Abstract
Corticotropin-releasing (CRF) factor plays a central role in the orchestration of behavioral and neuroendocrine responses to stress. The family of CRF-related peptides (CRF and paralogs: Urocortin (Ucn) -I,-II and -III) and associated receptors (CRF-R1 and CRF-R2) are also expressed in peripheral tissues such as the skin and gastrointestinal tract (GIT). Local signaling may exert multiple effects of stress-induced exacerbation of many complex syndromes including psoriasis and visceral hypersensitivity. Interstitial cystitis/painful bladder syndrome (IC/PBS), a chronic visceral pain syndrome characterized by urinary frequency, urgency and pelvic pain, is reported to be exacerbated by stress. Functional changes in the epithelial lining of the bladder, a vital blood-urine barrier called the urothelium, may play a role in IC/PBS. This study investigated the expression and functional activity of CRF-related peptides in the urothelium of normal cats and cats with feline interstitial cystitis (FIC), a chronic idiopathic cystitis exhibiting similarities to humans diagnosed with IC/PBS. Western blots showed urothelial (UT) expression of CRF-R1 and CRF-R2. Enzyme immunoassay revealed release of endogenous ligands (CRF and Ucn) by UT cells in culture. Evidence of functional activation of CRF-R1 and CRF-R2 by receptor selective agonists (CRF and UCN3 respectively) was shown by: (1)-measurement of ATP release using the luciferin-luciferase assay and (2)-the use of membrane impermeant fluorescent dyes (FM dyes) for fluorescence microscopy to assess membrane exocytotic responses in real-time. Our findings show evidence of CRF-related peptide signaling in the urothelium. Differences in functional responses between FIC and normal UT indicate that this system is altered in IC/PBS.
Keywords: Urothelium, CRF-signaling, Stress, Interstitial Cystitis
INTRODUCTION
Corticotropin-releasing factor (CRF), a 41-amino acid hypothalamic neuropeptide (Vale, et al. 1981) plays a central role in the orchestration of behavioral and neuroendocrine responses to stress (Stengel and Tache 2010). This complex process of stress adaptation is fine-tuned by several related peptides, called urocortins (UCNs; Ucn I, II, III) which exhibit various degrees of amino acid sequence homology to CRF. CRF and UCNs exert their actions in target cells via activation of the G protein-coupled receptors, CRF-R1 and CRF-R2, which are encoded by two separate genes (Grammatopoulos 2012). CRF-R1 and CRF-R2 have distinct pharmacological properties and agonist selectivity; CRF exhibits high affinity for CRF-R1 (with a ten-fold lower affinity for CRF-R2) (Jappelli, et al. 2014) and Ucn III preferentially binds and activates CRF-R2 (Lewis, et al. 2001).
In addition to their distribution in the central nervous system, CRF-related peptides and their receptors are widely expressed in peripheral tissues, including the skin, GIT, pancreas and adrenal glands where they are proposed to play a role in tissue homeostasis (Larauche, et al. 2009; Li, et al. 2007; Slominski, et al. 2001; Squillacioti, et al. 2011; Tsatsanis, et al. 2007). It is now well-established that CRF-related peptide receptors can activate a plethora of signal-transduction pathways, including PKA, PKC, PKB/Akt, ERK and p38 MAPK (Grammatopoulos 2012), which enables them to mediate diverse and sometimes opposing functions. For example, in human hepatocytes CRF-R1 and CRF-R2 mediate opposing pro- and anti-proliferative actions respectively (Paschos, et al. 2010). In the GIT, CRF-R1 drives gastric contractility while CRF-R2-activation down-regulates the activity (Nozu, et al. 2013).
In both humans and animals, physical and emotional stress appears to play a role in visceral dysfunction such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), overactive bladder (OAB) and interstitial cystitis/painful bladder syndrome (IC/PBS) (Klausner and Steers 2004; Lutgendorf, et al. 2001; Mayer 2010; Robbins and Ness 2008; Smith, et al. 2011; Stella, et al. 2011; Stengel and Tache 2010). There is now convincing evidence that local CRF-related peptide signaling within the intestinal tissues plays an important role in both the onset and exacerbation of IBS and IBD, involving changes in the permeability of the intestinal epithelial lining, impacting the barrier function (Chatzaki, et al. 2013; Larauche et al. 2009).
Although the etiology and pathogenesis of IC/PBS are incompletely understood, patients have an abnormal, leaky bladder epithelial lining, proposed to be a key event that initiates the cascade of nerve up-regulation, muscle reactions and tissue injury in IC/PBS (Parsons 2011). Very little is known as to whether there is a functioning CRF-related peptide signaling system in the urinary bladder and its potential role in the well-described correlation between stress and symptom exacerbation in bladder diseases, such as IC/PBS. IC/PBS is characterized by urinary frequency, urgency, and pelvic pain (Hanno et al.,2010) and patients are reported to display symptom exacerbation and a heightened sensitivity to pain after exposure to stressful situations (Rothrock, et al. 2001).
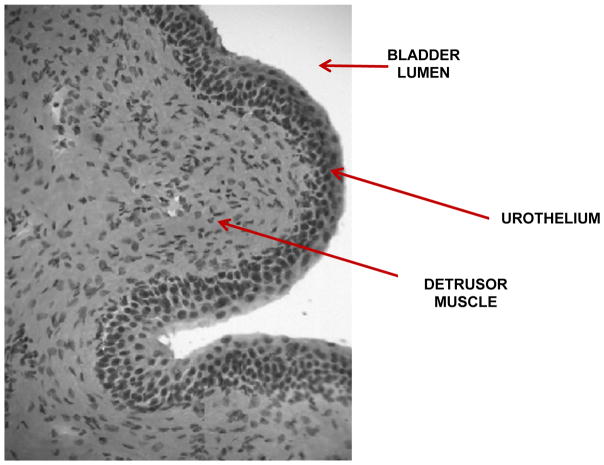
The mammalian urinary bladder is composed of the urothelium (a specialized stratified epithelial lining), and an outer layer of smooth muscle, called the detrusor muscle (Fig 1). It is innervated by sensory afferents, and efferent/motor nerves of the somatic and autonomic nervous system (Yoshimura and de Groat 1997). The urothelium is a vital blood-urine barrier, deficiency of which would allow the passage of water, urea and toxic substances into the underlying bladder tissue, affecting neural and/or muscle layers, resulting in symptoms of urgency and frequency as seen in IC/PBS (Birder and de Groat 2007; Lavelle, et al. 2000). The urothelium expresses a wide range of receptors and signaling molecules such as nitric oxide, ATP (Birder 2010) and acetylcholine (ACh)(Hanna-Mitchell, et al. 2007; Yoshida, et al. 2008), suggesting the potential for chemical dialogue with underlying sensory nerve endings (Birder 2010; Burnstock 2001; Vlaskovska, et al. 2001). Changes in this chemical dialogue could precipitate nociceptive signaling from the bladder and also play a role in IC/PBS.
Fig. 1. Hematoxylin and eosin (H&E)-stained cross-section of the feline urinary bladder.
Red arrows indicate (1): the urothelium which faces (2) the bladder lumen and the (3) underlying detrusor muscle.
We undertook this study to investigate for bladder urothelial (UT) CRF-R1 and CRF-R2 receptor expression and functional activity, as well as UT release of endogenous ligands, which might shed some light on potential mechanistic pathways linking stress to symptoms of IC/PBS. We chose to use tissue isolated from the bladders of normal healthy domestic cats and cats diagnosed with feline interstitial cystitis (FIC), a clinically recognized naturally-occurring animal model of IC/PBS (Buffington 2011; Malykhina and Hanno 2014), in order to gain a potential insight into alterations in this system under conditions of pathophysiology. We chose the following methodological approaches to assess for evidence of functional responses in UT cells to CRF-receptor-selective pharmacological ligands: 1) measurement of UT cell ATP release using the luciferin-luciferase assay (Schwiebert and Zsembery 2003) and, 2) measurement of UT cell exocytotic/secretory responses in real time (by microscopy), using the membrane-impermeant fluorescent dye, FM1-43 (Gaffield and Betz 2006). In addition, molecular biological techniques were used to assess for: 1) endogenous release on CRF-related peptides by UT cells (in vitro; Elisa), and 2): mucosal expression of CRF-R1 and -R2 (western blot).
MATERIALS AND METHODS
Animals
All procedures were conducted in accordance with Ohio State University and University of Pittsburgh Institutional Animal Care and Use Committee policies. Adult male and female domestic cats (Felis catus) were used for this study. The diagnosis of FIC was based on compatible history and consideration of standard National Institutes of Health inclusion and exclusion criteria. Healthy, age-matched cats obtained from commercial vendors which were determined to be free of disease and signs referable to the lower urinary tract according to the same diagnostic criteria as cats with FIC, were used as controls. The animals were housed in stainless steel cages and allowed to acclimatize to their environment for at least 3 months before the study (Buffington, et al. 1999).
Primary Cell Culture
Urinary bladders were removed under anesthesia, (isoflurane; 4%), after which the animals were euthanized. Excised bladders were cut open, gently stretched and pinned on a Sylgard-coated plate, with the mucosal side up. Following overnight incubation in minimum essential medium (MEM; Invitrogen, Life Technologies, NY, USA) containing 2.5 mg/ml dispase, the urothelium was gently scraped from underlying tissue, treated briefly with 0.25% trypsin, and triturated. Dissociated urothelial cells were plated (30–40,000 cells) onto either six-well collagen-coated dishes (Enzyme immunoassay) or onto collagen-coated glass cover slips (functional experiments). Cells were grown in protein-free CnT-16 culture medium (CELLnTEC, Zenbio, NC, USA) in an incubator with an atmosphere of 5% CO2 at 37°C.
Molecular Physiology
Western Blotting
Bladder mucosa was carefully dissected away from the underlying smooth muscle and homogenized in Hanks’ Balanced Salt Solution (HBSS: 5 mM KCl, 0.3 mM KH2PO4, 138 mM NaCl, 4 mM NaHCO3, 0.3 mM Na2HPO4, 5.6 mM glucose, and 10 mM Hepes, pH 7.4), containing complete protease inhibitor cocktail (1 tablet/10 ml; Roche, IN, USA) and phosphatase inhibitor cocktail (1:100; Sigma-Aldrich, St. Louis, USA). The homogenate was centrifuged (13,000 g; 15 min). Whole cell protein lysates were obtained and proteins were analyzed for relative expression levels using a standard immuno-blotting protocol as follows. Proteins were separated on a 10–20% PageR gel (Lonza, Walkersville, USA) and subsequently transferred to polyvinylidene fluoride membranes (Bio-Rad, Hercules, USA). The membranes were blocked with 5% milk in Tris-Buffered Saline Tween-20 (TBS-T) for 1 hour. After a brief rinse in TBS-T, the membranes were incubated 48–72 hr at 4°C with goat anti-CRF-R1 antibody (1:1000, C-20, Santa Cruz, Dallas, USA) and rabbit anti-CRF-R2 (1:2000, Abcam, Cambridge, USA) with and without pre-incubation with the respective blocking peptide as control. Santa Cruz anti-CRF-R1 antibody (C-20) was developed against the C-terminus of CRF-R1 of human origin and produces a single immunoreactive band at 66kDa which is blocked by the peptide. The validity of this antibody has been tested in HEK-293 and CHO-1 cells transfected with full length cDNA encoding rat CRF1 or CRF2B and in a rat GH4 cell line that endogenously expresses CRF1 by Western blot and immunohistochemistry (Dr. Y. Tache, personal communication). C-20 preferentially reacted with CRF-R1, with a very weak cross reaction with CRF-R2. Abcam CRF-R2 antibody was developed against the N-terminus of CRF-R2a/b (synthetic peptide). Peptide experiments showed a single immunoreactive band at 48kDa in addition to a non-specific band at 70kDa which is not blocked by the peptide. Following extensive washing, the membranes were incubated in secondary antibody (rabbit anti-goat Horseradish Peroxidase (HRP), Everest, Ramona, USA and goat anti-rabbit poly HRP, Pierce, Rockford, USA respectively) for 1hr in TBS-T and subsequently washed. The membranes were developed with Enhanced Chemiluminescence Plus (ECL Plus; GE Healthcare, USA) and exposed to film. The membranes were stripped (membrane recycling kit from Alpha Diagnostic International, San Antonio, USA) and reprobed with anti β-actin (Abcam) in 5% milk TBS-T as a loading control. Receptor expression was quantified by densitometry (Personal Densitometer SI; Molecular Probes) normalized by β-actin.
Enzyme Immunoassay
Primary cultured urothelial cells (UT) isolated from normal and FIC cat bladders (n=3 per group) were assessed for evidence of endogenous release of stress-related peptides CRF and Ucn. The plated cells were allowed to stabilize and grow for two days in vitro (2DIV) prior to commencement. The culture medium was carefully removed from the culture wells, replaced with sterile filtered HBSS and the dishes replaced in the incubator. At 30 minutes the medium was removed to investigate the presence of CRF and urocortin. Enzyme Immunoassay (EIA) kits from Phoenix Pharmaceuticals (Burlingame, CA; EK-019-06 and EK-019-15, detect CRF and non-selectively Ucn I, II and III, respectively) following the manufacturer’s instructions. The linear detection range of both kits was 0.1–4ng/ml. CRF Immunoassay has been validated against prepro-CRF (125–151), PACAP-38, LH-RH, ACTH, [Arg8] Vasopressin, and BNP45. UCN Immunoassay is 100% cross-reactive with UCN I, II, and III and has been validated against cortistatin-14, CRF, MCH, LH-RH, NPY, and somatostatin. Prior to assay, peptides were extracted from the cell culture supernatant using C-18 SEP Columns (Phoenix Pharmaceuticals, Burlingame, USA) following the manufacturer’s instructions.
Functional Studies
ATP Release
Normal and FIC UT cells, grown on collagen-coated cover slips (2–3 DIV), were transferred into a perfusion chamber and superfused with an oxygenated physiological saline solution (flow rate=0.5ml. min−1) until a stable baseline level of ATP release was measured (Control Phase). The perfusate was modified by the addition of the chemical agents (Test Phase). Perfusate was collected (100μl) in the Control and Test phases at 30–60s intervals. The agents used were: CRF (CRF-R1 agonist; 0.01μM; Tocris, Minneapolis, USA) and UCN3 (CRF-R2 receptor agonist; 0.1μM; Sigma-Aldrich, St. Louis, USA). CRF-R1 and CRF-R2 antagonists used were: Antalaramin (0.1μM) and Astressin-2B (Ast2B; 1.0μM), respectively (Tocris, Minneapolis, USA). ATP levels were quantified using a luciferin-luciferase reagent (Adenosine Triphosphate Assay Kit; Sigma-Aldrich, St. Louis, USA) and ATP concentrations were extrapolated from a standard-curve (ATP assay, Sigma-Aldrich, St. Louis, USA). Data are expressed as mean percentage change in ligand-evoked ATP release with respect to basal; taking basal as 100%.
Fluorescence Microscopy (live-cell imaging)
The fluorescent membrane-impermeant dye, FM1-43 (Molecular Probes, Life Technologies, NY, USA) was used as an “activity marker” in order to track membrane exocytosis from the cytoplasm to the urothelial cell plasma membrane in response CRF receptor-selective pharmacological ligands. FM dyes are non-toxic water-soluble, styryl pyridinium dyes, which fluoresce in a lipid environment but not an aqueous one. They reversibly stain but do not penetrate the plasma membranes of cells and have been used to study vesicle movement in a number of cell types (Gaffield and Betz 2006). When cells are incubated with FM dye, the dye partitions into the outer leaflet of the plasma membrane and the cells become visible when excited at the appropriate excitation wavelength (465nm) (Gaffield and Betz 2006). Membrane exocytosis is detected by an overall increase in the measured fluorescence intensity with dye in the bath. This is due to the addition of untagged (naïve) membrane from newly exocytosed vesicles (i.e. the FM dye in the bath now has more membrane to tag and the fluorescence intensity of the cell membrane increases). The dye can be internalized by endocytosis, evidenced by the presence of fluorescent punctae in the cytoplasm.
Coverslips of normal and FIC UT cells at 2–3 DIV were placed into a flow chamber specifically designed to fit the stage of an inverted epifluorescence microscope (Olympus IX70) equipped with a 40X oil immersion lens and connected to a Leica DC 200 digital camera (Leica Heerbrugg, Switzerland). All solutions were added to the dish via a gravity-fed perfusion system (flow rate: 1.5 ml/min). Following perfusion with HBSS alone for 10 minutes to allow for equilibration, the perfusate was changed to FM1-43 (5μM) in HBSS for 10 minutes, to allow the dye to partition into the plasma membranes of the cells. Images were taken of the cells at 30 second intervals during the partition-equilibration phase, and then at 10 second intervals upon change of the perfusate to one containing both FM1-43 and the chemical agents. The agonists were: CRF (CRF-R1 selective; 0.01μM) and UCN3 (CRF-R2 selective; 0.1μM). An average of 3 coverslips per culture (n=3) per group, were used for each experimental protocol. Post-analysis data (corrected for background fluorescence) were quantified as mean intensity (arbitrary units; AU) per experimental phase using Simple PCI Imaging software (Hamamatsu, Sewickley, USA). Results are expressed as percentage change in fluorescence following addition of agonist, relative to basal intensity-a measurement of exocytotic activity.
Data Analysis
Data are expressed as mean ± SEM and analyzed using Student’s unpaired t-test; statistical significance was accepted when p≤0.05
RESULTS
UT cells express CRF-R1 and CRF-R2
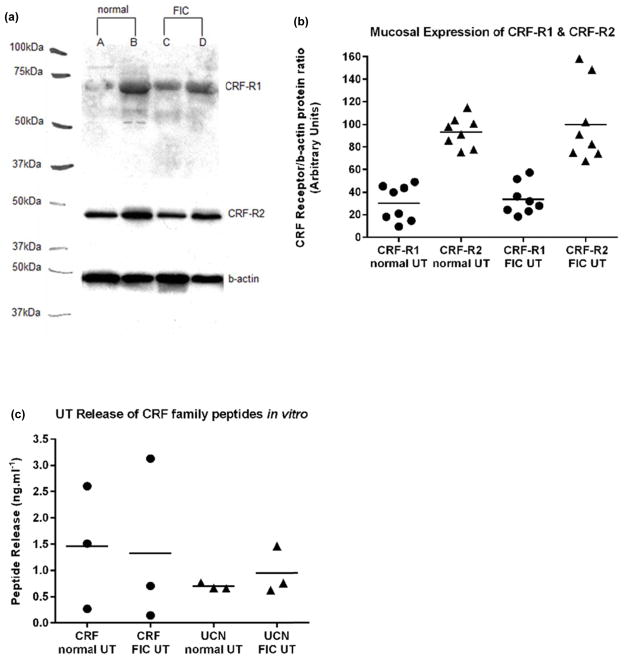
Normal and FIC mucosa expresses positive immuno-reactive bands for both CRF-R1 (66kDa) and CRF-R2 (48 kDa) (Fig. 2a); this was eliminated in the presence of respective blocking peptide (data not shown). In both normal and FIC tissue, CRF-R2 protein expression was significantly higher than CRF-R1 (3X); p<0.005; paired t-test, n=8 (Fig. 2b).
Fig. 2. Mucosal expression of CRF receptors and in vitro UT release of endogenous ligands.
(a): Representative immunoblot of CRF-R1 and CRF-R2 in normal and FIC cat mucosa lysates. When pre-incubated with competing peptide, the CRF-R1 immunoreactive band (66kDa) was greatly reduced and the CRF-R2 immunoreactive band (48kDa) was completely blocked. (b): In both normal and FIC mucosae, CRF-R2 (closed triangles) protein expression is significantly higher than CRF-R1 (closed circles; p<0.005; n=8). Mean expression is indicated by horizontal bars. (c): normal and FIC UT cells exhibited (1): endogenous release of CRF into the bathing medium of a similar magnitude (closed circles; p>0.5; n=3); (2): While endogenous release of UCN (non-selective Ucn I. II, II) appeared greater, no significant difference (closed triangles; p>0.05; n=3) was found between the two groups. Mean release is indicated by horizontal bars.
Urothelial expression of endogenous ligands
Normal and FIC UT cells cultured for 2 days exhibited endogenous release of CRF and UCN (non-selective Ucn I. II, II) into the bathing medium of a similar magnitude (p>0.05; n=3) (Fig 2c).
CRF-R1- and CRF-R2-evoked ATP release
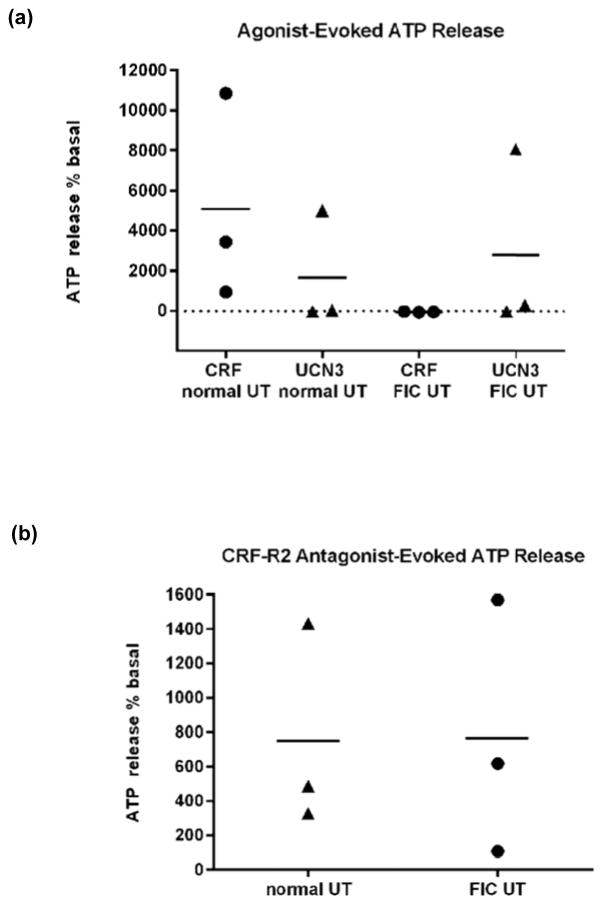
We examined for functional evidence of the presence of CRF-R1 and CRF-R2 in cultured UT cells by assessing receptor selective, agonist-evoked release of ATP, a major UT cell-signaling molecule. ATP release in response to CRF (CRF-R1 agonist; 0.01μM) and UCN3 (CRF-R2 agonist; 0.1μM) occurred in both normal and FIC UT. In normal UT, CRF induced a larger though not statistically significant, ATP-release compared with UCN3 (Fig. 3a; P>0.05; n=3). By contrast, in FIC UT, UCN3 induced a larger (though not statistically significant) ATP-release compared with CRF, which in turn inhibited basal/constitutive ATP release (Fig 3a; p>0.05, n=3). The release of ATP occurred with a consistent time lag of 3–5 minutes following addition of agent to bath in all experiments. Selectively of CRF for CRF-R1 was confirmed using the selective CRF-R1 antagonist Antalaramin (0.1μM), which significantly inhibited (p<0.05, n=3) CRF-evoked ATP release in normal UT (data not shown). Astressin-2B/Ast2B (CRF-R2 antagonist; 1.0μM) alone evoked ATP release in both normal and FIC UT, which did not differ significantly (Fig. 3b; P>0.05; n=3).
Fig. 3. Selective CRF-R1- and CRF-R2- agonist evoked ATP release by UT cells in vitro.
(a) In normal UT, CRF-R1 activation (CRF; 0.01μM; closed circles) evoked a larger ATP release (albeit not significant) compared with CRF-R2 (UCN3; 0.1μM; closed triangles) evoked release (p>0.05; n=3). By contrast, in FIC UT, CRF-R2 activation evoked a larger ATP release relative to CRF-R1 activation (albeit not significant; p>0.05; n=3). (b): Astressin-2B/Ast2B (CRF-R2 antagonist) alone, evoked ATP release in both normal and FIC UT, of a similar magnitude (p>0.05; n=3). Mean release is indicated by horizontal bars.
CRF-R1 and CRF-R2 agonists evoke UT membrane traffic (vesicular movement)
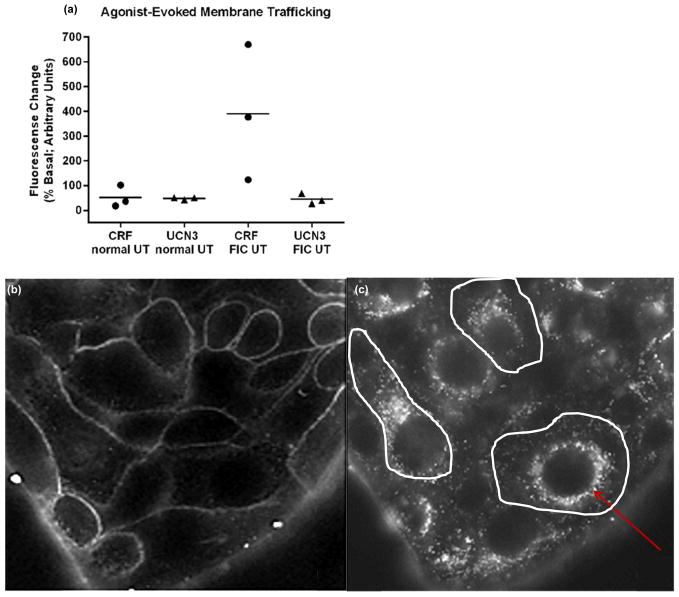
In normal UT both CRF and UCN3 evoked membrane exocytosis of a similar magnitude (Fig. 4a; p>0.05; n=3). By contrast, in FIC UT, CRF-evoked membrane exocytosis was significantly larger compared with responses to UCN3 (Fig. 4a; p<0.05; n=3). All membrane exocytotic responses were typically slow, indicative of GPCR (metabotropic) type response (Saini and Gautam 2010), with a characteristic temporal signature of a 3–5 minute time lag between entry of agent to the bath and onset of membrane trafficking, evidenced by an increase in fluorescence intensity (exocytosis) and the appearance of fluorescent punctae in the cytoplasm (endocytosis). Characteristic image stills (shown from FIC UT) from real-time experiments are depicted in Fig. 4b–c.
Fig. 4. Selective CRF-R1- and CRF-R2- agonist evoked UT membrane trafficking in vitro.
(a): In normal UT, CRF-R1 and CRF-R2 activation (CRF 0.01μM; closed circles and UCN3 0.1μM; closed triangles, respectively) evoked membrane exocytosis of a similar magnitude (p>0.05; n=3). By contrast in FIC UT, membrane exocytosis in response to CRF-R1 activation was significantly greater than that evoked by CRF-R2 activation (p<0.05; n=3). Mean release is indicated by horizontal bars.
(b–c): Characteristic image stills from time-lapse experiments using FM1-43 (5μM). In b: the cells are bathed in HBSS+FM-1-43(5μM) and unstimulated; in c: the UT cells exhibited a large membrane exocytosis in response to CRF-R1 receptor activation (CRF 0.01μM) as shown by the large number of fluorescent punctae in the cytoplasm. Following washout of dye from the bath, the plasma membrane is no longer visible-white lines delineate its presence. Endocytosed dye-containing vesicles gather around the nuclei in almost all cells, shown by the red arrow.
DISCUSSION
While peripheral CRF-related peptide signaling is reported to contribute to pathological symptoms in tissues such as the GIT (Teitelbaum, et al. 2008), esophagus (Cho, et al. 2011) and skin (Slominski 2007), very little is known about this system in the bladder and whether it plays a role in stress-related exacerbation of lower urinary tract disorders such as IC/PBS (Rothrock et al. 2001). Studies using animal models of chemically-induced cystitis report the presence and upregulation of CRF and CRF-R2, but no evidence of CRF-R1 in adult rat bladder (LaBerge, et al. 2006). In contrast, CRF-R1 presence and upregulation is reported in the mouse bladder (Saban, et al. 2002). These studies focused on gene expression in whole bladder and not specifically within the urothelium. The differential findings may be due to experimental approach and/or species differences.
Although the etiology and pathogenesis of IC/PBS are incompletely understood, deficiency in the epithelial lining of the bladder is proposed to be a causative factor (Hanno, et al. 2011; LaBerge et al. 2006). Due to the strong evidence of CRF-related peptide signaling locally impacting epithelial cell function (e.g., GIT, skin) we focused on investigation for the expression and functional activity of this system in the bladder urothelium and whether there was evidence of change in IC/PBS. As FIC is a naturally occurring animal model of interstitial cystitis and an important translational model in the study of IC/PBS (Buffington et al. 1999; Stella et al. 2011), we chose to examine this in feline bladder urothelium. Evidence of CRF-R1 and -R2 presence was investigated directly by assessing protein expression in isolated bladder mucosa (western blot). As the urothelium releases a wide array of signaling molecules, exocytotic membrane movement in response to CRF-R1 and –R2 selective agents was chosen as an indirect indicator of CRF receptor presence and activity. In addition, as ATP is well-established to be a urothelial signaling molecule, released in response to both mechanical and chemical stimuli with changes in pathology (Birder 2010), ATP release in response to these agents was also measured.
To our knowledge, this is the first study to report a functioning CRF-related peptide signaling system involving both CRF-R1/CRF-R2 receptors in the epithelial lining of the mammalian urinary bladder. Here we report our findings of mucosal expression of both CRF-R1 and CRF-R2 receptors, which are functionally responsive to selective ligands in in vitro studies using primary cultures of UT cells. We find that functional activity of these receptors is altered by pathology, in contrast to relative protein expression, which remained unchanged. In addition, we report the release of endogenous ligands, CRF and Ucn, by UT cells.
Our finding that CRF-R2 antagonism (Ast2B, in the absence of exogenous (bath) agonist) evoked ATP release in both normal and FIC UT cells (Fig. 3b), suggests an endogenous, possibly constitutive activation of CRF-R2 receptors to allow persistent dampening of constitutive (basal) ATP release by the urothelium. Our finding that UT cells release the endogenous ligands, CRF and Ucn is supportive of this theory. A similar counter-regulatory input mediated by CRF-R2 on CRF-R1 has been reported in the amygdala (Fu and Neugebauer 2008). Overstimulation by increased levels of local or systemic (serum) CRF may account for the observed hyposensitivity of CRF-R1-ATP pathway in FIC UT compared with normal UT (Fig. 3a).
The use of FM dyes for real-time imaging of membrane trafficking responses in bladder urothelial cells, as reported in this study, is novel. Existing information on the roles and dynamics of membrane-bound cytoplasmic vesicles in UT cell physiology is from (real-time) capacitance studies and microscopical analysis of fixed tissue and cells (electron and light microscopy) (Khandelwal, et al. 2009; Wang, et al. 2005). Our real-time experiments revealed not only that membrane exocytosis was evoked by both CRF-R1 and CRF-R2 selective ligands indicating functional activity of both CRF receptor types in UT cells but also that ATP release under these circumstances may include a non-vesicular component, as membrane exocytosis did not always correlate with ATP release. For example, CRF caused a much larger membrane exocytotic event in FIC UT compared with normal UT (Fig. 4a), while in contrast, CRF-evoked ATP release was larger in normal UT (Fig. 3a). One possible non-vesicular route of exit for ATP is via the membrane channel pannexin1 (Panx1). Panx1 has recently emerged as a candidate ATP release channel (Sandilos and Bayliss 2012) and GPCR-mediated activation of Panx1 has been reported (Billaud, et al. 2011). Panx1 is expressed by bladder urothelium and reported to play a role in bladder dysfunction (Negoro, et al. 2013; Timoteo, et al. 2014).
Conclusion
CRF-related peptides and receptors are phylogenetically ancient and well-preserved across species; they exert a wide spectrum of actions in the central nervous system (CNS) and the periphery that underpin their critical role in integrating and coordinating the activity of diverse physiological systems (Hillhouse and Grammatopoulos 2006). The wide range of activity of this group of peptides is reflected in the complexity of their physiology and pathophysiology. The pattern of G-protein activation by CRF-Rs appears to be unique for each tissue and controlled by as yet undefined mechanism (Hillhouse and Grammatopoulos 2006). This study is an initial investigation into the presence of CRF-related peptide signaling in the epithelial lining of the urinary bladder, which in turn is known to play an important role in bladder function and dysfunction. Our findings show that both receptor types and in addition, their endogenous ligands, CRF and Ucn, are expressed by the urothelium and show differences in function in health and disease. Differential functional responses of CRF-R1 and CRF-R2 to pharmacological ligands both in normal and FIC UT indicate a similar level of complexity reported in other tissues. Further studies will explore signal transduction pathways involved in CRF receptor signaling in order to gain a better understanding of the physiological role for CRF-related peptides within the bladder mucosa and whether they play a role in bladder pathophysiology. IC/PBS affects both women and men (~ 1.5:1) (Berry, et al. 2011; Suskind, et al. 2013); the associated symptoms seriously interfere with daily work and activities with a devastating impact on quality of life. The aim is to uncover novel therapeutic strategies for the treatment of this debilitating disease.
Acknowledgments
FUNDING
This work was supported by National Institutes of Health Grants: K01DK080184 (A. Hanna-Mitchell); R37DK54824 & R01DK57284 (L. Birder); P50DK64539 (L. Birder and C.A.T. Buffington).
Footnotes
DECLARATION OF INTEREST:
The Authors have no conflict of interest to declare.
References
- Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. The Journal of Urology. 2011;186:540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, Best AK, Chekeni FB, Ravichandran KS, Penuela S, et al. Pannexin1 regulates alpha1-adrenergic receptor- mediated vasoconstriction. Circulation Research. 2011;109:80–85. doi: 10.1161/CIRCRESAHA.110.237594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA. Urothelial signaling. Autonomic Neuroscience: Basic & Clinical. 2010;153:33–40. doi: 10.1016/j.autneu.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nature Clinical Practice Urology. 2007;4:46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington CA. Idiopathic cystitis in domestic cats--beyond the lower urinary tract. Journal of Veterinary Internal Medicine/American College of Veterinary Internal Medicine. 2011;25:784–796. doi: 10.1111/j.1939-1676.2011.0732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington CAT, Chew DJ, Woodworth BE. Feline Interstitial Cystitis. Journal of the American Veterinary Medical Association. 1999;215:682–687. [PubMed] [Google Scholar]
- Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends in Pharmacol Sciences. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- Chatzaki E, Anton PA, Million M, Lambropoulou M, Constantinidis T, Kolios G, Tache Y, Grigoriadis DE. Corticotropin-releasing factor receptor subtype 2 in human colonic mucosa: down-regulation in ulcerative colitis. World Journal of Gastroenterology: WJG. 2013;19:1416–1423. doi: 10.3748/wjg.v19.i9.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YJ, Kim JH, Yim HE, Lee dM, Im SK, Lee KJ. Role of corticotrophin-releasing factor in the stress-induced dilation of esophageal intercellular spaces. Journal of Korean Medical Science. 2011;26:279–283. doi: 10.3346/jkms.2011.26.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Neugebauer V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. The Journal of Neuroscience: the official Journal of the Society for Neuroscience. 2008;28:3861–3876. doi: 10.1523/JNEUROSCI.0227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffield MA, Betz WJ. Imaging synaptic vesicle exocytosis and endocytosis with FM dyes. Nature Protocols. 2006;1:2916–2921. doi: 10.1038/nprot.2006.476. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK. Insights into mechanisms of corticotropin-releasing hormone receptor signal transduction. British Journal of Pharmacology. 2012;166:85–97. doi: 10.1111/j.1476-5381.2011.01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna-Mitchell AT, Beckel JM, Barbadora S, Kanai AJ, de Groat WC, Birder LA. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sciences. 2007;80:2298–2302. doi: 10.1016/j.lfs.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanno P, Lin A, Nordling J, Nyberg L, van Ophoven A, Ueda T, Wein A. Bladder Pain Syndrome Committee of the International Consultation on Incontinence. Neurourology and Urodynamics. 2010;29:191–198. doi: 10.1002/nau.20847. [DOI] [PubMed] [Google Scholar]
- Hanno P, Nordling J, Fall M. Bladder pain syndrome. The Medical Clinics of North America. 2011;95:55–73. doi: 10.1016/j.mcna.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocrine Reviews. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- Jappelli R, Perrin MH, Lewis KA, Vaughan JM, Tzitzilonis C, Rivier JE, Vale WW, Riek R. Expression and functional characterization of membrane-integrated mammalian corticotropin releasing factor receptors 1 and 2 in Escherichia coli. PloS one. 2014;9:e84013. doi: 10.1371/journal.pone.0084013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. American Journal of Physiology-Renal Physiology. 2009;297:F1477–1501. doi: 10.1152/ajprenal.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner AP, Steers WD. Corticotropin releasing factor: a mediator of emotional influences on bladder function. Journal of Urology. 2004;172:2570–2573. doi: 10.1097/01.ju.0000144142.26242.f3. [DOI] [PubMed] [Google Scholar]
- LaBerge J, Malley SE, Zvarova K, Vizzard MA. Expression of corticotropin-releasing factor and CRF receptors in micturition pathways after cyclophosphamide-induced cystitis. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2006;291:R692–703. doi: 10.1152/ajpregu.00086.2006. [DOI] [PubMed] [Google Scholar]
- Larauche M, Kiank C, Tache Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. Journal of Physiology and Pharmacology. 2009;60(Suppl 7):33–46. [PMC free article] [PubMed] [Google Scholar]
- Lavelle JP, Meyers SA, Ruiz WG, Buffington CA, Zeidel ML, Apodaca G. Urothelial pathophysiological changes in feline interstitial cystitis: a human model. American Journal of Physiology-Renal Physiology. 2000;278:F540–553. doi: 10.1152/ajprenal.2000.278.4.F540. [DOI] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen P, Vaughan J, Lee KF, Vale W. Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4206–4211. doi: 10.1073/pnas.0611641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Kreder KJ, Rothrock NE, Ratliff TL, Zimmerman B. A laboratory stress model for examining stress and symptomatology in interstitial cystitis patients. Urology. 2001;57:122. doi: 10.1016/s0090-4295(01)01076-7. [DOI] [PubMed] [Google Scholar]
- Malykhina A, Hanno P. How are we going to make progress treating bladder pain syndrome? ICI-RS 2013. Neurourology and urodynamics 2014. 2014 Feb 24; doi: 10.1002/nau.2257. [DOI] [PubMed] [Google Scholar]
- Mayer EA. Pathophysiology of Persistent Abdominal and Pelvic Pain. In: Mogil JS, editor. PAIN 2010-An Updated Review: Refreshere Course Syllabus. Seattle: IASP Press; 2010. pp. 337–344. [Google Scholar]
- Negoro H, Lutz SE, Liou LS, Kanematsu A, Ogawa O, Scemes E, Suadicani SO. Pannexin 1 involvement in bladder dysfunction in a multiple sclerosis model. Scientific Reports. 2013;3:2152. doi: 10.1038/srep02152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozu T, Tsuchiya Y, Kumei S, Takakusaki K, Okumura T. Peripheral corticotropin-releasing factor (CRF) induces stimulation of gastric contractions in freely moving conscious rats: role of CRF receptor types 1 and 2. Neurogastroenterology and Motility: the official journal of the European Gastrointestinal Motility Society. 2013;25:190–197. doi: 10.1111/nmo.12050. [DOI] [PubMed] [Google Scholar]
- Parsons CL. The role of a leaky epithelium and potassium in the generation of bladder symptoms in interstitial cystitis/overactive bladder, urethral syndrome, prostatitis and gynaecological chronic pelvic pain. British Journal of Urology International. 2011;107:370–375. doi: 10.1111/j.1464-410X.2010.09843.x. [DOI] [PubMed] [Google Scholar]
- Paschos KA, Charsou C, Constantinidis TC, Anagnostoulis S, Lambropoulou M, Papachristou F, Simopoulos K, Chatzaki E. Corticotropin-releasing hormone receptors mediate opposing effects in cholestasis-induced liver cell apoptosis. Endocrinology. 2010;151:1704–1712. doi: 10.1210/en.2009-1208. [DOI] [PubMed] [Google Scholar]
- Robbins MT, Ness TJ. Footshock-induced urinary bladder hypersensitivity: role of spinal corticotropin-releasing factor receptors. Journal of Pain. 2008;9:991–998. doi: 10.1016/j.jpain.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothrock NE, Lutgendorf SK, Kreder KJ, Ratliff T, Zimmerman B. Stress and symptoms in patients with interstitial cystitis: a life stress model. Urology. 2001;57:422–427. doi: 10.1016/s0090-4295(00)00988-2. [DOI] [PubMed] [Google Scholar]
- Saban MR, Nguyen NB, Hammond TG, Saban R. Gene expression profiling of mouse bladder inflammatory responses to LPS, substance P, and antigen-stimulation. The American Journal of Pathology. 2002;160:2095–2110. doi: 10.1016/S0002-9440(10)61159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini DK, Gautam N. Live cell imaging for studying g protein-coupled receptor activation in single cells. Methods in Molecular Biology. 2010;617:191–207. doi: 10.1007/978-1-60327-323-7_16. [DOI] [PubMed] [Google Scholar]
- Sandilos JK, Bayliss DA. Physiological mechanisms for the modulation of pannexin 1 channel activity. The Journal of Physiology. 2012;590:6257–6266. doi: 10.1113/jphysiol.2012.240911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochimica et Biophysica Acta. 2003;1615:7–32. doi: 10.1016/s0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- Slominski A. A nervous breakdown in the skin: stress and the epidermal barrier. The Journal of Clinical Investigation. 2007;117:3166–3169. doi: 10.1172/JCI33508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton EA, Mazurkiewicz JE, Wei ET. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB Journal. 2001;15:1678–1693. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- Smith AL, Leung J, Kun S, Zhang R, Karagiannides I, Raz S, Lee U, Glovatscka V, Pothoulakis C, Bradesi S, et al. The effects of acute and chronic psychological stress on bladder function in a rodent model. Urology. 2011;78(967):e961–967. doi: 10.1016/j.urology.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squillacioti C, De Luca A, Liguori G, Paino S, Mirabella N. Expression of urocortin and corticotropin-releasing hormone receptors in the bovine adrenal gland. General and Comparative Endocrinology. 2011;172:416–422. doi: 10.1016/j.ygcen.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Stella JL, Lord LK, Buffington CA. Sickness behaviors in response to unusual external events in healthy cats and cats with feline interstitial cystitis. Journal of the American Veterinary Medical Association. 2011;238:67–73. doi: 10.2460/javma.238.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Tache Y. Corticotropin-releasing factor signaling and visceral response to stress. Exp Biology and Medine (Maywood) 2010;235:1168–1178. doi: 10.1258/ebm.2010.009347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskind AM, Berry SH, Ewing BA, Elliott MN, Suttorp MJ, Clemens JQ. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND Interstitial Cystitis Epidemiology male study. The Journal of Urology. 2013;189:141–145. doi: 10.1016/j.juro.2012.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum AA, Gareau MG, Jury J, Yang PC, Perdue MH. Chronic peripheral administration of corticotropin-releasing factor causes colonic barrier dysfunction similar to psychological stress. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2008;295:G452–459. doi: 10.1152/ajpgi.90210.2008. [DOI] [PubMed] [Google Scholar]
- Timoteo MA, Carneiro I, Silva I, Noronha-Matos JB, Ferreirinha F, Silva-Ramos M, Correia-de-Sa P. ATP released via pannexin-1 hemichannels mediates bladder overactivity triggered by urothelial P2Y6 receptors. Biochemical Pharmacology. 2014;87:371–379. doi: 10.1016/j.bcp.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Tsatsanis C, Dermitzaki E, Venihaki M, Chatzaki E, Minas V, Gravanis A, Margioris AN. The corticotropin-releasing factor (CRF) family of peptides as local modulators of adrenal function. Cellular and Molecular Life Sciences: CMLS. 2007;64:1638–1655. doi: 10.1007/s00018-007-6555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. The Journal of Neuroscience: the official journal of the Society for Neuroscience. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. The Journal of Clinical Investigation. 2005;115:2412–2422. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Masunaga K, Satoji Y, Maeda Y, Nagata T, Inadome A. Basic and clinical aspects of non-neuronal acetylcholine: expression of non-neuronal acetylcholine in urothelium and its clinical significance. Journal of Pharmacological Sciences. 2008;106:193–198. doi: 10.1254/jphs.fm0070115. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Neural control of the lower urinary tract. International Journal of Urology: official journal of the Japanese Urological Association. 1997;4:111–125. doi: 10.1111/j.1442-2042.1997.tb00156.x. [DOI] [PubMed] [Google Scholar]