Abstract
The epidermis of skin is the first line of defense against the environment. A three dimensional model of human skin was used to investigate tissue-specific phenotypes induced by the environmental contaminant, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Continuous treatment of organotypic cultures of human keratinocytes with TCDD resulted in intracellular spaces between keratinocytes of the basal and immediately suprabasal layers as well as thinning of the basement membrane, in addition to the previously reported hyperkeratinization. These tissue remodeling events were preceded temporally by changes in expression of the extracellular matrix degrading enzyme, matrix metalloproteinase-10 (MMP-10). In organotypic cultures MMP-10 mRNA and protein were highly induced following TCDD treatment. Q-PCR and immunoblot results from TCDD-treated monolayer cultures, as well as indirect immunofluorescence and immunoblot analysis of TCDD-treated organotypic cultures, showed MMP-10 was specifically contributed by the epidermal keratinocytes but not the dermal fibroblasts. Keratinocyte-derived MMP-10 protein accumulated over time in the dermal compartment of organotypic cultures. TCDD-induced epidermal phenotypes in organotypic cultures were attenuated by the keratinocyte-specific expression of tissue inhibitor of metalloproteinase-1, a known inhibitor of MMP-10. These studies suggest that MMP-10 and possibly other MMP-10-activated MMPs are responsible for the phenotypes exhibited in the basement membrane, the basal keratinocyte layer, and the cornified layer of TCDD-treated organotypic cultures. Our studies reveal a novel mechanism by which the epithelial-stromal microenvironment is altered in a tissue-specific manner thereby inducing structural and functional pathology in the interfollicular epidermis of human skin.
Keywords: Keratinocytes, NIKS, TCDD, MMP-10, dermis, TIMP-1
Introduction
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most well studied congener of a family of halogenated aromatic hydrocarbons known as dioxins. Due to their high lipophilicity and long half-life, dioxins are persistent in the environment and accumulate in biological systems. Dioxin exposure evokes a wide range of toxic effects in animals, but the skin condition known as chloracne is the most specific and sensitive biomarker of TCDD exposure in humans. The clinical manifestation of chloracne is described as a non-inflammatory alteration of keratinization in the pilosebaceous unit in skin (1). TCDD effects on skin have historically been studied using monolayer cultures of human keratinocytes (2–5). Though important in elucidating the biochemical and molecular mechanisms of the disease, these experiments are unable to answer the complex questions pertaining to the pathophysiologic effects of TCDD in human skin. Previous studies in our laboratory have used a three dimensional organotypic model of skin to investigate the tissue-specific effects of TCDD (6). These studies showed that organotypic cultures of human keratinocytes exposed to TCDD exhibited accelerated terminal differentiation of the interfollicular epidermis mimicking the keratinization phenotype observed in the pilosebaceous appendage of human skin during development of chloracne.
TCDD exposure has been shown to result in pathological lesions involving extracellular matrix (ECM) remodeling in organs such as seminal vesicle, prostate, mammary gland and palate (7–9). Although the mechanisms of how TCDD mediates these pathologies have not been elucidated, it is generally accepted that the majority of these effects are mediated through the activation of the AhR/ARNT pathway. Evidence points to the AhR/ARNT pathway as a potential regulator of genes involved in ECM remodeling (10). For example, MMP-13 and MMP-14 mRNA levels were upregulated in murine fetal hearts following TCDD exposure (11) while another study showed that TCDD exposure resulted in increased levels of MMP-1 mRNA in monolayer cultures of human keratinocytes (12), although MMP protein and biological activity were not demonstrated in these reports.
Human MMPs are a family of zinc-containing enzymes that currently constitute 23 endopeptidases (13). The primary function of these enzymes is to degrade the ECM, and recent studies have shown that this proteolytic activity leads to important downstream effects in numerous tissues. These include creating space for cell migration, producing novel biologicallyactive substrate-cleavage fragments, regulating tissue architecture by altering ECM components, and deactivating or modifying the activity of signaling molecules (14).
In skin, ECM remodeling is most frequently studied in the context of wound healing. Wound healing consists of three phases, inflammation, tissue formation and tissue remodeling, which temporally overlap (15). Degradation of the basement membrane (BM) and remodeling of the ECM is linked to all three of these phases through spatially and temporally-controlled expression of MMPs (16). MMPs are expressed by the different cell types in skin and have specific and overlapping functions during the wound healing process (15,17). Interfollicular keratinocytes express MMP-1, MMP-2, MMP-3, MMP-9 and MMP-10 during the various phases of wound healing (18).
An important mechanism for the regulation of MMP activities in tissues is a class of proteins known as tissue inhibitor of metalloproteinases (TIMPs) (19). The TIMP family is comprised of at least 4 distinct members that possess 12 conserved cysteine residues. Each member exhibits binding specificity towards individual MMPs and distinctive patterns of expression in tissues (19–21). TIMPs inhibit MMPs by either binding to the zinc-binding domain of active MMPs, or by binding to the inactive proMMP zymogen, thereby slowing the process of activation (21,22). The earliest reported member of this family, TIMP-1 is an extensively glycosylated protein produced and secreted by a variety of cell types including keratinocytes, fibroblasts, smooth muscle cells and endothelial cells (20). TIMP-1 is capable of inhibiting the activity of all soluble MMPs (22–24).
In the present study, the role of MMPs in the TCDD-mediated hyperkeratinization phenotype observed in organotypic cultures of human keratinocytes was investigated. We show that MMP-10 mRNA and protein are robustly induced by TCDD in these three dimensional cultures. Although the TCDD-mediated increase in MMP-10 gene expression is keratinocyte-specific in our organotypic model, MMP-10 protein accumulates in the dermal compartment. We demonstrate for the first time that increased MMP-10 is associated spatially with abnormalities in the basal layer and BM of organotypic cultures. To directly test the link between TCDD-induced skin-specific MMP levels and phenotypes, TIMP-1 expression was targeted to the basal keratinocytes using the K14 promoter. Human keratinocytes genetically modified to overexpress TIMP-1 in organotypic tissue results in the ablation of the TCDD-induced hyperkeratinization and alteration of the BM phenotypes. This is the first study to directly link MMPs to TCDD-induced phenotypes in a three dimensional human skin model. These findings suggest that the TCDD-induced skin phenotypes in this model are the consequence of altered epidermal-stromal interactions. Our studies point to the importance of three dimensional models of human organs in the study of tissue-specific toxicity.
Materials and Methods
Cell treatments
TCDD was prepared in dimethyl sulfoxide (DMSO) such that the final concentration of DMSO in the treatment medium did not exceed 0.1%. The final concentration of TCDD in the culturing medium was 10 nM (10−8M). Solvent control was 0.1% DMSO.
Monolayer cell culture
Near Diploid Human Keratinocyte (NIKS) cells (25) were cultivated on 100 mm tissue culture dishes in the presence of mitomycin C-treated Swiss mouse 3T3 fibroblast feeder layers as previously described (26). Standard keratinocyte culture medium was composed of a mixture of Ham’s F-12 medium and Dulbecco’s modified Eagle’s medium (3:1 F-12:DME, final calcium concentration 0.66 mM) supplemented with 2.5% Fetal Clone II serum (Hyclone, Logan, UT), 0.4 µg/ml hydrocortisone, 8.4 ng/ml cholera toxin, 5 µg/ml insulin, 24 µg/ml adenine and 10 ng/ml human recombinant epidermal growth factor. Keratinocytes were plated directly onto 6 well tissue culture plates in the absence of a feeder layer at 2×105 cells/well, medium was replaced 24 hrs post-plating and treated to a final concentration of 10 nM TCDD. Two ml of fresh treatment medium/well was provided on days 2 and 4 and increased to 4 ml on days 6 and 8, 6 ml on days 10 and 12 and 8 ml on days 14 and 16 to provide adequate nutrients for larger cell numbers. Total RNA was isolated at days 6, 8, 12, and 18 post-treatment for Q-PCR analysis or processed for immunoblot on day 12 post-treatment. To obtain conditioned media, cultures were rinsed twice with 8 ml of 3:1 F-12:DME and conditioned for 12 hrs with 4 ml of 3:1 F-12:DME at day 12 post-treatment.
Organotypic cell culture
Composite human skin substitutes were prepared using NIKS keratinocytes. Human skin substitutes were generated by organotypic culture of keratinocytes on HA (mixed cellulose esters)-membrane Milli cell culturing systems (Millipore, Billerica, MA). Briefly, normal human fibroblasts at a density of 3.6×104 cells/well were cultured with type I collagen for 4 days to form a cellularized dermal compartment. The proprietary Stratalife media series (Stratatech Corp. Madison, WI) was used for organotypic culture and contains approximately 2 mM Ca2+. Stratalife Medium 2 and 3 contains less than 1ng/ml EGF. The cellularized dermis was raised to the air-medium interface, Stratalife Medium 1 was added and keratinocytes were seeded onto the surface of the dermis at a density of 3.75×105 cells/well in StrataLife Medium 1. Two hours post-plating cultures were treated in media with TCDD or DMSO to a final concentration of 10 nM or 0.1% respectively. Forty-eight hours post-plating medium was replaced with StrataLife Medium 2. Ninety six hours postplating and every 48 hrs subsequent to this medium was replaced with StrataLife Medium 3. Cultures were retreated at each media change and maintained at the air-medium interface throughout the stratification process. Full stratification was typically present by day 14 of organotypic culture. Cultures were fixed at day 10 and 18 for hematoxylin and eosin (H&E) staining, at day 18 for indirect immunofluorescence (IIF) and processed at days 8, 12, 15 and 18 post-treatment for Q-PCR.
Quantitative PCR (Q-PCR) analysis
Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA). One microgram of total RNA was used to prepare cDNA using the manufacturer’s protocol (Invitrogen, Carlsbad, CA). The Q-PCR mix was composed of 0.6 µl of 1:10 diluted cDNA, 1 µl each of 5 µM forward and reverse primer, 10 µl of 2X SYBR Green Supermix (Bio-Rad, Hercules, CA) and DEPC H2O for a final volume of 20 µl. Q-PCR was performed on a Chromo 4 Detector (Bio-Rad, Hercules, CA) using the following conditions. Initial denaturation at 95°C for 5 min and 40 cycles of 94°C for 10 sec, 60°C for 30 sec and 72°C for 30 sec followed by an incubation at 72°C for 10 min. All PCR products were confirmed by a melting curve of 65°C to 95°C read every 0.2°C. Gene expression was calculated using a standard curve for both gene of interest and a reference gene (cyclophilin A). All values were normalized to cyclophilin A and expressed as relative fold-induction. All calculations were done using Opticon Monitor 3.1 software (Bio-Rad, Hercules, CA). Statistical analysis was done by unpaired t-test using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego CA). Results were considered significant when at least a two-fold difference in expression levels was detected and statistical analysis revealed P-values < 0.05 by t-test. Primer sequences are available upon request.
Immunoblot analysis
Monolayer cultures treated with either TCDD or DMSO were lysed in Cytobuster (Novagen, San Diego, CA) containing protease inhibitor cocktail set III (Calbiochem, San Diego, CA). Cell lysates were run through a 23 gauge needle 5 times, and centrifuged at 12,000 × g for 15 min at 4°C. Protein concentrations were determined by BCA. Equal amounts of protein or equal volume of conditioned medium were separated on a NuPAGE 10% Bis-Tris gel (Invitrogen, Carlsbad, CA) per the manufacturer’s protocol and proteins were electrophoretically transferred onto a 0.45 µm PVDF membrane (Millipore, Billerica, MA) using a XCell blot module (Invitrogen, Carlsbad, CA). Following transfer the membrane was blocked in 2% ECL Advance blocking reagent (block) (Amersham Biosciences, Pittsburgh, PA) in phosphate-buffered saline with 0.1% Tween-20 (PBST) overnight. Following the block, the blot was probed using a monoclonal antibody against recombinant human MMP-10 (R&D Systems, Minneapolis, MN) in 2% block in PBST for 1 hr at 25°C, washed 3 times with PBST and reprobed with a horseradish peroxidase-coupled anti-mouse IgG antibody (Roche, Indianapolis, IN) in 2% block PBST for 1 hr at 25°C. The antibody hybridized membrane was then developed with an ECL Advance system (Amersham Biosciences, Pittsburgh, PA) and visualized using a Kodak Image Station 2000R (Kodak, New Haven, CT). Band intensity was calculated using ImageJ software.
Indirect immunofluorescence analysis of organotypic cultures
Cryopreserved organotypic cultures were sectioned, mounted on glass slides, and fixed for 5 min in ice-cold acetone. Sections were then washed with PBS and blocked with 3% normal goat serum (Sigma, St. Louis, MO). Following the block, sections were incubated with 1:25 dilution of an anti-human MMP-10 monoclonal antibody (R&D Systems, Minneapolis, MN) for 1 hr at room temperature and rinsed twice with PBS. Sections were then incubated with Alexa 488-conjugated goat anti-mouse IgG (Molecular Probes, Eugene, OR) for 30 min at room temperature. All sections were counterstained with 5µg/ml Hoechst 33258 (Sigma, St. Louis, MO). Samples were viewed with an IX-70 inverted fluorescence microscope (Olympus, Center Valley, PA) equipped with FITC (470 ± 20nm), Hoechst (525 ± 20nm) band-pass filters and an image capture camera. Analysis was conducted with Image Pro Plus 5.1 software (Media Cybernetics, Inc. Silver Spring, MD).
TIMP-1 expression vector construction and clone selection
A 670 bp human TIMP-1 cDNA was isolated by PCR using commercially available cDNA (Clontech, Palo Alto, CA) and TIMP-1-specific primers. Amplified TIMP-1 cDNA was inserted into the pCR2.1-TOPO vector (Invitrogen, Carlsbad, CA) and confirmed by DNA sequencing. Tissue-specific expression of TIMP-1 was achieved using the K14 promoter cloned into the pUb-Bsd expression vector (Invitrogen, Carlsbad, CA). NIKS cells were either electroporated with the TIMP-1 construct using a Gene Pulser Xcell System (Bio-Rad, Hercules, CA) or transfected using TransIT reagent (Mirus Bio Corporation, Madison, WI) for the empty vector (EV) construct following the manufacturer’s instructions. Cells were subsequently cultured on a mitomycin C-treated 3T3 feeder layer in blasticidin-containing medium for three weeks. Independent NIKSTIMP-1 and NIKSEV clones were isolated, expanded for further characterization, and cryopreserved.
TIMP-1 protein detection
Concentrations of TIMP-1 protein present in 24 hr, phenol red-free HAM’s F-12 (Biosource, Rockville, MD): HyQ DMEM/ High Modified (Hyclone, Logan, UT) (3:1) conditioned media of monolayer keratinocytes were quantitated with a commercially available TIMP-1 ELISA kit (R&D Systems, Minneapolis, MN). All data was standardized to the cell number present when conditioned media was collected.
TIMP-1 activity assay
TIMP-1 activity was determined using the EnzChek Gelatinase/Collagenase assay (Molecular Probes, Carlsbad, CA). Triplicate samples of conditioned medium (phenol red-free HAM’s F-12 (Biosource, Rockville, MD):HyQ DMEM/ High Modified (Hyclone, Logan, UT) (3:1) from NIKSTIMP-1, or NIKSEV cultures were collected after 24 hrs. Serum-free medium was used to ensure that TIMP-1 from serum components did not interfere with the assay. 20µl of DQ Gelatin combined with 100 µl of 24 hr conditioned media was added to each well of a 96 well flat bottom assay plate (Corning, NY). A standard concentration of 5nM Active Human MMP-2 (Calbiochem, La Jolla, CA) was used to prepare the proteinase/substrate suspension for a total of 200µl/well. The plate was incubated at room temperature for 5hrs 45 min with shaking and a final measurement was taken according manufacturer’s instructions using a GeniosPlus plate reader (TECAN, Austria). To calculate TIMP-1 activity, the relative fluorescent unit (RFU) value for each clone or control sample was subtracted from the RFU value from the maximum MMP-2 activity (the negative control). The remaining value correlates to the TIMP-1 activity. These values were then normalized to cell recovery and the level of TIMP-1 activity/cell for the control NIKSEV culture was set to 1. To compare the TIMP-1 activity in conditioned media samples obtained from NIKSTIMP-1 clones, final values were recorded as a fold difference relative to the NIKSEV controls. A substantial inhibition (~85%) of MMP activity has been consistently obtained with the positive control (4 nM 1,10-phenanthroline).
TIMP-1 mediated inhibition of MMP-10 was measured using the SensoLyte™ 520 MMP-10 Assay Kit *Fluorimetric* (AnaSpec, Inc., San Jose, CA). Reactions utilized 25ng recombinant MMP-10 in test samples. Forty microliters of 3:1 phenol red-free HAM’s F-12 (Caisson Laboratories, Inc, North Logan, UT):HyQ DMEM/ High Modified (Hyclone, Logan, UT) medium condition by NIKS or NIKSTIMP-1 for 24 hrs were added to test samples in a 96 well flat bottom plate (Costar, Corning, NY). The plate was incubated at room temperature for 1 hr with shaking and a final measurement was taken using a GeniosPlus plate reader (TECAN, Austria). Results were obtained from samples from three independent experiments.
Results
TCDD results in intercellular spaces and a diffuse basement membrane morphology in organotypic cultures of human keratinocytes
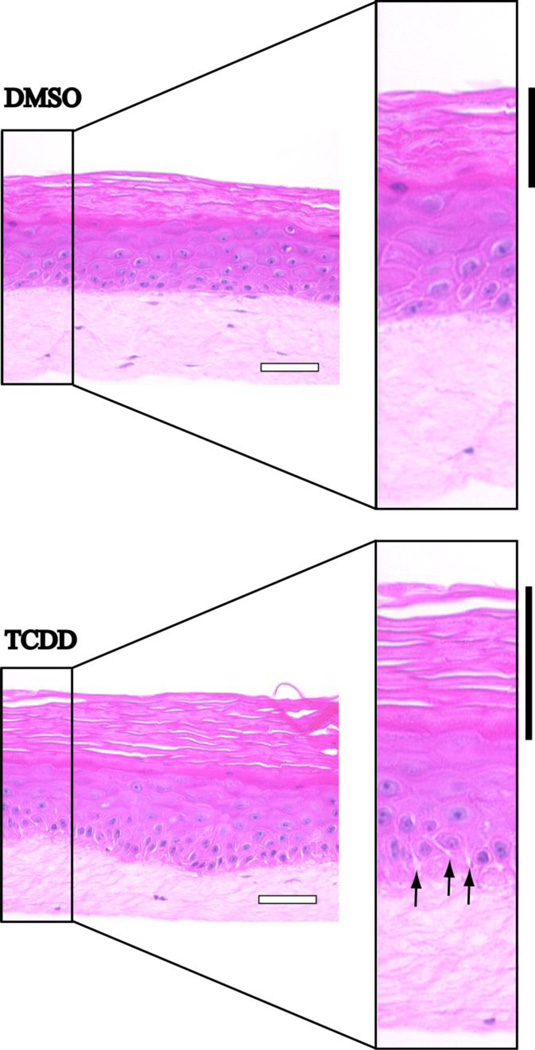
It has previously been demonstrated that an organotypic model of human skin containing both epidermal and dermal compartments is a powerful in vitro system in which to investigate TCDD-induced dermatopathology (6). Following a similar protocol, we plated keratinocytes on a fibroblast-containing dermal equivalent raised to the air medium interface and exposed developing skin tissue to 10 nM TCDD for 18 days. On day 18 cultures were fixed and counterstained with H&E for histological analysis. Morphological differences observed in TCDD-treated organotypic cultures were restricted to the basal layer and stratum corneum. Intercellular spaces were observed between keratinocytes in the basal and immediately suprabasal layers of TCDD-treated organotypic cultures but not DMSO-treated cultures (Fig. 1, arrows). In addition the BM region juxtaposed to basal keratinocytes appeared irregular and less dense. The previously reported hyperkeratotic phenotype (6) was readily evident (Fig. 1, black bars). These histological observations suggest that tissue remodeling events involving cell-cell and cell-substratum adhesion are induced by TCDD exposure.
Figure 1. TCDD results in intercellular spaces and a diffuse basement membrane morphology in organotypic cultures of human keratinocytes.
NIKS were grown in organotypic culture in the presence of DMSO (upper panel) or 10 nM TCDD (lower panel) for 18 days. Cultures were then fixed, paraffin embedded, sectioned and stained with hematoxylin and eosin. Black bars designate the cornified layer while arrows designate intercellular spaces. White scale bars each represent 50 µm.
TCDD treatment increases the expression of MMP-10 in organotypic cultures of keratinocytes
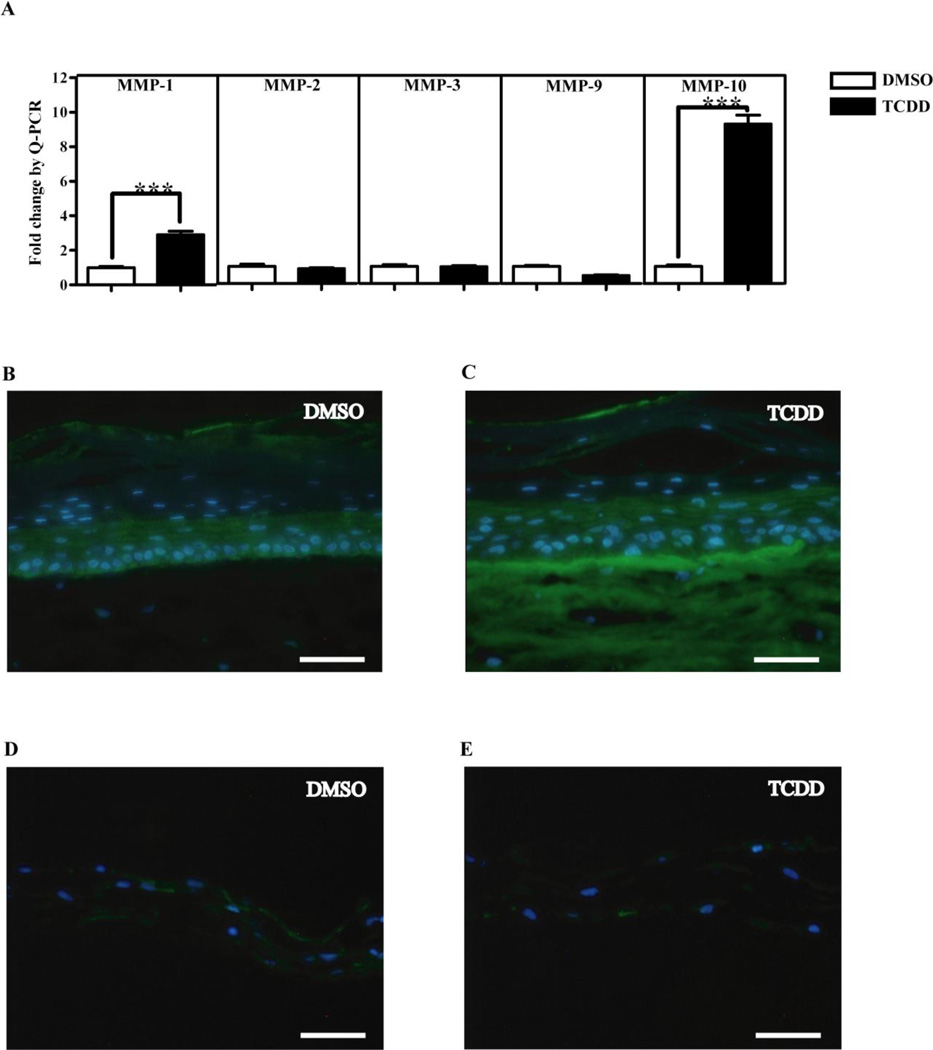
Unregulated turnover and remodeling of the ECM and BM contributes to the generation of pathological conditions in numerous tissues. The morphogenetic and molecular effects of environmental contaminants such as TCDD on ECM turnover and remodeling in human skin or in organotypic cultures containing human keratinocytes and dermal fibroblasts have not been previously described. Our initial observations of TCDD-treated organotypic cultures at day 18 were suggestive of ECM and BM degradation (Fig. 1) and prompted us to investigate MMP expression. Transcript levels of MMPs expressed in keratinocytes, MMP-1, MMP-2, MMP-3, MMP-9 and MMP-10, were measured by Q-PCR in 18 day-old organotypic cultures continuously exposed to either 10 nM TCDD or DMSO. A comparison of normalized CT values for the above mentioned MMPs in day 18 DMSO-treated control organotypic cultures showed that all of the skin-specific MMPs with the exception of MMP-3 have comparable constitutive mRNA expression levels (data not shown). MMP-3 exhibited a lower constitutive mRNA level. Following TCDD exposure, MMP-10 exhibited a 9-fold increase in mRNA expression (P< 0.001), while MMP-1 exhibited a more modest increase. MMP-2, MMP-3 and MMP-9 displayed no change in mRNA expression following TCDD treatment (Fig. 2A). The increases in MMP-1 and MMP-10 mRNA levels following TCDD treatment suggest a role for MMPs in tissue remodeling in organotypic culture. Further studies were focused on MMP-10 which exhibited the highest fold difference following TCDD exposure in these bioengineered human tissues.
Figure 2. TCDD treatment increases the expression of MMP-10 in organotypic cultures of keratinocytes.
(A) NIKS were grown in organotypic culture in the presence of DMSO or 10nM TCDD. Total RNA was isolated on day 18 post-treatment. Relative expression level of MMP-1, MMP-2, MMP-3 MMP-9 and MMP-10 mRNA were determined by Q-PCR analysis. MMP transcript levels were normalized to the level of cyclophilin-A transcript, and expressed as fold induction of the level found in day 18 DMSO-treated organotypic cultures for each gene. Data shown are mean ± SEM from a single experiment performed in triplicate and are representative of at least 3 independent experiments. *** P<0.001 by t-test. (B,C) NIKS cells or (D,E) dermises lacking keratinocytes were grown in organotypic culture in the presence of DMSO or 10 nM TCDD. Eighteen days post-treatment, cultures were sectioned and stained using a monoclonal antibody against human MMP-10 (green). Sections were counterstained with Hoechst to visualize the nuclei (blue). Scale bars equal 50 µm.
To test if elevated levels of MMP-10 mRNA translated into increased protein levels, IIF was performed on organotypic cultures treated with either DMSO or TCDD. (Fig. 2B, C, D, E). MMP-10 expression in control, day 18 DMSO-treated cultures was apparent in the basal layer of the epidermis but not in the dermis of these tissues (Fig. 2 B). In contrast, TCDD-treated organotypic cultures exhibited pronounced MMP-10 staining at the basolateral surface of the basal keratinocytes and in the dermis (Fig. 2C). To determine if the dermal fibroblasts of these tissues were contributing to the overall MMP-10 expression, dermises lacking keratinocytes were treated with 10 nM TCDD or DMSO for 18 days. These cultures displayed little detectable MMP-10 following DMSO or TCDD treatment (Fig. 2D, E). Therefore, these results suggest that the keratinocytes of the epidermal component of the organotypic cultures are the key contributor of MMP-10 observed at the BM and in the dermis of TCDD-treated organotypic cultures.
TCDD treatment increases expression of MMP-10 in monolayer cultures of keratinocytes
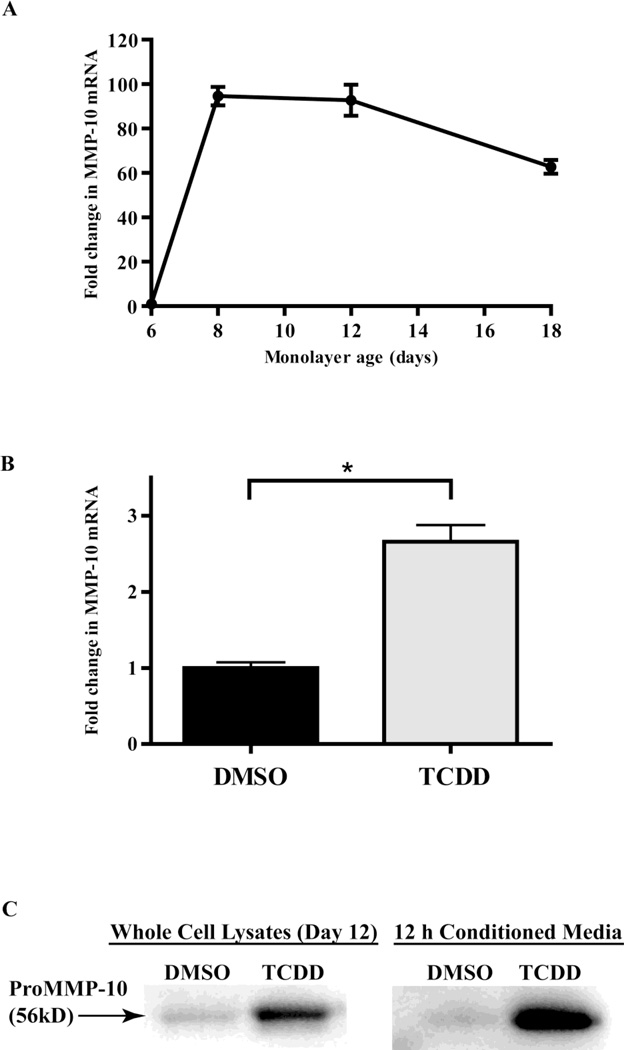
To determine the cellular origin of MMP-10 in TCDD-treated organotypic cultures, MMP-10 mRNA levels were measured in monolayer cultures of keratinocytes and fibroblasts treated with TCDD. Keratinocytes were directly plated onto 6 well plates and treated every two days with either 10nM TCDD or DMSO and total RNA was isolated on day 6, 8, 12 and 18 post plating. Q-PCR analysis showed a sharp 94-fold increase in MMP-10 mRNA expression for control DMSO cultures between days 6 and 8 at the time the keratinocytes cultures become confluent (Fig. 3A). The increase reached a plateau and was maintained in control cultures throughout the remainder of the time course. This result provides evidence that MMP-10 expression may be cell contact-dependent in human keratinocytes. TCDD treatment of monolayer keratinocyte cultures further increased MMP-10 levels at all time points tested (data not shown). Relative to time matched controls, TCDD-treatment resulted in a 2.6-fold increase in keratinocyte MMP-10 mRNA levels at day 12 (Fig. 3B).
Figure 3. TCDD increases expression of MMP-10 in monolayer cultures of keratinocytes.
(A) NIKS cells were grown without feeder layers in 6 well plates and treated with DMSO. The relative expression level of MMP-10 mRNA was determined by Q-PCR at day 6, 8, 12 and 18 post-treatment. Values were normalized to cyclophilin-A, then expressed as fold induction of the level found in day 6 DMSO treated cells. Data shown are means ± SEM from a single experiment performed in triplicate and are representative of at least 3 independent experiments. (B) The relative expression level of MMP-10 mRNA was calculated by Q-PCR for NIKS treated with DMSO control, or 10nM TCDD, at 12 days post-treatment. The levels of MMP-10, were normalized to cyclophilin-A, then expressed as fold induction over day 6 DMSO treated cells. Data shown are means ± SEM from a single experiment performed in triplicate and are representative of at least 3 independent experiments. *P<0.05 by t-test (C) Whole cell lysates of NIKS treated with DMSO or 10 nM TCDD for 12 days (left panel) or 12 hr conditioned media from these cultures (right panel) were resolved by SDS-polyacrylamide gel electrophoresis and probed using a monoclonal antibody against human recombinant MMP-10 (R&D systems, Minneapolis, MN).
We next determined if elevated levels of MMP-10 mRNA in monolayer keratinocytes following TCDD treatment resulted in increased MMP-10 protein synthesis and secretion. Monolayer keratinocytes were exposed every two days to TCDD for a total of 12 days and whole cell lysates and 12 hr conditioned media samples were harvested. Whole cell lysates and conditioned medium from TCDD and DMSO-treated samples were compared by immunoblot. Both whole cell lysates and conditioned media from the TCDD-treated samples exhibited a more intense proMMP-10 band compared to their respective DMSO controls (Fig. 3C). Whole cell lysates from TCDD treated samples display 3.5 fold greater band intensity than DMSO treated samples. Similar experiments done with TCDD-treated monolayer human fibroblasts showed no detectable MMP-10 expression in either whole cell lysates or conditioned media (data not shown). These results suggest that fibroblasts do not contribute to the MMP-10 pool in organotypic cultures. Our findings confirm that MMP-10 mRNA, as well as cellular and secreted MMP-10 protein, are increased following TCDD-treatment of human keratinocytes.
TIMP-1 overexpressing keratinocytes show no change in tissue architecture following TCDD treatment in organotypic culture
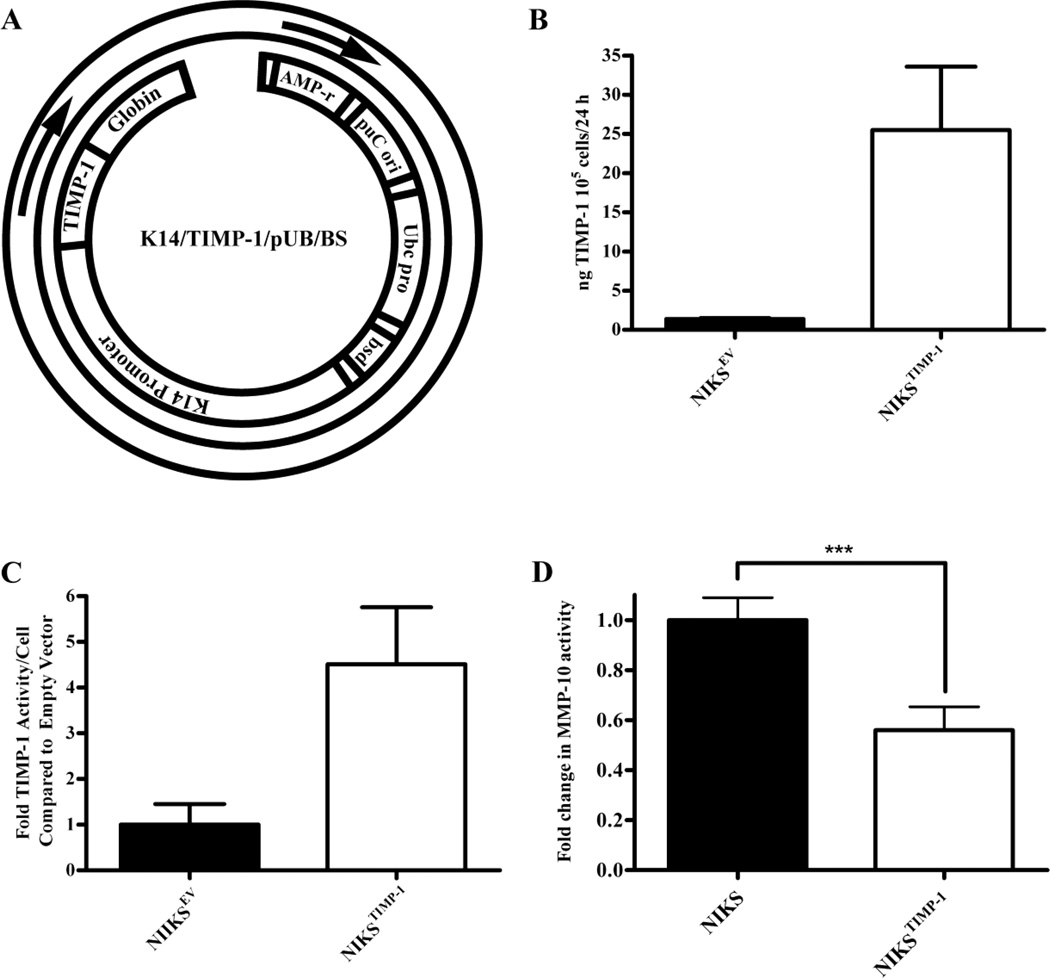
TIMP-1 is a well-studied endogenous inhibitor of MMP activities including MMP-10 (22,23). To test the hypothesis that TCDD-induced MMP expression contributed to the accelerated terminal differentiation and altered morphology of basal keratinocytes and BM of tissue, we directly inhibited MMP activity in keratinocytes by targeted overexpression TIMP-1. Clonal NIKS cell lines overexpressing active TIMP-1 (NIKSTIMP-1) were generated and characterized (Fig. 4). Since MMP-10 staining was observed predominantly in the basal keratinocyte layer of control organotypic cultures, we targeted TIMP-1 to the same area of the tissue in order to neutralize MMP-mediated effects. To accomplish this, we designed a TIMP-1 construct driven by the basal layer-specific promoter, Keratin 14 (K14) (Fig. 4A). The NIKSTIMP-1 clone used in these experiments expressed 25.5 ng TIMP-1/105 keratinocytes/24 hrs and inhibited MMP activity 4.5-fold more than the EV control NIKS cell line (NIKSEV) (Fig. 4B, C). To confirm inhibition of MMP-10 activity, we used an MMP-10-specific substrate-based assay. Conditioned medium from monolayer cultures of NIKSTIMP-1 possessed approximately half as much MMP-10 activity as control NIKS (Fig. 4D).
Figure 4. NIKSTIMP-1 exhibits elevated TIMP-1 protein expression and activity.
(A) Diagram of K14/TIMP-1/pUB/BS vector. NIKS cells transfected with either empty vector (NIKSEV) or K14/TIMP-1/pUB/BS (NIKSTIMP-1) were grown without feeder layers in 60 mm dishes and conditioned with 3:1 phenol red-free HAM’s F-12:HyQ DMEM/High Modified medium for 24 hr. (B) TIMP-1 protein expression was quantified by ELISA. (C) TIMP-1 activity was determined using a fluorescent substrate assay. Results were standardized to the cell number present at time of conditioned medium collection, and are expressed as fold induction of the EV control cells. (D) NIKSTIMP-1 shows greater inhibition of MMP-10 activity than unmodified NIKS. Data shown are means ± SEM from 3 independent experiments. *** P<0.05 by t-test.
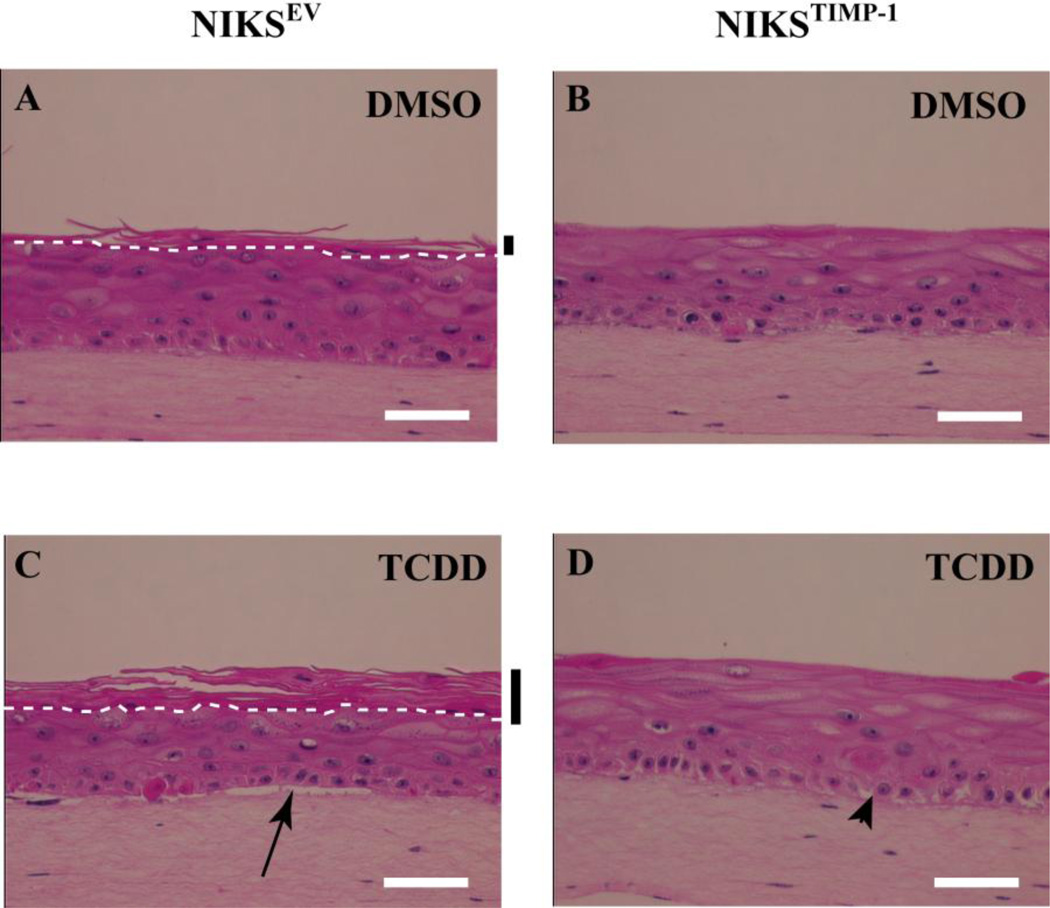
NIKSTIMP-1 and NIKSEV cells were grown in organotypic culture and continuously treated with either 10 nM TCDD or DMSO for 12 days. If MMP activity was required for the TCDD-mediated morphological changes in the BM, basal and stratum corneum layers, we predicted that TCDD-treated NIKSTIMP-1 organotypic cultures would exhibit an attenuated response. NIKSEV organotypic cultures treated with DMSO exhibited a fully differentiated morphology with a cornified layer (Fig. 5A) while TCDD-treated NIKSEV tissue exhibited accelerated terminal differentiation with a more pronounced cornified layer consistent with our earlier findings (6) (Fig. 5C). TCDD treatment of NIKSEV cultures also affected the BM and basal layer, where visible separation of the basal keratinocyte layer from the underlying dermis (Fig. 5C, arrow) and intercellular spaces between the basal keratinocytes were consistently noted. In contrast, TCDD-treated NIKSTIMP-1 tissue was comparable to the DMSO-treated controls with no observable cornified layer (Fig. 5B,D). These results directly demonstrate that the inhibition of MMP activity by TIMP-1 dramatically attenuates the TCDD-induced hyperkeratinization of the cornified layer. In addition, TCDD-treated NIKSTIMP-1 tissue exhibited no separation of the basal layer from the underlying dermis and appeared to have less prominent intercellular spaces between the cells of the basal layer (Fig. 5D). The intercellular spaces noted are likely due to incomplete MMP inhibition by the NIKSTIMP-1 organotypic cultures. Overall these studies showed that inhibition of MMP activity by TIMP-1 abolishes the TCDD-mediated hyperkeratinization of the cornified layer and decreases the abnormalities in the basal keratinocytes and BM in these tissues.
Figure 5. TIMP-1 overexpressing keratinocytes show no change in tissue architecture following TCDD treatment in organotypic culture.
NIKSEV (A,C) and NIKSTIMP-1 (B,D) cells were grown in organotypic culture in the presence of DMSO (A,B) or 10nM TCDD (C,D) for 10 days. Cultures were then fixed, paraffin embedded, sectioned and stained with hematoxylin and eosin. White dashed lines designate the boundary between the stratum corneum and stratum granulosum. Black bars indicate the cornified layer. The arrow highlights separation of the dermis from basal layer. The arrow head designates intercellular spaces. White scale bars each equal 50 µm.
Discussion
In this study TCDD exposure of skin tissue not only resulted in premature cornification and flattening of the suprabasal keratinocytes as previously reported (6), but also resulted in morphological changes in the basal keratinocyte layer and BM of organotypic cultures. Q-PCR analysis on a panel of MMPs expressed in skin showed that MMP-10 was significantly and robustly upregulated following TCDD treatment of the organotypic cultures. This result was confirmed by IIF. These studies also demonstrate that TCDD exposure increases MMP-10 mRNA and protein expression in monolayer keratinocytes and conditioned media, providing compelling evidence in two different experimental model systems that MMP-10 is upregulated following TCDD treatment. MMP-10 upregulation after ARNT1 knockdown in monolayer cultures suggests a role for the Ahr/ARNT pathway in the repression of this metalloproteinase in skin during physiologically normal conditions. IIF and immunoblot data showed that TCDD-mediated induction of MMP-10 is epidermal-specific, resulting in keratinocyte secretion of MMP-10 and deposition in the dermis. In order to investigate the role of MMP-10 in the observed tissue abnormalities, MMP activity was inhibited in the basal layer. TCDD exposure of TIMP-1 overexpressing tissues resulted in the ablation of the TCDD-mediated hyperkeratinization phenotype, as well as the attenuation of basal layer and BM abnormalities. Taken together, these results suggest a novel role for MMP-10 in TCDD-induced skin pathology.
Chloracne is considered to be the most specific and sensitive biomarker of TCDD intoxication. This condition results in a series of histopathological transformations which begin as early as 5 days post-exposure and can last up to 30 years (1). The disease is highlighted by a series of changes that occur in the pilosebaceous unit and surrounding tissue. During the early stage of the disease keratinocytes of the outer root sheath and sebaceous duct become hyperplastic, followed by squamous metaplasia and involution of the sebaceous acini. Later, small comedones start to appear and are filled by the products of keratinized cells and sebum. In the final stages of the disease, the larger comedones rupture due to thinning of the outer root sheath and the keratin lipid mass within is extruded into the dermis (1,29). These observations point to a series of tissue remodeling events following TCDD exposure in human skin which results in the disruption of the ECM and BM through the actions of serine proteases and MMPs (9). In this study, MMP-10 protein levels were upregulated following TCDD exposure in our organotypic culture model of human skin.
MMP-10 is a 56 kD member of the stromelysin subset of MMPs that is secreted in a proform and later cleaved and activated to a 47 kD form (23). This enzyme is expressed in basal keratinocytes and in keratinocytes of the migrating edge of the epidermis (16,18). Overexpression of MMP-10 results in disorganization of the migrating edge of the epidermis and is detrimental to wound healing (30). To date no studies have investigated effects on MMP-10 following TCDD-treatment in tissues from any species. In our studies, TCDD-treated organotypic cultures containing both epidermal and dermal compartments displayed intense MMP-10 staining in the BM region and upper dermis. Organotypic cultures containing the dermal compartment only showed no detectable MMP-10 RNA or protein following TCDD treatment. Furthermore, TCDD treatment of monolayer keratinocytes increased MMP-10 expression while TCDD treatment of fibroblasts did not. These results suggest that the TCDD-induced expression of MMP-10 occurs in the epidermal compartment of skin tissue and accumulates in the dermis. Since a large portion of the ECM proteins in our tissue are concentrated in the dermis component of the organotypic culture, it is reasonable to consider that MMP-10 targets this area of the tissue following overexpression in the epidermal compartment in addition to its effects on the basal layer cells and BM. A study done by Windsor et al. (24) illustrates similar results where human foreskin keratinocytes in monolayer culture treated with phorbol ester selectively expressed MMP-10 while fibroblasts did not. Transgenic mice overexpressing a constitutively active MMP-10 mutant exhibited histological abnormalities in the organization of the wound epidermis (30). In these studies the migrating edge of wounded skin was scattered and the investigators attributed this observation to MMP-mediated degradation of laminin 5, an ECM component of the BM required for the normal migration of keratinocytes (30).
The cellularized dermal compartment of our organotypic cultures is a simplified model for human dermis consisting of fibroblasts and type 1 collagen. ECM and BM components, including laminin 5 as well as proteases such as MMPs, may be secreted by keratinocytes and fibroblasts during tissue maturation. These substrates could serve as targets for numerous MMPs including MMP-10, all of which could contribute to the overall TCDD-induced tissue phenotype seen here. This modified dermis is still a gross simplification of in vivo human dermis in terms of the magnitude and diversity of the ECM constituents. In addition, TCDD-exposed tissues exhibited abnormalities in the basal layer, where cells assumed a more columnar shape and displayed increasing disorganization with numerous spaces between the cells. These observations could be attributed to increased degradation of ECM components by MMP-10. MMP-10 has a broad substrate specificity including collagen III and IV, gelatin, nidogen, laminin, proteoglycans and elastin (16). Moreover, MMP-10 has the ability to activate pro-MMP-1, pro-MMP-7, pro-MMP-8 and pro-MMP-9 (23) thereby amplifying the proteolytic milieu. These MMPs affect additional components of the ECM and BM that are not directly targeted by MMP-10 and could also contribute to the BM, basal layer and stratum corneum morphology observed in TCDD-treated organotypic cultures. Various cell-cell adhesion molecules are important for the alteration of skin tissue organization. For example; MMP-dependent E-cadherin shedding is responsible for various remodeling events of neoplastic epithelium (31). Such a mechanism could be involved in producing the phenotype following TCDD exposure in our tissue. Furthermore, the complex biological interactions between the appendages of skin and ECM components are absent in this model of skin. Hence it is reasonable to conjecture that the abnormalities observed in organotypic cultures would be more pronounced in human skin exposed to TCDD due to the more complex nature of the in vivo tissue and its appendages.
Studies by Murphy and coworkers have shown increased MMP-1 mRNA expression in monolayer cultures of human keratinocytes following TCDD treatment (12). In our organotypic culture experiments, mRNA levels of MMP-1 were also upregulated following TCDD-treatment. MMP-1 and MMP-10 are both located on chromosome 11q22-q23 (32,33). MMP-3, another MMP mapping to this gene cluster, was not upregulated following TCDD treatment in this study. MMP-13, an additional member of this gene cluster not considered in this study, is upregulated in murine fetal hearts following TCDD exposure (11). It is possible that TCDD treatment targets this genetic locus in human keratinocytes. A more comprehensive study involving all MMPs of this cluster is needed to test this hypothesis.
TCDD-induced aberrations in tissue were attenuated by targeting TIMP-1 expression to the basal keratinocyte layer of organotypic cultures. While the most logical explanation for this result is an inactivation of MMP-10, TIMP-1, which has the ability to inhibit all soluble MMPs, could also be neutralizing MMP-1 as well as other MMPs such as MMP-7, MMP-8 and MMP-9, all of which are activated by MMP-10. Further studies are needed to answer more specific questions pertaining to the precise inhibition of MMPs in our tissue.
In conclusion, we have shown for the first time that TCDD treatment induces MMP-10 expression in organotypic culture of keratinocytes. This TCDD-mediated increase in MMP-10 was keratinocyte-specific. This finding suggests a role for MMP-10 in the hyperkeratinization, abnormal morphology of basal layer keratinocytes, and altered BM morphology of organotypic cultures following TCDD treatment. Moreover, basal layer-specific overexpression of MMP-10 inhibitor TIMP-1 in these cultures attenuated these effects, supporting this theory. These studies would not have been feasible in standard monolayer keratinocyte culture and confirm the importance of using tissue-like, stratified co-culture systems for the study of complex interactions such as TCDD-mediated effects on MMP-10 expression. Our findings suggest that MMP-10 and other MMPs have a critical role in the TCDD-mediated pathology in skin and its appendages.
Highlights.
TCDD causes hyperkeratosis and basement membrane changes in a model of human skin
TCDD induces MMP-10 expression in organotypic cultures of human keratinocytes
Keratinocyte-expressed MMP-10 accumulates in the dermal compartment
Keratinocyte K14 promoter-driven TIMP-1 expression ablates TCDD-induced phenotypes
Acknowledgements
We thank Dr. Jens Eickhoff, Department of Biostatistics and Medical Informatics at the University of Wisconsin School of Medicine and Public Health, for statistical analysis, Heather Coon for technical assistance with the TIMP-1 ELISA and activity assays, Satoshi Kinoshita for preparation and processing of histological samples, and Erin Gill for assistance with utilizing ImageJ software for analyzing immunoblot band intensity. This work was supported by National Institutes of Health Grants R01 AR42853, HL074284 (to L. A.-H.) and R41 AG026174 (to C. T.-V.). This report is contribution 371, Molecular and Environmental Toxicology Center, University of Wisconsin.
The abbreviations used are
- MMP
matrix metalloproteinase
- BM
basement membrane
- TIMP-1
tissue inhibitor of metalloproteinase-1
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- NIKS
neonatal human keratinocyte cell line
- ECM
extracellular matrix
- Q-PCR
quantitative polymerase chain reaction
- AhR
aryl hydrocarbon receptor
- ARNT
aryl hydrocarbon receptor nuclear translocator
- K14
keratin 14
- DMSO
dimethyl sulfoxide
- DME
Dulbecco’s modified Eagle’s media
- ELISA
enzyme-linked immunosorbent assay
- IIF
indirect immunofluorescence
- PBS
phosphate buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None to declare.
References
- 1.Panteleyev AA, Bickers DR. Exp Dermatol. 2006;15:705–730. doi: 10.1111/j.1600-0625.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- 2.Hudson LG, Toscano WA, Jr., Greenlee WF. Toxicol Appl Pharmacol. 1986;82:481–492. doi: 10.1016/0041-008x(86)90283-8. [DOI] [PubMed] [Google Scholar]
- 3.Milstone LM, LaVigne JF. J Invest Dermatol. 1984;82:532–534. doi: 10.1111/1523-1747.ep12261149. [DOI] [PubMed] [Google Scholar]
- 4.Osborne R, Greenlee WF. Toxicol Appl Pharmacol. 1985;77:434–443. doi: 10.1016/0041-008x(85)90183-8. [DOI] [PubMed] [Google Scholar]
- 5.Greenlee WF, Dold KM, Osborne R. In Vitro Cell Dev Biol. 1985;21:509–512. doi: 10.1007/BF02620843. [DOI] [PubMed] [Google Scholar]
- 6.Loertscher JA, Sattler CA, Allen-Hoffmann BL. Toxicol Appl Pharmacol. 2001;175:121–129. doi: 10.1006/taap.2001.9202. [DOI] [PubMed] [Google Scholar]
- 7.Brown NM, Lamartiniere CA. Environ Health Perspect. 1995;103:708–713. doi: 10.1289/ehp.95103708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roman BL, Timms BG, Prins GS, Peterson RE. Toxicol Appl Pharmacol. 1998;150:254–270. doi: 10.1006/taap.1998.8395. [DOI] [PubMed] [Google Scholar]
- 9.Hillegass JM, Murphy KA, Villano CM, White LA. Biol Chem. 2006;387:1159–1173. doi: 10.1515/BC.2006.144. [DOI] [PubMed] [Google Scholar]
- 10.Gu YZ, Hogenesch JB, Bradfield CA. Annu Rev Pharmacol Toxicol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 11.Thackaberry EA, Jiang Z, Johnson CD, Ramos KS, Walker MK. Toxicol Sci. 2005;88:231–241. doi: 10.1093/toxsci/kfi301. [DOI] [PubMed] [Google Scholar]
- 12.Murphy KA, Villano CM, Dorn R, White LA. J Biol Chem. 2004;279:25284–25293. doi: 10.1074/jbc.M402168200. [DOI] [PubMed] [Google Scholar]
- 13.Ganz T. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 14.Page-McCaw A, Ewald AJ, Werb Z. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer AJ, Clark RA. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 16.Rechardt O, Elomaa O, Vaalamo M, Paakkonen K, Jahkola T, Hook-Nikanne J, Hembry RM, Hakkinen L, Kere J, Saarialho-Kere U. J Invest Dermatol. 2000;115:778–787. doi: 10.1046/j.1523-1747.2000.00135.x. [DOI] [PubMed] [Google Scholar]
- 17.Hunt TK, Hopf H, Hussain Z. Adv Skin Wound Care. 2000;13:6–11. [PubMed] [Google Scholar]
- 18.Parks WC. Wound Repair Regen. 1999;7:423–432. doi: 10.1046/j.1524-475x.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- 19.Brew K, Dinakarpandian D, Nagase H. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 20.Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Eur J Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- 21.Baker AH, Edwards DR, Murphy G. J Cell Sci. 2002;115:3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 22.Handsley MM, Edwards DR. Int J Cancer. 2005;115:849–860. doi: 10.1002/ijc.20945. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura H, Fujii Y, Ohuchi E, Yamamoto E, Okada Y. Eur J Biochem. 1998;253:67–75. doi: 10.1046/j.1432-1327.1998.2530067.x. [DOI] [PubMed] [Google Scholar]
- 24.Windsor LJ, Grenett H, Birkedal-Hansen B, Bodden MK, Engler JA, Birkedal-Hansen H. J Biol Chem. 1993;268:17341–17347. [PubMed] [Google Scholar]
- 25.Allen-Hoffmann BL, Schlosser SJ, Ivarie CA, Sattler CA, Meisner LF, O’Connor SL. J Invest Dermatol. 2000;114:444–455. doi: 10.1046/j.1523-1747.2000.00869.x. [DOI] [PubMed] [Google Scholar]
- 26.Allen-Hoffmann BL, Rheinwald JG. Proc Natl Acad Sci U S A. 1984;81:7802–7806. doi: 10.1073/pnas.81.24.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geng S, Mezentsev A, Kalachikov S, Raith K, Roop DR, Panteleyev AA. J Cell Sci. 2006;119:4901–4912. doi: 10.1242/jcs.03282. [DOI] [PubMed] [Google Scholar]
- 28.Loertscher JA, Lin TM, Peterson RE, Allen-Hoffmann BL. Toxicol Sci. 2002;68:465–472. doi: 10.1093/toxsci/68.2.465. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto O, Tokura Y. J Dermatol Sci. 2003;32:85–94. doi: 10.1016/s0923-1811(03)00097-5. [DOI] [PubMed] [Google Scholar]
- 30.Krampert M, Bloch W, Sasaki T, Bugnon P, Rulicke T, Wolf E, Aumailley M, Parks WC, Werner S. Mol Biol Cell. 2004;15:5242–5254. doi: 10.1091/mbc.E04-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, Hudson LG, Stack MS. Cancer Res. 2007;67:2030–2039. doi: 10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- 32.Visse R, Nagase H. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 33.Pendas AM, Santamaria I, Alvarez MV, Pritchard M, Lopez-Otin C. Genomics. 1996;37:266–268. doi: 10.1006/geno.1996.0557. [DOI] [PubMed] [Google Scholar]