparisons between two groups, T-test or Mann–Whitney test were used depending on the distribution of the data. Comparisons between three or more groups were performed with either a one-way analysis of variance or repeated measures analysis of variance followed by the Bonferroni post test where appropriate. In cases of repeated, nonparametric data, the Friedman test followed by Dunn's multiple comparison post test was used. In the situation of two independent parameters, a two-way analysis of variance was applied (either regular or repeated measures) with the Bonferroni post test.
Acknowledgments
We would like to thank Gerard Hofman (Department of Pharmaceutical Sciences, Utrecht University) for his help with several animal procedures, Debby Smits (Central Animal Laboratory, Radboud University, Nijmegen Medical Centre) for her help in organizing animal experiments and Rob Bleumink (The Center for Cell Imaging, Faculty of Veterinary Medicine, Utrecht University) for his help with the fluorescent imaging. This study was performed and supported within the framework of Dutch Top Institute Pharma (project number D1-101).
The authors declared no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/mi
Supplementary Material
References
- Takeuchi O., et al. TLR6: a novel member of an expanding toll-like receptor family. Gene. 1999;231:59–65. doi: 10.1016/s0378-1119(99)00098-0. [DOI] [PubMed] [Google Scholar]
- Hajjar A.M., et al. Cutting edge: functional interactions between toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J. Immunol. 2001;166:15–19. doi: 10.4049/jimmunol.166.1.15. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., et al. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 2001;13:933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- Ozinsky A., et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl Acad. Sci. USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depaolo R.W., et al. Toll-like receptor 6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host Microbe. 2008;4:350–361. doi: 10.1016/j.chom.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaolo R.W., Kamdar K., Khakpour S., Sugiura Y., Wang W., Jabri B. A specific role for TLR1 in protective T(H)17 immunity during mucosal infection. J. Exp. Med. 2012;209:1437–1444. doi: 10.1084/jem.20112339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pot C., Apetoh L., Kuchroo V.K. Type 1 regulatory T cells (Tr1) in autoimmunity. Semin. Immunol. 2011;23:202–208. doi: 10.1016/j.smim.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira A.P., et al. The protective role of TLR6 in a mouse model of asthma is mediated by IL-23 and IL-17A. J. Clin. Invest. 2011;121:4420–4432. doi: 10.1172/JCI44999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P., Kolls J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- Ahern P.P., et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostins L., et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschek M.A., et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J. Exp. Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastaff-Leung N., Mabarrack N., Barbour A., Cummins A., Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J. Clin. Immunol. 2010;30:80–89. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- Doherty G.A., et al. CD73 is a phenotypic marker of effector memory Th17 cells in inflammatory bowel disease. Eur. J. Immunol. 2012;42:3062–3072. doi: 10.1002/eji.201242623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen T., Rismo R., Cui G., Goll R., Christiansen I., Florholmen J. TH1 and TH17 interactions in untreated inflamed mucosa of inflammatory bowel disease, and their potential to mediate the inflammation. Cytokine. 2011;56:633–640. doi: 10.1016/j.cyto.2011.08.036. [DOI] [PubMed] [Google Scholar]
- Ghoreschi K., Laurence A., Yang X.P., Hirahara K., O'Shea J.J. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol. 2011;32:395–401. doi: 10.1016/j.it.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong D.J., Hogaboam C.M., Matsuno Y., Akira S., Uematsu S., Joshi A.D. Toll-like receptor 6 drives interleukin-17A expression during experimental hypersensitivity pneumonitis. Immunology. 2010;130:125–136. doi: 10.1111/j.1365-2567.2009.03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst R., Durak D., Roos A., Luhrmann A., Tschernig T. TLR2/6 stimulation of the rat lung: effects on lymphocyte subsets, natural killer cells and dendritic cells in different parts of the air-conducting compartments and at different ages. Immunology. 2009;126:132–139. doi: 10.1111/j.1365-2567.2008.02886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg H.H., Ly T.T., Ussat S., Meyer T., Kabelitz D., Wesch D. Differential but direct abolishment of human regulatory T cell suppressive capacity by various TLR2 ligands. J. Immunol. 2010;184:4733–4740. doi: 10.4049/jimmunol.0804279. [DOI] [PubMed] [Google Scholar]
- Nyirenda M.H., et al. TLR2 stimulation drives human naive and effector regulatory T cells into a Th17-like phenotype with reduced suppressive function. J. Immunol. 2011;187:2278–2290. doi: 10.4049/jimmunol.1003715. [DOI] [PubMed] [Google Scholar]
- Zheng B., Morgan M.E., van de Kant H.J., Garssen J., Folkerts G., Kraneveld A.D. Transcriptional modulation of pattern recognition receptors in acute colitis in mice. Biochim. Biophys. Acta. 2013;1832:2162–2172. doi: 10.1016/j.bbadis.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Ivanov I.I., et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Ruan Q., et al. The Th17 immune response is controlled by the Rel-RORgamma-RORgamma T transcriptional axis. J. Exp. Med. 2011;208:2321–2333. doi: 10.1084/jem.20110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur. J. Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- Gao Z., et al. Synergy between IL-6 and TGF-beta signaling promotes FOXP3 degradation. Int. J. Clin. Exp. Pathol. 2012;5:626–633. [PMC free article] [PubMed] [Google Scholar]
- Kryczek I., et al. Cutting edge: IFN-gamma enables APC to promote memory Th17 and abate Th1 cell development. J. Immunol. 2008;181:5842–5846. doi: 10.4049/jimmunol.181.9.5842. [DOI] [PubMed] [Google Scholar]
- Komai-Koma M., Jones L., Ogg G.S., Xu D., Liew F.Y. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc. Natl Acad. Sci. USA. 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka M.K., Whitton J.L. Antigen-specific regulation of T cell-mediated cytokine production. Immunity. 2000;12:451–457. doi: 10.1016/s1074-7613(00)80197-1. [DOI] [PubMed] [Google Scholar]
- Walker J.A., Barlow J.L., McKenzie A.N. Innate lymphoid cells—how did we miss them. Nat. Rev. Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- Li H., Wojciechowski W., Dell'Agnola C., Lopez N.E., Espinoza-Delgado I. IFN-gamma and T-bet expression in human dendritic cells from normal donors and cancer patients is controlled through mechanisms involving ERK-1/2-dependent and IL-12-independent pathways. J. Immunol. 2006;177:3554–3563. doi: 10.4049/jimmunol.177.6.3554. [DOI] [PubMed] [Google Scholar]
- Gessani S., Belardelli F. IFN-gamma expression in macrophages and its possible biological significance. Cytokine Growth Factor Rev. 1998;9:117–123. doi: 10.1016/s1359-6101(98)00007-0. [DOI] [PubMed] [Google Scholar]
- Bradley L.M., Dalton D.K., Croft M. A direct role for IFN-gamma in regulation of Th1 cell development. J. Immunol. 1996;157:1350–1358. [PubMed] [Google Scholar]
- Hall L.J., Faivre E., Quinlan A., Shanahan F., Nally K., Melgar S. Induction and activation of adaptive immune populations during acute and chronic phases of a murine model of experimental colitis. Dig. Dis. Sci. 2011;56:79–89. doi: 10.1007/s10620-010-1240-3. [DOI] [PubMed] [Google Scholar]
- Gao Q., et al. Expression of matrix metalloproteinases-2 and -9 in intestinal tissue of patients with inflammatory bowel diseases. Dig. Liver Dis. 2005;37:584–592. doi: 10.1016/j.dld.2005.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.