Summary
An essential requisite for transmission of Plasmodium, the causative agent of malaria, is the successful completion of a complex developmental cycle in its mosquito vector. Of hundreds of ookinetes that form in the mosquito midgut, only few transform into oocysts, a loss attributed to the action of the mosquito immune system. However, once oocysts form, they appear to be resistant to mosquito defences. During oocyst development, a thick capsule forms around the parasite and appears to function as a protective cover. Little information is available about the composition of this capsule. Here we report on the identification and partial characterization of the first Plasmodium oocyst capsule protein (PbCap380). Genetic analysis indicates that the gene is essential and that PbCap380(−) mutant parasites form oocysts in normal numbers but are gradually eliminated. As a result, mosquitoes infected with PbCap380(−) parasites do not transmit malaria. Targeting of the oocyst capsule may provide a new strategy for malaria control.
Introduction
Together with AIDS and tuberculosis, malaria is one of the major infectious disease killers in the world, accounting for one to three million deaths per year (Breman et al., 2001; Greenwood and Mutabingwa, 2002). In contrast to the other two diseases, transmission of Plasmodium, the causative agent of malaria, occurs only via a mosquito vector. Thus, strategies that interfere with Plasmodium development in its insect host will result in reduced spread of the disease. After mating of male and female gametes in the midgut lumen, the resulting zygotes differentiate into ookinetes. These traverse the midgut epithelium to form sessile oocysts (Ghosh et al., 2000). Interaction of the ookinete with midgut basal lamina proteins is thought to trigger this transformation (Arrighi and Hurd, 2002). The oocyst remains sandwiched between the midgut epithelial cells and the basal lamina for 10–14 days. During this period the parasite grows in size and undergoes numerous nuclear divisions resulting in the formation of 2000–8000 haploid nuclei (Howells and Davies, 1971a, b; Canning and Sinden, 1973). As development proceeds, a distinct capsule secreted by the oocyst separates it from surrounding mosquito tissues (Aikawa, 1971). The molecular composition of the capsule remains unknown although its proteins have been speculated to interact with mosquito components (Adini and Warburg, 1999). Recently, a transglutaminase activity has been reported in oocysts and may function in cross-linking parasite and mosquito proteins (Adini et al., 2001).
The ookinete elicits strong immune responses during the initial stages of its development in the mosquito midgut (Dong et al., 2006) that include lysis of ookinetes during their invasion of midgut epithelial cells (Vernick et al., 1995) and melanotic encapsulation of ookinetes as they emerge on the basal side of the midgut epithelium (Collins et al., 1986). Once formed, the oocyst appears to be more resistant to the mosquito defences and as suggested by the present results, the capsule may play an important protective role. At the end of parasite development, each oocyst releases thousands of sporozoites that find their way into the salivary gland lumen and are transferred to a new vertebrate host when the mosquito takes another blood meal. In the field, of the hundreds of ookinetes that form, only few (usually less than five) succeed to differentiate into oocysts (Pringle, 1966). Targeting these remaining few oocysts may be part of an efficient strategy to control malaria.
Here we report on the functional characterization of PbCap380, the first Plasmodium capsule protein gene identified. PbCap380 is expressed exclusively during Plasmodium oocyst development and is essential for parasite survival in the mosquito.
Results and discussion
Identification of the gene encoding Plasmodium berghei capsule protein 380 (PbCap380)
PbCap380 was identified from a subtraction library enriched for genes expressed at the oocyst stage of Plasmodium development (Srinivasan et al., 2004). PbCap380 has orthologues in every sequenced Plasmodium species but has no similarity with any other protein or with any functional domain in the available databases. All Plasmodium orthologues are predicted to be transcribed from a single exon encoding a protein with putative signal sequence at the N-terminus (Fig. S1). The partial P. berghei nucleotide and predicted amino acid sequence available in the database is > 90% identical to the P. yoelli orthologue, another rodent malaria parasite. The P. falciparum and P. vivax orthologues share overall 25% and 22% amino acid identity with the P. yoelli protein respectively (Fig. S1; PlasmoDB accession numbers PFC0905c, Pv095215, PY00597, PB000071.00.0 and PB300510.00.0). However, similarity at N-terminal half of the predicted proteins is higher than the C-terminal half among multiple Plasmodium species (Fig. S1).
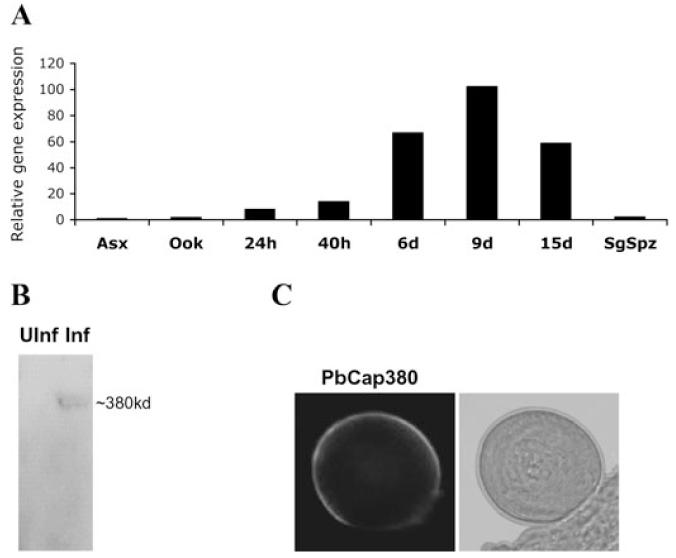
Quantitative RT-PCR analysis shows that PbCap380 is expressed only during oocyst differentiation in the mosquito (Fig. 1A). mRNA abundance mirrors oocyst growth in the midgut where it attains its maximum size around day 12 after infection. Moreover, PbCap380 expression can be detected neither in blood stage parasites nor in purified ookinetes and sporozoites (Fig. 1A). This is consistent with P. falciparum microarray data showing that the PbCap380 orthologue is expressed neither in gametocytes and sporozoites nor in blood stage parasites (Le Roch et al., 2003).
Fig. 1.
Initial characterization of PbCap380.
A. Quantification of gene transcription. Quantitative RT-PCR was performed using cDNAs prepared from blood stage parasites (Asx), in vitro-cultured mature ookinetes (ook), midguts dissected at different times after infection (24 h to 15 days) and salivary gland sporozoites (SgSpz). Data shown are from one representative experiment. Analysis of two other sets of independently obtained RNAs gave similar results.
B. Western blot analysis. A rabbit anti-PbCap380 polyclonal antibody recognizes a high molecular weight protein from mosquito guts dissected 15 days after infection (Inf). Guts from non-infected control mosquitoes (UInf) gave no signal at this position.
C. Indirect immunofluorescence assay. The anti-PbCap380 antibody labels the oocyst periphery. No signal was detected with any other parasite forms including asexual and sexual stage parasites other than oocysts (data not shown).
The PbCap380 protein is expressed throughout oocyst development
For analysis of protein expression we produced a rabbit polyclonal antibody against a recombinant PbCap380 protein fragment. The antibody recognizes a single protein in the expected size range on Western blots of day 15 infected midguts (Fig. 1B) and specifically labels the peripheral region of mature oocysts (Fig. 1C).
Oocyst development in the mosquito is asynchronous. To investigate the time-course of protein expression, we labelled mosquito midguts with anti-PbCap380 antibodies at different times after infection. PbCap380 can be detected soon after the ookinete transforms into an oocyst (36 h) and remains on the oocyst periphery till sporozoites begin to be released at around day 15 (Fig. 2A).
Fig. 2.
The PbCap380 protein is expressed throughout oocyst development.
A. Developmental profile of PbCap380 protein expression. The PbCap380 protein can be detected soon after oocyst formation (36 h) and continues till the end of oocyst development (day 15).
B. Transition between ookinete surface proteins and oocyst-specific proteins. Oocysts were double labelled with anti-PbCap380 (green) and anti-P28 (a major ookinete surface protein) (red) antibodies and visualized by confocal microscopy. Day-3 oocysts (single optical section) contain both PbCap380 and P28 on the surface. P28 can also be seen inside the oocyst. In day 5 oocysts (single optical section), P28 is largely internalized, while PbCap380 covers the entire oocyst.
Transition between ookinete and oocyst surface protein expression
Major ookinete surface proteins, such as P25 (del Carmen Rodriguez et al., 2000) and P28 (same as Pbs21, Tomas et al., 2001) (Fig. 2B), persist during the first few days of oocyst development. On the other hand, capsule protein expression starts soon after ookinete emergence from the midgut epithelium (Fig. 2A), and therefore both proteins initially coexist in the developing oocyst (Fig. 2B, upper panels). By day 5 post infection, a substantial proportion of the P28 protein is found inside the oocyst, presumably internalized for degradation (Fig. 2B, lower panels). P28 is not detectable after day 6 (data not shown). In addition to the role of P25 and P28 in interaction with the midgut (Siden-Kiamos et al., 2000; Vlachou et al., 2001), these surface proteins may also serve a protection function (Saxena et al., 2006). X-ray crystal structure of P25 and P28 proteins identified tile-like rectangular prisms possibly forming protective sheets on the parasite surface (Saxena et al., 2006). As the oocyst matures, new capsule proteins such as PbCap380 are synthesized and may take over such a protective function.
PbCap380 localizes to the oocyst capsule
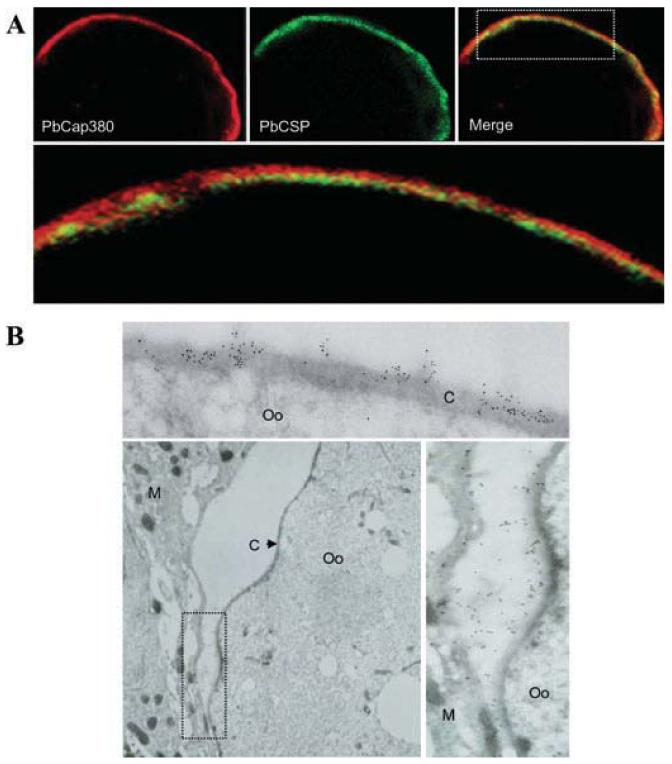
Circumsporozoite protein (CSP), the most abundant sporozoite surface protein, also localizes to the plasma membrane surrounding the oocyst cytoplasm (Nagasawa et al., 1987; Wang et al., 2005). Confocal microscopy of day 15 oocysts double-labelled with anti-PbCap380 and anti-PbCSP antibodies show that PbCap380 localizes externally to the CS protein, suggesting that it is part of the protective capsule (Fig. 3A). Immuno-electron microscopy confirmed that PbCap380 actually localizes to the capsule (Fig. 3B, upper panel).
Fig. 3.
PbCap380 localizes to the oocyst capsule.
A. Confocal microscopy of a day-15 oocyst. The parasite was double labelled with antibodies against PbCap380 (red) and PbCSP (circumsporozoite surface protein) (green). The lower panel shows a higher magnification of the area demarcated by the rectangle in the merged image. PbCap380 appears to be located externally relative to CSP.
B. Immunoelectron microscopy labelling with PbCap380 antibody. Immunogold labelling localizes PbCap380 to the oocyst capsule (upper panel). We also consistently observed immunogold labelling in regions of the mosquito tissue, which was originally in contact with the oocyst capsule. The lower right panel shows at higher magnification, the region demarcated by the rectangle on the lower left panel. C, capsule; Oo, oocyst; M, mosquito tissue.
PbCap380 labelling could also be detected in regions where the mosquito tissues were originally in contact with the oocyst capsule but probably detached as a result of tissue sectioning (Fig. 3B, lower panels). This is consistent with the idea of parasite capsule proteins interacting with mosquito-derived proteins.
Generation of PbCap380(−) parasites
To gain further insights into PbCap380 function, we disrupted the gene in P. berghei parasites (Fig. 4A). This task was facilitated by the fact that PbCap380 is specifically expressed in the mosquito stages of parasite development, and is thus expected not to be required for asexual growth in the mouse. Independent clonal parasite lines generated from the transfection were confirmed for gene disruption by insertion-specific PCR (Fig. 4B). As expected PbCap380(−) oocysts do not express PbCap380 (Fig. 4C).
Fig. 4.
Targeted disruption of the PbCap380 gene.
A. Schematic representation of the targeting strategy. The wild-type PbCap380 genomic locus (WT) was targeted with an NdeI-linearized plasmid (pCap380) carrying a truncated PbCap380 open reading frame and the TgDHFR-positive selection marker. Upon a single cross-over event, the region of homology is duplicated, resulting in two truncated, disrupted copies of the gene [PbCap380(−)]. The homologous regions in the disruption plasmid are shaded grey. Arrowheads indicate PCR primer pairs used to confirm gene disruption.
B. PCR analysis of the disrupted parasite. Genomic DNA was prepared from drug-resistant parasite clones and PCR was performed using the primer pairs indicated in A. The presence of the 2358 bp integration-specific PCR product (P1/P3) but not the 2063 bp WT locus-specific PCR product (P1/P2) in the PbCap380(−) lanes confirm gene disruption. Control reactions show that the WT-specific primers, but not the integration-specific primers, produce a product with the WT DNA.
C. PbCap380 protein cannot be detected in the knockout parasite. WT and PbCap380(−) oocysts (day 3) were double labelled with P28 and PbCap380 antibodies. Whereas both WT and knockout parasites express P28, only the WT expresses the PbCap380 protein.
D. Morphology of day-15 WT and PbCap380(−) oocysts. WT parasites develop normally and form mature, sporozoite-containing (arrow) oocysts, while PbCap380(−) oocysts die and are eliminated. Very rarely, abnormal oocyst-like structures (arrow head) are observed in some PbCap380(−)-infected mosquitoes.
PbCap380 is not required for oocyst formation but is essential for oocyst development
To study the role of PbCap380 in sexual stage development, PbCap380(−) parasites were fed to mosquitoes. Formation of male and female gametocytes and the subsequent formation of male gametes (exflagellation) were not affected in the knockout parasites (data not shown). The ability of the knockout parasites to form ookinetes in vivo was assessed by smearing the mosquito midgut contents 24 h after an infectious blood meal and determining the number of ookinetes in each midgut. PbCap380(−) parasites were able to form ookinetes in normal numbers (Table 1). However, when analysed 15 days later, neither mature oocysts nor sporozoites could be detected (Table 1). In a few mosquitoes we observed what appeared to be small, disintegrating oocysts (Fig. 4D). In contrast, midgut of mosquitoes that fed on WT parasites carried numerous oocysts that produced sporozoites (Table 1). Not surprisingly, mosquitoes carrying PbCap380(−) parasites were unable to transmit the parasite to other mice (data not shown).
Table 1.
PbCap380(−) parasites differentiate into ookinetes normally but do not form mature oocysts.
| Experiment | Ookinetes/mosquitoa | Oocysts/mosquitob | Sporozoites/mosquitoc |
|---|---|---|---|
| WT | 4915 (333–26900) | 153 (0–520) | 161 000 |
| PbCap380(−) | 3832 (2000–10714) | 0 | 0 |
Mosquitoes were fed an infected blood meal and 24 h later, the midgut contents were smeared and the number of ookinetes per mosquito was determined. Results shown are pooled from two independent experiments with wild-type parasites (10 mosquitoes total) and three independent experiments with PbCap380(−) (15 mosquitoes total).
Oocysts were counted 15 days after mosquitoes ingested an infectious blood meal. Results shown are pooled from four independent experiments with the wild-type parasite (73 mosquitoes) and 10 independent experiments with PbCap380(−) (103 mosquitoes). A mature oocyst was never found in PbCap380(−)-infected mosquitoes.
Sporozoites were prepared from midguts dissected 15 days after mosquitoes ingested an infectious blood meal. Results shown are pooled from two independent experiments with the wild-type parasite (30 mosquitoes) and two independent experiments with PbCap380(−) (30 mosquitoes).
At least three possible explanations exist for the failure to produce mature oocysts: PbCap380(−) ookinetes may not be able to invade the midgut, ookinetes may not be able to differentiate into oocysts after invasion or PbCap380(−) parasites can form oocysts but these are unable to survive in the mosquito. To test these possibilities, we infected mosquitoes with PbCap380(−) parasites and followed their development in the mosquito after staining with a mixture of anti-P28 and anti-PbCSP antibodies. P28 is expressed until days 5–6 after infection while PbCSP begins to be expressed from day 6 (Fig. 2B; P. Srinivasan and M. Jacobs-Lorena, unpub. results). In this way we were able to follow the development of early and late oocysts without using the PbCap380 marker. As shown in Fig. 5A, ookinetes were able to invade the midgut epithelium. This was perhaps not surprising, taking into account that PbCap380 is not expressed in ookinetes (Fig. 1B). While both PbCap380(+) and PbCap380(−) parasites efficiently transform into oocysts, the number of Cap380(−) oocysts declined as development proceeded (Fig. 5A and B). These results suggest that PbCap380 is not required for oocyst formation but that in its absence the developing oocysts are eliminated from the mosquito.
Fig. 5.
PbCap380(−) form oocysts that do not survive in the mosquito.
A. Immunofluorescence of developing oocysts.
B. Quantification. Midguts were dissected at different times after an infectious blood meal and labelled with a mixture of anti-P28 (a major ookinete surface protein) and anti-PbCSP (circumsporozoite surface protein) antibodies. The number of labelled oocysts was determined for each time point. The highest number of parasites among all time points was considered to be 100%. The number of PbCap380(−) oocysts decreased drastically as a function of time, whereas the number of WT oocysts did not change substantially. Results shown are from one of two independent experiments (5–15 midguts per time point). The ‘100% values’ for the two experiments were as follows. Cap380(+): 554 and 389 oocysts/mosquito on day 6 and day 5 respectively; Cap380(−): 236 and 110 oocysts/mosquito on day 2.
The Plasmodium oocyst capsule forms soon after the ookinete-to-oocyst transition and must accompany the dramatic increase in size during the differentiation of the oocyst (Fig. 2A). The newly formed oocyst is ~5 μm and the mature oocyst is ~50 μm in diameter, corresponding to a 1000-fold increase in volume. It is intriguing how this might happen, as there is no evidence for the presence of a capsule lipid bilayer. Simple addition of proteins to the capsule would result in increasing thickness without allowing for increase of cytoplasm volume. Hence, capsule structure must be dynamic, possibly with active turnover of the bonds that link the capsule components and perhaps with turnover of capsule components themselves. Moreover, in addition to allowing for growth, the capsule must have an ordered structure to allow for precursors and nutrients that support parasite growth and differentiation to enter the oocyst and metabolites to exit it (Vanderberg and Rhodin, 1967). To clarify these important issues it will be necessary to define the molecular nature of the remaining capsule components.
As discussed earlier, the capsule might function to protect the parasite. We speculate that capsule proteins such as PbCap380 interact with one another as well as with mosquito proteins. Interactions with mosquito proteins may ‘coat’ the oocyst surface and render the parasite ‘invisible’ to the mosquito immune system, and interference with such interactions would result in killing of the parasite. In support of this hypothesis, oocysts lacking a functional PbCap380 protein do not survive in the mosquito. Disruption of PbCap380 may interfere with capsule assembly resulting in a ‘naked’ oocyst, which would be recognized and destroyed by the mosquito. In principle, structural changes in the capsule could play a role in nutrient acquisition by the parasite. We believe this is less likely as the protein involved in nutrient transport would be expected to be part of the parasite plasma membrane and PbCap380 is clearly not (it localizes to the capsule). Furthermore, the predicted PbCap380 protein does not have any known nutrient transport domains or transmembrane domains as observed in proteins involved in nutrient transport. Another possibility would be that PbCap380 is involved in providing critical structural support for the oocyst. However, we did not see any gross difference in the morphology of PbCap380(−) early oocysts compared with wild type as observed by immunofluorescence.
In conclusion, mosquitoes infected with PbCap380(−) parasites do not produce sporozoites and can not transmit malaria. Elucidating the mechanisms by which defective oocysts are killed is an important outstanding question. As demonstrated here, interference with a single capsule component effectively kills the parasite and makes the Plasmodium oocyst a potential target for disease control.
Experimental procedures
Parasite maintenance and mosquito infections were performed as described (Srinivasan et al., 2004).
PbCap380 gene expression analysis
Total RNA was isolated with Trizol (Molecular Research Center) from mixed blood stage parasites, ookinetes, infected midguts and salivary gland sporozoites. PCR was performed using gene-specific primers PbCap380F: 5′-GAAATCACCATTTAATTTCTCCAATGGGT-3′ and PbCap380R: 5′-TGTAGTTCGA AAAGG ATGGTTTTGATTGT-3′. Plasmodium ribosomal RNA (rRNA) (PbrRNA1: 5′-TGGGAGATTGGTTTTGACGTTTATGT-3′ and PbrRNA2: 5′-AAGCATTAAATAA AGCGAATACATCCTTAC-3′) was used for normalization (Arreaza et al., 1991). The rRNA primers amplify PbrRNA from all parasite developmental stages.
Antibody synthesis and Western blot analysis
A polyclonal antibody was raised against a region of PbCap380 protein corresponding to amino acids 1946–2304 of the P. yoelli orthologue (Fig. S1). For detection of PbCap380 protein by Western blotting, the lysates from 40 midguts from either uninfected or day 15 infected mosquitoes midguts were run on a SDS-PAGE gel and blotted. The protein was detected with the anti-PbCap380 (1:1000) antibody and a horseradish peroxidase-conjugated anti-rabbit secondary antibody (Sigma).
Oocyst immunofluorescence
To detect PbCap380 in oocysts, infected midguts were fixed in 4% paraformaldehyde for 2 h and then permeabilized in PBS containing 0.2% Triton X-100. The midguts were then incubated with the primary antibody (1:1000) and detected using a rhodamine-conjugated anti-rabbit IgG. Pre-immune serum from the same rabbit was used as a control. To count early oocysts, midguts were dissected, the blood meal was carefully removed and the resulting midgut sheets were processed as described above. Oocysts were counted after staining with anti-P28 (same as Pbs21, Tomas et al., 2001) (1:1000) and anti-PbCSP (3D11, 1:1000) monoclonal antibodies and detected with FITC-conjugated anti-mouse secondary antibody (Sigma).
Confocal microscopy
Midguts were dissected 15 days after infection and processed as described above. Antibodies against PbCap380, P28 and PbCSP were used for oocyst double-labelling experiments and were detected using either rhodamine-conjugated or FITC-conjugated anti-rabbit and anti-mouse secondary antibodies.
Immunoelectron microscopy
Infected midguts were fixed for 20 min at 4°C with 1% formaldehyde and 0.2% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4. Fixed samples were washed, dehydrated and embedded in LR White resin (Polysciences). After blocking the thin sections in phosphate-buffered saline containing 5% w/v non-fat dry milk and 0.01% v/v Tween 20 (PBTM), the grids were incubated with anti-PbCap380 primary antibody. Following labelling of the sections with 5 nm gold-conjugated goat anti-rabbit IgG (Amersham Biosciences), samples were stained with uranyl acetate and lead citrate and then examined in a Zeiss CEM902 electron microscope (Oberkochen, Germany).
Generation of PbCap380(−) knockout parasites
For targeted disruption of the PbCap380 locus, a disruption plasmid was generated by amplification of a PCR fragment using primers IntgF (5′-GCATGTAAAGTAATAAAACCATCTACA-3′) and IntgR (5′-AGGTGTAAATAATGATATGAAACCT-3′) and P. berghei genomic DNA as template. Cloning into the P. berghei transfection vector (van Dijk et al., 1995) resulted in plasmid pCap380. The disruption plasmid was linearized at the unique NdeI site, transfected into P. berghei schizonts and disruptants were selected and cloned as described previously (Waters et al., 1997). To confirm disruption of the PbCap380 locus, integration-specific PCR was performed using the following primers, P1: 5′-TGTAGTTCGAAAAGGATGGTTTTGATTGTTT-3′, P2: 5′-ACATACTTGATTTC AGCACCTTATCAGA-3′ and P3: 5′-GAGTTCATTTTACACAATCC-3′. Two independent PbCap380(−) clones were obtained which were phenotypically identical. Results from one representative clone are discussed.
Phenotypic analysis of PbCap380(−) parasites
Development of wild-type (WT) and PbCap380(−) parasites in mice was assessed by injecting a known number of parasites in Swiss Webster mice. The ability of parasites to differentiate into gametocytes and form male gametes (exflagellation) was assessed as described previously (Dearsly et al., 1990). Infected mice were fed to Anopheles stephensi mosquitoes and the ability of the mutant parasites to form ookinetes (24 h) and oocysts (days 2–15) were examined microscopically. For counting ookinetes, individual midguts were dissected 24 h after an infectious blood meal and counted after Giemsa staining. We counted ookinetes/100 RBCs by counting at least 2500 RBCs and assumed that each mosquito ingested 2 μl (Clements, 1992) and that mouse blood has 4 × 109 RBCs ml−1 (Brodsky et al., 1966). Developing oocysts were counted by immunostaining the parasites with anti-P28 and anti-PbCSP antibodies as described above. Mature oocysts were counted on day15 with a light microscope. Sporozoites were isolated from midguts of infected mosquitoes 15 days after infection and counted using a haemocytometer.
Supplementary Material
Fig. S1. Sequence comparison of predicted Cap380 orthologues. Sequence identity between the available sequences of the two rodent malaria parasites is greater than 90%, while identity between rodent and human parasites is 25% or less. However, there is higher sequence identity in the N-terminal half (48% identical) of the predicted proteins compared to the C-terminal half (20% identical). The arrow indicates the predicted signal sequence cleavage site. Region of PbCap380 used for generating antibody is shaded yellow. Amino acid sequence information not available for PbCap380 is shaded grey. This alignment was generated using the ClustalW software. Red: identical amino acid residues in all four Plasmodium species; green: strongly similar; blue: weakly similar. PF, P. Falciparum; PV, P. Vivax; PB, P. Berghei; PY, P. yoelli.
Acknowledgements
We thank Dr Robert Sinden for providing the P28 antibody and Dr Victor Nussenzweig for the PbCSP antibodies as well as allowing us to use P. berghei infected mosquito midgut sections for immunoelectron microscopy. This research was funded by Grant R01 AI031478 from the National Institutes of Health.
Footnotes
The following supplementary material is available for this article online:
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1462-5822.2008.01127.x
References
- Adini A, Warburg A. Interaction of Plasmodium gallinaceum ookinetes and oocysts with extracellular matrix proteins. Parasitology. 1999;119:331–336. doi: 10.1017/s0031182099004874. [DOI] [PubMed] [Google Scholar]
- Adini A, Krugliak M, Ginsburg H, Li L, Lavie L, Warburg A. Transglutaminase in Plasmodium parasites: activity and putative role in oocysts and blood stages. Mol Biochem Parasitol. 2001;117:161–168. doi: 10.1016/s0166-6851(01)00345-0. [DOI] [PubMed] [Google Scholar]
- Aikawa M. Plasmodium: the fine structure of malarial parasites. Exp Parasitol. 1971;30:284–320. doi: 10.1016/0014-4894(71)90094-4. [DOI] [PubMed] [Google Scholar]
- Arreaza G, Corredor V, Zavala F. Plasmodium yoelii: quantification of the exoerythrocytic stages based on the use of ribosomal RNA probes. Exp Parasitol. 1991;72:103–105. doi: 10.1016/0014-4894(91)90127-i. [DOI] [PubMed] [Google Scholar]
- Arrighi RB, Hurd H. The role of Plasmodium berghei ookinete proteins in binding to basal lamina components and transformation into oocysts. Int J Parasitol. 2002;32:91–98. doi: 10.1016/s0020-7519(01)00298-3. [DOI] [PubMed] [Google Scholar]
- Breman JG, Egan A, Keusch GT. The intolerable burden of malaria: a new look at the numbers. Am J Trop Med Hyg. 2001;64:4–7. doi: 10.4269/ajtmh.2001.64.iv. [DOI] [PubMed] [Google Scholar]
- Brodsky I, Dennis LH, Kahn SB, Brady LW. Normal mouse erythropoiesis. I. The role of the spleen in mouse erythropoiesis. Cancer Res. 1966;26:198–201. [PubMed] [Google Scholar]
- Canning EU, Sinden RE. The organization of the ookinete and observations on nuclear division in oocysts of Plasmodium berghei. Parasitology. 1973;67:29–40. doi: 10.1017/s0031182000046266. [DOI] [PubMed] [Google Scholar]
- del Carmen Rodriguez M, Gerold P, Dessens J, Kurtenbach K, Schwartz RT, Sinden RE, et al. Characterisation and expression of P25, a sexual and sporogonic stage specific protein of Plasmodium berghei. Mol Biochem Parasitol. 2000;110:147–159. doi: 10.1016/s0166-6851(00)00265-6. [DOI] [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes: Adult Food and Feeding Mechanisms. Vol. 1. Oxford University press; Oxford: 1992. [Google Scholar]
- Collins FH, Sakai RK, Vernick KD, Paskewitz S, Seeley DC, Miller LH, et al. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science. 1986;234:607–610. doi: 10.1126/science.3532325. [DOI] [PubMed] [Google Scholar]
- Dearsly AL, Sinden RE, Self IA. Sexual development in malarial parasites: gametocyte production, fertility and infectivity to the mosquito vector. Parasitology. 1990;3:359–368. doi: 10.1017/s0031182000078628. [DOI] [PubMed] [Google Scholar]
- van Dijk MR, Waters AP, Janse CJ. Stable transfection of malaria parasite blood stages. Science. 1995;268:1358–1362. doi: 10.1126/science.7761856. [DOI] [PubMed] [Google Scholar]
- Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, Dimopoulos G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Edwards MJ, Jacobs-Lorena M. The journey of the malaria parasite in the mosquito: hopes for the new century. Parasitol Today. 2000;16:196–201. doi: 10.1016/s0169-4758(99)01626-9. [DOI] [PubMed] [Google Scholar]
- Greenwood B, Mutabingwa T. Malaria in 2002. Nature. 2002;415:670–672. doi: 10.1038/415670a. [DOI] [PubMed] [Google Scholar]
- Howells RE, Davies EE. Post-meiotic nuclear divisions in the oocyst of Plasmodium berghei. Trans R Soc Trop Med Hyg. 1971a;65:421. doi: 10.1016/0035-9203(71)90119-2. [DOI] [PubMed] [Google Scholar]
- Howells RE, Davies EE. Nuclear division in the oocyst of Plasmodium berghei. Ann Trop Med Parasitol. 1971b;65:451–459. doi: 10.1080/00034983.1971.11686777. [DOI] [PubMed] [Google Scholar]
- Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301:1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Procell PM, Atkinson CT, Campbell GH, Collins WE, Aikawa M. Localization of circumsporozoite protein of Plasmodium ovale in midgut oocysts. Infect Immun. 1987;55:2928–2932. doi: 10.1128/iai.55.12.2928-2932.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle G. A quantitative study of naturally-acquired malaria infections in Anopheles gambiae and Anopheles funestus in a highly malarious area of East Africa. Trans R Soc Trop Med Hyg. 1966;60:626–632. doi: 10.1016/0035-9203(66)90009-5. [DOI] [PubMed] [Google Scholar]
- Saxena AK, Singh K, Su H, Klein MM, Stowers AW, Saul AJ, et al. The essential mosquito-stage P25 and P28 proteins from Plasmodium form tile-like triangular prisms. Nat Strucl Mol Biol. 2006;13:90–91. doi: 10.1038/nsmb1024. [DOI] [PubMed] [Google Scholar]
- Siden-Kiamos I, Vlachou D, Margos G, Beetsma A, Waters AP, Sinden RE, Louis C. Distinct roles for pbs21 and P25 in the in vitro ookinete to oocyst transformation of Plasmodium berghei. J Cell Sci. 2000;113:3419–3426. doi: 10.1242/jcs.113.19.3419. [DOI] [PubMed] [Google Scholar]
- Srinivasan P, Abraham EG, Ghosh AK, Valenzuela J, Ribeiro JM, Dimopoulos G, et al. Analysis of the Plasmodium and Anopheles transcriptomes during oocyst differentiation. J Biol Chem. 2004;279:5581–5587. doi: 10.1074/jbc.M307587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas AM, Margos G, Dimopoulos G, van Lin LH, de Koning-Ward TF, Sinha R, et al. P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. EMBO J. 2001;20:3975–3983. doi: 10.1093/emboj/20.15.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderberg J, Rhodin J. Differentiation of nuclear and cytoplasmic fine structure during sporogonic development of Plasmodium berghei. J Cell Biol. 1967;32:C7–C10. doi: 10.1083/jcb.32.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernick KD, Fujioka H, Seeley DC, Tandler B, Aikawa M, Miller LH. Plasmodium gallinaceum: a refractory mechanism of ookinete killing in the mosquito, Anopheles gambiae. Exp Parasitol. 1995;80:583–595. doi: 10.1006/expr.1995.1074. [DOI] [PubMed] [Google Scholar]
- Vlachou D, Lycett G, Siden-Kiamos I, Blass C, Sinden RE, Louis C. Anopheles gambiae laminin interacts with the P25 surface protein of Plasmodium berghei ookinetes. Mol Biochem Parasitol. 2001;112:229–237. doi: 10.1016/s0166-6851(00)00371-6. [DOI] [PubMed] [Google Scholar]
- Wang Q, Fujioka H, Nussenzweig V. Mutational analysis of the GPI-anchor addition sequence from the circumsporozoite protein of Plasmodium. Cell Microbiol. 2005;7:1616–1626. doi: 10.1111/j.1462-5822.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- Waters AP, Thomas AW, van Dijk WR, Janse CJ. Transfection of malaria parasites. Methods. 1997;13:134–147. doi: 10.1006/meth.1997.0506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Sequence comparison of predicted Cap380 orthologues. Sequence identity between the available sequences of the two rodent malaria parasites is greater than 90%, while identity between rodent and human parasites is 25% or less. However, there is higher sequence identity in the N-terminal half (48% identical) of the predicted proteins compared to the C-terminal half (20% identical). The arrow indicates the predicted signal sequence cleavage site. Region of PbCap380 used for generating antibody is shaded yellow. Amino acid sequence information not available for PbCap380 is shaded grey. This alignment was generated using the ClustalW software. Red: identical amino acid residues in all four Plasmodium species; green: strongly similar; blue: weakly similar. PF, P. Falciparum; PV, P. Vivax; PB, P. Berghei; PY, P. yoelli.