Abstract
The genetic manipulation of mosquito vectors is an alternative strategy in the fight against malaria. It was previously shown that bee venom phospholipase A2 (PLA2) inhibits ookinete invasion of the mosquito midgut although mosquito fitness was reduced. To maintain the PLA2 blocking ability without compromising mosquito biology, we mutated the protein-coding sequence to inactivate the enzyme while maintaining the protein’s structure. DNA encoding the mutated PLA2 (mPLA2) was placed downstream of a mosquito midgut-specific promoter (Anopheles gambiae peritrophin protein 1 promoter, AgPer1) and this construct used to transform Aedes fluviatilis mosquitoes. Four different transgenic lines were obtained and characterized and all lines significantly inhibited Plasmodium gallinaceum oocyst development (up to 68% fewer oocysts). No fitness cost was observed when this mosquito species expressed the mPLA2.
Keywords: transformation, bee venom phospholipase A2, malaria, piggyBac, fitness
Introduction
Malaria remains a major global problem, causing an unacceptable toll on the health and economic welfare of the world’s poorest communities. An estimated 350–500 million clinical malaria episodes occur annually; most of these caused by infection with Plasmodium falciparum and Plasmodium vivax. P. falciparum malaria causes more than one million deaths a year, mainly in sub-Saharan Africa. It also contributes indirectly to many additional deaths, mainly in young children, through synergy with other infections and illnesses (WHO, 2005).
Problems related to parasite drug resistance, mosquito resistance to insecticides and the lack of an effective vaccine increase the need for alternative strategies for malaria control. By genetically manipulating the mosquito vector it is possible to insert effector molecules that block Plasmodium development in the insect (Ito et al., 2002; Kim et al., 2004). Various candidates, including the anticircumsporozoite protein antibody (de Lara Capurro et al., 2000), a dodecapeptide SM1 (Ghosh et al., 2001), cecropin-like peptides (Arrighi et al., 2002), gomesin (Moreira et al., 2007) and the bee venom phospholipase A2 (PLA2) (Zieler et al., 2001), have been studied.
Phospholipase A2 enzymes belong to a protein family which catalyses the hydrolysis of the two-acyl ester bond of glycerophospholipids, leading to the production of free fatty acids and lysophospholipids. Secreted phospholipases A2 (sPLA2) have been identified in venoms, but are also distributed in mammalian tissues, fluids, and secretions (Kudo & Murakami, 2002), plants (Lee et al., 2003), bacteria (Sugiyama et al., 2002) and viruses (Canaan et al., 2004; Nagiec et al., 2004). Venom PLA2s cause diverse pharmacological effects such as neurotoxic, myotoxic or cardiotoxic reactions, or have anticoagulant properties, and some sPLA2s have been shown to display antibacterial (Buckland & Wilton, 2000; Koduri et al., 2002), antiviral (HIV) (Fenard et al., 1999) or antiPlasmodium activities (Deregnaucourt & Scrével, 2000; Guillaume et al., 2004).
PLA2s from snake, scorpion and bee venoms are potent in vitro inhibitors of the intraerythrocytic development of P. falciparum: their antiPlasmodium activity is more dependent on the presence of exogenous phospholipids in serum than on the hydrolysis of the erythrocyte membrane (Guillaume et al., 2004, 2006). When administered via blood feeding, venom PLA2s inhibit the P. falciparum and P. gallinaceum sporogonic cycle by associating with the mosquito midgut epithelium and blocking ookinete adhesion and invasion. This mechanism is independent of PLA2 catalytic activity (Zieler et al., 2001).
Transgenic Anopheles stephensi mosquitoes expressing the bee venom PLA2 blocked both the development of Plasmodium berghei oocysts and transmission to naïve mice (Moreira et al., 2002). However, the effector protein reduced female fertility, probably because of damage to midgut epithelial cells (Abraham et al., 2005) that reduced uptake of blood nutrients, and consequently, egg production (Moreira et al., 2004). Fitness of transgenic vectors is a crucial feature when one considers their use in disease control.
Previous studies have shown that histidine at position 48 of pancreatic PLA2 is crucial for enzymatic activity. Substitution of this residue to asparagine does not affect the enzyme’s structural integrity although it reduces its activity fivefold (Janseen et al., 1999).
To maintain the Plasmodium blocking ability of the bee venom phospholipase A2 without causing any fitnessrelated problem to mosquitoes, we generated point mutations, changing the conserved histidine in its active site (H67), and consequently inactivating the enzyme. We expressed this new protein (mPLA2) in transgenic mosquitoes and evaluated its effect on the progression of the P. gallinaceum cycle.
Results
Using site-directed mutagenesis, we generated two point mutations in the bee venom phospholipase A2 coding sequence, which resulted in amino acids changes and enzyme inactivation (Riehle et al., 2007). This approach was pursued because Zieler et al. (2001) demonstrated that even inactive venom PLA2 is able to block Plasmodium spp.
The recombinant protein was expressed in Escherichia coli, purified and dialysed in order to decrease the concentration of urea used for its purification. Its effect on P. gallinaceum was tested in comparison with the commercial bee venom PLA2. Recombinant mPLA2 inhibited P. gallinaceum oocyst formation up to 80% when compared with the control (buffer) (P < 0.05), while the commercial PLA2 inhibited parasite development by 91 to 99% when compared to the control (P < 0.01). Additionally, the prevalence of infected mosquitoes was always lower when either protein was present in comparison to the control (Table 1).
Table 1.
Effect of recombinant mutated phospholipase A2 (mPLA2) or commercial bee venom PLA2 on Plasmodium gallinaceum oocyst formation†
| Exp. | Samples | Infection prevalence‡ | Oocyst number (range) | Mean oocyst number | Inhibition§ |
|---|---|---|---|---|---|
| 1 | Buffer | 74% (20/27) | (0–122) | 32.4 | – |
| mPLA2 | 67% (12/28) | (0–72) | 10.7 | 70%* | |
| PLA2 | 40% (8/20) | (0–21) | 2.8 | 91%** | |
| 2 | Buffer | 90% (18/20) | (0–120) | 21.3 | – |
| mPLA2 | 40% (8/20) | (0–34) | 4.5 | 76.8%* | |
| PLA2 | 15% (3/20) | (0–2) | 0.2 | 93%** | |
| 3 | Buffer | 70% (17/27) | (0–277) | 33.7 | – |
| mPLA2 | 33% (8/24) | (0–45) | 6.6 | 80.4%* | |
| PLA2 | 25% (6/24) | (0–5) | 0.4 | 98.8%** |
Infected blood was mixed with recombinant mPLA2 or commercial PLA2 (0.1 μmol/l) or buffer alone (100 mM NaH2PO4, 10 mM Tris, 0.5 M urea, pH 6.9) prior to feeding. The midguts were dissected 7 days after the bloodmeal and checked for oocysts in order to measure prevalence and parasite intensity. Mean oocyst number was significantly different from mosquitoes fed on buffer, according to Mann–Whitney U test (*P < 0.05) (**P < 0.01). Data are from three independent experiments.
Percentage of infected mosquitoes (number of infected mosquitoes/total number of mosquitoes).
100 – [(mean oocyst number per midgut in experimental mosquitoes/mean oocyst number per midgut in control mosquitoes) × 100].
We used a mosquito midgut-specific promoter [Anopheles gambiae peritrophin protein 1 promoter (AgPer1); Abraham et al., 2005] to drive the expression of the mPLA2 in four independent transgenic lines. Expression of the 3 × P3-EGFP (enhanced green fluorescent protein) marker gene was detected by fluorescence microscopy by screening the G1 larvae and had the same pattern as previously observed in other mosquito species (Kokoza et al., 2001; Ito et al., 2002; Kim et al., 2004; Rodrigues et al., 2006). By PCR, it was possible to amplify both marker (EGFP: 645 bp) and effector (mPLA2: 480 bp) genes in transgenic lines, but not in control lines. The Aedes aegypti actin control (Krebs et al., 2002) was amplified in both transgenic and control mosquitoes (Fig. 1A). The number of transgene copies that integrated was assessed by Southern blot analysis. Genomic DNA was digested with the BglII enzyme, blotted and a probe comprising the left arm of the piggyBac transposable element was used. We observed different transposition events in our four transgenic lines, confirming the integration of the foreign genes in the germline of Ae. fluviatilis. As expected, there was no hybridization of the radioactive probe in the wild-type mosquitoes’ DNA (Fig. 1B).
Figure 1.
PCR amplification of the EGFP, mutated phospholipase A2 (mPLA2) and Actin genes from genomic DNA isolated from nontransgenic and transgenic Aedes fluviatilis larvae. (A) Genomic DNA from nontransgenic (NT) and transgenic larvae from 4T, 8T, 9T and 10T lines were amplified by PCR with primers specific to each target gene (EGFP; mPLA2; Actin). (B) About 10 μg of transgenic and wild-type mosquito genomic DNA was digested with BglII and transferred to a nylon membrane. The blot was hybridized with a 32P-labelled piggyBac left arm probe (0.8 kb SalI fragment). M, 100 bp DNA marker; c(−), no DNA; 4T, 8T, 9T and 10T, transgenic lines; c(+), pBacEGFPAgPermPLA2 (EGFP and mPLA2) and Ae. aegypti genomic DNA (Actin).
To check whether the AgPer1 promoter could drive the expression of the mPLA2 in mosquito midguts, we performed RT-PCR using RNA prepared from midgut samples collected prior to (sugar-fed midguts) and after blood feeding (8 and 48 h post bloodmeal). The AgPer1 promoter drove the production of mPLA2 in mosquito midguts of all four transgenic lines (Fig. 2). Devenport et al. (2004) reported that there were no significant changes in Ag-Aper1 mRNA abundance after a bloodmeal. Our RT-PCR data on expression of AgPer-mPLA22, although not strictly quantitative, are consistent with similar behaviour for this recombinant construct in Ae. fluviatilis (Supplementary Material S1). In other mosquito tissues no mPLA2 mRNA was detected (Supplementary Material Fig. S2).
Figure 2.
Mutated phospholipase A2 (mPLA2) mRNA expression in Aedes fluviatilis transgenic lines. Total RNA samples isolated from nontransgenic (NT) and transgenic mosquito (4T, 8T, 9T and 10T) midguts (before and after a bloodmeal) were subjected to RT-PCR analysis using primers specific to the mPLA2 gene (A), to the RP49 gene (ribosomal protein) (B) or as a control, without the reverse transcriptase enzyme (C). M1, 100 bp DNA marker; M2, 1 kb plus DNA marker; c(−), no cDNA; c(+), pBacEGFPAgPermPLA2 (in A).
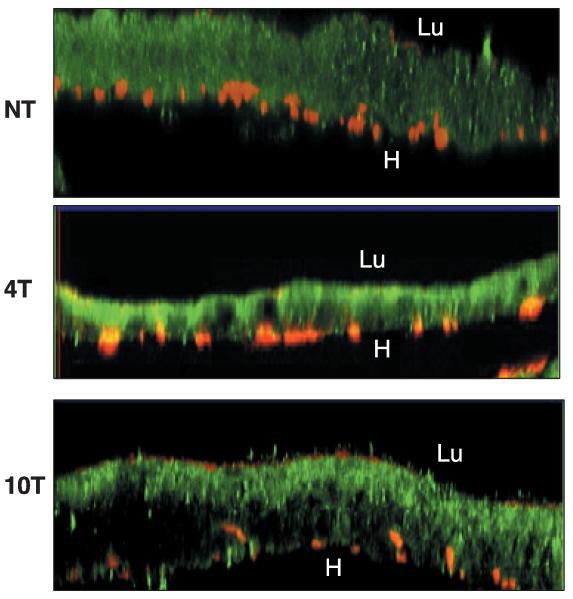
The presence of mPLA2 protein was investigated by immunostaining of mosquito midguts followed by confocal microscopy. The mutated PLA2 was detected on epithelial cells of midguts from blood-fed mosquitoes using a commercial polyclonal antibee venom PLA2. A fluorescent signal was detected towards the lumenal side of midgut epithelial cells, where the cell secretory vesicles are also localized. There, one could expect to find AgPer1 in equivalent cells from An. gambiae. In nontransgenic mosquito midguts a light green background signal was detected (Fig. 3). The specificity of the polyclonal antibody was verified by Western blots using commercial bee venom PLA2 in which our recombinant mPLA2 protein was easily recognized (data not shown).
Figure 3.
Immunofluorescence staining of transgenic mosquitoes midguts by antimutated phospholipase A2 (antimPLA2). Midgut from nontransgenic mosquito (NT), 4T and 10T transgenic mosquito lines 24 h postbloodmeal treated with antirabbit PLA2 and Alexa 488 secondary antibody (green) plus rhodamine phalloidin (red), to label the muscle cells (400× magnification). Lu, midgut lumenal side; H, haemocoel.
The effect of the mPLA2 expression on the development of P. gallinaceum in the mosquito was assessed by simultaneously feeding transgenic and nontransgenic female mosquitoes on the same infected chickens and by counting the number of Plasmodium oocysts in mosquito midguts 7 days after infection. At least three independent experiments were performed for each transgenic line. All transgenic lines significantly reduced oocyst numbers compared to nontransgenic siblings (17.5 to 68.5% inhibition), although the level of inhibition was not as high as when recombinant protein was added to the bloodmeal (Table 2). When infected mosquitoes were maintained for 15 days and had their salivary glands removed to check for the presence of sporozoites, transgenic mosquitoes had significantly fewer sporozoites than nontransgenic adult females (Supplementary Material Table S1).
Table 2.
Effect of expression of mutated phospholipase A2 (mPLA2) in transgenic Aedes fluviatilis on Plasmodium gallinaceum oocyst formation
| Experiment | Mosquitoes | Prevalence† | Oocyst‡ | Inhibition§ |
|---|---|---|---|---|
| 1 | Control | 96% (43/45) | 51.9 (0–170) | – |
| 4T | 86.2%(25/29) | 26 (0–63) | 49.9%* | |
| 2 | Control | 96.4% (27/28) | 41.9 (0–148) | – |
| 4T | 75% (21/28) | 13.4 (0–136) | 68%* | |
| 3 | Control | 90% (29/32) | 83.6 (0–287) | – |
| 4T | 86% (19/22) | 40.1 (0–168) | 52%* | |
| 4 | Control | 87.5% (35/40) | 104.75 (0–240) | – |
| 4T | 71% (16/21) | 65.6 (0–259) | 37.3%* | |
| 5 | Control | 80.6% (25/31) | 26.4 (0–136) | – |
| 8T | 75.6% (31/41) | 19.4 (0–120) | 26.5%* | |
| 6 | Control | 74% (20/27) | 12.7 (0–50) | – |
| 8T | 59% (13/22) | 4 (0–44) | 68.5%* | |
| 7 | Control | 85.2% (41/49) | 48 (0–213) | – |
| 8T | 75% (23/30) | 34 (0–200) | 21.9%* | |
| 8 | Control | 83.3% (30/36) | 34.8 (0–160) | – |
| 9T | 71.4% (15/21) | 20.5 (0–64) | 41.2%* | |
| 9 | Control | 100% (39/39) | 49.8 (1–172) | – |
| 9T | 80.9% (17/21) | 41.2 (0–184) | 17.5%* | |
| 10 | Control | 78.3% (18/23) | 11.9 (0–71) | – |
| 9T | 65% (13/20) | 8.6 (0–40) | 27.7%* | |
| 11 | Control | 87.5% (21/24) | 7.5 (0–56) | – |
| 10T | 81.8% (9/11) | 3.8 (0–9) | 49.3%* | |
| 12 | Control | 93% (38/40) | 63.8 (0–212) | – |
| 10T | 50% (10/20) | 27.1 (0–124) | 57.5%* | |
| 13 | Control | 100% (32/32) | 120.4 (12–291) | – |
| 10T | 85.7% (19/21) | 84.4 (0–198) | 30%* |
Percentage of infected (actual numbers in parentheses).
Arithmetic mean of oocyst number per mosquito gut. The range of observed values is indicated in parentheses.
100 – [(mean oocyst number in transgenic mosquitoes/mean oocyst number in control mosquitoes) × 100].
Reduction of oocyst number/gut was significant (P-value < 0.05) for all lines, as calculated by the Mann–Whitney U test.
To check whether the expression of mPLA2 had any influence on mosquito overall fitness, we performed some experiments to assess the stability of the transgene through generations and measured biological parameters such as fertility and survivorship. The proportion of transgenic mosquitoes from two different lines was compared with nontransgenic counterparts and, as shown in Supplementary Material Fig. S3, the number of transgenic individuals was significantly higher than nontransgenic mosquitoes for several generations. In addition, there was no difference in egg production between nontransgenic and transgenic mosquitoes; survival was significantly longer in most of the lines for both male and female mosquitoes (Supplementary Material Table S2).
Discussion
This is the first report on the generation of transgenic Ae. fluviatilis mosquitoes bearing a parasite-blocking molecule. The idea of expressing the inactive bee venom PLA2 was based on our previous observations that transgenic An. stephensi mosquitoes expressing the active enzyme significantly reduced their egg production (Moreira et al., 2004), probably because of midgut epithelia damage (Abraham et al., 2005) which may have compromised nutrient absorption. That effect was not observed by Zieler et al. (2001), who were the first to report the Plasmodium blocking feature of venom PLA2 in mosquitoes. When those authors chemically inactivated the enzyme, mixed with a parasite-enriched bloodmeal and fed female mosquitoes, no fitness-related problems were detected (Zieler et al., 2001). We presume that damage occurs when the wildtype enzyme is expressed intracellularly.
We expressed the mutated bee venom PLA2 (mPLA2) in bacteria as a recombinant protein. This protein had similar in vitro parasite-blocking ability when compared to our control, the commercial bee venom PLA2, which encouraged us to express this molecule in mosquitoes. The idea of using midgut-specific promoters to express potential parasite effector molecules has been pursued in different studies (Ito et al., 2002; Moreira et al., 2002; Kim et al., 2004; Abraham et al., 2005; Franz et al., 2006). As the parasite’s early sporogonic stages develop within the mosquito midgut environment, the expression of a blocking molecule in that tissue could target gametocytes, gametes, zygotes and ookinetes (Baton & Ranford-Cartwright, 2005).
The AgPer1 protein is a major constituent of the peritrophic matrix, which protects the midgut during a bloodmeal (Shen et al., 1998). In anopheline mosquitoes, peritrophic matrix proteins are stored in vesicles within the mosquito midgut epithelial cells (Devenport et al., 2004) and are immediately released upon blood feeding. In culicine mosquitoes (Aedes spp.), epithelial cells store peritrophin mRNA and protein translation is initiated upon blood feeding (Devenport et al., 2006). Although we used an anopheline promoter to express a transgene in Aedes, which last shared a common ancestor at least 140 million years ago (Service, 1993), the Anopheles regulatory sequences was recognized by Ae. fluviatilis. Previously, another gut-specific promoter from an anopheline mosquito (An. gambiae carboxypeptidase) was also able to drive a transgene expression in Ae. aegypti (Moreira et al., 2000).
The effect of phospholipase A2 towards Plasmodium development has been extensively studied (Deregnaucourt & Scrével, 2000; Zieler et al., 2001; Guillaume et al., 2004), although the exact mechanism of action is not completely understood. In culture, PLA2s have an indirect effect by degrading lipoproteins present in serum, which in turn produce components (eg arachidonic, linoleic and docosahex-aenoic acid) toxic to the parasite (Guillaume et al., 2006).
Confocal microscopy was used to localize the transgenic protein in blood-fed mosquito midguts presenting a homogeneous distribution throughout the tissue towards the lumenal side of the epithelial cells. The localization is similar to that of the peritrophic matrix protein (Ag-Aper1) in An. gambiae (Devenport et al., 2004), which demonstrates that the transgenic protein is being properly processed within the mosquito midgut.
Inhibition of oocyst development varied among different transgenic lines and even within lines, in different experiments. Those differences can be a result of various factors including degree of mosquito infection, chicken parasitemia upon infection and effector protein expression in different lines (Coates et al., 1998; Perera et al., 2002; Marcus et al., 2004). Furthermore, the location of transgene insertion can have a strong influence on the gene expression, whether lying on a euchromatic or heterochromatic region (Boulesteix & Biémont, 2005). It is important to note that in all experiments we worked with heterozygous transgenic lines, as we have been crossing transgenic mosquitoes with colony nontransgenic adults for several generations in order to reduce the possibility of associating the transgene with deleterious alleles that might become fixed when one establishes homozygous lines early in the process (Catteruccia et al., 2003; Marrelli et al., 2006). When working with homozygous lines, which should have higher levels of the effector molecule, we would expect a higher degree of inhibition but that has yet to be tested.
This effector molecule is a strong candidate to be tested in human malaria vectors as Zieler et al. (2001) have already demonstrated the effect of PLA2 towards P. falciparum infecting An. stephensi and An. gambiae mosquitoes, and also because we have shown here that mosquito fitness was not compromised with the expression of mPLA2.
We are aware that transgenic mosquitoes bearing only one effector molecule will not be sufficient to block parasite transmission. For P. gallinaceum it has been experimentally demonstrated that mosquitoes bearing only 20 sporozoites can infect a naïve chick (Jasinskiene et al., 2007). Other blocking molecules must be studied in order to generate a mosquito that, for instance, would block parasites within their midguts (by using midgut-specific promoters) and also when traversing the salivary glands (molecules driven by salivary gland-specific promoters). In that way, the possibility of plasmodia overcoming those barriers would be reduced.
The genetic modification of mosquito vectors is important not only for generating parasite-blocking mosquitoes for the control of malaria, but also as a tool for studying parasite-mosquito interactions.
Experimental procedures
Mutated phospholipase A2 (mPLA2) and protein expression
Point mutations relative to the original PLA2 cDNA sequence (Moreira et al., 2002) were obtained by PCR site-directed mutagenesis (Riehle et al., 2007). The histidine (codon CAC) residue in the active site was modified to asparagine (AAC) in order to inactivate the bee venom phospholipase A2 without altering its conformation (mPLA2). An extra point mutation was made on position 62 [codon GCA (A) was changed to ACA (T)].
The mPLA2 coding sequence was cloned into the pET32a expression vector. Recombinant protein was expressed in E. coli BL21(DE3)pLysS strain and induced with 1 mM IPTG for 4 h. After induction, cells were pelleted and resuspended in 250 ml chilled lysis buffer (50 mM Tris-HCl pH 7.5, 2 mM EDTA, 1 mM DTT, 2 mg/ml lysozyme), frozen in dry ice/ethanol bath and then sonicated. Lysed cells were centrifuged (10 000 g for 16 min, at 4 °C) and after washing, the pellet was resuspended in milli-Q water (50 ml). Protein purification was performed by affinity chromatography in a column packed with 1.0 ml nickel-nitrilotriacetic acid (Ni-NTA; Qiagen, Valencia, CA, USA) resin and eluted according to the manufacturer’s protocol. Purified fractions were collected and dialysed (12 kDa cutoff) to decrease urea concentration and analysed by SDS-PAGE.
Construction for mosquito transgenesis
The promoter region, 5′ UTR and 3′ UTR sequences from An. gambiae peritrophic matrix protein 1 (AgAper1) were cloned in pUC19 (Abraham et al, 2005). The mutated bee venom PLA2 replaced the original PLA2 by restriction digesting the pUC19 (AgPer1/PLA2) plasmid with KpnI and BamHI. The mPLA2 was inserted into KpnI BamHI sites, between the 5′ and 3′ fragments of AgAper1, in pUC19. A PstI site 610 bp downstream from the 5′ end of the AgAper1 cloned promoter and pUC19 multiple cloning site PstI were used to release the fragment carrying 2570 bp 5′-AgAper1 + 480 bp mPLA2 sequence + 907 bp AgAper1 3′ sequence. This PstI fragment was subcloned into the PstI site of the pSLfa1180fa shuttle vector, which has unique FseI and AscI sites. The plasmid was digested with both enzymes and the fragment was inserted into FseI/AscI sites of piggyBac[3 × P3-EGFPPafm] to generate piggyBac[3 × P3-EGFP(AgAper1PLA2 m)]. The final plasmid was sequenced to verify the inserts before microinjection.
Mosquitoes
Ae. fluviatilis were reared at 27 °C and 80% humidity under a 12 h light/dark cycle, and adults were fed on 10% sucrose solution ad libitum. For egg production female mosquitoes were blood fed on anaesthetized Swiss Webster mice.
Microinjection and mosquito rearing
Ae. fluviatilis embryos were injected, as previously described (Rodrigues et al., 2006), with a mixture of piggyBac[3′ P3-EGFP(AgPerPLA2m)] plasmid (0.3 μg/μl) and piggyBac helper plasmid (0.2 μg/μl), which encodes the pBac transposase (Handler & Harrell, 1999). Transformants were selected by screening the G1 larvae for the presence of green fluorescent protein (GFP)-positive individuals using a fluorescent stereomicroscope. Individually transformed adult mosquitoes were mated with nontransgenic mosquitoes to establish stable transgenic lines. Transgenic mosquito lines have been maintained for several generations as heterozygotes.
Detection of the transgene
Larval genomic DNA was isolated by an adapted protocol (Black & Munstermann, 1996). Briefly, fourth instar larvae from all transgenic lines or nontransgenic siblings were frozen in dry ice: ethanol bath, homogenized in grinding buffer (0.1 M NaCl; 0.2 M sucrose; 0.1 M Tris-HCl pH 9.1; 0.05 M EDTA; SDS 0.05%) and incubated at 65 °C for 30 min. Following proteinase K digestion for 3 h at 52 °C, the DNA was extracted with phenol and phenol:chloroform:isoamylic alcohol. After air drying, the DNA pellet was solubilized in 10 mM Tris-HCl, pH 8.5. For PCR analysis, primers specific to EGFP marker (EGFPF: 5′-GGTGCCCATCCTGGTCGAGC-3′ and EGFPR: 5′-GCGGTCACGAACTCCAGCAGG-3′); mPLA2 (PLAMF: 5′-GGTACCTGGCAAATCAGGGATAGG-3′ and PLAMR: 5′-GGATCCCTATCAGGCGTAGTCGG-3′) and actin (ActinF: 5′-AAGGTGTGATGGTCGGTA-3′ and actinR: 5′-TCATTGCCCCACCAGAAC-3′; Krebs et al., 2002) genes were used.
For Southern blot analysis, 10 μg of genomic DNA was digested with 10 U of BglII (Promega, Madison, WI, USA), separated on a 0.7% agarose gel and the blot hybridized with a 32P-labelled piggyBac left arm probe (0.8 kb SalI fragment). Blots were exposed to x-ray film for 1 week, at −80 °C.
Transgenic PLA2 mRNA expression
Mosquito midguts were dissected from transgenic and wildtype mosquitoes at several time-points after blood feeding. All tissues were immediately frozen in an ethanol/dry ice bath and stored at −80 °C. Total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. First-strand cDNA synthesis was performed with SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer’s protocol with 0.5 μg total RNA from each sample as a template for the reactions and oligo-dT primer. To avoid genomic DNA carry over, total RNA was treated with RQ1 RNase-free DNase (Promega). After cDNA synthesis, oligonucleotides specific to the mPLA2 fragment [AGPERF–5′-GTGTGGTGCTACTGTTGGC-3′; PLAMR (see above)] were used to amplify the mPLA2 gene by PCR (95 °C for 2 min and 30 cycles of 95 °C for 1 min; 55 °C for 1 min; 72 °C for 1 min). As a control, we used a ribosomal specific gene (Gentile et al., 2005) (RP49 F: 5′-GTGAAGAAGCGGACGAAGAAGTT-3′ and RP49 R: 5′-TGCATCATCAGCACCTCCAGC-3′) (95 °C for 2 min and 30 cycles of 95 °C for 1 min; 61 °C for 1 min; 72 °C for 1 min).
Midgut immunostaining
Midguts from transgenic and nontransgenic mosquitoes fed on naïve mice were dissected in phosphate-buffered saline (PBS; 130 mM NaCl, 7 mM Na2HPO4, 3 mM NaH2PO4·H2O, pH 7.4) and fixed for 2 h in 4% paraformaldehyde. Fixed tissues were washed with PBS three times for 5 min and blocked in RPMI (Roswell Park Memorial Institute) medium for 2 h at room temperature. In between each of these steps, midguts were washed twice for 5 min with PBS, following incubation with PBT/BSA (1% bovine serum albumin, 0.2% Triton in PBS pH 7.5) for 30 min. The antirabbit PLA2 (Nordic, The Netherlands) was diluted in blocking solution AC Dako (1 : 2000) (Dakocytomation, Denmark) and incubated with the midguts at 4 °C overnight. Secondary antibody conjugated to Alexa 488 (Molecular Probes, Eugene, OR, USA) was used at a 1 : 500 dilution in Ac Dako for 2 h at room temperature. After each antibody incubation, the tissue was washed three times for 5 min with PBT. The tissues were then incubated with rhodamine/phaloidin (diluted in PBT) for 2 h following another washing step. Immunostainings were analysed with a confocal microscope (LSM510, Zeiss, Jena, Germany) at a magnification of 400×, using a 505–530 nm filter.
Plasmodium gallinaceum blocking experiments
The effect of the recombinant protein (mPLA2) on the development of the bird malaria parasite was tested, in vitro, in three independent experiments. mPLA2 was added to P. gallinaceum-infected chicken blood (0.1 μmol/l) and offered to Ae. fluviatilis female mosquitoes through a membrane feeding apparatus. Buffer alone (100 mM NaH2PO4, 10 mM Tris, 0.5 M urea, pH 6.9) and native PLA2 (0.1 μmol/l, Sigma, St Louis, MO, USA) [able to inhibit the development of the parasite as demonstrated by Zieler et al. (2001)], were used as controls.
The effect of the mutated protein towards parasite development was also tested in transgenic mosquitoes. P. gallinaceum 8A parasite strain was inoculated intra-peritoneally into 2-day-old chicks and parasitemia checked daily via Giemsa-stained blood smears. Transgenic and nontransgenic mosquitoes (3–5 days old) maintained with sugar supplemented with 0.5 g/l p-aminobenzoic acid plus 0.025% gentamicin, were allowed to feed on gametocytepositive chicks (10–15% parasitemia). Fully engorged mosquitoes were separated after 24 h and kept at 27 °C with 10% sugar solution. After 7 days, midguts were dissected, stained with mercurochrome (2% solution) and the number of oocysts per midgut was determined by microscopy (DIC-100×). The head was separated from the thorax in order to screen, on a fluorescence stereoscope, which midguts belonged to each transgenic or nontransgenic mosquitoes. The procedure was carried out by another person in a double blind assay to avoid any bias with oocyst counting.
To check for sporozoites, transgenic and nontransgenic sibling mosquitoes were infected with P. gallinaceum and after 7 days some mosquitoes were dissected to check for oocysts. Fifteen days postinfection the thoraces were separated from the abdomens. Twelve mosquito thoraces and heads were directly transferred to a 0.5 ml tube (previously perforated with a 25 × 7 mm needle and containing glass wool on the bottom) that was then placed inside a 1.5 ml microcentrifuge tube. One hundred microlitres of RPMI 1640 was added to each tube and centrifuged at 3000 g for 5 min at 4 °C. The bottom tube was replaced and this last step was repeated twice, after which all the collected flow-through was mixed and centrifuged at 3000 g for 15 min. The supernatant was removed, leaving about 10 μL inside each tube. Forty microlitres of RPMI 1640 were added and after resuspension 10 μL was transferred to a hemacitometer and counted using a DIC-microscope at 400× magnification.
Fitness of transgenic mosquitoes
Fifty heterozygous virgin transgenic female mosquitoes were crossed with 50 nontransgenic male mosquitoes. One hundred larvae of each progeny were randomly selected in order to check the proportion of transgenic individuals. Subsequent crosses were made, with the same number of randomly chosen male and female individuals. The proportion of male and female adults was recorded. These experiments were followed for five generations.
For egg production, 3-day-old females from nontransgenic and transgenic lines were blood fed and after 3 days their wings were removed and the insects confined in 4 cm Petri dishes containing wet filter paper to collect the eggs. These experiments were repeated four times. To check mosquito survivorship, 1-day-old male and female mosquitoes were grouped in cages, offered sucrose ad libitum and mortality was observed every day until all had died. Three independent experiments using female mosquitoes and two with male individuals were performed.
Statistical analysis
The blocking ability of both the recombinant protein and the transgenic mosquitoes expressing the mPLA2 towards P. gallinaceum was analysed with the nonparametric Mann–Whitney U test. Biological parameters were analysed with Kruskal–Wallis and Mann–Whitney U tests (Dawson & Trapp, 2001) and chi-square analysis was performed (5% probability) to check whether the population reached the Hardy–Weinberg equilibrium after the second generation.
Supplementary Material
Figure S1. Mutated phospholipase A2 (mPLA2) mRNA time-course expression in Aedes fluviatilis in a transgenic line. Total RNA samples from nontransgenic (NT) and transgenic (4T) mosquito midguts dissected at different time-points after a bloodmeal were subjected to RT-PCR using oligonucleotides specific to the mPLA2 gene (B) and to the RP49 gene (ribosomal protein) (C) or as a control, without the reverse transcriptase enzyme (A). M1, 100 pb marker; M2, 1 kb plus DNA marker; c(-), no cDNA; c(+), pBacEGFPAgPermPLA2.
Figure S2. Tissue and sex specificity of mutated phospholipase A2 (mPLA2) mRNA expression in Aedes fluviatilis transgenic lines. Total RNA samples from nontransgenic (NT) and transgenic (4T and 8T) mosquito midguts or carcasses were subjected to RT-PCR using oligonucleotides specific to the mPLA2 gene (A) and to the RP49 gene (ribosomal protein) (B). As a negative control, the reactions were carried out without RT enzyme (C). M1, 100 bp marker; M2, 1 kb plus DNA marker; c(-), no DNA; c(+), pBacEGFPAgPermPLA2; (1, 4, 7), carcasses; (2, 5, 8), female midguts 24 h after bloodmeal; (3, 6, 9), male midguts.
Figure S3. Fitness of mutated phospholipase A2 (mPLA2) transgenic mosquitoes. Heterozygous virgin transgenic (T) females were crossed with nontransgenic (NT) males and fed on mouse blood. At each generation, 100 larvae were randomly selected to determine the proportion of transgenic (fluorescent) to nontransgenic individuals. Subsequent crosses were made with the same number of randomly chosen male and female individuals. The proportion of transgenic to nontransgenic was compared by the chi-square test, at 5% probability (*shows difference from the expected frequency: 50% of each on the first generation and 56% NT and 44% T for F2 through F5).
Table S1. Number of Plasmodium gallinaceum sporozoites in nontransgenic and transgenic mosquitoes expressing mutated phospholipase A2 (mPLA2).
Table S2. Life table parameters of transgenic and nontransgenic Aedes fluviatilis expressing mutated phospholipase A2 (mPLA2).
Acknowledgments
We are in debt to Tatiana A. da Silva for technical support on confocal analysis, Fernanda O. Rezende on sporozoite assays, Ana Paula M. Rebouças for statistical analysis and Paula M. Nogueira on fitness experiments. F.G.R., T.X.T.C., B.C.R. and L.A.M. received fellowships from CNPq. This work was partially funded by TDR/WHO and FAPEMIG.
Footnotes
The following supplementary material is available for this article:
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/full/10.1111/j.1365-2583.2008.00791.x.
References
- Abraham EG, Donnelly-Doman M, Fujioka H, Ghosh A, Moreira LA, Jacobs-Lorena M. Driving midgut-specific expression and secretion of a foreign protein in transgenic mosquitoes with AgPer1 regulatory elements. Insect Mol Biol. 2005;14:271–279. doi: 10.1111/j.1365-2583.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- Arrighi RB, Nakamura C, Miyake J, Hurd H, Burgess JG. Design and activity of antimicrobial peptides against sporogonic-stage parasites causing murine malarias. Antimicrob Agents Chemother. 2002;46:2104–2110. doi: 10.1128/AAC.46.7.2104-2110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baton LA, Ranford-Cartwright LC. Spreading the seeds of million-murdering death: metamorphoses of malaria in the mosquito. Trends Parasitol. 2005;21:573–580. doi: 10.1016/j.pt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Black WC, Munstermann LE. Molecular taxonomy and systematics of arthropod vectors. In: Beaty BJ, Marquardt WC, editors. The Biology of Disease Vectors. University Press of Colorado; Niwot: 1996. pp. 438–470. [Google Scholar]
- Boulesteix M, Biémont C. Transposable elements in mosquitoes. Cytogenet Genome Res. 2005;110:500–509. doi: 10.1159/000084983. [DOI] [PubMed] [Google Scholar]
- Buckland AG, Wilton DC. The antibacterial properties of secreted phospholipases A2. Biochim Biophys Acta. 2000;1488:71–82. doi: 10.1016/s1388-1981(00)00111-6. [DOI] [PubMed] [Google Scholar]
- Canaan S, Zadori Z, Ghomashchi F, Bollinger J, Sadilek M, Moreau ME, et al. Interfacial enzymology of parvovirus phospholipases A2. J Biol Chem. 2004;279:14502–14508. doi: 10.1074/jbc.M312630200. [DOI] [PubMed] [Google Scholar]
- Catteruccia F, Godfray HC, Crisanti A. Impact of genetic manipulation on the fitness of Anopheles stephensi mosquitoes. Science. 2003;299:1225–1227. doi: 10.1126/science.1081453. [DOI] [PubMed] [Google Scholar]
- Coates CJ, Jasinskiene N, Miyashiro L, James AA. Mariner transposition and transformation of the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci USA. 1998;95:3748–3751. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson B, Trapp RG. Basic & Clinical Biostatistics. 3rd edn. New York; Lange Medical Books/McGraw-Hill: 2001. [Google Scholar]
- De Lara Capurro M, Coleman J, Beerntsen BT, Myles KM, Olson KE, Rocha E, et al. Virus-expressed, recombinant single-chain antibody blocks sporozoite infection of salivary glands in Plasmodium gallinaceum-infected Aedes aegypti. Am J Trop Med Hyg. 2000;62:427–433. doi: 10.4269/ajtmh.2000.62.427. [DOI] [PubMed] [Google Scholar]
- Deregnaucourt C, Schrével J. Bee venom phospholipase A2 induces stage-specific growth arrest of the intraerytrocytic Plasmodium falciparum via modifications of human serum components. J Biol Chem. 2000;275:39973–39980. doi: 10.1074/jbc.M006712200. [DOI] [PubMed] [Google Scholar]
- Devenport M, Fujioka H, Jacobs-Lorena M. Storage and secretion of the peritrophic matrix protein Ag-Aper1 and trypsin in the midgut of Anopheles gambiae. Insect Mol Biol. 2004;13:349–358. doi: 10.1111/j.0962-1075.2004.00488.x. [DOI] [PubMed] [Google Scholar]
- Devenport M, Alvarenga PH, Shao L, Fujioka H, Bianconi ML, Oliveira PL, et al. Identification of the Aedes aegypti peritrophic matrix protein AeIMUCI as a heme-binding protein. Biochemistry. 2006;45:9540–9549. doi: 10.1021/bi0605991. [DOI] [PubMed] [Google Scholar]
- Fenard D, Lambeau G, Valentin E, Lefebvre JC, Lazdunski M, Doglio A. Secreted phospholipase A(2), a new class of HIV inhibitors that block virus entry into host cells. J Clin Invest. 1999;104:611–618. doi: 10.1172/JCI6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz AW, Sanchez-Vargas I, Adelman ZN, Blair CD, Beaty BJ, James AA, et al. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc Natl Acad Sci. 2006;103:4198–4203. doi: 10.1073/pnas.0600479103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile C, Lima JB, Peixoto AA. Isolation of a fragment homologous to the rp49 constitutive gene of Drosophila in the Neotropical malaria vector Anopheles aquasalis (Diptera: Culicidae) Mem Inst Oswaldo Cruz. 2005;100:545–547. doi: 10.1590/s0074-02762005000600008. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Ribolla PE, Jacobs-Lorena M. Targeting Plasmodium ligands on mosquito salivary glands and midgut with a phage display peptide library. Proc Natl Acad Sci USA. 2001;98:13278–13281. doi: 10.1073/pnas.241491198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume C, Deregnancourt C, Clavey V, Schrével J. Anti-Plasmodium properties of group IA, IB, IIA, and III secreted phospholipases A2 are serum-dependent. Toxicon. 2004;43:311–318. doi: 10.1016/j.toxicon.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Guillaume C, Calzada C, Lagarde M, Schrével J, Deregnancourt C. Interplay between lipoproteins and bee venom phospholipase A2 in relation to their anti-Plasmodium toxicity. J Lipid Res. 2006;47:1493–1506. doi: 10.1194/jlr.M600111-JLR200. [DOI] [PubMed] [Google Scholar]
- Handler AM, Harrell RA., 2nd. Germline transformation of Drosophila melanogaster with the piggyBac transposon vector. Insect Mol Biol. 1999;8:449–457. doi: 10.1046/j.1365-2583.1999.00139.x. [DOI] [PubMed] [Google Scholar]
- Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature. 2002;417:452–455. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- Janseen MJW, Van de Wiel WA, Beiboer SH, Van Kampen MD, Verheij HM, Slotboom AJ, et al. Catalytic role of the active site histidine of porcine pancreatic phospholipase A2 probed by the variants H48Q, H48N and H48K. Protein Eng. 1999;12:497–503. doi: 10.1093/protein/12.6.497. [DOI] [PubMed] [Google Scholar]
- Jasinskiene N, Coleman J, Ashikyan A, Salampessy M, Marinotti O, James AA. Genetic control of malaria parasite transmission: threshold levels for infection in an avian model system. Am J Trop Med Hyg. 2007;76:1072–1078. [PubMed] [Google Scholar]
- Kim W, Koo H, Richman AM, Seeeley D, Vizioli J, Klocko AD, et al. Ectopic expression of a cecropin transgene in the human malaria vector mosquito Anopheles gambiae (Diptera: Culicidae): Effects on susceptibility to Plasmodium. J Med Entomol. 2004;41:447–455. doi: 10.1603/0022-2585-41.3.447. [DOI] [PubMed] [Google Scholar]
- Koduri RS, Gronroos JO, Laine VJ, Le Calvez C, Lambeau G, Nevalainen TJ, et al. Bactericidal properties of human and murine group I, II, V, X, and XII secreted phospholipases A2. J Biol Chem. 2002;277:5849–5857. doi: 10.1074/jbc.M109699200. [DOI] [PubMed] [Google Scholar]
- Kokoza V, Ahmed A, Wimmer EA, Raikhel AS. Efficient transformation of the yellow fever mosquito Aedes aegypti using the piggyBac transposable element vector pBac[3′P3-EGFPafm] Insect Biochem Mol Biol. 2001;31:1137–1143. doi: 10.1016/s0965-1748(01)00120-5. [DOI] [PubMed] [Google Scholar]
- Krebs KC, Brzoza KL, Lan Q. Use of subtracted libraries and macroarray to isolate developmentally specific genes from the mosquito, Aedes aegypti. Insect Biochem Mol Biol. 2002;32:1757–1767. doi: 10.1016/s0965-1748(02)00116-9. [DOI] [PubMed] [Google Scholar]
- Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins Other Lipids Mediat. 2002:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Lee HY, Bahn SC, Kang YM, Lee KH, Kim HJ, Nohn EK, et al. Secretory low molecular weight phospholiase A2 plays important roles in cell elongation and shoot gravitropism in Arabidopsis. Plant Cell. 2003;15:1990–2002. doi: 10.1105/tpc.014423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JM, Ramos DM, Monteiro A. Germline transformation of the butterfly Bicyclus anynana. Proc Biol Sci. 2004;271:263–265. doi: 10.1098/rsbl.2004.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelli MT, Moreira CK, Kelly D, Alphey L, Jacobs-Lorena M. Mosquito transgenesis: what is the fitness cost? Trends Parasitol. 2006;22:197–202. doi: 10.1016/j.pt.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Moreira CK, Rodrigues FG, Ghosh A, Varotti FP, Miranda A, Daffre S, et al. Broad effect of the antimicrobial peptide gomesin against different life stages of Plasmodium spp. Exp Parasitol. 2007;116:346–353. doi: 10.1016/j.exppara.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira LA, Edwards MJ, Adhami F, Jasinskiene N, James AA, Jacobs-Lorena M. Robust gut-specific gene expression in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci USA. 2000;97:10895–10898. doi: 10.1073/pnas.97.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira LA, Ito J, Ghosh A, Devenport M, Zieler H, Eappen GA, et al. Bee venom phospholipase inhibits malaria parasite development in transgenic mosquitoes. J Biol Chem. 2002;277:40839–40843. doi: 10.1074/jbc.M206647200. [DOI] [PubMed] [Google Scholar]
- Moreira LA, Wang J, Collins FH, Jacobs-Lorena M. Fitness of anopheline mosquitoes expressing transgenes that inhibit Plasmodium development. Genetics. 2004;166:1337–1341. doi: 10.1534/genetics.166.3.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiec MJ, Lei B, Parker SK, Vasil ML, Matsumoto M, Ireland RM, et al. Analysis of a novel prophage-encoded group A Streptococcus extracelular phospholipase A2. J Biol Chem. 2004;279:45909–45918. doi: 10.1074/jbc.M405434200. [DOI] [PubMed] [Google Scholar]
- Perera OP, Harrell RA, II, Handler AM. Germ-line transformation of the South American malaria vector, Anopheles albimanus, with a piggyBac/EGFP transposon vector is routine and highly efficient. Insect Mol Biol. 2002;11:291–297. doi: 10.1046/j.1365-2583.2002.00336.x. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Moreira CK, Lampe D, Lauzon C, Jacobs-Lorena M. Using bacteria to express and display anti-Plasmodium molecules in the mosquito midgut. Int J Parasitol. 2007;37:595–603. doi: 10.1016/j.ijpara.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Rodrigues FG, Oliveira SB, Rocha BC, Moreira LA. Germline transformation of Aedes fluviatilis (Diptera:Culicidae) with the piggyBac transposable element. Mem Inst Oswaldo Cruz. 2006;101:755–757. doi: 10.1590/s0074-02762006000700008. [DOI] [PubMed] [Google Scholar]
- Service MW. Mosquitoes (Culicidae) In: Lane RP, Crosskey RW, editors. Medical Insects and Arachnids. Chapman & Hall; London: 1993. pp. 120–240. [Google Scholar]
- Shen Z, Jacobs-Lorena M. A type I peritrophic matrix protein from the malaria vector Anopheles gambiae binds to chitin. J Biol Chem. 1998;273:17665–17670. doi: 10.1074/jbc.273.28.17665. [DOI] [PubMed] [Google Scholar]
- Sugiyama MK, Ohtani K, Izuhara M, Koike T, Susuki K, Imamura S, et al. A novel prokaryotic phospholipase A2. Characterization, gene cloning, and solution structure. J Biol Chem. 2002;277:20051–20058. doi: 10.1074/jbc.M200264200. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) World malaria report 2005. Geneva Switzerland: 2005. http://www.rbm.who.int/wmr2005 Online. [Google Scholar]
- Zieler H, Keister DB, Dvorak JA, Ribeiro JM. A snake venom phospholipase A(2) blocks malaria parasite development in the mosquito midgut by inhibiting ookinete association with the midgut surface. J Exp Biol. 2001;204:4157–4167. doi: 10.1242/jeb.204.23.4157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Mutated phospholipase A2 (mPLA2) mRNA time-course expression in Aedes fluviatilis in a transgenic line. Total RNA samples from nontransgenic (NT) and transgenic (4T) mosquito midguts dissected at different time-points after a bloodmeal were subjected to RT-PCR using oligonucleotides specific to the mPLA2 gene (B) and to the RP49 gene (ribosomal protein) (C) or as a control, without the reverse transcriptase enzyme (A). M1, 100 pb marker; M2, 1 kb plus DNA marker; c(-), no cDNA; c(+), pBacEGFPAgPermPLA2.
Figure S2. Tissue and sex specificity of mutated phospholipase A2 (mPLA2) mRNA expression in Aedes fluviatilis transgenic lines. Total RNA samples from nontransgenic (NT) and transgenic (4T and 8T) mosquito midguts or carcasses were subjected to RT-PCR using oligonucleotides specific to the mPLA2 gene (A) and to the RP49 gene (ribosomal protein) (B). As a negative control, the reactions were carried out without RT enzyme (C). M1, 100 bp marker; M2, 1 kb plus DNA marker; c(-), no DNA; c(+), pBacEGFPAgPermPLA2; (1, 4, 7), carcasses; (2, 5, 8), female midguts 24 h after bloodmeal; (3, 6, 9), male midguts.
Figure S3. Fitness of mutated phospholipase A2 (mPLA2) transgenic mosquitoes. Heterozygous virgin transgenic (T) females were crossed with nontransgenic (NT) males and fed on mouse blood. At each generation, 100 larvae were randomly selected to determine the proportion of transgenic (fluorescent) to nontransgenic individuals. Subsequent crosses were made with the same number of randomly chosen male and female individuals. The proportion of transgenic to nontransgenic was compared by the chi-square test, at 5% probability (*shows difference from the expected frequency: 50% of each on the first generation and 56% NT and 44% T for F2 through F5).
Table S1. Number of Plasmodium gallinaceum sporozoites in nontransgenic and transgenic mosquitoes expressing mutated phospholipase A2 (mPLA2).
Table S2. Life table parameters of transgenic and nontransgenic Aedes fluviatilis expressing mutated phospholipase A2 (mPLA2).