The primate order is a monophyletic group thought to have diverged from the Euarchonta more than 65 million years ago (mya).1 Recent paleontological and molecular evolution studies place the last common ancestor of primates even earlier (≥ 85 mya).2 More than 300 extant primate species are recognized today,3,4 clearly emphasizing their diversity and success. Our understanding of the evolution of primates and the composition of their genomes has been revolutionized within the last decade through the increasing availability and analyses of sequenced genomes. However, several aspects of primate evolution have yet to be resolved. DNA sequencing of a wider array of primate species now underway will provide an opportunity to investigate and expand upon these questions in great detail. One of the most surprising findings of the human (Homo sapiens) genome project was the high content of repetitive sequences, in particular of mobile DNA.5 This finding has been replicated in all available and analyzed primate draft genome sequences analyzed to date.5–7 In fact, transposable elements (TEs) contribute about 50% of the genome size of humans,5 chimpanzees (Pan troglodytes),6 and rhesus macaques (Macacca mulatta).7 The proportion of TEs among the overall genome content is likely even higher due to the decay of older mobile elements beyond recognition, rearrangements of genomes over the course of evolution, and the challenge of sequencing and assembling repeat-rich regions of the genome.8,9 Retrotransposons (see glossary) – in particular L1, long interspersed element 1 (LINE1), and Alu, a short interspersed element (SINE) – are prominent in primate genomes, and have played a major role in genome evolution and architecture. The evolution and success of the primate-specific LINE and SINE subfamilies (L1 and Alu in particular), their application in phylogenetic studies, and their impact on the architecture of primate genomes will be the focus of this review. In addition, we will briefly cover the emergence and impact of SVA (SINE-R/VNTR/Alu) – a composite retrotransposon of relatively recent origin – and of other SINEs that are not common to all primates.
LINE AND SINE BIOLOGY
The evolution of retrotransposons has been impacted not only by commonly considered aspects such as population genetics and genetic selection, but also by their amplification mode and insertion mechanism. Consequently, it is important to have some general understanding about the unique features and biology of retrotransposons. We discuss this briefly in the following sections. For further details we refer to other recent reviews.10–14 Occasionally, LINEs and SINEs are referred to as retrotransposons and retroposons, respectively. In this review, we use the term retrotransposon for all non-LTR (Long Terminal Repeat) retroelements, if not otherwise indicated.
TEs are classified into different groups on the basis of their transposition mechanism and family-specific characteristics. Primate-specific non-LTR retrotransposons, such as L1, Alu, and SVA (see Text Box and Fig. A) belong to the group of Class I elements and propagate via a “copy and paste” mechanism using an RNA intermediate.13,15 Newly integrated retrotransposon insertions are usually flanked by a short stretch (6–20bp) of duplicated unique host DNA called target site duplications (TSDs) (see glossary, Box 1).16,17 In primates, L1 appears to be the only currently active autonomous retrotransposon. Autonomous retrotransposons encode the required enzymatic machinery to copy themselves.18 L1 shows a strong cis preference in vitro, meaning that the L1 RNA recruits its own translated proteins during retrotransposition.19–21 However, the enzymatic machinery of L1 is also known to insert non-autonomous retrotransposons such as Alu elements into the genome.13,22 The vast majority of retrotransposon insertions in primate genomes are believed to insert into a genome via target-primed reverse transcription (TPRT; Fig. 1, see also glossary).23,24 However, non-classical insertion pathways have also been identified that are far less frequently utilized.25–27
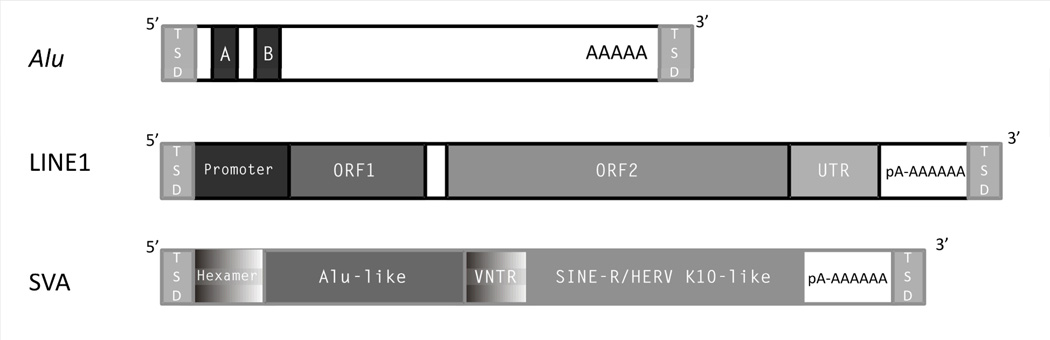
Box 1 (with Fig. A).
A full-length L1 is ~6kb in length, contains an internal polymerase II promoter, two ORFs, 3’ and 5’ UTRs, and terminates in a polyadenylation signal (indicated as pA in Fig. A) followed by a polyA-tail (Fig. A). L1s are often 5’ truncated, inverted, rearranged, and involved in transduction events.5–7,12,59 Most L1 insertions are severely truncated upon insertion.5,93 Alu elements are dimeric, ~300bp long elements that do not encode proteins, contain a polymerase III promoter, and end in a polyA-tail (Fig. A).12,13,51 Full-length SVA elements are composite elements named after its main components SINE, VNTR (variable number of tandem repeats), and Alu.140 They are non-autonomous retrotransposons composed of five different segments (Fig. A). From 5’ to 3’, they contain a hexamer simple repeat region of variable length; an Alu homologous region composed of two antisense Alu fragments, including additional sequence of unknown origin; a VNTR region; a SINE region derived from the 3’end of the env-gene and the 3’LTR-region of HERV-K10, an endogenous retrovirus; and a polyadenylation signal followed by a polyA-tail.123,124 As a consequence of the VNTR region, full-length SVA elements can vary greatly in size. Due to similar insertion characteristics SVA elements are thought to use the L1 machinery for retrotransposition.123,124 At present, SVA elements are not very well studied, and the concrete transcription mechanism (e.g. polymerase preference) and promoter site are subject to debate.
Box 1, Figure A. Illustration of L1, Alu, and SVA.
Full-length retrotransposons are not drawn to scale. The 5’ region of Alu elements contain an internal RNA polymerase III promoter (A and B boxes). The internal polymerase II promoter of L1s is located within the 3’UTR. The ORF1 of L1 elements encodes for an RNA-binding protein (ORF1p), and ORF2 encodes a protein (ORF2p) with endonuclease and reverse transcriptase activities. The SVA element represents a composite retrotransposon without coding sequence. TSD means target site duplication, pA stands for polyadenylation site.
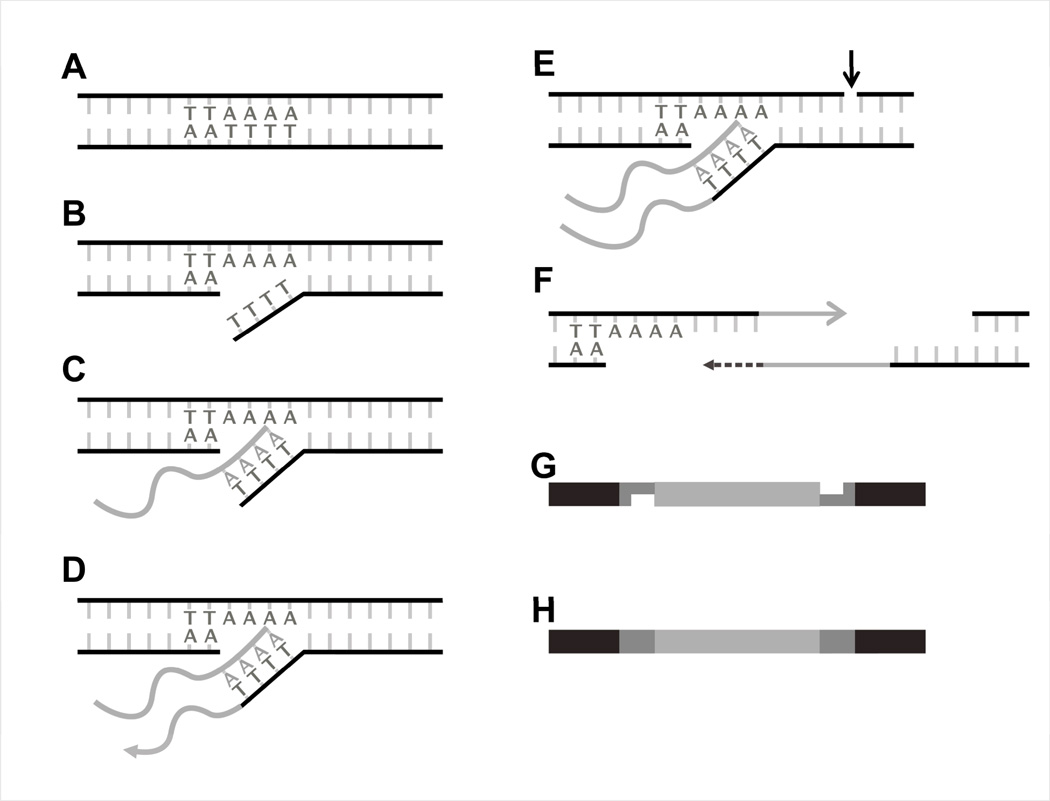
Figure 1. Retrotransposition via target-primed reverse transcription (TPRT).
L1, SVA, and Alu elements are thought to insert into the genome through a mechanism called target-primed reverse transcription (TPRT). (A) Shown is host DNA with a predicted target site. (B) The L1 endonuclease encoded by the ORF2 loosely recognizes a target site (5’-TTTTAA-3’) and nicks the bottom strand of the host DNA.16,141 (C) The polyA-tail of an mRNA intermediate (grey line with As) of an L1, SVA, or Alu element binds complementary to the cleaved TTTT overhang and (D) is reverse transcribed by the enzymatic activity of ORF2 protein (D).142 (E) The following steps of second-strand DNA cleavage and (F) second strand DNA synthesis are not well understood. (G) Illustration of the integration of the new retrotransposon insertion (light grey) into the host DNA (black). Medium grey are incomplete TSDs (H) The retrotransposon insertion is flanked by TSDs (medium grey).
L1 AND Alu ARE DRIVERS OF GENOME EXPANSION
With the availability of completed genome sequences, our understanding of the evolution and impact of retrotransposons upon primate genomes has been revolutionized. However, even a fully sequenced genome reveals only selective information and allows – at best – a narrow window into the current state of a genome. Most recently integrated “young” elements are subject to neutral selection strongly suggesting that the vast majority of retrotransposon insertions are neutral residents in primate genomes.28 Under neutral selection, only 1/(2Ne) new insertions (with Ne being the effective population size) reach fixation in a population.10 Consequently, a large fraction of novel retrotransposon insertions are lost over the course of evolution. At present, three primate genomes – H. sapiens, P. troglodytes, and M. mulatta – have been sequenced and analyzed. An assembled draft genome sequence derived from an orangutan of Sumatran origin (Pongo abelii) is already available and expected to join the analyzed genomes in the near future. In addition, several smaller scale retrotransposon studies using more diverged primate species have provided insights into retrotransposon evolution and amplification patterns.29–32 The overall physical expansion of primate genomes is driven by repeats, with L1 and Alu elements being the major contributors.31 Retrotransposons accumulate in primate genomes, due to the imbalance between their insertion and removal rates such as ectopic recombination. Accordingly, the retrotransposon composition of primate genomes is composed of both old and new elements.
In general, L1 and Alu elements appear to have remained active throughout primate evolution.5–7,30,31,33,34 As L1 originated well before the origin of primates (at least 170 mya),35 primate genomes contain L1 insertions predating the origin of primates, as well as more recent primate-specific insertions. In contrast, Alu elements are unique to primate genomes. Despite their relatively recent origin, Alu elements have amplified to more than one million copies and account for ~10% of the genome mass in all three sequenced primate genomes.5–7 With ~17% of the overall genome content, L1 is arguably the most successful and only currently known active autonomous retrotransposon in primates. L1 is responsible not only for its own retrotransposition, but also for the insertion of non-autonomous elements and processed pseudogenes.5,19,36 Consequently, about one third of the genome mass of all primate genomes analyzed to date is derived from L1 retrotransposition related events.37 In addition, in some primate species (e.g., human) L1 is at present the only active driver of retrotransposition, due to the lack of LTR retrotransposon activity (i.e. endogenous retroviruses).12
NUCLEOTIDE SUBSTITUTIONS AND CONCEPT OF RETROTRANSPOSON SUBFAMILIES
Retrotransposons have evolved continuously throughout primate evolution
Sequence alterations of retrotransposons are caused by random mutations at a neutral substitution rate upon insertion and/or nucleotide substitutions after insertion.28 Consequently, older retrotransposons contain on average more substitutions than younger insertions. Thus, the average substitution rate can be utilized to estimate the age of retrotransposon insertions in primate lineages. To estimate the age of retrotransposon insertions, it is crucial to distinguish between CpG (see glossary) and non-CpG bases because CpG sites have a higher mutation rate.38–42 This is of particular interest for Alu elements, as 30% of all CpG sites reside within them.43 Altogether, more than 40% of CpG dinucleotides are found within TEs in primate genomes.5
Nucleotide substitutions can alter the ability of retrotransposons to mobilize and create new copies. It has been proposed that host selective pressure (e.g. host defense mechanisms) against retrotransposons is a driver of retrotransposon evolution.44 This scenario, similar in nature to infectious disease host interactions, creates a constant loop of repression and escape. Host factors evolve constantly to keep retrotransposons in check, and selection pressure drives the evolution of retrotransposons and the creation of new subfamilies. The concept of subfamilies within a retrotransposon family was first suggested after the identification of species-specific substitutions.45,46 Subfamilies can be constructed through the identification of diagnostic mutations, which are shared by more retrotransposons than expected through random mutations.8 Reconstruction of retrotransposon subfamily interrelationships indicates hierarchical characteristics, with the youngest subfamilies containing the most, and oldest subfamilies the least, diagnostic mutations.13 Some subfamilies have been identified that likely arose through gene conversion (e.g. in some platyrrhines),30 a mechanism that had been suggested previously.47,48 Considering the average random substitution rate within each subfamily and the range of divergence from the consensus sequence between members of a particular subfamily, we are able to reconstruct its reproductive history. Network phylogenetic analyses seem beneficial for the reconstruction of retrotransposon relationships as they allow for persisting nodes, leading to multiple branching events commonly observed in retrotransposon phylogenies, in particular with Alu elements.49,50
EVOLUTION OF ALU AND L1 SUBFAMILIES IN PRIMATE GENOMES
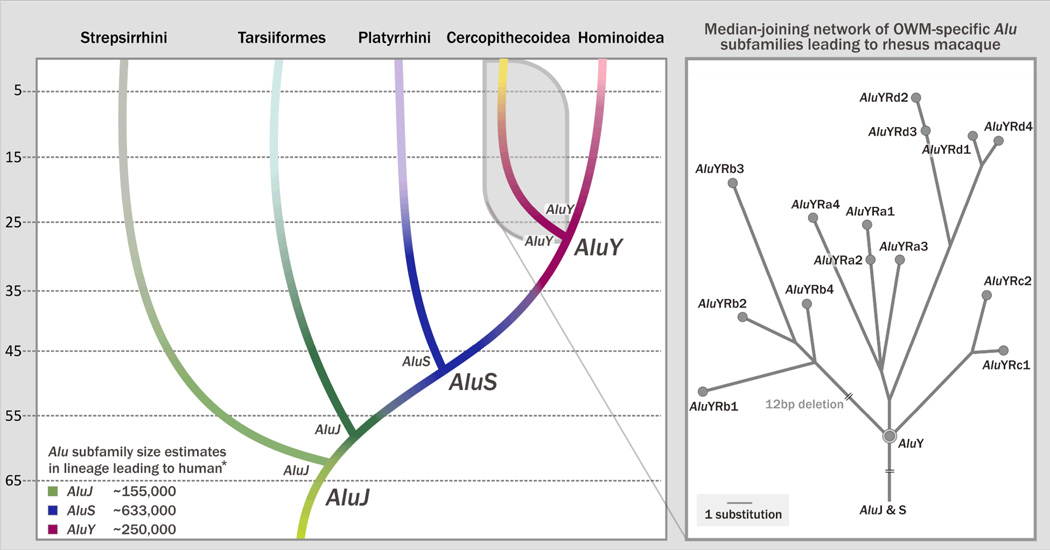
The identification of subfamily structure has led to a better understanding of the relationship between retrotransposon and primate genome evolution. Alu elements are specific to primates. (The origin of Alu elements is for instance reviewed in detail in Roy-Engel et al.)51 Alu subfamilies have been grouped together into three major subfamilies. The oldest subfamilies belong to AluJ; intermediates are members of AluS; and the youngest insertions belong to AluY (Fig. 2).13,52,53 AluJ subfamilies were actively amplifying early in primate evolution and can be detected in all primates. The deepest primate divergence falls into the period when the AluJ subfamilies were expanding.29,34 The Alu lineage in the Tarsiiformes, a sister group to anthropoid primates, might also have been derived from AluJ.54–56 Prior to the divergence of platyrrhines and catarrhines, AluS derived from AluJ and successively took over amplification approximately 55 mya. More recently, AluY evolved from AluS subfamily members and succeeded in the catarrhine lineage.57,58 Detailed reconstruction of Alu subfamilies shows parallel retrotransposition activity of several different subfamilies in any given primate species.13,49,50,59 Some of these subfamilies can be short- or long-lived, with or without generation of new subfamilies. Consequently, the parallel evolution of several Alu subfamilies and lineages throughout primate evolution has created a diverged, “bush-like” picture with several branches and sub-branches, and each primate lineage possessing its own unique network of Alu subfamilies (Fig. 2).
Figure 2. Alu subfamily evolution in primates.
The evolution of Alu elements in primate genomes is roughly illustrated. The left panel shows the three major Alu subfamilies AluJ (green), S (blue), and Y (red). The range of their activity and continuous evolution is indicated through a color gradient. The estimated sizes of AluY, S, and J subfamilies, drawn from Wang et al.,143 are given at the bottom left. The major Alu subfamily thought to be active at the time of divergence of each lineage is shown at the base of each lineage branch. Lineage specific subfamilies are likely derived from that subfamily. The color gradient within each lineage branch indicates that Alu subfamilies continued to evolve in each lineage and created lineage-specific subfamilies. Each major subfamily contains several subfamilies. Several different Alu subfamilies are commonly active in parallel and often evolve, causing diverged Alu subfamily networks. On the right the evolution of lineage specific Alu subfamilies in the Cercopithecoidea lineage leading to rhesus macaque (M. mulatta) is exemplified. The network was reconstructed with Alu subfamily data from Han et al. with permission from the original publisher (Science).59
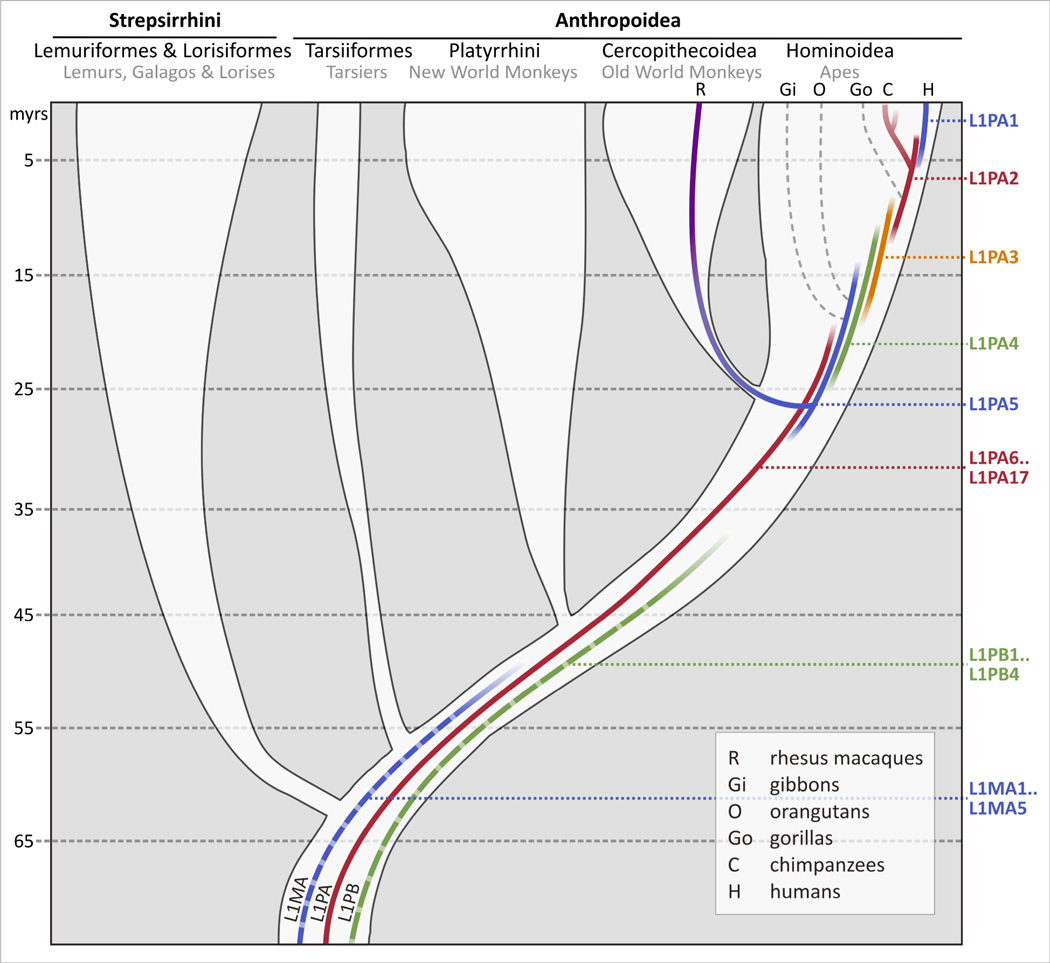
The evolution of L1 in diverse primates is altogether less well characterized than that of Alu, with most of our understanding derived from detailed analyses of the three sequenced genomes – in particular the human genome. While the existence of more than one L1 subfamily within a species is common, most studies point toward the propagation of a single L1 lineage with a linear evolution pattern in mammalian genomes over prolonged time periods.60–65 However, the coexistence of two or more L1 lineages over prolonged periods of time has been reported in some primates.8 Early in primate evolution as many as three L1 lineages – L1MA4-1, L1PB3-1, and L1PA17-1 – have been active in parallel for up to 30 million years (myrs, Fig. 3).60 Intriguingly, the 5’UTRs (untranslated regions) of these three lineages were clearly distinct and the overall combined retrotransposition rate was not exceedingly high, indicating that these L1 lineages might have competed for host factors.60 L1PA succeeded and has remained active within the anthropoid lineage leading to human (Fig. 3).8
Figure 3. Evolution of L1 in primates.
The evolution of L1 in primates on the basis of analyses of the human, chimpanzee, and rhesus macaque genome sequences is loosely illustrated. Fading of the lines indicates that the time span of subfamily activity is roughly estimated. In general, average age estimates of the different subfamilies were taken from Khan et al.;60 the activity range of L1PA1-5 was estimated on the basis of lineage-specific analyses.59,78,144 The L1PB and L1MA lineages are not shown as separate subfamilies, and L1PA6-17 subfamilies have been combined. Subfamilies L1PA1-5 are shown as separate lines to illustrate a typical pattern for the evolution of L1 subfamilies. All lineages show a similar pattern of overlapping activity of different subfamilies. The figure shows that L1PA1 is presently active in human; chimpanzee-specific subfamilies are derived from L1PA2, with parallel evolution of two L1 lineages over time (branched line); and L1PA5 was the founder for OWM-specific subfamilies including rhesus macaque (shown).
An analysis of orthologous L1 sites through the lens of the human genome has indicated the absence of L1PA5 insertions in baboon, and activity of L1PA7 before and after the divergence of the Cercopithecidae (Old World monkeys, OWMs) from the hominoid lineage.60 However, a subsequent analysis of the M. mulatta genome revealed that L1PA5 gave rise to the OWM-specific L1 lineage.7,59 The origin of lineage-specific L1 insertions in the OWM lineage may have occurred early in L1PA5 evolution, causing mostly lineage-specific insertions in both OWMs and hominoids. This illustrates, conclusions extrapolated from the perspective of one genome onto another need to be regarded with caution.
RETROTRANSPOSITION INSERTION RATE VARIATION DURING PRIMATE EVOLUTION
The propagation of lineage-specific retrotransposon subfamilies and the accumulation of their respective copy numbers in different primate taxa vary greatly over the evolution of primates. Retrotransposition rates have varied widely over the last 65 myrs of primate evolution, with periods of low and high activity.8,53,60,66 Moreover, the retrotransposition rate varied greatly between different lineages. For example, the Lemur catta (ringtailed lemur) genome appears to contain the lowest Alu density yet identified in primates whereas the Callithrix jacchus (common marmoset) genome shows evidence for the highest Alu density.32,34 A burst of both L1 and Alu insertions occurred ~35–40 mya in anthropoid primates.60,66 Since then, overall the collective retrotransposition rate seems to have decelerated in anthropoids. The propagation rate of both L1 and Alu appears to be higher in OWMs compared to human and chimpanzee; and in humans, the retrotransposition rate of Alu elements appears to be higher than that of chimpanzee.6,31,34,67,68
Many factors can impact the viability of actively mobilizing retrotransposons and their propagation rates. Highly active retrotransposons are very susceptible to loss or saturation during speciation events or population bottlenecks (see glossary), as they are commonly polymorphic within a population.10,69 Consequently, the number of active retrotransposons can vary greatly and affect the amplification rate (increase, decrease, or no change) after speciation or a bottleneck. In addition, it has been proposed that interaction of host factor(s) with the enzymatic L1 machinery could cause periods of high and low activity.60,70 For example, members of the ABOBEC family (see glossary) have been found to inhibit L1 and Alu.71–73 Conceivably, environmental stress factors could alter the retrotransposition rate.74 Different factors may have contributed to retrotransposition rate variations during primate evolution.
RETROTRANSPOSON AMPLICATION MODEL IN PRIMATE GENOMES
The previous sections describing the dynamics of retrotransposons have provided the framework to address these primary questions: How do we distinguish active from inactive retrotransposons? How can this be utilized to study primate evolution? Based on the typical distribution pattern of SINEs and LINEs observed in primate genomes, we know that only a small fraction of retrotransposons are capable of retrotransposition at any given time. This is best characterized by a modified “master-gene” model.49,50,75 In this model, “master” elements of a subfamily create copies over a prolonged time period with a few offspring elements that generate the bulk of de novo insertions.75,76 These highly active elements are usually relatively short-lived due to their highly deleterious nature to their host.75
The identification of potentially active L1s is relatively straightforward, as only full-length elements with intact open reading frames (ORFs, see glossary) are capable of retrotransposing themselves. In primate genomes, only a small number of L1 insertions satisfy these requirements as the majority of L1s are truncated upon insertion and/or have accumulated random mutations. For example, in the human diploid genome, only about 80–100 L1s are considered retrotranspositionally competent on the basis of their nucleotide sequence.77 This number appears even lower for the chimpanzee and rhesus macaque genomes with five and nine intact elements, respectively.59,78 Consequently, only a limited number of L1s – in particular, members of the youngest subfamilies – are active, and an even smaller number of L1s contribute to the bulk of novel insertions.
The identification of Alu source elements13,79 (see glossary) is far more demanding than for L1, as they do not contain coding sequence and are highly similar to each other. Recent research efforts have identified several factors that alter the retrotransposition activity of Alu elements. These include polyA-tail length, nucleotide substitutions within the polyA-tail, distance of the polymerase III TTTT termination signal from the end of an Alu element, sequence variation from the consensus sequence of an active subfamily, interaction ability of SRP9/14 (see glossary) to build RNA/protein complexes, and 5’flanking sequence.14,80–84 In addition, while not required, ORF1p increases the retrotransposition rate of Alu elements.85 The interplay of the different factors has not yet been studied in detail, and conceivably not all factors are required simultaneously for source drivers. The combination of varying mobilization rates of source elements and their continued evolution has shaped each primate genome uniquely. Retrotransposons that have reached fixation can be utilized for phylogenetic studies to denote branching events, whereas polymorphic insertions within a species can be used to study the population genetic structure.
RETROTRANSPOSONS AS PHYLOGENETIC AND POPULATION GENETIC MARKERS
Phylogenetics reconstructs evolutionary relationships between various species. It has been shown that retrotransposons represent highly valuable genetic systems to infer the relationships of different species.86–88 Consequently, these markers – in particular Alu elements and (to a lesser extent) L1 – are now commonly used to investigate phylogenetic and population genetic relationships within the order of primates.29,30,55,88–97 Alu elements are used more commonly, as they are relatively easy to genotype with a single PCR reaction due to their relatively small size (~300bp). In contrast, the insertion size of L1 varies widely, from about 50bp up to larger than 6kb.5,98 Accordingly, more than one PCR reaction is often required to genotype larger L1 insertions. This makes them less convenient than Alu elements; but they provide equal phylogenetic value, and can be used in conjunction with or as alternatives to Alu elements.
Retrotransposons are compelling genetic markers, with unique properties relative to other commonly used systems (e.g. single nucleotide polymorphisms, microsatellites, and restriction fragment length polymorphisms). Retrotransposons insert quasi-randomly into the host genome and create unique TSDs specific to the insertion site. Consequently, parallel insertions (see glossary) of two independent retrotransposons within the amplicon represent uncommon events. About 0.4% of more than 11,000 primate-specific retrotransposon insertions were identified as parallel insertions, with all but five insertions caused by so-called near-parallel insertion events (see glossary).95 No parallel L1 insertions have been identified to date, likely due to the size variability of L1 insertions, resulting from their frequent 5’ truncation.92,99,100 Consequently, in contrast to many other DNA markers, retrotransposon insertions can be considered as nearly homoplasy free markers.13,89,95–97,99
A shared retrotransposon insertion between two species (or two individuals) more than likely indicates a common ancestor. Thus, in contrast to most other commonly used marker systems, retrotransposon markers indicate identity by descent as opposed to identity by state (as reviewed in13,95,100). In general, precise deletions of retrotransposons represent very rare and unlikely events.91,95,101 Consequently, the ancestral state is marked by absence of the retrotransposon.13,96 This is in contrast to other commonly used marker systems within which the ancestral state cannot be unambiguously predicted. Like other markers, polymorphic retrotransposon insertions are not immune to incomplete lineage sorting. Different scenarios can commonly result in incomplete lineage sorting. Examples include two species with a prolonged divergence time over several million years due to, for instance, a large ancestral population size; recurrent re-introduction of populations to the gene pool; or divergence of several species over a very short time period. In primates, incomplete lineage sorting has been described, but altogether it appears to be a minor problem.95,102 In general, the use of several markers for each branch is recommended to determine lineage sorting events.
RETROTRANSPOSON-BASED PRIMATE PHYLOGENETIC STUDIES
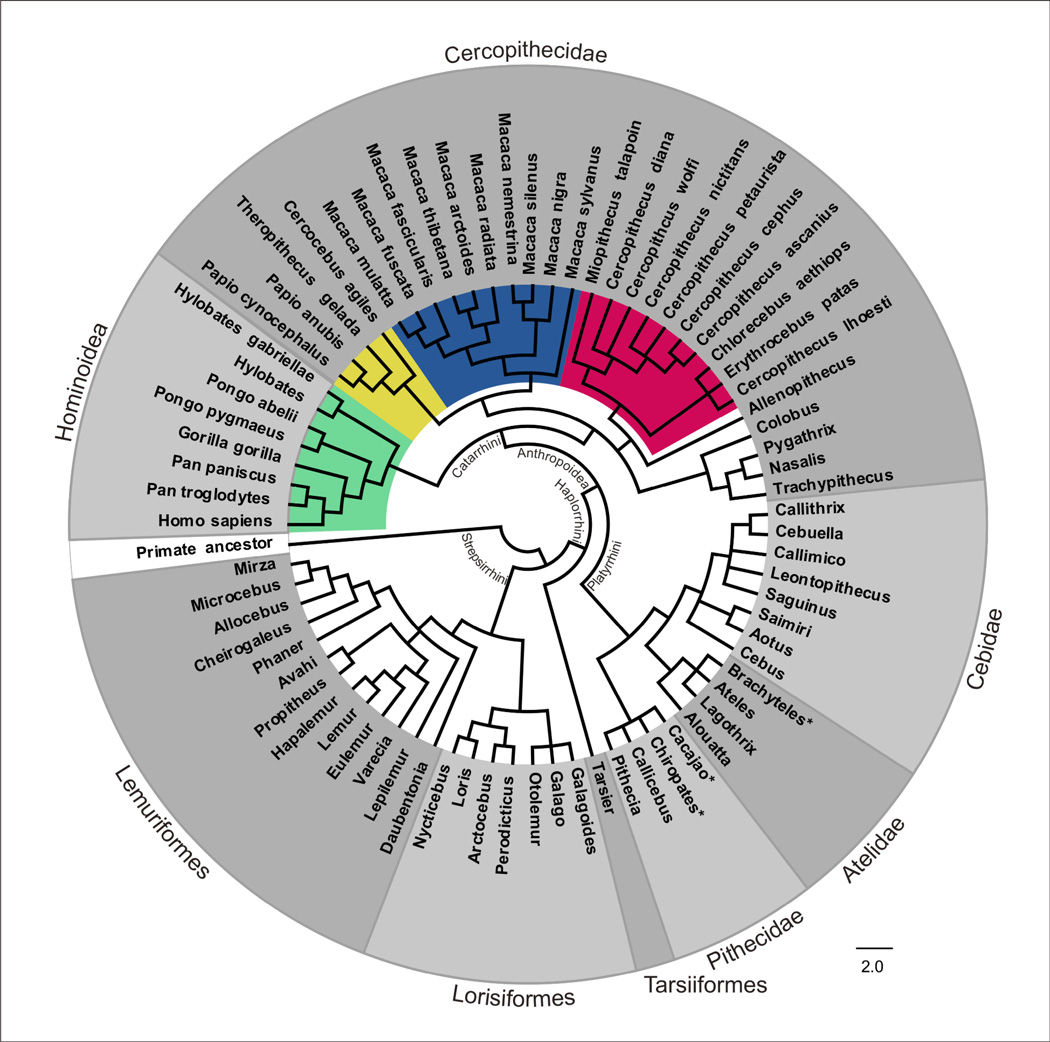
In this review we have outlined the evolutionary mechanisms by which different primate taxa accumulate a unique pattern of retrotransposon insertions, with some shared by other closely related taxa and others specific to that lineage. This hierarchical accumulation of “identical by descent” retrotransposon markers allows researchers to target subfamilies that were active during the evolutionary period of interest to identify candidate loci with phylogenetic value. On the basis of these retrotransposon insertion patterns (presence/absence among different species) numerous phylogenetic relationships have been successfully reconstructed across almost the entire order of primates (reviewed in91). Figure 4 illustrates the to-date use of retrotransposon markers to infer primate phylogeny.
Figure 4. Primate retrotransposon-based phylogenetic Tree.
Illustrated is a phylogenetic cladogram of primates that is supported by retrotransposon markers. Where possible, we show resolution up to the species level. The core of the cladogram (indicted in white) was reconstructed from a recent review in the Yearbook of Physical Anthropology.91 More detailed information on the species level has been integrated for Hominidae (green)109 and Cercopithecidae with Cercopithecinae and Colobinae (yellow)107, Macaca (blue)102, and Cercopithecini (guenon, red).108 Three platyrrhine branching events (shown by an asterisk) were resolved by Osterholz et al.106 We followed the nomenclature of Groves’ Primate Taxonomy.3
The availability of sequenced primate genomes – in particular the human genome – has revolutionized the field of phylogenetics. Over the last decade or so, several heavily debated questions have been successfully resolved with retrotransposon markers in the primate order. For example, a phylogenetic study using Alu elements unequivocally resolved the human-chimpanzee-gorilla trichotomy.103 Three separate studies, confirmed monophyly of platyrrhines and determined the branching order of various families of platyrrhine primates.30,104,105 This work was recently confirmed and expanded upon by Osterholz et al.106 using a total of 128 retrotransposon integrations from across all platyrrhine genera. In addition, several studies have used Alu elements extensively to refine the branching pattern of OWMs.59,102,107,108 Xing et al.107 reported a mobile element based phylogeny of OWMs using 285 novel Alu insertions. This work was further refined within subfamily Cercopithecinae (tribe Cercopithecini) using 151 novel Alu insertion loci from 11 species.108 Recently, Li et al. identified 298 new Alu insertion loci from the genus Macaca within OWMs and reported a comprehensive and robust resolution of macaque phylogeny with higher statistical support than previous studies.102 Roos et al.29 used SINE insertions to construct a strongly supported phylogenetic tree representing 20 strepsirrhine species. This work is supported by Herke et al.109 in a comprehensive SINE-based dichotomous key for the identification of primates. In this study, a total of 443 Alu loci (81 of which were novel) were evaluated to characterize some of the deepest nodes of the primate phylogenetic tree and to refine a number of previously unresolved terminal branches.109 Moreover, this dichotomous key is highly valuable to confirm a species and/or to identify an unknown species.
Retrotransposons have also been used to exclude species from the primate order. Schmitz and colleagues110,111 presented clear evidence separating dermopterans (colugos or flying lemurs) from primates. In this case, the absence of Alu elements universal to all primates from the Cynocephalus variegatus (flying lemur) genome placed the flying lemur outside the primate order.91,110 The complete relationship among Primates, Scandentia and Dermoptera, (also known as the “primate-tree shrew-colugo trichotomy”) has yet to be satisfactorily resolved. The Tu Type I and Type II families of SINEs identified in the tree shrew (Tupaia belangeri) derived from 7SL RNA, as are Alu SINEs in primates and B1 SINEs in rodents, but as yet there is no conclusive evidence placing tree shrews either closer to primates or to rodents.112 The CYN-SINE family identified in the C. variegatus genome is specific to Dermoptera and thus uninformative for resolving the phylogeny of Dermoptera in relation to Scandentia, Primates, and Rodentia.111 As more sequencing data become available, future studies may identify phylogenetically-informative retrotransposon markers that were active during this evolutionary time period.
RETROTRANSPOSONS IN PRIMATE POPULATION GENETIC STUDIES
The same properties of retrotransposons that make them useful phylogenetic markers (homoplasy free and identical by descent characters) also make them ideal for population genetic studies. However, instead of targeting fixed insertions, population studies focus on recently integrated insertions that are still polymorphic and belong to subfamilies with a low divergence from their respective subfamily consensus sequences. Individuals within a species remain polymorphic for insertion presence/absence and create discrete differences within the gene pool. This can be used to reconstruct the population structure of that species. Detailed knowledge about population dynamics is of great interest for understanding the diversity within a species, complexity of intra-species relationships, and for conservation efforts (e.g. re-introduction of a species in the wild).
Retrotransposons have been commonly used to infer the population structure of humans as well as non-human primates and to determine human geographic origins for forensics.7,89,91,96,97,113–116 The population structure of human populations and their history, in addition to the population architecture of the human population worldwide has been investigated intensively with the sole use of Alu retrotransposon markers or in combination with other markers.115–117 Most population structure research has focused on humans due to the broad geographic distribution of the species and the abundant available genetic information for humans. However, retrotransposons have proven successful across the mammalian lineage to infer the population structure of marsupials (i.e., Monodelphis domestica, opossum)118 and monotremes (i.e., Ornithorhynchus anatinus, platypus)119. The only non-human primate population genetic study using retrotransposon markers (Alu and L1) published to date investigated the population structure of rhesus macaques.7 In this study, Chinese rhesus macaques could be clearly distinguished from Indian rhesus macaques.7
To infer the population structure, the use of more than 50 (better 75 to 100) polymorphic retrotransposon loci is required.116,120 The minimal number of insertions necessary to reliably analyze the population structure depends on the level of genetic similarity of the populations (reviewed in91). Fewer loci are required to infer the population structure of two distinct (often geographically more removed) populations than for populations with more similar gene pools. The success of retrotransposon based population genetic studies, the unique characteristics of retrotransposon markers, and the relative ease of use make them an attractive marker system to investigate the population structure of other primate species.
LINEAGE-SPECIFIC NON-AUTONOMOUS RETROTRANSPOSONS
In this section, we will briefly discuss the emergence of two less common primate SINEs as well as SVA elements (SINE-R/VNTR/Alu) – a composite retrotransposon of relatively recent origin. One SINE, first discovered in Galago crassicaudatus, is termed Type III and is a monomeric element derived from tRNA.121 Type III elements have been shown by Southern blot analyses to be present in galagos and lorises but absent from lemur species29 indicating lineage-specificity and origin after the divergence of Lorisiformes and Lemuriformes.29,122 The second SINE recognized in the galago genome, a Type II element, represents a chimeric SINE most likely created by the integration of a Type III element into the center of an Alu element.121 Both retrotransposons contain typical hallmarks of SINEs: TSDs flanking the insertion, an A-rich 3’ terminus, and a split intragenic RNA polymerase III promoter.122 Type II elements appear to have been highly active in galagos (G. crassicaudatus and Galago senegalensis).122 To our knowledge, there is no further information available about the distribution of Type II elements in other closely related species.
Another example of lineage-specific non-autonomous retrotransposons is the SVA family of elements, which are specific to the hominoid lineage and are most prevalent in their current form in the great apes.123,124 However, precursors of SVA have been identified in OWMs, indicating that SVA evolved over several million years before mobilizing in its current state.59 In the public human genome, ~3000 insertions have been identified, indicating their successful propagation in spite of their relatively recent origin.123 Quantitative PCR analyses indicate a similar number of SVA insertions in the chimpanzee, gorilla, and human genomes, a lower number in the orangutan (Pongo pygmaeus) (~1000 insertions), and near absence in the siamang (Symphalangus syndactylus, ~40 insertions).123 There is clear evidence of active SVA retrotransposition in the human genome, as de novo SVA insertions have been identified as the underlying cause for some human diseases (reviewed in10,125,126). Whole genome analyses will prove useful in confirming these copy number estimates, as it is conceivable that in more diverged species the copy number is underestimated by quantitative PCR experiments that used human reference sequences. Conceivably, even more lineage-specific retrotransposon families will be identified in the future as more sequenced primate genomes become available, allowing for exhaustive comparative genomics studies.
IMPACT ON GENOME ARCHITECTURE
Retrotransposons are major contributors to structural variation that has shaped the landscape of primate genomes. Primates regularly experience de novo retrotransposon insertions, occasionally resulting in disease (reviewed in10,125,126). For example, the latest estimates for Alu, L1, and SVA insertions within the human species are one in 21, 212, and 916 live births, respectively.127 This is in good agreement with previous estimates for Alu insertion rates.28 Earlier estimates for de novo L1 insertion rates on the basis of transgenic mice models indicated a roughly 4× higher activity rate.12,128 Occasionally, genomic deletions are associated with retrotransposon insertions, potentially resulting in the loss of important genetic information such as exons.129,130 Apart from insertional mutagenesis, which in itself represents a major impact on primate genomes, the accumulation of very similar sequences makes the genome more susceptible to non-allelic homologous recombination events that can cause genome rearrangements including deletions and duplications.131–133 Other types of recombination events, such as Alu-mediated gene conversion (see glossary), have been shown to alter gene function (reviewed in14). An example of this is the Alu-mediated loss of the agouti signaling protein gene in gibbons.134 Exonization (see glossary) of retrotransposons is another mechanism retrotransposons contribute to structural variation (e.g.,135, reviewed in14) and has taken place occasionally during the course of primate evolution.136 Although exonization is not widespread, it is estimated that about 5% of alternatively spiced exons in humans are derived from Alu elements.14 Occasionally, molecular domestication (see glossary) of retrotransposons occurs as demonstrated for the SETMAR gene.14,137 In addition, L1 and SVA have been identified in 3’ and 5’ transduction events that occasionally can give rise to a new functional gene.12,17,138
CONCLUSIONS
Retrotransposons have had a major influence on primate genomes, and have contributed to the expansion of primate genome sizes. In addition, retrotransposons have shaped each primate genome uniquely and have had a major influence on the genome architecture. Due to their continuous insertion throughout primate evolution and their unique features, retrotransposons serve as valuable markers for the investigation of phylogenetic, population genetic, and forensic relationships. With some evidence of varying retrotransposition rates in different primate lineages, the evolution of retrotransposons might vary considerably. As more sequenced primate genomes become available we will be able to draw a more complete picture of retrotransposon evolution in the whole primate lineage.
ACKNOWLEDGEMENTS
We like to thank G. Cook, T. J. Meyer, and D. Srikanta for critical discussion during the manuscript preparation. Special thanks to B. Ullmer for his advice on figure designs and manuscript edits. We also want to thank the four anonymous reviewers for their thoughtful advice. Research in the Batzer laboratory on retrotransposons has been supported by grants from the State of Louisiana Board of Regents Governor’s Biotechnology Initiative GBI (2002-005) (M.A.B.), National Science Foundation BCS-0218338 (M.A.B.), and National Institutes of Health PO1 AG022064 and RO1 GM59290 (M.A.B.).
GLOSSARY
- DNA
desoxyribonucleic acid; two anti-parallel backbones comprised of the sugar deoxyribose and phosphoric acid joined by phosphodiester bonds; attached to each sugar is one of four nucleotides (adenine (A), guanine (G), thymine (T), or cytosine (C)). The nucleotides encode the genetic information.
- mRNA
messenger ribonucleic acid; similar to DNA but contains ribose instead of deoxyribose, and uracil (U) instead of thymine (T).
- CpG dinucleotide
a 5’ cytosine (C) nucleotide followed 3’ by a guanine (G) nucleotide within a linear DNA sequence. The cytosines of CpG dinucleotides are targets of DNA methylation resulting in 5-methylcytosine. Deamination of 5-methylcytosine results in thymine. In general, CpG sites mutate ~10 times faster than other dinucleotide combinations.38–41 For Alu insertions less than 50 myrs in age, the CpG mutation rate is ~6 times faster compared to non-CpG sites.42
- Homopolymeric tract
stretch of DNA sequence containing identical nucleotides; simplest form of a repetitive sequence.
- PolyA-tail
homopolymeric tract of adenosine nucleotides; here at the 3’ end of non-LTR retrotransposons.
- Retrotransposon
Class I elements (including endogenous retroviruses and retrotransposons) that move in a genome via a “copy and paste” mechanism through an RNA intermediate and are reverse transcribed into DNA by reverse transcriptase.
- LTR retrotransposon
retrotransposons with long terminal repeats; e.g. endogenous retroviruses.
- Non-LTR retrotransposon
retrotransposons lacking LTRs; SINEs, LINEs, and SVAs.
- Autonomous element
element that provides its own machinery for amplification; e.g. full-length LINEs with intact ORFs.
- Non-autonomous element
dependent on enzymatic machinery from autonomous elements; e.g. Alu and SVA.
- SINEs
short interspersed elements; originally defined by their interspersed nature and length (75–500bp), but also further characterized by their RNA polymerase III transcription.
- LINEs
Long interspersed elements; full-length elements are ~6kb in length, contain an internal promoter for Polymerase II, two ORFs, and end in a polyA-tail.
- SVA
composite elements named after its main components SINE, variable number of tandem repeats (VNTR), and Alu.
- TSD
target site duplication; short stretch (generally 6–20bp in length) of identical DNA generated at each end of a retrotransposon integration event as a result of the staggered cut in the target site DNA; TSDs are a hallmark of TPRT-mediated retrotransposition.
- ORF
open reading frame; a portion of a DNA sequence in which there are no termination codons (stop codons) in at least one of the possible reading frames; begins with a start codon (initiation codon) and ends with a stop codon; ORFs potentially encode for protein or polypeptide. L1 elements contain ORF1 and ORF2; the product of ORF1 is an RNA-binding protein (ORF1p), and ORF2 encodes a protein (ORF2p) with endonuclease and reverse transcriptase activities.
- TPRT
target-primed reverse transcription (Fig. 1); term for the integration mechanism of non-LTR retrotransposons into the genome; the bottom strand of chromosomal DNA is cut at a target site (5’-TTTT/AA-3’) by an endonuclease encoded by L1, followed by binding of non-LTR retrotransposon RNA at the DNA cleavage site, and reverse transcription by L1-encoded reverse transcriptase. Following steps, such as generation of second strand nick and second-strand DNA synthesis, are not well understood.
- SRP9/14
subunit of the human Signal Recognition Particle 9/14; SRP9 and SRP14 proteins form a stable heterodimer (SRP9/14) that bind to 7SL RNA of Alu elements; impaired binding reduces Alu mobilization.
- APOBEC3
Apolipoprotein B mRNA Editing Complex 3; believed to inhibit L1 and Alu retrotransposition.
- Bottleneck
substantial reduction in size of a population over a short period of time; potentially results in radical changes of allele frequencies and reduced genetic variation.
- Source element
element that is both transcriptionally and retrotranspositionally active and able to generate copies.
- Precise parallel insertion
independent retrotransposon insertions at exactly the same target site.
- Near-parallel insertion
independent retrotransposon insertions within the PCR amplicon or genomic region, but not at identical insertion sites.
- Homoplasy
shared genetic state or allele that is not inherited from a common ancestor but rather is due to independent events.
- Incomplete lineage sorting
a marker (e.g. an Alu element) polymorphic at the time of the divergence of several species gets randomly distributed in the emerging taxa.
- Gene conversion
unequal non-reciprocal recombination of homologous sequence (e.g. between Alu elements).
- Exonization
a transposable element residing in an intron is recruited into the coding sequence and thus exonized. In particular Alu elements have been commonly identified in alternatively spliced exons.139
- Molecular domestication
sequence of a transposable element is incorporated into a novel function within a genome.
Biographies
Mark A. Batzer is currently LSU System Boyd Professor and Dr. Mary Lou Applewhite Distinguished Professor, Department of Biological Sciences at Louisiana State University, Baton Rouge, Louisiana. Dr. Batzer’s research interests focus on comparative genomics of human and non-human primates, mobile DNA, forensic genomics, and computational biology. Additional research interests include the identification of genes related to healthy aging in humans.
Miriam K. Konkel, M.D., is a postdoctoral researcher in Dr. Batzer’s laboratory. Her current research focuses on the evolution of retrotransposons in diverse species, with emphasis on primates and other mammals; her background also includes clinical practice and infectious diseases research.
Jerilyn A. Walker, M.S., is a research associate in the Batzer laboratory. Her work centers on the development of quantitative assays for species-specific DNA identification through detection of retrotransposons. Both Konkel and Walker have been involved in retrotransposon studies and population genetic analyses for several whole genome sequencing and analysis projects. In addition, they are actively involved in the Louisiana Healthy Aging Study.
References
- 1.Chatterjee H, Simon H, Ian B, Colin G. Estimating the phylogeny and divergence times of primates using a supermatrix approach. BMC Evolutionary Biology. 2009;9:259–278. doi: 10.1186/1471-2148-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavaré S, Marshall C, Will O, Soligo C, Martin R. Using the fossil record to estimate the age of the last common ancestor of extant primates. Nature. 2002;416:726–729. doi: 10.1038/416726a. [DOI] [PubMed] [Google Scholar]
- 3.Groves C. Primate Taxonomy. Washington, D.C: Smithsonian Press; 2001. [Google Scholar]
- 4.Goodman M, Grossman LI, Wildman DE. Moving primate genomics beyond the chimpanzee genome. Trends Genet. 2005;21:511–517. doi: 10.1016/j.tig.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 6.CSAC. Initial sequence of the chimpanzee genome and comparison with the human genome. Nature. 2005;437:69–87. doi: 10.1038/nature04072. [DOI] [PubMed] [Google Scholar]
- 7.RMGSAC. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 8.Smit A, Tóth G, Riggs A, Jurka J. Ancestral, mammalian-wide subfamilies of LINE-1 repetitive sequences. Journal of molecular biology. 1995;246:401–417. doi: 10.1006/jmbi.1994.0095. [DOI] [PubMed] [Google Scholar]
- 9.Smit AF. The origin of interspersed repeats in the human genome. Curr Opin Genet Dev. 1996;6:743–748. doi: 10.1016/s0959-437x(96)80030-x. [DOI] [PubMed] [Google Scholar]
- 10.Belancio V, Hedges D, Deininger P. Mammalian non-LTR retrotransposons: For better or worse, in sickness and in health. Genome Research. 2008;18:343–358. doi: 10.1101/gr.5558208. [DOI] [PubMed] [Google Scholar]
- 11.Goodier J, Kazazian H. Retrotransposons revisited: the restraint and rehabilitation of parasites. Cell. 2008;135:23–35. doi: 10.1016/j.cell.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Kazazian HH., Jr Mobile elements: drivers of genome evolution. Science. 2004;303:1626–1632. doi: 10.1126/science.1089670. [DOI] [PubMed] [Google Scholar]
- 13.Batzer MA, Deininger PL. Alu repeats and human genomic diversity. Nat Rev Genet. 2002;3:370–379. doi: 10.1038/nrg798. [DOI] [PubMed] [Google Scholar]
- 14.Cordaux R, Batzer MA. The impact of retrotransposons on human genome evolution. Nature Reviews Genetics. 2009;10:691–703. doi: 10.1038/nrg2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostertag EM, Kazazian HH., Jr Biology of mammalian L1 retrotransposons. Annu Rev Genet. 2001;35:501–538. doi: 10.1146/annurev.genet.35.102401.091032. [DOI] [PubMed] [Google Scholar]
- 16.Feng Q, Moran JV, Kazazian HH, Jr, Boeke JD. Human L1 retrotransposon encodes a conserved endonuclease required for retrotransposition. Cell. 1996;87:905–916. doi: 10.1016/s0092-8674(00)81997-2. [DOI] [PubMed] [Google Scholar]
- 17.Szak ST, Pickeral OK, Makalowski W, Boguski MS, Landsman D, Boeke JD. Molecular archeology of L1 insertions in the human genome. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-10-research0052. research0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dombroski BA, Mathias SL, Nanthakumar E, Scott AF, Kazazian HH., Jr Isolation of an active human transposable element. Science. 1991;254:1805–1808. doi: 10.1126/science.1662412. [DOI] [PubMed] [Google Scholar]
- 19.Esnault C, Maestre J, Heidmann T. Human LINE retrotransposons generate processed pseudogenes. Nat Genet. 2000;24:363–367. doi: 10.1038/74184. [DOI] [PubMed] [Google Scholar]
- 20.Wei W, Gilbert N, Ooi SL, Lawler JF, Ostertag EM, Kazazian HH, Boeke JD, Moran JV. Human L1 retrotransposition: cis preference versus trans complementation. Mol Cell Biol. 2001;21:1429–1439. doi: 10.1128/MCB.21.4.1429-1439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulpa DA, Moran JV. Cis-preferential LINE-1 reverse transcriptase activity in ribonucleoprotein particles. Nat Struct Mol Biol. 2006;13:655–660. doi: 10.1038/nsmb1107. [DOI] [PubMed] [Google Scholar]
- 22.Dewannieux M, Esnault C, Heidmann T. LINE-mediated retrotransposition of marked Alu sequences. Nat Genet. 2003;35:41–48. doi: 10.1038/ng1223. [DOI] [PubMed] [Google Scholar]
- 23.Luan DD, Korman MH, Jakubczak JL, Eickbush TH. Reverse transcription of R2Bm RNA is primed by a nick at the chromosomal target site: a mechanism for non-LTR retrotransposition. Cell. 1993;72:595–605. doi: 10.1016/0092-8674(93)90078-5. [DOI] [PubMed] [Google Scholar]
- 24.Cost GJ, Feng Q, Jacquier A, Boeke JD. Human L1 element target-primed reverse transcription in vitro. Embo J. 2002;21:5899–5910. doi: 10.1093/emboj/cdf592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrish TA, Gilbert N, Myers JS, Vincent BJ, Stamato TD, Taccioli GE, Batzer MA, Moran JV. DNA repair mediated by endonuclease-independent LINE-1 retrotransposition. Nat Genet. 2002;31:159–165. doi: 10.1038/ng898. [DOI] [PubMed] [Google Scholar]
- 26.Sen SK, Huang CT, Han K, Batzer MA. Endonuclease-independent insertion provides an alternative pathway for L1 retrotransposition in the human genome. Nucleic Acids Res. 2007;35:3741–3751. doi: 10.1093/nar/gkm317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srikanta D, Sen S, Huang C, Conlin E, Rhodes R, Batzer M. An alternative pathway for Alu retrotransposition suggests a role in DNA double-strand break repair. Genomics. 2009;93:205–212. doi: 10.1016/j.ygeno.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cordaux R, Lee J, Dinoso L, Batzer MA. Recently integrated Alu retrotransposons are essentially neutral residents of the human genome. Gene. 2006;373:138–144. doi: 10.1016/j.gene.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Roos C, Schmitz J, Zischler H. Primate jumping genes elucidate strepsirrhine phylogeny. Proc Natl Acad Sci U S A. 2004;101:10650–10654. doi: 10.1073/pnas.0403852101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray DA, Batzer MA. Tracking Alu evolution in New World primates. BMC Evol Biol. 2005;5:51. doi: 10.1186/1471-2148-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu G, Zhao S, Bailey JA, Sahinalp SC, Alkan C, Tuzun E, Green ED, Eichler EE. Analysis of primate genomic variation reveals a repeat-driven expansion of the human genome. Genome Res. 2003;13:358–368. doi: 10.1101/gr.923303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boissinot S, Roos C, Furano A. Different rates of LINE-1 (L1) retrotransposon amplification and evolution in New World monkeys. Journal of Molecular Evolution. 2004;58:122–130. doi: 10.1007/s00239-003-2539-x. [DOI] [PubMed] [Google Scholar]
- 33.Boissinot S, Furano A. The recent evolution of human L1 retrotransposons. Cytogenet Genome Res. 2005;110:402–406. doi: 10.1159/000084972. [DOI] [PubMed] [Google Scholar]
- 34.Liu G, Alkan C, Jiang L, Zhao S, Eichler E. Comparative analysis of Alu repeats in primate genomes. Genome Research. 2009;19:876–885. doi: 10.1101/gr.083972.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Hedges S. Amolecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- 36.Dewannieux M, Heidmann T. LINEs, SINEs and processed pseudogenes: parasitic strategies for genome modeling. Cytogenet Genome Res. 2005;110:35–48. doi: 10.1159/000084936. [DOI] [PubMed] [Google Scholar]
- 37.Han J, Boeke J. LINE-1 retrotransposons: modulators of quantity and quality of mammalian gene expression? Bioessays. 2005;27:775–784. doi: 10.1002/bies.20257. [DOI] [PubMed] [Google Scholar]
- 38.Nachman MW, Crowell SL. Estimate of the mutation rate per nucleotide in humans. Genetics. 2000;156:297–304. doi: 10.1093/genetics/156.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyamoto MM, Slightom JL, Goodman M. Phylogenetic relations of humans and African apes from DNA sequences in the psi eta-globin region. Science. 1987;238:369–373. doi: 10.1126/science.3116671. [DOI] [PubMed] [Google Scholar]
- 40.Labuda D, Striker G. Sequence conservation in Alu evolution. Nucleic Acids Res. 1989;17:2477–2491. doi: 10.1093/nar/17.7.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batzer MA, Kilroy GE, Richard PE, Shaikh TH, Desselle TD, Hoppens CL, Deininger PL. Structure and variability of recently inserted Alu family members. Nucleic Acids Res. 1990;18:6793–6798. doi: 10.1093/nar/18.23.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xing J, Hedges DJ, Han K, Wang H, Cordaux R, Batzer MA. Alu element mutation spectra: molecular clocks and the effect of DNA methylation. J Mol Biol. 2004;344:675–682. doi: 10.1016/j.jmb.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 43.Schmid CW. Human Alu subfamilies and their methylation revealed by blot hybridization. Nucleic Acids Res. 1991;19:5613–5617. doi: 10.1093/nar/19.20.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furano AV, Duvernell DD, Boissinot S. L1 (LINE-1) retrotransposon diversity differs dramatically between mammals and fish. Trends Genet. 2004;20:9–14. doi: 10.1016/j.tig.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Daniels GR, Fox GM, Loewensteiner D, Schmid CW, Deininger PL. Species-specific homogeneity of the primate Alu family of repeated DNA sequences. Nucleic Acids Res. 1983;11:7579–7593. doi: 10.1093/nar/11.21.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers J. The origin and evolution of retroposons. Genome Evolution in Prokaryotes and Eukaryotes. 1985;187 [Google Scholar]
- 47.Batzer MA, Rubin CM, Hellmann-Blumberg U, Alegria-Hartman M, Leeflang EP, Stern JD, Bazan HA, Shaikh TH, Deininger PL, Schmid CW. Dispersion and insertion polymorphism in two small subfamilies of recently amplified human Alu repeats. J Mol Biol. 1995;247:418–427. doi: 10.1006/jmbi.1994.0150. [DOI] [PubMed] [Google Scholar]
- 48.Kass DH, Batzer MA, Deininger PL. Gene conversion as a secondary mechanism of short interspersed element (SINE) evolution. Mol Cell Biol. 1995;15:19–25. doi: 10.1128/mcb.15.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cordaux R, Hedges DJ, Batzer MA. Retrotransposition of Alu elements: how many sources? Trends Genet. 2004;20:464–467. doi: 10.1016/j.tig.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 50.Price AL, Eskin E, Pevzner PA. Whole-genome analysis of Alu repeat elements reveals complex evolutionary history. Genome Res. 2004;14:2245–2252. doi: 10.1101/gr.2693004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy-Engel AM, Batzer MA, Deininger PL. Encyclopedia of Life Sciences. Chichester, UK: John Wiley & Sons, Ltd.; 2008. Evolution of human retrosequences: Alu. [Google Scholar]
- 52.Jurka J, Smith T. A fundamental division in the Alu family of repeated sequences. Proc Natl Acad Sci U S A. 1988;85:4775–4778. doi: 10.1073/pnas.85.13.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen MR, Batzer MA, Deininger PL. Evolution of the master Alu gene(s) J Mol Evol. 1991;33:311–320. doi: 10.1007/BF02102862. [DOI] [PubMed] [Google Scholar]
- 54.Zietkiewicz E, Richer C, Sinnett D, Labuda D. Monophyletic origin of Alu elements in primates. J Mol Evol. 1998;47:172–182. doi: 10.1007/pl00006374. [DOI] [PubMed] [Google Scholar]
- 55.Schmitz J, Ohme M, Zischler H. SINE insertions in cladistic analyses and the phylogenetic affiliations of Tarsius bancanus to other primates. Genetics. 2001;157:777–784. doi: 10.1093/genetics/157.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitz J, Roos C, Zischler H. Primate phylogeny: molecular evidence from retroposons. Cytogenet Genome Res. 2005;108:26–37. doi: 10.1159/000080799. [DOI] [PubMed] [Google Scholar]
- 57.Batzer MA, Deininger PL, Hellmann-Blumberg U, Jurka J, Labuda D, Rubin CM, Schmid CW, Zietkiewicz E, Zuckerkandl E. Standardized nomenclature for Alu repeats. J Mol Evol. 1996;42:3–6. doi: 10.1007/BF00163204. [DOI] [PubMed] [Google Scholar]
- 58.Kapitonov V, Jurka J. The age of Alu subfamilies. J Mol Evol. 1996;42:59–65. doi: 10.1007/BF00163212. [DOI] [PubMed] [Google Scholar]
- 59.Han K, Konkel MK, Xing J, Wang H, Lee J, Meyer TJ, Huang CT, Sandifer E, Hebert K, Barnes EW, et al. Mobile DNA in Old World monkeys: a glimpse through the rhesus macaque genome. Science. 2007;316:238–240. doi: 10.1126/science.1139462. [DOI] [PubMed] [Google Scholar]
- 60.Khan H, Smit A, Boissinot S. Molecular evolution and tempo of amplification of human LINE-1 retrotransposons since the origin of primates. Genome Res. 2006;16:78–87. doi: 10.1101/gr.4001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Furano AV, Hayward BE, Chevret P, Catzeflis F, Usdin K. Amplification of the ancient murine Lx family of long interspersed repeated DNA occurred during the murine radiation. J Mol Evol. 1994;38:18–27. doi: 10.1007/BF00175491. [DOI] [PubMed] [Google Scholar]
- 62.Boissinot S, Entezam A, Young L, Munson PJ, Furano AV. The insertional history of an active family of L1 retrotransposons in humans. Genome Res. 2004;14:1221–1231. doi: 10.1101/gr.2326704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pascale E, Liu C, Valle E, Usdin K, Furano AV. The evolution of long interspersed repeated DNA (L1, LINE 1) as revealed by the analysis of an ancient rodent L1 DNA family. J Mol Evol. 1993;36:9–20. doi: 10.1007/BF02407302. [DOI] [PubMed] [Google Scholar]
- 64.Boissinot S, Furano AV. Adaptive Evolution in LINE-1 Retrotransposons. Mol Biol Evol. 2001;18:2186–2194. doi: 10.1093/oxfordjournals.molbev.a003765. [DOI] [PubMed] [Google Scholar]
- 65.Pascale E, Valle E, Furano AV. Amplification of an ancestral mammalian L1 family of long interspersed repeated DNA occurred just before the murine radiation. Proc Natl Acad Sci U S A. 1990;87:9481–9485. doi: 10.1073/pnas.87.23.9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Britten RJ. Evidence that most human Alu sequences were inserted in a process that ceased about 30 million years ago. Proc Natl Acad Sci U S A. 1994;91:6148–6150. doi: 10.1073/pnas.91.13.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watanabe H, Fujiyama A, Hattori M, Taylor TD, Toyoda A, Kuroki Y, Noguchi H, BenKahla A, Lehrach H, Sudbrak R, et al. DNA sequence and comparative analysis of chimpanzee chromosome 22. Nature. 2004;429:382–388. doi: 10.1038/nature02564. [DOI] [PubMed] [Google Scholar]
- 68.Hedges DJ, Callinan PA, Cordaux R, Xing J, Barnes E, Batzer MA. Differential alu mobilization and polymorphism among the human and chimpanzee lineages. Genome Res. 2004;14:1068–1075. doi: 10.1101/gr.2530404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hedges DJ, Batzer MA. From the margins of the genome: mobile elements shape primate evolution. Bioessays. 2005;27:785–794. doi: 10.1002/bies.20268. [DOI] [PubMed] [Google Scholar]
- 70.Furano AV. The biological properties and evolutionary dynamics of mammalian LINE-1 retrotransposons. Prog Nucleic Acid Res Mol Biol. 2000;64:255–294. doi: 10.1016/s0079-6603(00)64007-2. [DOI] [PubMed] [Google Scholar]
- 71.Hulme AE, Kulpa DA, Perez JLG, Moran JV. The Impact of LINE-1 Retro transposition on the Human Genome. Genomic Disorders. 2006:35–55. [Google Scholar]
- 72.Bogerd H, Wiegand H, Hulme A, Garcia-Perez J, O’Shea K, Moran J, Cullen B. Cellular inhibitors of long interspersed element 1 and Alu retrotransposition. Proceedings of the National Academy of Sciences. 2006;103:8780–8785. doi: 10.1073/pnas.0603313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schumann G. APOBEC3 proteins: major players in intracellular defence against LINE-1-mediated retrotransposition. Biochemical Society Transactions. 2007;35:637–642. doi: 10.1042/BST0350637. [DOI] [PubMed] [Google Scholar]
- 74.Farkash EA, Prak ET. DNA damage and l1 retrotransposition. J Biomed Biotechnol. 2006;2006:37285. doi: 10.1155/JBB/2006/37285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han K, Xing J, Wang H, Hedges DJ, Garber RK, Cordaux R, Batzer MA. Under the genomic radar: The Stealth model of Alu amplification. Genome Res. 2005;15:655–664. doi: 10.1101/gr.3492605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leeflang EP, Liu WM, Chesnokov IN, Schmid CW. Phylogenetic isolation of a human Alu founder gene: drift to new subfamily identity [corrected] J Mol Evol. 1993;37:559–565. doi: 10.1007/BF00182741. [DOI] [PubMed] [Google Scholar]
- 77.Brouha B, Schustak J, Badge RM, Lutz-Prigge S, Farley AH, Moran JV, Kazazian HH., Jr Hot L1s account for the bulk of retrotransposition in the human population. Proc Natl Acad Sci U S A. 2003;100:5280–5285. doi: 10.1073/pnas.0831042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee J, Cordaux R, Han K, Wang J, Hedges DJ, Liang P, Batzer MA. Different evolutionary fates of recently integrated human and chimpanzee LINE-1 retrotransposons. Gene. 2007;390:18–27. doi: 10.1016/j.gene.2006.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deininger PL, Batzer MA, Hutchison CA, 3rd, Edgell MH. Master genes in mammalian repetitive DNA amplification. Trends Genet. 1992;8:307–311. doi: 10.1016/0168-9525(92)90262-3. [DOI] [PubMed] [Google Scholar]
- 80.Roy AM, West NC, Rao A, Adhikari P, Aleman C, Barnes AP, Deininger PL. Upstream flanking sequences and transcription of SINEs. J Mol Biol. 2000;302:17–25. doi: 10.1006/jmbi.2000.4027. [DOI] [PubMed] [Google Scholar]
- 81.Chesnokov I, Schmid CW. Flanking sequences of an Alu source stimulate transcription in vitro by interacting with sequence-specific transcription factors. J Mol Evol. 1996;42:30–36. doi: 10.1007/BF00163208. [DOI] [PubMed] [Google Scholar]
- 82.Roy-Engel AM, Salem AH, Oyeniran OO, Deininger L, Hedges DJ, Kilroy GE, Batzer MA, Deininger PL. Active Alu element "A-tails": size does matter. Genome Res. 2002;12:1333–1344. doi: 10.1101/gr.384802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Comeaux M, Roy-Engel A, Hedges D, Deininger P. Diverse cis factors controlling Alu retrotransposition: What causes Alu elements to die? Genome Research. 2009;19:545. doi: 10.1101/gr.089789.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bennett E, Keller H, Mills R, Schmidt S, Moran J, Weichenrieder O, Devine S. Active Alu retrotransposons in the human genome. Genome Research. 2008;18:1875–1883. doi: 10.1101/gr.081737.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wallace N, Wagstaff B, Deininger P, Roy-Engel A. LINE-1 ORF1 protein enhances Alu SINE retrotransposition. Gene. 2008;419:1–6. doi: 10.1016/j.gene.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murata S, Takasaki N, Saitoh M, Okada N. Determination of the phylogenetic relationships among Pacific salmonids by using short interspersed elements (SINEs) as temporal landmarks of evolution. Proc Natl Acad Sci U S A. 1993;90:6995–6999. doi: 10.1073/pnas.90.15.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okada N, Shedlock AM, Nikaido M. Retroposon mapping in molecular systematics. Methods Mol Biol. 2004;260:189–226. doi: 10.1385/1-59259-755-6:189. [DOI] [PubMed] [Google Scholar]
- 88.Shedlock AM, Okada N. SINE insertions: powerful tools for molecular systematics. Bioessays. 2000;22:148–160. doi: 10.1002/(SICI)1521-1878(200002)22:2<148::AID-BIES6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 89.Batzer MA, Stoneking M, Alegria-Hartman M, Bazan H, Kass DH, Shaikh TH, Novick GE, Ioannou PA, Scheer WD, Herrera RJ, et al. African origin of human-specific polymorphic Alu insertions. Proc Natl Acad Sci U S A. 1994;91:12288–12292. doi: 10.1073/pnas.91.25.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Minghetti P, Dugaiczyk A. The emergence of new DNA repeats and the divergence of primates. Proceedings of the National Academy of Sciences. 1993;90:1872–1876. doi: 10.1073/pnas.90.5.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xing J, Witherspoon DJ, Ray DA, Batzer MA, Jorde LB. Mobile DNA elements in primate and human evolution. Am J Phys Anthropol Suppl. 2007;45:2–19. doi: 10.1002/ajpa.20722. [DOI] [PubMed] [Google Scholar]
- 92.Konkel MK, Wang J, Liang P, Batzer MA. Identification and characterization of novel polymorphic LINE-1 insertions through comparison of two human genome sequence assemblies. Gene. 2007;390:28–38. doi: 10.1016/j.gene.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 93.Vincent BJ, Myers JS, Ho HJ, Kilroy GE, Walker JA, Watkins WS, Jorde LB, Batzer MA. Following the LINEs: an analysis of primate genomic variation at human-specific LINE-1 insertion sites. Mol Biol Evol. 2003;20:1338–1348. doi: 10.1093/molbev/msg146. [DOI] [PubMed] [Google Scholar]
- 94.Ryan SC, Dugaiczyk A. Newly arisen DNA repeats in primate phylogeny. Proc Natl Acad Sci U S A. 1989;86:9360–9364. doi: 10.1073/pnas.86.23.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ray DA, Xing J, Salem AH, Batzer MA. SINEs of a nearly perfect character. Syst Biol. 2006;55:928–935. doi: 10.1080/10635150600865419. [DOI] [PubMed] [Google Scholar]
- 96.Batzer MA, Deininger PL. A human-specific subfamily of Alu sequences. Genomics. 1991;9:481–487. doi: 10.1016/0888-7543(91)90414-a. [DOI] [PubMed] [Google Scholar]
- 97.Stoneking M, Fontius JJ, Clifford SL, Soodyall H, Arcot SS, Saha N, Jenkins T, Tahir MA, Deininger PL, Batzer MA. Alu insertion polymorphisms and human evolution: evidence for a larger population size in Africa. Genome Res. 1997;7:1061–1071. doi: 10.1101/gr.7.11.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Myers JS, Vincent BJ, Udall H, Watkins WS, Morrish TA, Kilroy GE, Swergold GD, Henke J, Henke L, Moran JV, et al. A comprehensive analysis of recently integrated human Ta L1 elements. Am J Hum Genet. 2002;71:312–326. doi: 10.1086/341718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ho HJ, Ray DA, Salem AH, Myers JS, Batzer MA. Straightening out the LINEs: LINE-1 orthologous loci. Genomics. 2005;85:201–207. doi: 10.1016/j.ygeno.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 100.Salem AH, Ray DA, Batzer MA. Identity by descent and DNA sequence variation of human SINE and LINE elements. Cytogenet Genome Res. 2005;108:63–72. doi: 10.1159/000080803. [DOI] [PubMed] [Google Scholar]
- 101.van de Lagemaat LN, Gagnier L, Medstrand P, Mager DL. Genomic deletions and precise removal of transposable elements mediated by short identical DNA segments in primates. Genome Res. 2005;15:1243–1249. doi: 10.1101/gr.3910705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li J, Han K, Xing J, Kim H, Rogers J, Ryder O, Disotell T, Yue B, Batzer M. Phylogeny of the macaques (Cercopithecidae: Macaca) based on Alu elements. Gene. 2009;448:242–249. doi: 10.1016/j.gene.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Salem AH, Ray DA, Xing J, Callinan PA, Myers JS, Hedges DJ, Garber RK, Witherspoon DJ, Jorde LB, Batzer MA. Alu elements and hominid phylogenetics. Proc Natl Acad Sci U S A. 2003;100:12787–12791. doi: 10.1073/pnas.2133766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Singer SS, Schmitz J, Schwiegk C, Zischler H. Molecular cladistic markers in New World monkey phylogeny (Platyrrhini, Primates) Mol Phylogenet Evol. 2003;26:490–501. doi: 10.1016/s1055-7903(02)00312-3. [DOI] [PubMed] [Google Scholar]
- 105.Ray DA, Xing J, Hedges DJ, Hall MA, Laborde ME, Anders BA, White BR, Stoilova N, Fowlkes JD, Landry KE, et al. Alu insertion loci and platyrrhine primate phylogeny. Mol Phylogenet Evol. 2005;35:117–126. doi: 10.1016/j.ympev.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 106.Osterholz M, Walter L, Roos C. Retropositional events consolidate the branching order among New World monkey genera. Molecular Phylogenetics and Evolution. 2009;50:507–513. doi: 10.1016/j.ympev.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 107.Xing J, Wang H, Han K, Ray D, Huang C, Chemnick L, Stewart C, Disotell T, Ryder O, Batzer M. A mobile element based phylogeny of Old World monkeys. MOLECULAR PHYLOGENETICS AND EVOLUTION. 2005;37:872–880. doi: 10.1016/j.ympev.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 108.Xing J, Wang H, Zhang Y, Ray D, Tosi A, Disotell T, Batzer M. A mobile element-based evolutionary history of guenons(tribe Cercopithecini) BMC biology. 2007;5:5. doi: 10.1186/1741-7007-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Herke S, Xing J, Ray D, Zimmerman J, Cordaux R, Batzer M. A SINE-based dichotomous key for primate identification. Gene. 2007;390:39–51. doi: 10.1016/j.gene.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 110.Schmitz J, Ohme M, Suryobroto B, Zischler H. The colugo (Cynocephalus variegatus, Dermoptera): the primates' gliding sister? Molecular Biology and Evolution. 2002;19:2308–2312. doi: 10.1093/oxfordjournals.molbev.a004054. [DOI] [PubMed] [Google Scholar]
- 111.Schmitz J, Zischler H. A novel family of tRNA-derived SINEs in the colugo and two new retrotransposable markers separating dermopterans from primates. Molecular Phylogenetics and Evolution. 2003;28:341–349. doi: 10.1016/s1055-7903(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 112.Nishihara H, Terai Y, Okada N. Characterization of novel Alu-and tRNA-related SINEs from the tree shrew and evolutionary implications of their origins. Molecular biology and evolution. 2002;19:1964. doi: 10.1093/oxfordjournals.molbev.a004020. [DOI] [PubMed] [Google Scholar]
- 113.Batzer MA, Gudi VA, Mena JC, Foltz DW, Herrera RJ, Deininger PL. Amplification dynamics of human-specific (HS) Alu family members. Nucleic Acids Res. 1991;19:3619–3623. doi: 10.1093/nar/19.13.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ray DA, Walker JA, Hall A, Llewellyn B, Ballantyne J, Christian AT, Turteltaub K, Batzer MA. Inference of human geographic origins using Alu insertion polymorphisms. Forensic Sci Int. 2005;153:117–124. doi: 10.1016/j.forsciint.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 115.Watkins WS, Rogers AR, Ostler CT, Wooding S, Bamshad MJ, Brassington AM, Carroll ML, Nguyen SV, Walker JA, Prasad BV, et al. Genetic variation among world populations: inferences from 100 Alu insertion polymorphisms. Genome Res. 2003;13:1607–1618. doi: 10.1101/gr.894603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bamshad MJ, Wooding S, Watkins WS, Ostler CT, Batzer MA, Jorde LB. Human population genetic structure and inference of group membership. Am J Hum Genet. 2003;72:578–589. doi: 10.1086/368061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bamshad M, Kivisild T, Watkins WS, Dixon ME, Ricker CE, Rao BB, Naidu JM, Prasad BV, Reddy PG, Rasanayagam A, et al. Genetic evidence on the origins of Indian caste populations. Genome Res. 2001;11:994–1004. doi: 10.1101/gr.173301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mikkelsen T, Wakefield M, Aken B, Amemiya C, Chang J, Duke S, Garber M, Gentles A, Goodstadt L, Heger A. Genome of the marsupial Monodelphis domestica reveals innovation in non-coding sequences. Nature. 2007;447:167–177. doi: 10.1038/nature05805. [DOI] [PubMed] [Google Scholar]
- 119.Warren W, Hillier L, Graves J, Birney E, Ponting C, Gr¸tzner F, Belov K, Miller W, Clarke L, Chinwalla A. Genome analysis of the platypus reveals unique signatures of evolution. Nature. 2008;453:175–183. doi: 10.1038/nature06936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Witherspoon D, Marchani E, Watkins W, Ostler C, Wooding S, Anders B, Fowlkes J, Boissinot S, Furano A, Ray D. Human population genetic structure and diversity inferred from polymorphic L1 (LINE-1) and Alu insertions. Hum Hered. 2006;62:30–46. doi: 10.1159/000095851. [DOI] [PubMed] [Google Scholar]
- 121.Daniels GR, Deininger PL. Repeat sequence families derived from mammalian tRNA genes. Nature. 1985;317:819–822. doi: 10.1038/317819a0. [DOI] [PubMed] [Google Scholar]
- 122.Daniels GR, Deininger PL. Characterization of a third major SINE family of repetitive sequences in the galago genome. Nucleic Acids Res. 1991;19:1649–1656. doi: 10.1093/nar/19.7.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang H, Xing J, Grover D, Hedges DJ, Han K, Walker JA, Batzer MA. SVA Elements: A Hominid-specific Retroposon Family. J Mol Biol. 2005;354:994–1007. doi: 10.1016/j.jmb.2005.09.085. [DOI] [PubMed] [Google Scholar]
- 124.Ostertag EM, Goodier JL, Zhang Y, Kazazian HH., Jr SVA elements are nonautonomous retrotransposons that cause disease in humans. Am J Hum Genet. 2003;73:1444–1451. doi: 10.1086/380207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Callinan P, Batzer MA. Retrotransposable Elements and Human Disease. Genome Dynamics. 2006;1:104–115. doi: 10.1159/000092503. [DOI] [PubMed] [Google Scholar]
- 126.Chen JM, Stenson PD, Cooper DN, Ferec C. A systematic analysis of LINE-1 endonuclease-dependent retrotranspositional events causing human genetic disease. Hum Genet. 2005;117:411–427. doi: 10.1007/s00439-005-1321-0. [DOI] [PubMed] [Google Scholar]
- 127.Xing J, Zhang Y, Han K, Salem A, Sen S, Huff C, Zhou Q, Kirkness E, Levy S, Batzer M. Mobile elements create structural variation: analysis of a complete human genome. Genome Research. 2009;19:1516–1526. doi: 10.1101/gr.091827.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ostertag EM, DeBerardinis RJ, Goodier JL, Zhang Y, Yang N, Gerton GL, Kazazian HH., Jr A mouse model of human L1 retrotransposition. Nature Genetics. 2002;32:655–660. doi: 10.1038/ng1022. [DOI] [PubMed] [Google Scholar]
- 129.Callinan PA, Wang J, Herke SW, Garber RK, Liang P, Batzer MA. Alu Retrotransposition-mediated Deletion. J Mol Biol. 2005;348:791–800. doi: 10.1016/j.jmb.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 130.Han K, Sen SK, Wang J, Callinan PA, Lee J, Cordaux R, Liang P, Batzer MA. Genomic rearrangements by LINE-1 insertion-mediated deletion in the human and chimpanzee lineages. Nucleic Acids Res. 2005;33:4040–4052. doi: 10.1093/nar/gki718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bailey JA, Liu G, Eichler EE. An Alu transposition model for the origin and expansion of human segmental duplications. Am J Hum Genet. 2003;73:823–834. doi: 10.1086/378594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sen SK, Han K, Wang J, Lee J, Wang H, Callinan PA, Dyer M, Cordaux R, Liang P, Batzer MA. Human genomic deletions mediated by recombination between Alu elements. Am J Hum Genet. 2006;79:41–53. doi: 10.1086/504600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Han K, Lee J, Meyer TJ, Remedios P, Goodwin L, Batzer MA. L1 recombination-associated deletions generate human genomic variation. Proc Natl Acad Sci U S A. 2008;105:19366–19371. doi: 10.1073/pnas.0807866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nakayama K, Ishida T. Alu-mediated 100-kb deletion in the primate genome: The loss of the agouti signaling protein gene in the lesser apes. Genome research. 2006;16:485–490. doi: 10.1101/gr.4763906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lev-Maor G, Sorek R, Shomron N, Ast G. The birth of an alternatively spliced exon: 3' splice-site selection in Alu exons. Science. 2003;300:1288–1291. doi: 10.1126/science.1082588. [DOI] [PubMed] [Google Scholar]
- 136.Krull M, Brosius J, Schmitz J. Alu-SINE exonization: en route to protein-coding function. Mol Biol Evol. 2005;22:1702–1711. doi: 10.1093/molbev/msi164. [DOI] [PubMed] [Google Scholar]
- 137.Cordaux R, Udit S, Batzer MA, Feschotte C. Birth of a chimeric primate gene by capture of the transposase gene from a mobile element. Proc Natl Acad Sci U S A. 2006;103:8101–8106. doi: 10.1073/pnas.0601161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xing J, Wang H, Belancio VP, Cordaux R, Deininger PL, Batzer MA. Emergence of new primate genes by retrotransposon-mediated sequence transduction. Proc Natl Acad Sci U S A. 2006;103:17608–17613. doi: 10.1073/pnas.0603224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sorek R, Ast G, Graur D. Alu-containing exons are alternatively spliced. Genome Res. 2002;12:1060–1067. doi: 10.1101/gr.229302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shen L, Wu LC, Sanlioglu S, Chen R, Mendoza AR, Dangel AW, Carroll MC, Zipf WB, Yu CY. Structure and genetics of the partially duplicated gene RP located immediately upstream of the complement C4A and the C4B genes in the HLA class III region. Molecular cloning, exonintron structure, composite retroposon, and breakpoint of gene duplication. J Biol Chem. 1994;269:8466–8476. [PubMed] [Google Scholar]
- 141.Jurka J. Sequence patterns indicate an enzymatic involvement in integration of mammalian retroposons. Proc Natl Acad Sci U S A. 1997;94:1872–1877. doi: 10.1073/pnas.94.5.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mathias SL, Scott AF, Kazazian HH, Jr, Boeke JD, Gabriel A. Reverse transcriptase encoded by a human transposable element. Science. 1991;254:1808–1810. doi: 10.1126/science.1722352. [DOI] [PubMed] [Google Scholar]
- 143.Wang J, Song L, Gonder M, Azrak S, Ray DA, Batzer M, Tishkoff S, Liang P. Whole genome computational comparative genomics: A fruitful approach for ascertaining Alu insertion polymorphisms. Gene. 2006;365:11–20. doi: 10.1016/j.gene.2005.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Boissinot S, Chevret P, Furano AV. L1 (LINE-1) retrotransposon evolution and amplification in recent human history. Mol Biol Evol. 2000;17:915–928. doi: 10.1093/oxfordjournals.molbev.a026372. [DOI] [PubMed] [Google Scholar]