Abstract
This study investigated potential cumulative effects of multiple pregnancy and multigenerational exposure to dietary ZEA (0, 0.8, 4, or 20 ppm) on female puberty and reproduction in C57BL/6J mice. Multiple pregnancies did not significantly affect litter size or offspring puberty. Significant effects were observed in 20 ppm ZEA-treated females: advanced puberty onset in F0, F1, and F2 generations; decreased implantation rate, pregnancy rate, and litter size, and increased pregnancy gap and gestation period in F1 and F2 generations; and reduced fertility index in F2 generation. F3 females from 0 and 20 ppm groups were split into 0 or 20 ppm ZEA diets at weaning, with advanced puberty onset seen in 0-20 and 20-20 groups and decreased implantation rate observed in 20-20 group. In summary, 20 ppm dietary ZEA advanced puberty onset without obvious cumulative effect and impaired fertility with multigenerational cumulative effect, which could be partially alleviated upon exposure cessation.
Keywords: Zearalenone, multipregnancy, multigeneration, vaginal opening, embryo implantation, litter size, female reproduction
Introduction
Mycotoxin zearalenone (ZEA) is commonly found in livestock feed and in human food. Its contamination levels are in the range of ppb to low ppm with the highest reported at 600 ppm [1, 2]. Plant foods (e.g., corn and wheat) can contain ZEA through fungal contamination. Animal foods (e.g., meat and dairy products) can be contaminated with ZEA via intake of fungus contaminated feedstuff by livestock or can contain zeranol (α-zearalanol), a derivative of ZEA used as a growth promoter in livestock [3, 4]. Contaminated food is the main source of human exposure to ZEA [1–3]. The median and 95th percentile daily dietary ZEA exposure in European population were estimated to be <0.1 μg/kg body weight and <0.3 μg/kg body weight, respectively [2]. The tolerable daily intake (TDI) for ZEA established by the Panel on Contaminants in the Food Chain in Europe is 0.25 μg/kg body weight [2].
A study on 76 girls/2 boys with idiopathic precocious puberty (IPP) and 99 girls/1 boy in the control indicated positive correlation between ZEA and IPP, with an odds ratio of 8.833 (95% confidence interval: 2.281–34.208) [5]. Epidemiological studies support ZEA and its derivative mycotoxins as a triggering factor for precocious pubertal development in prepubertal exposed girls (reviewed in [4]). Although human data on definitive causative effects of ZEA and its metabolites on puberty and female reproductive system are unavailable, the estrogenicity of ZEA and its metabolites renders them the potential to influence puberty and functions of the female reproductive system [1].
Female puberty and reproduction are regulated by estrogen [6–8], therefore, these processes could be affected by ZEA. Our previous study showed that postweaning exposure to 10 ppm or 40 ppm dietary ZEA promoted premature onset of puberty and 40 ppm dietary ZEA also disrupted early pregnancy events in female mice [9]. ZEA is quickly absorbed and the unconjugated ZEA has an elimination half-life of 16.8 hours after oral administration in male rats [2]. Placental transfer of ZEA and its metabolites in rats and pigs, as well as lactational transfer of ZEA and its metabolites in the bovine milk, have been demonstrated (reviewed in [2]). These observations suggest that placental and lactational transfer of ZEA and its metabolites may also occur in other species, such as human and mouse. Given that ZEA is a common contaminant in the human diet [2], which is consumed daily across generations, it is natural to ask if there are any cumulative effects of ZEA on female puberty and reproduction during multigenerational exposure.
Multigenerational studies on reproduction usually cover the F0 (parental), F1 and F2 generations, with exposure commencing from the F0 generation prior to mating and continuing through the F2 generation until the F2 offspring are weaned [10], allowing the investigation of reproduction upon exposure for one or two generations. A survey of multigenerational studies of 316 chemicals in rats indicates that more chemicals have reproductive effects on the F1 generation than on the F0 generation and more adult reproductive effects are seen in the F1 generation than in the F0 generation [11]. Therefore, it is necessary to assess the effects of ZEA on female puberty and reproduction in a multigenerational setting. It was hypothesized that ZEA in the diet could have cumulative adverse effects on female puberty and reproduction. This hypothesis was tested in C57BL/6J mice. Vaginal opening, pregnancy rate, and litter size were among the parameters used to determine effects of ZEA treatments on female puberty and reproduction.
Materials and Methods
Animals
The C57BL/6J mice were initially derived from animals at Jackson Laboratories (Bar Harbor, ME) [9]. All mice were housed in polypropylene cages with free access to a casein-based phytoestrogen-free AIN-93G diet (Bio-Serv, Frenchtown, NJ) or a ZEA in AIN-93G diet and to water in polypropylene water bottles. Polypropylene containers were not significant sources for endocrine disruption [12]. Generally, ZEA levels in rodent diets were undetectable or very low (<30 ppb) [13, 14]. The animal facility was maintained on a 12-h light/dark cycle (0600 h to 1800 h) at 23 ± 1°C with 30–50% relative humidity. All methods used were approved by the Animal Subjects Programs of the University of Georgia and conform to National Institutes of Health guidelines and public law.
ZEA treatment, mating, and data collection
Homemade diets containing 0, 0.8, 4, and 20 ppm ZEA (Fermentek, Israel) were prepared as previously described [9]. These ZEA levels were within the range of levels in the highly contaminated food [1, 2]. The treatment regimen is outlined in Figure 1. F0 females were treated from weaning (3 weeks old) to dissection; F1 and F2 females were exposed to ZEA diets during their entire lives, from gestation to dissection (Fig. 1A).
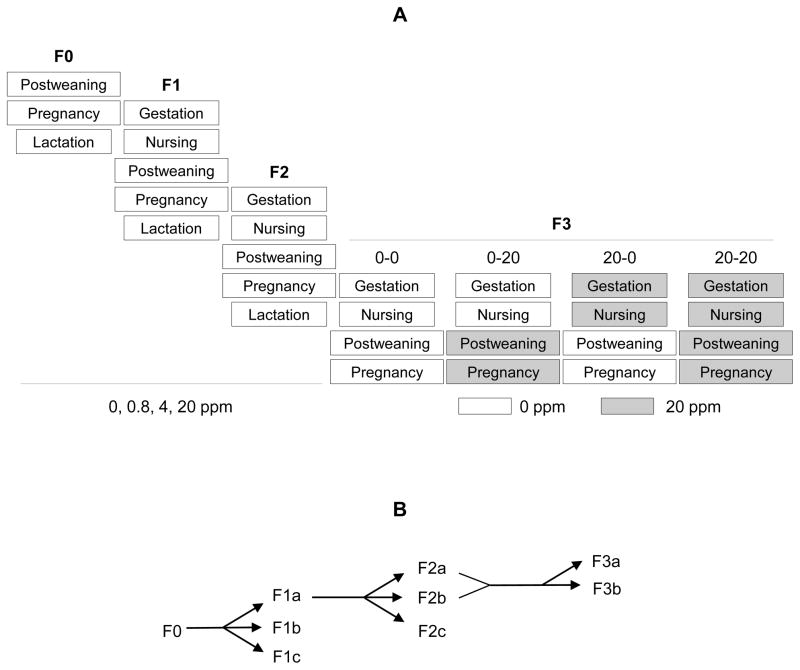
Figure 1.
Treatment and breeding regimens. A. Treatment protocols. F0 generation was treated with 0, 0.8, 4, or 20 ppm ZEA diets during the postweaning period, pregnancy, and lactation; F1 and F2 generations were exposed to 0, 0.8, 4, or 20 ppm ZEA diets during gestation and nursing via maternal exposure and treated with the same ZEA diets during the postweaning period, pregnancy, and lactation via direct dietary exposure. Females in the 0.8 and 4 ppm ZEA-treated F2 generation were dissected on D4.5 without producing F3 generation. In the F3 generation, 0 ppm and 20 ppm ZEA-treated groups were split into two groups each at weaning, and then treated with 0 ppm or 20 ppm ZEA during the postweaning period and pregnancy. B. Breeding regimen. F0 dams produced three litters: F1a, F1b, and F1c; F1a dams produced three litters: F2a, F2b, and F2c; F2a and F2b dams produced two litters: F3a and F3b.
Newly weaned F0 generation females were randomly assigned into 0, 0.8, 4 and 20 ppm ZEA-treated groups with littermates assigned into different groups. At 8 weeks old, the F0 females in each group were mated with fresh stud males, which were exposed to the same ZEA diets as the females only during mating, to produce three consecutive litters, F1a, F1b and F1c (Fig. 1B). Another set of F0 females were mated and dissected on gestation day 4.5 (D4.5) to determine embryo implantation in the F0 generation. At 8 weeks old, F1a and F1b females were mated with fresh stud males. The F1a females would eventually produce three consecutive F2 litters, F2a, F2b, and F2c. The F1b females were dissected on D4.5 to determine embryo implantation in the F1 generation (Fig. 1B).
At 8 weeks old, F2a and F2b females were mated with fresh stud males. Since no obvious adverse effects on puberty and fertility were observed in the 0.8 and 4 ppm ZEA-treated F0 and F1 females, F2a and F2b females in the 0.8 and 4 ppm ZEA-treated groups were dissected on D4.5 to determine implantation without producing an F3 generation for these two dose groups. F1c and F2c pups were sacrificed before or at weaning. In the 0 ppm ZEA group, 11 F2a and 2 F2b females were mated to produce the F3a and F3b litters; in the 20 ppm ZEA groups, 3 F2a and 8 F2b females were mated to produce the F3a and F3b litters (Fig. 1B). The rest of the F2b females in the 0 and 20 ppm ZEA were dissected on D4.5 to determine embryo implantation in the F2 generation.
At weaning, F3 female littermates from each dam in the 0 and 20 ppm ZEA groups were randomly split into 0 or 20 ppm ZEA-treated groups, resulting in a total of four groups: 0-0, 0-20, 20-0, and 20-20 (Fig. 1A). At 8 weeks old, all F3 females were mated with fresh stud males and dissected on D4.5 to determine embryo implantation in the F3 generation.
During mating in each generation, each female was checked every morning for the presence of a vaginal plug to determine mating in the previous night. They were separated from the mating males ~D15.5 and housed individually when an enlarging belly (to indicate pregnancy) was clearly observed. Some females had demonstrated more than one mating plug before a pregnancy was evident. Dams that lost all pups before weaning or had pups weaned at three weeks old were back to mating after one week of rest if they were scheduled to produce more litters.
Water consumption and food consumption were recorded weekly during postweaning (F0 and F1) and lactation (F0, F1, and F2). Postnatal body weights were monitored at 7, 14, 21, 22, 29, 36, 43, 50, and 57 days of age. Body weights during pregnancy were recorded every 5 days from D2.5 to D17.5 for F0, F1, and F2 dams and their body weights during lactation were measured weekly. Litter size was recorded at birth. Gender ratio of the pups was determined at weaning (3 weeks old).
Female pups were monitored daily after weaning for signs of vaginal opening; the age at vaginal opening was recorded as an indication of puberty onset [9, 15, 16]. As previously described [9, 17], embryo implantation and pregnancy status on D4.5 were determined by using Evans blue dye reaction, or for those without implantation sites, by uterine flushing to detect the presence of healthy-looking embryos. All male pups were sacrificed at weaning without further study.
The number of animals in each group was indicated in the figure legends. All groups began with at least 6 mice. However, since not all mated mice were pregnant, not all F0 and F1 pregnant mice produced three litters as planned, and there was further impaired fertility in the F1, F2, and F3 20 ppm ZEA-treated groups, the number of pregnant females per group analyzed for food and water consumption during lactation, body weight during gestation and lactation, length of the gap between mating and delivery (pregnancy gap), age of dams at delivery, and number of implantation sites could be less than 6.
Statistical analysis
ANOVA analyses were done using SigmaPlot 12.0. ANOVA on ranks followed by Dunnett’s test was used for analyzing the age at vaginal opening and the number of implantation sites. One way ANOVA followed by Dunnett’s test was used for analyzing food and water consumption, body weight during gestation and lactation, mating duration, and pregnancy gap. Two-way repeated measures ANOVA followed by Dunnett’s method was used for analyzing gestational period and litter size from the females that gave birth two (F2) or three (F0 & F1) times and postweaning body weight for each mouse that was repeatedly measured weekly. Two-way ANOVA followed by the Holm-Sidak test was used for analyzing preweaning body weight. Fisher’s exact test was used to analyze pregnancy rate, implantation rate, mating index, and fertility index. The significance level was set at P<0.05 and two-tailed tests were used.
Results
Food consumption, water consumption, and body weight
Although the numbers fluctuated, no consistent differences in either food consumption or water consumption were observed among 0, 0.8, 4 and 20 ppm ZEA-treated groups at the same ages in the same generation during postweaning or lactation (Tables S1 & S2). The only consistent difference observed in body weight was a significantly higher body weight in the F1 and F2 20 ppm ZEA-treated groups during weeks 6–8 (Fig. S1 and data not shown). Based on food consumption and body weight, the estimated ZEA doses from postweaning growth (lowest) to the end of lactation (highest) were: 0.1~0.24 mg/kg body weight per day in the 0.8 ppm ZEA group; 0.5~1.2 mg/kg body weight per day in the 4 ppm ZEA group; and 2.5 to 6 mg/kg body weight per day in the 20 ppm ZEA group. The gender ratios at weaning were comparable among all treatment groups at different generations (data not shown).
Vaginal opening in F0, F1, and F2 females
No significant difference in the age at vaginal opening was observed among all F0, F1, and F2 females in the 0, 0.8, and 4 ppm ZEA treated groups (Fig. 2). However, the females in the 20 ppm ZEA-treated group in each generation had significantly reduced ages at vaginal opening compared with the control in each generation, indicating accelerated puberty onset upon 20 ppm ZEA dietary treatment. Among the F0, F1, and F2 generations treated with 20 ppm ZEA, no significant difference in the age at vaginal opening was observed (Fig. 2). The females in different litters from the same dams (litters F1a, F1b from the F0 dams; and litters F2a, F2b from the F1a dams (Fig. 1B)) in the same treatment group had comparable ages at vaginal opening (data not shown).
Figure 2.
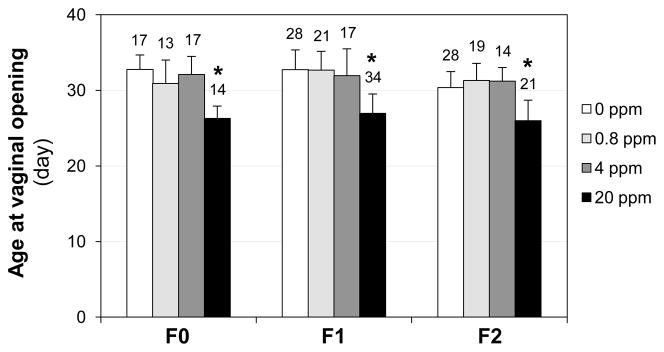
Effect of ZEA on the ages at vaginal opening in F0, F1, and F2 female mice. ANOVA on ranks followed by Dunnett’s test was used for analyzing the differences among groups. The numbers above bars, the total numbers of females in the indicated groups; * P<0.05, compared with the 0 ppm group in the same generation; error bar, standard deviation; N=13–34.
Mating behavior, 1st pregnancy rate, fertility index, litter size, gestation period, and pregnancy gap in F0, F1, and F2 females
ZEA treatment did not affect the mating behavior of female mice among different treatment groups or different generations as indicated by a comparable mating index (number of vaginal plug-positive females/number of mating females × 100%) and comparable mating duration (time from cohabitation to detection of a vaginal plug) (data not shown).
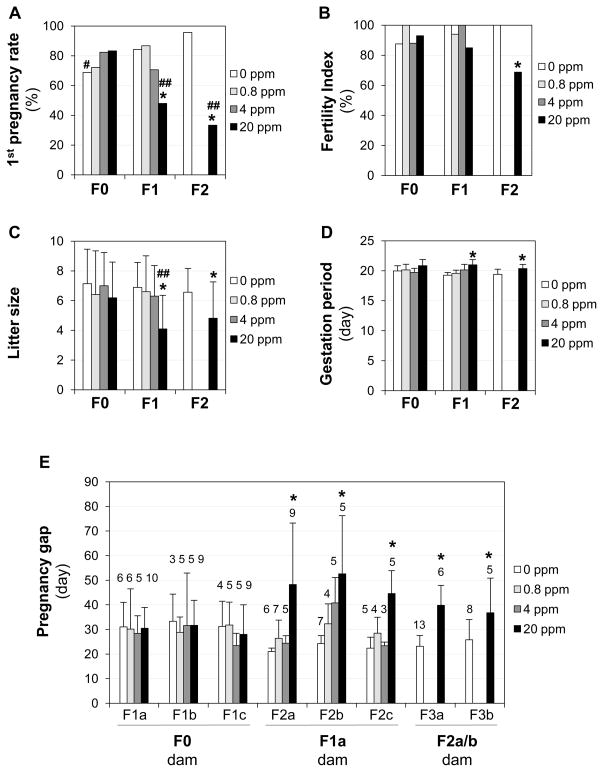
The 1st pregnancy rate was determined as the percentage of the first mating, indicated by a vaginal plug, leading to a full term pregnancy (number of females producing litters/number of females with 1st vaginal plugs × 100%) in the same group. The F0 and F1 dams potentially had three 1st vaginal plugs and the F2 dams potentially had two 1st vaginal plugs during the production of three and two litters, respectively. Results revealed that 20 ppm ZEA-treated F1 and F2 females, but not the F0 females or the 0.8 and 4 ppm ZEA-treated F0, F1, and F2 females, had significantly reduced 1st pregnancy rates compared with the control females in the same generation (Fig. 3A). No significant difference was observed between the 1st pregnancy rates of F1 and F2 20 ppm ZEA-treated groups, both of which were significantly lower than that in the F0 20 ppm ZEA-treated group (Fig. 3A). Interestingly, there was a significant increase in the 1st pregnancy rate of the F2 control compared with the F0 control (Fig. 3A).
Figure 3.
Effect of ZEA on fertility in F0, F1, and F2 female mice. A. Pregnancy rate following the first mating (number of females producing litters/number of females with 1st vaginal plugs × 100%). N=15–29 first vaginal plugs from 6–13 dams. B. Fertility index (number of females ever producing litters/number of females ever mated × 100%) from all matings. N=16–29 from 6–13 dams. C. Litter size. N=10–26 litters from 6–13 dams. D. Gestation period. N=7–24 from 6–13 dams. E. Pregnancy gap from cohabitation to delivery. The numbers above bars, the total numbers of dams in the indicated groups; N=3–13. A & B, Fisher’s exact test; C & D, Two-way repeated measures ANOVA followed by Dunnett’s test; E, One-way ANOVA followed by Dunnett’s test. A–E: * P<0.05, compared with respective 0 ppm ZEA control group; # P<0.05, compared with the F2 0 ppm ZEA group (A); ## P<0.05, compared with F0 20 ppm ZEA group (A & C); error bar (C~E), standard deviation. A–D: X-axis, dams’ generation.
The fertility index is the percentage of females in which vaginal plugs were detected (some plugged multiple times) that then produced litters. The only difference was seen in the F2 20 ppm ZEA-treated group, in which 5 out of 11 females with vaginal plugs never became pregnant. The other 6 females had full term 1st pregnancies (F3a). However, one of them had to be sacrificed due to a delivery problem. The remaining 5 females continued to give birth to a second litter each (F3b). The fertility index in the F2 20 ppm ZEA-treated group was significantly lower than that in the F2 control group (P=0.0232) but was not significantly different from the F0 (P=0.0788) or the F1 (P=0.2565) 20 ppm ZEA-treated groups (Fig. 3B).
No obvious effect of birth order (F1a, F1b, and F1c from the F0 dams; F2a, F2b, and F2c from the F1a dams; F3a and F3b from the F2a and F2b dams) on litter sizes and gestation periods were observed in each treatment group (data not shown), indicating that increased exposure time of the dams did not significantly alter any effect of ZEA on their fertility. When all the litters from the same dams were counted, no significant difference in litter sizes was observed among all treatment groups in different generations except the 20 ppm ZEA-treated F1 and F2 dams, which produced significantly smaller litter sizes compared with the control F1 or F2 dams. There was no significant difference in litter sizes produced by 20 ppm ZEA-treated F1 or F2 dams. However, the litter size from the F1 dams was significantly smaller than that from the F0 dams in the 20 ppm ZEA-treated group (Fig. 3C). In addition, F1 and F2 dams in the 20 ppm ZEA-treated groups also had prolonged gestation periods compared with their respective control groups in the same generation (Fig. 3D). The pregnancy gaps (duration between cohabitation and delivery) in the F1a dams producing F2a, F2b, and F2c litters and the F2a/b dams producing F3a and F3b litters in the 20 ppm ZEA-treated groups (Fig. 1) were significantly longer than those in their respective control groups (Fig. 3E). The ages of F1a dams at delivering F2a and F2b litters and F2a/b dams at delivering F3a litters in the 20 ppm ZEA-treated groups were significantly older than those in their respective control groups (Fig. S2).
Embryo implantation in F0, F1 and F2 females
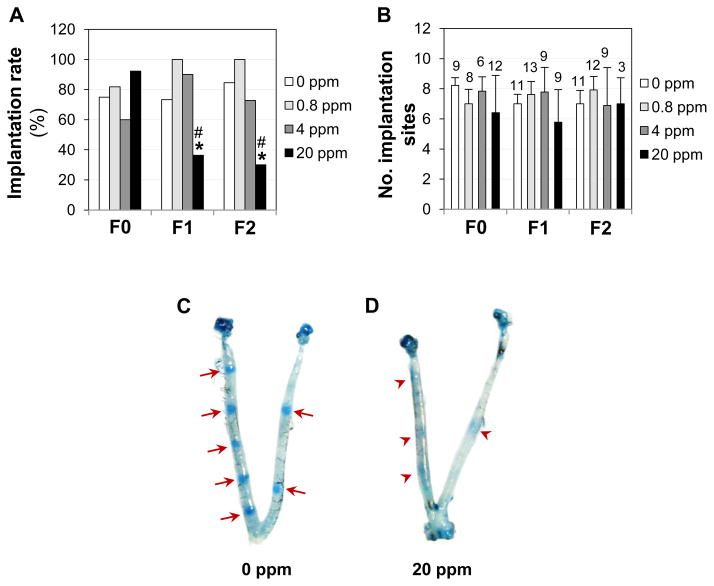
Embryo implantation was examined on D4.5 to investigate the mechanism of impaired fertility upon 20 ppm ZEA diet treatment. Among all groups in the F0, F1, and F2 generations, only the F1 and F2 females in the 20 ppm ZEA-treated groups had significantly lower implantation rates (number of females with implantation sites/number of females with vaginal plugs in the same group × 100%) compared with their respective controls (Fig. 4A). However, the dams with implantation sites had comparable numbers of implantation sites among all four different treatment groups in all three generations (F0, F1, and F2) (Fig. 4B). It was noticed that all the mice in the 0, 0.8, and 4 ppm ZEA-treated groups had distinct blue bands (Fig. 4C and data not shown), but about 1/3 of the pregnant mice with implantation sites in the F0 (4/12) and the F1 (3/9) 20 ppm ZEA-treated groups had faint blue bands (Fig. 4D), an indication of delayed implantation [18]. In the F2 generation, only 3 out of 10 females had implantation sites on D4.5 and all of them had distinct implantation sites (data not shown) as seen in the control (Fig. 4C).
Figure 4.
Effect of ZEA on embryo implantation detected on gestation day 4.5 (D4.5) in F0, F1, and F2 generations. A. Implantation rate. Fisher’s exact test. * P<0.05, compared with the control group in the same generation; # P<0.05, compared with the F0 20 ppm ZEA-treated group; N=10–22. B. Average number of implantation sites per mouse with implantation sites. ANOVA on ranks. Error bar, standard deviation; the numbers above bars, the numbers of females with implantation sites; N=3–14. C. A representative D4.5 uterine image from a control mouse (0 ppm ZEA). Red arrow, on-time implantation site. D. A D4.5 uterine image from the F1 20 ppm ZEA-treated group showing faint blue bands. Red arrowhead, delayed implantation site.
Vaginal opening and embryo implantation in F3 females
The treatment regimen in the F3 females consisted of four groups designated as 0-0, 0-20, 20-0, and 20-20 (Fig. 1A). This treatment regimen could potentially provide clues to the following questions: Would generational cumulative effects continue upon 20 ppm ZEA treatment (20-20 group)? Would any adverse effects lessen if the treatment was discontinued (20-0 group)? Or, would some adverse effects be associated with only immediate exposure (0-20 group)?
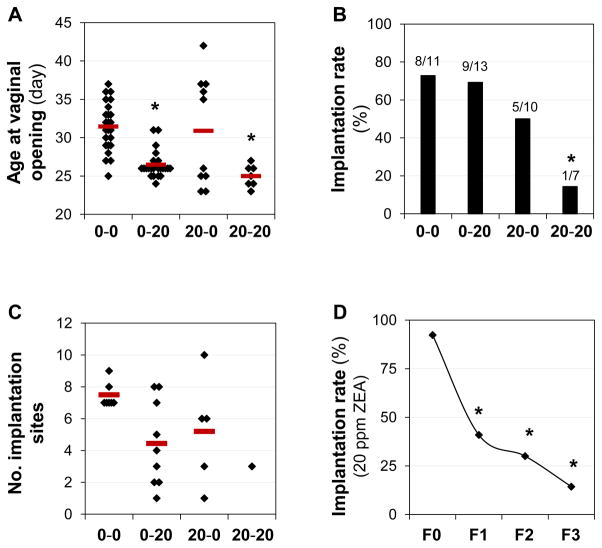
Significantly advanced ages at vaginal opening were observed in the 0-20 and the 20-20 groups compared with the 0-0 control group, and there was no significant difference between the 0-20 and the 20-20 groups (Fig. 5A). Interestingly, although the average age at vaginal opening in the 20-0 group was comparable with that of the 0-0 group, the ages at vaginal opening in the 20-0 group were shown in two distinct clusters, a phenomenon not observed in their littermates in the 20-20 group (Fig. 5A).
Figure 5.
Effects of switched ZEA exposure on age at vaginal opening and embryo implantation at gestation day 4.5 in the F3 generation. A. Ages at vaginal opening. ANOVA on ranks followed by Dunnett’s test. Black diamond, individual data point; red line, average age in each group; * P<0.05, compared with the 0-0 group; N=7–24. B. Implantation rate. Fisher’s exact test. The ratio above each bar, the number of females with implantation sites over the total number of plugged females; N=7–13; * P<0.05, compared with the 0-0 group. C. Number of implantation sites. ANOVA on ranks. Black diamond, individual data point; red line, average number of implantation sites in each group; only mice with implantation sites were included. D. Progressively decreasing implantation rates in the F0, F1, F2, and F3 20 ppm ZEA-treated groups. Fisher’s exact test. * P<0.05, compared with F0.
The implantation rate in the 0-20 group was comparable with those in the 0-0 group (Fig. 5B) and the 20 ppm ZEA-treated group in F0 generation (Fig. 4A), which also had the treatment only after weaning (Fig. 1A). A significantly decreased implantation rate was observed in the 20-20 group (F3 20 ppm ZEA-treated group) compared with the 0-0 group. The implantation rate in the 20-0 group (5/10=50%) fell in between the 0-0 (8/11=72.7%) and the 20-20 (1/7=14.3%) groups, although there was no significant difference between the 20-0 and the 0-0 groups or the 20-0 and the 20-20 groups due to the small sample sizes. Females without detectable implantation sites had no embryos detected in the reproductive tracts except for one animal in the 0-20 treated group, which had three embryos flushed from the oviduct but none from the uterus, indicating delayed embryo transport [9].
The average numbers of implantation sites in the mice with implantation sites were 7.5±0.8 (N=8) in the 0-0 group, 4.4±2.7 (N=9) in the 0-20 group, 5.2±3.4 (N=5) in the 20-0 group, and 3 (N=1) in the 20-20 group (Fig. 5C). Although there was no significant difference among the 0-0, 0-20 and 20-0 groups (P=0.066, ANOVA on ranks), large variations among animals in the 0-20 and the 20-0 groups were noticed. In addition, females with implantation sites in the 0-20 group had a significantly higher percentage of mice (8/9, compared to 0/8 in the 0-0 group, P<0.05) with only faint blue bands in the uterus, indicating delayed implantation as seen in Fig. 4D, while all mice in the 0-0 and the 20-0 groups had distinct blue bands as seen in Fig. 4C, indicating on-time implantation. The only pregnant mouse in the 20-20 group had distinct implantation sites.
Discussion
This multiple pregnancy and multigenerational study revealed a promoting effect of 20 ppm ZEA diet on female puberty. However, it did not show any obvious cumulative effect either via multiple pregnancies or through multiple generations under the experimental setting in this study. It also showed an adverse effect of 20 ppm ZEA diet on female reproduction, which was cumulative in multiple generations but not over multiple pregnancies in the same generation. Cessation of exposure to the 20 ppm ZEA diet in the F3 generation after three generations (F0, F1, and F2) of exposure could partially reverse these adverse effects.
Vaginal opening is an early indication of puberty onset in rodents [9]. The lack of a difference among different litters from the same dams (data not shown) or among different generations under the same ZEA treatment (Fig. 2) suggested the absence of a cumulative effect of ZEA on female puberty onset. However, this study design was not capable of revealing any cumulative shortening of the time to pubertal onset if an average of 25~26 days old was the earliest possible age for vaginal opening in C57BL/6J mice upon postweaning ZEA treatment. This possibility was supported by our previous published observation [9] and unpublished observations that stronger estrogenic treatments of newly-weaned C57BL/6J females mice with a 40 ppm ZEA diet, a 0.05 ppm diethylstilbestrol (DES) diet, or with daily i.p. injection of 12.5 μg 17β-estradiol (E2, a dose equivalent to ~10 ppm E2 in the diet) all led to a similar age (25~26 days old) at vaginal opening. Decreased age at vaginal opening was mainly seen in the mice with direct exposure to 20 ppm ZEA during the postweaning period (Figs. 2, 5A), a phenomenon also seen in rats treated with 500 ppm dietary genistein for multiple generations [19]. These observations suggested that the period immediately prior to puberty onset was a vulnerable window for ZEA to influence puberty, something also observed in CD-1 mice subcutaneously injected with 10 mg/kg/day ZEA [20]. However, based on the switched exposure regimen in the F3 generation, the F3 females in the 20-0 group showed two distinct clusters of ages at vaginal opening (Fig. 5A). The observation suggested that gestational and lactational exposure to 20 ppm ZEA might have segregated effects on puberty onset of female offspring due to possible genetic segregation of capacity to clear ZEA. It also suggested that dam-mediated gestational and lactational exposure could contribute to the effects of estrogenic endocrine disruptors on offspring puberty onset [2, 16].
A generational cumulative effect on implantation rate was seen as a dramatic decrease from F0 (comparable to control) to F1 with a further gradual decline from F1 to F3 upon 20 ppm ZEA treatment (Fig. 5D). However, a more subtle effect, such as delayed implantation, was already evident in the F0 20 ppm ZEA-treated group. Since reduced pregnancy rates at term correlated with reduced implantation rates at D4.5 in the F1 and F2 20 ppm ZEA-treated groups (Figs. 3A, 4A), it suggested that one or more early pregnancy events, e.g., fertilization, embryo transport, preimplantation embryo development, or embryo implantation [9], or oocyte quality [21], were adversely affected by the continuous exposure to 20 ppm ZEA from gestation to pregnancy. One study in rats revealed reduced numbers of corpora lutea in the F1B female offspring from parents exposed to 10 mg/kg ZEA [22], suggesting that follicle development and/or ovulation might also be affected by a higher dose of ZEA exposure. Since no embryos were detected in the reproductive tracts of 6 out of 7 F3 20-20 females (P<0.05 compared with the 0-0 group, 3/11) on D4.5 in this study, it implied that ovulation and/or fertilization were adversely affected in the F3 20-20 females.
Litter sizes from F1 and F2 dams were also significantly reduced but not those from F0 dams exposed to 20 ppm ZEA (Fig. 3C). Reduced litter size from F1 but not F0 dams was also reported in rats exposed to another estrogenic endocrine disruptor, genistein [23]. However, the litter sizes from both F0 and F1 dams were reduced in rats treated with 10 mg/kg ZEA (~80 ppm in the diet for adult mice) [22]. This discrepancy was most likely caused by the use of higher ZEA doses because a higher ZEA dose at 40 ppm could also adversely affect fertility of the F0 females [9]. Despite reduced litter sizes (Fig. 3C) and reduced implantation rates (Fig. 4A) in the F1 and F2 20 ppm ZEA-treated groups, the numbers of implantation sites from the remaining pregnant F1 and F2 females were not significantly changed (Fig. 4B), indicating postimplantational lethality in these pregnant females. Increased postimplantational lethality upon treatment with 10 mg/kg ZEA was demonstrated in rats [22]. The generational cumulative effect of 20 ppm ZEA diet on female fertility was also manifested in a reduced fertility index in the F2 but neither the F0 nor the F1 generations (Fig. 3B) and increased pregnancy gap in the F1 and F2 generations (Fig. 3E).
The switched exposure regimen in the F3 females provided further information on those developmental windows sensitive to ZEA exposure. Since implantation rate, pregnancy rate, and litter size were not adversely affected in the F0 20 ppm ZEA-treated group (Figs. 3A, 3C, 4A) and implantation rate was unaffected in the F3 0-20 group (Fig. 5B), which all started ZEA treatment after weaning, the results imply that maternal exposure during mating, gestation, and lactation played an important role for the adverse effects of 20 ppm ZEA on F1 and F2 fertility. Although no significant difference in implantation rate or number of implantation sites was observed in the F3 20-0 group due to small sample sizes, both parameters fell in between those of the F3 0-0 control group and the F3 20-20 group (Figs. 5B, 5C), indicating that exposure cessation (20-0 group) could partially alleviate the adverse effects while extending exposure for one more generation continued the trend of diminished fertility (Fig. 5D). The impaired fertility might be caused by dam-mediated in-utero and/or lactational exposure [2], a phenomenon that could be influenced by epigenetic mechanisms [24, 25].
The contribution of the mating males to the adverse effects of 20 ppm ZEA is insignificant based on the following observations. First, male rats exposed to 10 mg/kg body weight of ZEA via diet for at least 10 weeks did not have any reported obvious pathological changes in the testis [22]. Second, male mice intraperitoneally injected with 15 mg/kg ZEA for 7 consecutive days did not have visible effects on sperm quality and testis histology [26]. Third, young adult C57BL/6J males treated with 40 ppm ZEA diets for three weeks plus one week with gavage during mating did not affect mating activity, testis weight, sperm counts, or the fertility of the mated females (data not shown). Since the effect of ZEA on female fertility was dose-dependent [9] and the mating males in this study were exposed to ZEA at lower doses (0.8~20 ppm, or <2.5 mg/kg body weight per day [9]) for shorter periods, the adverse effects on fertility observed in the 20 ppm ZEA treated females in this study were assumed to be mainly attributed by the effects of ZEA on female reproductive system.
In summary, our study demonstrated that exposure to a 20 ppm ZEA diet promoted female puberty onset without obvious cumulative effect and diminished female fertility over generations under the current experimental design. While direct exposure to ZEA immediately prior to puberty onset was a sensitive window for ZEA to affect female puberty, different exposure periods from gestation, lactation, to postweaning could all contribute to the adverse effects of ZEA on female fertility. Cessation of exposure to ZEA seemed to partially alleviate these adverse effects.
Supplementary Material
Highlights.
No significant effect of multiple pregnancies on litter size or offspring puberty.
Advanced puberty onset in F0, F1, and F2 females on 20 ppm ZEA diet.
Multigenerational cumulative impairment of fertility in females on 20 ppm ZEA diet.
Partial recovery from 20 ppm ZEA treatment upon exposure cessation.
Acknowledgments
The authors thank Ms. Elizabeth Dudley for proofreading the manuscript, Dr. Xiao Song and Mr. Xuedong Wu for statistical consultations, the Office of the Vice President for Research, Interdisciplinary Toxicology Program, and Department of Physiology and Pharmacology at the University of Georgia, and the National Institutes of Health (NIH R15HD066301 and NIH R01HD065939 (co-funded by ORWH and NICHD) to X.Y.) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Fei Zhao, Email: feizhao@uga.edu.
Rong Li, Email: lirong9@uga.edu.
Shuo Xiao, Email: shuoxiao@northwestern.edu.
Diao Honglu, Email: hldiao1976@gmail.com.
Ahmed E. El Zowalaty, Email: ahmdezat@uga.edu.
Xiaoqin Ye, Email: ye@uga.edu.
References
- 1.Zinedine A, Soriano JM, Molto JC, Manes J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogenic mycotoxin. Food Chem Toxicol. 2007;45:1–18. doi: 10.1016/j.fct.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 2.EFSA. Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA Journal. 2011;9:2197. [Google Scholar]
- 3.Bandera EV, Chandran U, Buckley B, Lin Y, Isukapalli S, Marshall I, et al. Urinary mycoestrogens, body size and breast development in New Jersey girls. Sci Total Environ. 2011;409:5221–7. doi: 10.1016/j.scitotenv.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massart F, Saggese G. Oestrogenic mycotoxin exposures and precocious pubertal development. Int J Androl. 2010;33:369–76. doi: 10.1111/j.1365-2605.2009.01009.x. [DOI] [PubMed] [Google Scholar]
- 5.Deng F, Tao FB, Liu DY, Xu YY, Hao JH, Sun Y, et al. Effects of growth environments and two environmental endocrine disruptors on children with idiopathic precocious puberty. Eur J Endocrinol. 2012;166:803–9. doi: 10.1530/EJE-11-0876. [DOI] [PubMed] [Google Scholar]
- 6.Delemarre EM, Felius B, Delemarre-van de Waal HA. Inducing puberty. Eur J Endocrinol. 2008;159 (Suppl 1):S9–15. doi: 10.1530/EJE-08-0314. [DOI] [PubMed] [Google Scholar]
- 7.Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, et al. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci U S A. 2010;107:22693–8. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Findlay JK, Liew SH, Simpson ER, Korach KS. Estrogen signaling in the regulation of female reproductive functions. Handb Exp Pharmacol. 2010:29–35. doi: 10.1007/978-3-642-02062-9_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao F, Li R, Xiao S, Diao H, Viveiros MM, Song X, et al. Postweaning exposure to dietary zearalenone, a mycotoxin, promotes premature onset of puberty and disrupts early pregnancy events in female mice. Toxicol Sci. 2013;132:431–42. doi: 10.1093/toxsci/kfs343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FDA. Guidance for Industry and Other Stakeholders Toxicological Principles for the Safety Assessment of Food Ingredients. 2000 [Google Scholar]
- 11.Martin MT, Mendez E, Corum DG, Judson RS, Kavlock RJ, Rotroff DM, et al. Profiling the reproductive toxicity of chemicals from multigeneration studies in the toxicity reference database. Toxicol Sci. 2009;110:181–90. doi: 10.1093/toxsci/kfp080. [DOI] [PubMed] [Google Scholar]
- 12.Chung BY, Kyung M, Lim SK, Choi SM, Lim DS, Kwack SJ, et al. Uterotrophic and Hershberger assays for endocrine disruption properties of plastic food contact materials polypropylene (PP) and polyethylene terephthalate (PET) J Toxicol Environ Health A. 2013;76:624–34. doi: 10.1080/15287394.2013.801767. [DOI] [PubMed] [Google Scholar]
- 13.Waldemarson AH, Hedenqvist P, Salomonsson AC, Haggblom P. Mycotoxins in laboratory rodent feed. Lab Anim. 2005;39:230–5. doi: 10.1258/0023677053739819. [DOI] [PubMed] [Google Scholar]
- 14.Boettger-Tong H, Murthy L, Chiappetta C, Kirkland JL, Goodwin B, Adlercreutz H, et al. A case of a laboratory animal feed with high estrogenic activity and its impact on in vivo responses to exogenously administered estrogens. Environ Health Perspect. 1998;106:369–73. doi: 10.1289/ehp.98106369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasier G, Toppari J, Parent AS, Bourguignon JP. Female sexual maturation and reproduction after prepubertal exposure to estrogens and endocrine disrupting chemicals: a review of rodent and human data. Mol Cell Endocrinol. 2006:254–255. 187–201. doi: 10.1016/j.mce.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Maranghi F, Mantovani A. Targeted toxicological testing to investigate the role of endocrine disrupters in puberty disorders. Reprod Toxicol. 2012;33:290–6. doi: 10.1016/j.reprotox.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–8. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diao H, Paria BC, Xiao S, Ye X. Temporal expression pattern of progesterone receptor in the uterine luminal epithelium suggests its requirement during early events of implantation. Fertil Steril. 2011;95:2087–93. doi: 10.1016/j.fertnstert.2011.01.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delclos KB, Weis CC, Bucci TJ, Olson G, Mellick P, Sadovova N, et al. Overlapping but distinct effects of genistein and ethinyl estradiol (EE(2)) in female Sprague-Dawley rats in multigenerational reproductive and chronic toxicity studies. Reprod Toxicol. 2009;27:117–32. doi: 10.1016/j.reprotox.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikaido Y, Danbara N, Tsujita-Kyutoku M, Yuri T, Uehara N, Tsubura A. Effects of prepubertal exposure to xenoestrogen on development of estrogen target organs in female CD-1 mice. In Vivo. 2005;19:487–94. [PubMed] [Google Scholar]
- 21.Malekinejad H, Schoevers EJ, Daemen IJ, Zijlstra C, Colenbrander B, Fink-Gremmels J, et al. Exposure of oocytes to the Fusarium toxins zearalenone and deoxynivalenol causes aneuploidy and abnormal embryo development in pigs. Biol Reprod. 2007;77:840–7. doi: 10.1095/biolreprod.107.062711. [DOI] [PubMed] [Google Scholar]
- 22.Becci PJ, Johnson WD, Hess FG, Gallo MA, Parent RA, Taylor JM. Combined two-generation reproduction-teratogenesis study of zearalenone in the rat. J Appl Toxicol. 1982;2:201–6. doi: 10.1002/jat.2550020406. [DOI] [PubMed] [Google Scholar]
- 23.NTP. Multigenerational reproductive study of genistein (Cas No. 446-72-0) in Sprague-Dawley rats (feed study) Natl Toxicol Program Tech Rep Ser. 2008:1–266. [PubMed] [Google Scholar]
- 24.Zeybel M, Hardy T, Wong YK, Mathers JC, Fox CR, Gackowska A, et al. Multigenerational epigenetic adaptation of the hepatic wound-healing response. Nat Med. 2012;18:1369–77. doi: 10.1038/nm.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118:3462–9. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Yang JY, Wang GX, Liu JL, Fan JJ, Cui S. Toxic effects of zearalenone and its derivatives alpha-zearalenol on male reproductive system in mice. Reprod Toxicol. 2007;24:381–7. doi: 10.1016/j.reprotox.2007.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.