Abstract
To accurately diagnose microbial infections in blood, it is essential to recover as many microorganisms from a sample as possible. Unfortunately, recovering such microorganisms depends significantly on their adhesion to the surfaces of diagnostic devices. Consequently, we sought to minimize the adhesion of methicillin-sensitive Staphylococcus aureus (MSSA) to the surface of polypropylene- and acrylic-based bacteria concentration devices. These devices were treated with 11 different coatings having various charges and hydrophobicities. Some coatings promoted bacterial adhesion under centrifugation, whereas others were more likely to prevent it. Experiments were run using a simple buffer system and lysed blood, both inoculated with MSSA. Under both conditions, Hydromer’s 7-TS-13 and Aqua65JL were most effective at reducing bacterial adhesion.
Introduction
The recovery of microorganisms from blood or other normally sterile fluids is crucial for proper diagnosis and treatment of infection. To obtain accurate results, it is necessary to maximize the number of organisms collected from a given sample. This can be challenging due to the fact that the concentrations of pathological organisms in the blood can vary enormously.1, 2 One example of this wide range of concentrations is the case of bacteremia, a condition where viable bacteria are present in the circulating blood. For this condition the concentration of bacteria is normally in the range of 1–100 cfu/mL, but can be up to 103 cfu/mL in severe cases.3–5
The prompt diagnosis and treatment of bacteremia is of significant interest to health care professionals. This condition is often the result of a severe infection introduced to the body by an infected catheter or other device. When left undiagnosed, bacteremia can lead to systemic inflammatory response syndrome (SIRS) and ultimately sepsis.6 The identification of SIRS is based upon the recognition of two or more of the following symptoms: fever (or hypothermia), accelerated heart rate or respiratory rate and abnormal white blood cell count.7 Symptom identification can often be slow and inaccurate, and once SIRS is correctly diagnosed, patients are often at high risk for developing sepsis, the 13th leading cause of death in the US.8 Consequently, it is necessary to develop quick and accurate diagnostics for detecting bacteria in blood, urine, and other normally sterile fluids.
When developing diagnostics for detecting bacteria, adhesion to the diagnostic device’s surface is an important factor to consider.9–11 Bacterial adhesion is a complex process that is affected by many factors.12, 13 Both specific and non-specific interactions affect the bacteria’s ability to attach to the surface, as well as surface properties (chemical composition, charge, roughness) and the associated flow conditions.14–18
The chemical composition of the surface can influence bacterial adherence to a surface.19–23 Materials with different functional groups change bacterial adhesion depending on material hydrophobicity and charge.24 For example, Chu and Williams studied the effects of physical configurations of suture materials on bacterial adhesion.25 They showed that polydioxanone sutures exhibited slight affinity towards the adherence of Escherichia coli and Staphylococcus aureus; however, Dexon sutures had much higher affinity towards the two bacteria. Additionally, if the surface chemistry of the material is modified or changed, bacterial adhesion can be affected. James and Jayakrishnan proved that surface thiocyanation of PVC decreased adhesion of S. aureus and Staphylococcus epidermidis because of the change in hydrophilicity of the native PVC from 72° to 50°.26
Surface charge can affect the bacterial adhesion. At a neutral pH, bacteria are commonly negatively charged; consequently, a slight repulsion on negatively charged surfaces is expected. For example, Kiremitci-Gumusderelioglu and Pesmen showed that bacterial adhesion was reduced on negatively-charged PMMA/AA, but it increased on positively-charged PMMA/DMAEMA.27 Similarly, Terada et al found that E. coli’s adhesion to modified PE sheets where the functional groups were positively charged was significantly higher than that of negatively charged functional groups on the modified PE sheets.28, 29
The material’s surface roughness plays a large role in bacterial adhesion. Surface irregularities promote bacterial adhesion whereas very smooth surfaces do not.30, 31 One reason could be that rough surfaces have a greater surface area and the depressions in the roughened surfaces provide favorable location for colonization. Taylor et al produced a range of roughness on PMMA and tested bacterial adhesion. Large augmentations in roughness produced by silicone carbide paper (grades P400 and P120) had no significant effect in adhesion compared to the smooth surface. But, a small increase in surface roughness using silicon carbide paper P1200 resulted in noteworthy increase in bacterial adhesion.32 In a comparison of polished, unpolished, and abraded stainless steel, it was shown that on the roughest surface (abraded), most of the S. aureus remained on the surface whereas on the smoothest surface (polished), significantly less cells present.33 This example illustrates the dependence of bacterial adhesion to surface roughness. Due to the multiplicity of factors that can affect the adhesion of bacteria, a survey studying the bacterial adhesion on a variety of surface coatings would be a useful resource to researchers developing bacterial diagnostics and other medical devices.
In this paper, we describe machined polypropylene and acrylic devices for concentrating bacteria and compare how various surface coatings impact recovery of low numbers of bacteria. We investigated the surface coatings in two model matrices: a simple buffer and whole blood. Ideally, the surface of the device would prevent bacterial adhesion, yet not be anti-microbial to facilitate a wide-range of downstream bacterial diagnostics (e.g. both genetic-based and culture-based methods). Additionally, the device surface would enable the recovery of very low numbers of bacteria in a complex matrix (e.g. blood) so as to be useful for the development of in vitro bacteremia diagnostics. Both commercially available and in-house coatings were tested for their bacterial adhesion.
Materials and methods
Blunt-nosed Devices
The devices were created from polypropylene or acrylic (MSC Industrial Supply Company). These materials were machined into the cone-shaped devices shown in Figure 1 with surface finishes (Ra) ranging from 0.1 – 0.5 μm on the interior surfaces. Details on the fabrication of these devices can be found in Supplementary Information.
Fig. 1.

Images of the blunt-nosed devices. Left: Angled top view. Right: Top view.
Polypropylene was the material of most interest due to its common use in laboratory consumables and all coatings were applied to it. However, one of the coating vendors suggested using acrylic to produce a more effective coating. As a result, some acrylic devices were also tested
Device Coatings
Hydrophobicity and charge were two factors likely to play a role in bacterial adhesion. To better understand these factors, coatings were chosen from four commercial vendors with a range of surface charges and hydrophobicities. Table 1 details these vendors and coatings. Blunt-nosed devices were shipped to these vendors for coating application. Upon return, the devices were sterilized with ethylene oxide gas (Andersen Products) prior to use.
Table 1.
Description of the commercial coatings.
| Company | Coatings | Hydrophilic or Hydrophobic | Charged | Main Structure | General Coating Process |
|---|---|---|---|---|---|
|
| |||||
| AST | HydroLAST | Slightly hydrophilic | Positive | ND | Dip Coated |
| Negative | ND | Dip Coated | |||
| Neutral | ND | Dip Coated | |||
|
| |||||
| BioCoat | Hydak B-23KX2/L-578 | Hydrophilic | Negative | Hyaluronic Acid | Dip Coated |
|
| |||||
| Hydromer | 7-TS-13 | Hydrophilic | Neutral | Polyvinylpyrrolidone | Dip Coated |
| Aqua65JL | Hydrophilic | Neutral | Polyvinylpyrrolidone | Dip Coated | |
|
| |||||
| IST | Philix | Hydrophilic | ND | SiO2 | Vacuum Vapor Delivery |
| Hydrophilic | ND | PEG | Vacuum Vapor Delivery | ||
| Hydrophilic | ND | Al2O3 | Vacuum Vapor Delivery | ||
| Repellix | Super-hydrophobic | ND | ND | Vacuum Vapor Delivery | |
All the devices treated with experimental coatings were compared to identical devices treated with the triblock copolymer Pluronic F127 (Sigma-Aldrich). This material has been used to reduce bacterial adhesion in other studies and could be easily applied in our laboratory.34 The blunt-nosed devices were coated by submerging them in a beaker of Pluronic solution (0.5 g/L) and placing the beaker in a sonic bath for 10 minutes.
Also, Pluronic F127 was used to coat the inside of 15 mL polypropylene centrifuge tubes (Fisher Scientific). It was determined that almost 100% of bacteria were recovered when the 15 mL conical tubes were coated with Pluronic F127, so these tubes were used as standards.
Bacterial Culture
Methicillin-sensitive S. aureus (MSSA) – strain Wichita (ATCC 29213) (a gram-positive bacteria with a dynamic surface which consists of proteins, carbohydrates, and lipids) was used in the study.35 It was grown in suspension in Luria-Bertani (LB) broth (Fischer Scientific) at 37 °C and 25 rpm for 16 h.34 Before use, it was diluted with water to approximately 103 cfu/mL.
Simple Buffer System Tests
Whole human blood is a very complex system containing various different types of cells, lipids, proteins and ions. Prior to working with blood, it was desirable to test a simple buffer system to reduce the number of variables that could influence bacterial adhesion. A solution of phosphate-buffered saline (PBS) (Fisher Scientific) and bovine serum albumin (BSA) (Sigma-Aldrich) was chosen for this system. PBS was used to mimic the ions found in blood and BSA was added to represent the most abundant blood protein, albumin.
A solution was made containing 1 part 0.05% BSA, 2 parts 1X PBS and 1 part 103 cfu/mL MSSA bacteria diluted with water. The final solution of BSA, PBS, and bacteria contained approximately 100 cfu/400 μL. This concentration was verified by plating 200 μL of the solution on an LB agar plate, incubating overnight at 37 °C, and counting the number of resulting colonies.
Each of the coated blunt-nosed devices and centrifuge tubes were filled with 400 μL of the above solution. The samples were spun in a swinging bucket centrifuge (Eppendorf 5810 R) at 3200 RCF for 5 minutes to concentrate the bacteria to the bottom of the devices/tubes. Next, 350 μL of the supernatant was drawn off and discarded. (In initial studies, the supernatant was also interrogated by quantitative plating, but few to no bacterial were ever found in the supernatant. To save on materials, the supernatant was not plated further). The remaining 50 μL (the “pellet”) was aspirated with a pipette and deposited on an LB agar plate. After adding 70 μL of sterile deionized water to each device/tube, the water was pipetted up and down to remove any loosely adhered bacteria (the “wash”) and deposited on a separate LB agar plate.
These plates were incubated at 37 °C overnight and the resulting colonies were counted the next day. Those numbers were compared to the number of colony forming units originally added to the devices/tubes to determine how much bacteria could be removed with just aspiration and how much needed an addition rinse to be removed.
Whole Blood and Lysis Buffer Tests
Additionally, tests were performed using whole human blood inoculated with bacteria. The procedure was identical to the simple buffer system solution, except a blood solution replaced the PBS and BSA solution. Each 400 μL of the blood solutions contained 360 μL 0.005% Tween-20 (Sigma-Aldrich), 40 μL pooled whole human blood (Golden West Biologicals) and 10 μL of 103 cfu/mL MSSA. The low concentration of the detergent Tween-20 was chosen based on ability to lyse blood cell without being antimicrobial. The in-house developed lysis solution is able to preferentially burst red blood cells by a combination of osmotic pressure and preferential solubilization of the blood cellular membranes, while maintaining the integrity of the bacterial cell walls.
Results and discussion
All of the coatings, except the coatings from IST, were produced via dip-coating (Table 1). Dip coating is a useful process to obtain thin uniform films on substrates of various shapes. The dip coating process is divided into five stages: immersion, start-up, deposition, drainage, and evaporation. The deposition stage is a key stage where the thin layer deposits itself on the substrate as the substrate is withdrawn from the solution of coating material. It is necessary to extract the substrate at a constant uniform speed since; in general, the speed determines the thickness of the coating. While this technique produces high quality, uniform coatings; it requires precise control and a clean environment. Comparatively, the environment in the vacuum vapor delivery system used for IST’s coatings is in a vacuum, which inherently is very clean and eliminates any moisture variation which could affect surface modification coatings. Also, this process produces very smooth and uniform coatings (in the nm range), even in small areas because of the vaporized precursors.
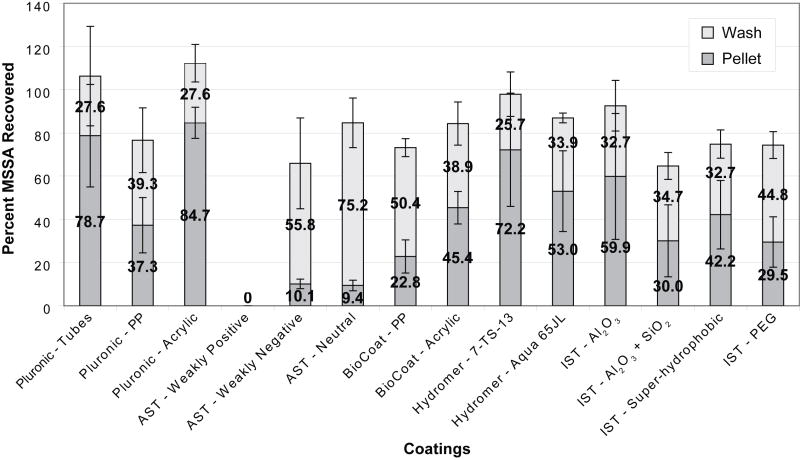
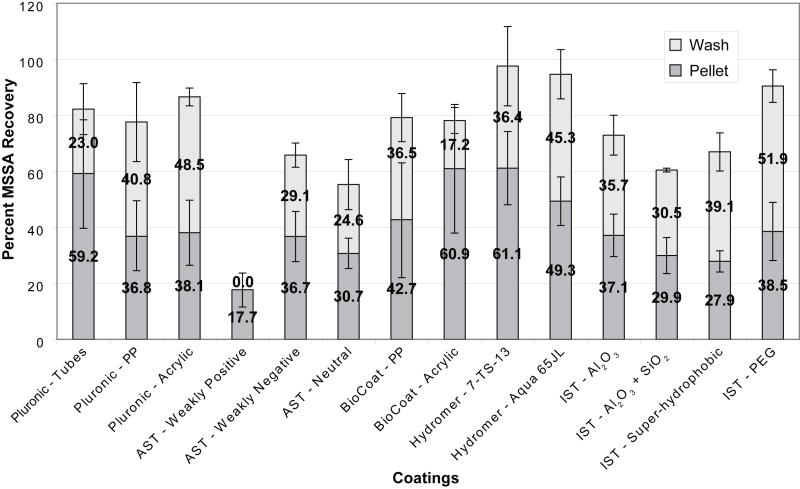
Figures 2 and 3 show the percentage of bacteria recovered from our coated devices and tubes when tests were run using the simple buffer system (Figure 2) and lysed whole blood (Figure 3). In these figures, each bar represents the average recovery observed for a single type of coating. The dark gray portions of the bars correspond to the amount of bacteria recovered from the pellet of each device or tube while the light gray portions of the bars show the additional residual bacteria recovered during the subsequent wash step. Since removal of the pellet involved a single aspiration and was less mechanically rigorous than the wash, higher pellet recovery rates were expected for coatings with greater resistances to bacterial adhesion.
Fig. 2.
Comparison of coatings in simple buffer system (PBS/BSA). The bottom (dark shading) bars represent the bacterial recovery from the pellet at the bottom of the disposable. The top (light shading) bars represent the bacterial recovery after 70 μL of deionized water were added to the chamber and pipetted up and down. Each bar represents the mean of at least three replicate experiments with a single standard deviation shown.
Fig. 3.
Comparison of coatings in pooled whole blood. The bottom (dark shading) bars represent the bacterial recovery from the pellet at the bottom of the disposable. The top (light shading) bars represent the bacterial recovery after 70 μL of deionized water were added to the chamber and pipetted up and down. Each bar represents the mean of at least three replicate experiments with a single standard deviation shown.
When assessing the performance of each coating, two main factors were considered: the overall recovery of bacteria as well as how much of the bacteria were recovered from the pellet versus from the wash. We rationalized that the bacteria collected in the pellet were less adhered to the surface than those recovered in the subsequent more vigorous wash step. Accordingly, the pellet recovery was an important factor to bear in mind and it was observed that the recovery varied considerably between coatings.
To determine whether the results were statistically significant, student t-tests were performed for each of the tested coatings against the controls (Pluronic-coated devices and Pluronic-coated commercial tubes). Summaries of the statistical analysis are shown in Tables 2 and 3.
Table 2.
This table summarizes the statistical significance of the recovery rates obtained from the simple buffer tests. Each row represents a single coating and the columns represent the control conditions to which they are being compared.
| Pellet Recoveries | Total Recoveries | |||
|---|---|---|---|---|
| Pluronic Coated Devices | Pluronic Coated Tubes | Pluronic Coated Devices | Pluronic Coated Tubes | |
|
| ||||
| AST - Weakly Positive | − | − | − | − |
| AST - Weakly Negative | − | − | 0 | − |
| AST - Neutral | − | − | 0 | − |
| BioCoat - PP | − | − | 0 | − |
| BioCoat - Acrylic | − | − | − | − |
| Hydromer - 7-TS-13 | + | 0 | + | 0 |
| Hydromer - Aqua 65JL | + | 0 | 0 | − |
| IST - Al2O3 | + | 0 | + | − |
| IST - Al2O3 + SiO2 | 0 | − | 0 | − |
| IST - Super-hydrophobic | 0 | − | 0 | − |
| IST - PEG | 0 | − | 0 | − |
Table 3.
This table summarizes the statistical significance of the recovery rates obtained from the lysed blood tests. Each row represents a single coating and the columns represent the control conditions to which they are being compared.
| Pellet Recoveries | Total Recoveries | |||
|---|---|---|---|---|
| Pluronic Coated Devices | Pluronic Coated Tubes | Pluronic Coated Devices | Pluronic Coated Tubes | |
|
| ||||
| AST - Weakly Positive | − | − | − | − |
| AST - Weakly Negative | 0 | − | 0 | 0 |
| AST - Neutral | 0 | − | − | − |
| BioCoat - PP | 0 | 0 | 0 | 0 |
| BioCoat - Acrylic | 0 | 0 | 0 | 0 |
| Hydromer - 7-TS-13 | + | 0 | + | 0 |
| Hydromer - Aqua 65JL | + | 0 | + | 0 |
| IST - Al2O3 | 0 | − | 0 | 0 |
| IST - Al2O3 + SiO2 | 0 | − | 0 | 0 |
| IST - Super-hydrophobic | 0 | − | 0 | 0 |
| IST - PEG | 0 | − | 0 | 0 |
Pluronic-coated Polypropylene Tubes and Blunt-nosed Devices (Control Conditions)
Polypropylene tubes and blunt-nosed devices were coated with Pluronic F127 and are considered our positive control conditions. Pluronic is a tri-block copolymer of poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO). Due to its PEOn-PPOm-PEOn configuration, the copolymers physically adsorb dissimilarly to surfaces of different hydrophobicities; on a hydrophilic surface the two terminal blocks of PEOn anchor to the surface compared to the attachment of the central block PPOm when the surface is hydrophobic.36 Since polypropylene is slightly hydrophobic, the PPOm block adheres to the surface, leaving the PEOn chains to be suspended in the adjacent solution, creating a brush conformation. The brush conformation of PEOn produces non-adhesive properties due to its highly hydrated polymer chains. These chains can be compacted by an approaching particle, which result in less-mobile polymer chains and a repulsive osmotic force. Both of these results discourage close contact of the particle and therefore, decrease adhesion.37, 38 Therefore, Pluronic-coated commercial polypropylene tubes and blunt-nosed devices are used as our positive controls.
The bars to the far left in each graph show the recovery obtained from 15 mL commercial polypropylene centrifuge tubes coated with Pluronic. Previous tests in our laboratory showed that these conditions yielded almost 100% recovery of MSSA, and subsequently we used these figures as the standards to which all the coatings would be compared. We found that approximately 100% of the bacteria were recovered from the control tubes when the simple buffer system was used (79% in the pellet and 28% in the wash). When lysed blood was used about 80% of the MSSA added was recovered (59% in the pellet and 23% in the wash).
In addition to the Pluronic-coated polypropylene tubes, we also coated polypropylene and acrylic blunt-nosed devices with Pluronic. These devices were machined, not molded like the commercial tubes, and were used as a second control condition to which we compared the coated devices’ recoveries. While the commercial tube recovery rates were considered to be the maximum possible under perfect conditions, the recovery rates from the Pluronic-coated blunt-nosed devices were in fact a more realistic control condition to which to compare the coatings because the surface was prepared in a non-proprietary method. The commercial tubes may have additional surface coatings or treatments that influence bacterial adhesion; our efforts at researching the manufacturing method were unproductive. Therefore, the machined blunt-nosed devices were used as our benchmarks.
When the simple buffer system was used with the polypropylene devices coated with Pluronic, we saw total recovery rates of approximately 80% with nearly equal amounts of bacteria coming from the pellet and wash. These figures remained roughly the same when blood was used, once again with similar recoveries coming from the pellet and wash.
When acrylic blunt-nosed devices were treated with Pluronic, recovery rates were comparable to those seen in the commercial tubes when both the simple buffer system and lysed blood were used. For the simple buffer system, recoveries were 85% in the pellet and 28% in the wash. For lysed blood, recoveries were 38% in the pellet and 49% in the wash. Overall, the Pluronic-coated acrylic devices performed better than the equivalent polypropylene devices. However, polypropylene is a much more common material in biomedical devices due to its ability to be autoclaved, its strength to withstand high centrifugation, and ease in molding. Subsequently, the polypropylene devices were of greater interest to us than the acrylic devices.
AST Coatings – Positive, Negative and Neutral Charged Coatings
In the simple buffer system, the weakly negative and neutral AST coatings had about 70% total recovery, which was comparable to Pluronic-coated devices. However, the weakly positive coating had 0% recovery. There were no bacteria in the wash or the pellet of the device coated with the weakly positive coating, presumably because the negatively-charged bacteria adhered to the positively-charged coating. Since MSSA has a slight negative charge in aqueous solutions, they most likely electrostatically adhered to the coating. Also, all AST coatings had very low pellet recoveries, indicating the bacteria were adhered to the coating surface, rather than concentrated into the pellet.
AST’s weakly positive coating rendered the lowest recovery rate of all the coatings when lysed blood was used with just 17% total recovery all from the pellet. AST’s other coatings also resulted in low overall recoveries when compared to Pluronic-coated devices using lysed blood. On the other hand, the neutral and negative coatings resulted in pellet recoveries statistically similar to those seen with the Pluronic-coated devices. More bacteria were collected in the pellet using pooled whole blood as compared to the simple buffer system presumably because of the additional components present in blood (such as cells, cell fragments, proteins, fat, etc). These components can interact with the bacteria and block the walls of the devices, which can interfere with the electrostatic association. Both of these factors could help to concentrate bacteria under centrifugation.
BioCoat Coatings – Hyaluronic Acid
The main component of BioCoat’s coatings is hyaluronic acid, a lubricant found in body tissue. Given its hydrophilic, biocompatibility, and non-thrombogenic nature, good interactions with the blood system were predicted. BioCoat’s coatings were applied to both polypropylene and acrylic blunt-nosed devices for the simple buffer system and the lysed blood experiments. An acrylic device was used in addition to polypropylene because the manufacturer advised that the coating might not adhere properly to polypropylene. In the simple buffer system, the acrylic device had a slightly better recovery in the pellet compared to the polypropylene device. However, the total recovery of the bacteria of both devices was around 75% when the wash was taken into account.
In the whole blood and lysis buffer test, little difference was observed between the polypropylene and acrylic devices. Both of the two materials had almost 80% recovery between the pellet and the wash. Once again, the acrylic device performed slightly better in the pellet recovery than the polypropylene device with 61% and 43%, respectively, confirming predictions of a better coating on an acrylic material.
Overall, regardless of the material or the experimental condition, devices treated with BioCoat’s coatings did not prevent bacterial adhesion better than equivalent devices treated with Pluronic. Under the best conditions, BioCoat’s coatings performed as well as Pluronic-coated controls. However, because this coating performed better on acrylic than the more common polypropylene, it is not as good of a candidate for devices that are required to be made of polypropylene.
Hydromer Coatings – Polyvinylpyrrolidone (Neutral and Hydrophilic Coatings)
Hydromer provided two coatings: 7-TS-13 and Aqua 65JL. The primary constituent of these coatings was stated to be polyvinylpyrrolidone, a transparent polymer which has chemical and biological inertness, low toxicity, and biocompatibility. Given these properties, the high pellet recoveries were expected. In the simple buffer system experiments, the 7-TS-13 coating gave very high bacterial recoveries in the pellet (72%) and when the pellet recovery was combined with the wash recovery (26%), almost 100% bacterial recovery was obtained. Both the pellet and total recoveries were comparable to those seen with the Pluronic-coated commercial tubes and significantly greater than the Pluronic-coated devices. The Aqua 65JL coating produced high recoveries; however, the total recovery was lower than that of the commercial tubes and was comparable to that of the Pluronic-coated devices.
Almost 100% total recovery was obtained from Hydromer’s two coatings when whole lysed blood was used. The pellet recovery for 7-TS-13 was 61% and Aqua65JL was 49%. Both the pellet and total recoveries were significantly better than the Pluronic-coated devices, and comparable to the commercial tubes treated with Pluronic.
IST Coatings – Hydrophilic and Super-hydrophobic Coatings
Four coatings were obtained from IST: Al2O3, SiO2 (atomic layer deposited), super-hydrophobic, and poly(ethylene glycol) (PEG). In the simple buffer test, the SiO2, super-hydrophobic and PEG coatings did not perform well (around 65% total recovery) when compared to the other IST coating, Al2O3, which was the only IST coating with higher recoveries than the Pluronic-coated devices (around 90% total recovery). The disappointing PEG results were surprising given its reputation as a biocompatible cell repellent surface. PEG is a water-soluble, nontoxic, and non-immunogenic polymeric material that reduces non-specific effects of protein adsorption and colloidal aggregation. The large number of hydrogen bonds between PEG and water molecules produce large repulsive forces with proteins, resulting in resistance to non-specific protein binding.39, 40 The degree to which a PEG coating successfully reduces bacterial adhesion is dependent on the length of the polymer. The PEG coating available through IST was mPEG (EGn where n = 9), which is a relatively short polymer with an average molecular weight of around 400 Da. Comparatively, PEG chains with high molecular weight (n ~= 30) are frequently grafted onto the surface of materials to improve the biocompatibility and decrease bacterial adhesion.41–44
In the lysed blood tests, IST’s PEG coating had the best result of the four IST coatings tested, which was more consistent with our hypothesis based on PEG’s exceptional resistance to protein adsorption. The PEG coated devices had almost 90% total recovery; however the pellet recovery was quite low at 39%, indicating most of the bacteria were weakly adhered to the surface and became dislodged during the wash step. The other three coatings (Al2O3, SiO2, super-hydrophobic) each had less than 80% total recovery and none of them had over 50% of the bacteria present in the pellet. The Al2O3 coating was tested because the devices could be coated quickly, even though Al2O3 has been found to attract bacteria.45 The SiO2 coating is essentially a glass coating on the polypropylene blunt-nosed device.46 Untreated glass can adhere biological organisms, suggesting that the bacteria could have adhered to the coating. The low recovery of the super-hydrophobic coating was unexpected since the aqueous solution should have very minimal interactions with the coating wall due to its high hydrophobicity. Super-hydrophobic coatings have contact angles greater than 150° but can have a low or high adhesive force.47 On these devices, the aqueous solutions had a low adhesive force, and adopted a non-wet-contact mode, so it was expected the bacterial solutions would have negligible contact with the surface. Some evidence exists to support the hypothesis that super-hydrophobic coatings can prevent biofilm formation and bacterial colonization.48, 49 However, with 67% total recovery and, of that, only 28% in the pellet, the performance of the super-hydrophobic coating was lack luster. It may be that in the conditions of our experiments, the centrifugal forces overcome the repulsive hydrophobic forces resulting in poorer performance than may be achieved in other experimental parameters (e.g. implantable devices).
Conclusions
In these experiments, commercial centrifuge tubes and machined polypropylene and acrylic devices were treated with several different coatings in an effort to reduce bacterial adhesion under centrifugation. It was found that the range of bacteria recovered from the devices and tubes varied considerably with some coatings resulting in almost no recovery, and others allowing almost 100% recovery.
Of all the coatings tested, two in particular showed the most promise: Hydromer’s 7-TS-13 and Aqua 65JL. The 7-TS-13 coating resulted in total recoveries comparable to those seen with Pluronic-coated commercial tubes or Pluronic-coated devices, when both lysed blood and the simple buffer system were used. This was also true for the Aqua 65JL coating except its total recoveries were slightly lower than those corresponding to Pluronic-coated commercial tubes when the lysed blood model was used.
When just the recovery rates from the pellets were considered, these coatings still outperformed the others tested. When either the simple buffer or lysed blood tests were used, both Hydromer coatings showed pellet recoveries similar to the coated commercial tubes and better than the Pluronic-coated blunt-nosed devices. Although the 7-TS-13 coating was not statistically better than the 65JL coating, its average recoveries were higher and it was the only coating of all those tested that out-performed the Pluronic-coated devices under the simple buffer and lysed blood conditions for both total recovery and pellet recovery. However, because both of these coatings were produced via dip coating, it could be challenging to use this type of coating for small areas or feature sizes.
By studying this array of coatings, we were able to gain insight as to the best coatings to minimize MSSA adhesion to coated polypropylene and acrylic devices. Based on the obtained data, we were able to determine that Hydromer’s 7-TS-13 coating was the most effective at resisting bacterial adhesion, even in devices in which the bacterial interactions with the surface was driven via centrifugation. In the development of in vitro diagnostic devices for the detection of bacteremia, coatings such as Hydromer’s 7-TS-13, are critical so that the low numbers of bacteria found in solution are not lost to non-specific binding to the surface. With the increasing focus on miniaturization and microfluidic devices that have high surface area to volume ratios, coatings that reduce bacterial adhesion are of even greater importance.
Supplementary Material
Acknowledgments
The authors thank Michael Zeiss and Jasmin Loeder for their technical support on this project; Holger Wirz for the design of the devices; Doug Foss and Felix Schmid for the fabrication of the devices; and Jean Lee for the use of the ethylene oxide sterilization system. We would also like to thank Dr. Jennifer Campbell for her contribution to the table of contents illustration. This work was supported by Grant R01AI090815 from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health. The NIH had no role in writing this paper; the study design; or the collection, analysis and interpretation of data.
Appendix A. Supplementary Data
Details on the fabrication of the blunt-nosed devices are specified in the Supplementary Data.
References
- 1.Dorn GL, Haynes JR, Burson GG. J Clin Microbiol. 1976;3:251–257. doi: 10.1128/jcm.3.3.251-257.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herlich MB, Schell RF, Francisco M, Le Frock JL. J Clin Microbiol. 1982;16:99–102. doi: 10.1128/jcm.16.1.99-102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Karlin A, Loike JD, Silverstein SC. Proc Natl Acad Sci USA. 2002;99(12):8289–8294. doi: 10.1073/pnas.122244799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werner AS, Cobbs CG, Kaye D, Hook EW. JAMA. 1967;202(3):199–203. [PubMed] [Google Scholar]
- 5.Wain J, Diep TS, Ho VA, Walsh AM, Hoa NTT, Parry CM, White NJ. J Clin Microbiol. 1998;36(6):1683–1687. doi: 10.1128/jcm.36.6.1683-1687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Brien JM, Ali NA, Aberegg SK, Abraham E. Am J Med. 2007;120(12):1012–1022. doi: 10.1016/j.amjmed.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 7.American Crit Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 8.Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, KLK, Parsonnet J, Panzer R, Orav EJ, Snydman DR, Black E, Schwartz JS, Moore R, Johnson BL, Platt R. JAMA. 1997;278(3):234–240. [PubMed] [Google Scholar]
- 9.Banerjee I, Pangule RC, Kane RS. Adv Mater. 2011;23(6):690–718. doi: 10.1002/adma.201001215. [DOI] [PubMed] [Google Scholar]
- 10.Klemm P, Vejborg RM, Hancock V. Appl Microbiol Biotechnol. 2010;88(2):451–459. doi: 10.1007/s00253-010-2805-y. [DOI] [PubMed] [Google Scholar]
- 11.Vasilev K, Cook J, Griesser HJ. Expet Rev Med Dev. 2009;6(5):553–567. doi: 10.1586/erd.09.36. [DOI] [PubMed] [Google Scholar]
- 12.Katsikogianni M, Missirlis YF. Eur Cell Mater. 2004;8:37–57. doi: 10.22203/ecm.v008a05. [DOI] [PubMed] [Google Scholar]
- 13.Boks NP, Norde W, van der Mei HC, Busscher HJ. Microbiology. 2008;154(10):3122–3133. doi: 10.1099/mic.0.2008/018622-0. [DOI] [PubMed] [Google Scholar]
- 14.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Gotz F. Mol Microbiol. 1996;20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 15.Morra M, Cassinelli C. J Biomater Sci Polymer Edn. 1997;9:55–74. doi: 10.1163/156856297x00263. [DOI] [PubMed] [Google Scholar]
- 16.Vaudaux P, Yasuda H, Velazco MI, Huggler E, Ratti I. J Biomater Appl. 1990;5:134–153. doi: 10.1177/088532829000500204. [DOI] [PubMed] [Google Scholar]
- 17.An YH, Friedman RJ. J Biomed Mater Res. 1998;43:338–348. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Abu-Lail NI, Camesano TA. Langmuir. 2006;22(17):7296–7301. doi: 10.1021/la0533415. [DOI] [PubMed] [Google Scholar]
- 19.Cordero J, Munuera L, Folgueira MD. Injury. 1996;27(Suppl 3):SC34–37. doi: 10.1016/0020-1383(96)89030-9. [DOI] [PubMed] [Google Scholar]
- 20.Speranza G, Gottardi G, Pederzolli C, Lunelli L, Canteri R, Pasquardini L, Carli E, Lui A, Maniglio D, Brugnara M, Anderle M. Biomaterials. 2004;25(11):2029–2037. doi: 10.1016/j.biomaterials.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 21.Gottenbos B, van der Mei HC, Busscher HJ. J Biomed Mater Res. 2000;50(2):208–214. doi: 10.1002/(sici)1097-4636(200005)50:2<208::aid-jbm16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 22.Gottenbos B, Busscher HJ, van der Mei HC, Nieuwenhuis P. J Mater Sci: Mater Med. 2002;13(8):717–722. doi: 10.1023/a:1016175502756. [DOI] [PubMed] [Google Scholar]
- 23.Henriques M, Azeredo J, Oliveira R. Col Surf B Biointerf. 2004;33:235–241. [Google Scholar]
- 24.Gorth DJ, Puckett S, Ercan B, Webster TJ, Rahaman M, Bal BS. Int J Nanomedicine. 2012;7:4829–4840. doi: 10.2147/IJN.S35190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu CC, Williams DF. Am J Surg. 1984;147:197–204. doi: 10.1016/0002-9610(84)90088-6. [DOI] [PubMed] [Google Scholar]
- 26.James NR, Jayakrishnan A. Biomaterials. 2003;24:2205–2212. doi: 10.1016/s0142-9612(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 27.Kiremitci-Gumusderelioglu M, Pesmen A. Biomaterials. 1996;17:443–449. doi: 10.1016/0142-9612(96)89662-1. [DOI] [PubMed] [Google Scholar]
- 28.Terada A, Okuyama K, Nishikawa M, Tsuneda S, Hosomi M. Biotechnol Bioeng. 2012;109(7):1745–1754. doi: 10.1002/bit.24429. [DOI] [PubMed] [Google Scholar]
- 29.Terada A, Yuasa A, Kushimoto T, Tsuneda S, Katakai A, Tamada M. Microbiology. 2006;152(12):3575–3583. doi: 10.1099/mic.0.28881-0. [DOI] [PubMed] [Google Scholar]
- 30.Scheuerman TR, Camper AK, Hamilton MA. J Col Interf Sci. 1998;208:23–33. doi: 10.1006/jcis.1998.5717. [DOI] [PubMed] [Google Scholar]
- 31.Morgan TD, Wilson M. J Appl Microbiol. 2001;91(1):47–53. doi: 10.1046/j.1365-2672.2001.01338.x. [DOI] [PubMed] [Google Scholar]
- 32.Taylor RL, Verran J, Lees GC, Ward AJP. J Mater Sci: Mater Med. 1998;9:17–22. doi: 10.1023/a:1008874326324. [DOI] [PubMed] [Google Scholar]
- 33.Boyd RD, Verran J, Jones MV, Bhakoo M. Langmuir. 2002;18:2343–2346. [Google Scholar]
- 34.Razatos A, Ong YL, Boulay F, Elbert DL, Hubbell JA, Sharma MM, Georgiou G. Langmuir. 2000;16(24):9155–9158. [Google Scholar]
- 35.Tortora GJ, Funke BR, Case CL. Microbiology: An Introduction. 9. Benjamin Cummings; 2006. [Google Scholar]
- 36.Schroen CGPH, Cohen Stuart MA, Maarschalk KV, van der Padt A, Vantriet K. Langmuir. 1995;11:3068–3074. [Google Scholar]
- 37.Nejadnik MR, Olsson ALJ, Sharma PK, van der Mei HC, Norde W, Busscher HJ. Langmuir. 2009;25(11):6245–6249. doi: 10.1021/la9001169. [DOI] [PubMed] [Google Scholar]
- 38.Nejadnik MR, van der Mei HC, Busscher HJ, Norde W. Appl Environ Microbiol. 2008;74(3):916–9. doi: 10.1128/AEM.01557-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langer R, Tirrell DA. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 40.Zhang M, Desai T, Ferrari M. Biomaterials. 1998;19:953–960. doi: 10.1016/s0142-9612(98)00026-x. [DOI] [PubMed] [Google Scholar]
- 41.Benhabbour SR, Sheardown H, Adronov A. Macromolecules. 2008;41:4817–4823. [Google Scholar]
- 42.Gombotz WR, Guanghui W, Horbett TA, Hoffman AS. J Biomed Mater Res. 1991;25(12):1547–1562. doi: 10.1002/jbm.820251211. [DOI] [PubMed] [Google Scholar]
- 43.Prime KLWGM. JACS. 1993;115:10714–10721. [Google Scholar]
- 44.Zhu B, Eurell T, Gunawan R, Leckband D. J Biomed Mater Res. 2001;56(3):406–416. doi: 10.1002/1097-4636(20010905)56:3<406::aid-jbm1110>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 45.Li B, Logan BE. Colloids Surf B: Biointerfaces. 2004;36:81–90. doi: 10.1016/j.colsurfb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Saldarriaga Fernández IC, van der Mei HC, Lochhead MJ, Grainger DW, Busscher HJ. Biomaterials. 2007;28(28):4105–4112. doi: 10.1016/j.biomaterials.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Jiang L. Adv Mater. 2007;19:3423–3424. [Google Scholar]
- 48.Everaert EPJM, Mahieu HF, van de Belt-Gritter B, Peeters AJGE, Verkerke GJ, van der Mei HC, Busscher HJ. Arch Otolaryngol Head Neck Surg. 1999;125:1329–1332. doi: 10.1001/archotol.125.12.1329. [DOI] [PubMed] [Google Scholar]
- 49.Tsibouklis J, Stone M, Thorpe AA, Graham P, Peters V, Heerlien R, Smith JR, Green KL, Nevell TG. Biomaterials. 1999;20(13):1229–1235. doi: 10.1016/s0142-9612(99)00023-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.