Abstract
Fentanyl and its analogs have been mainstays for the treatment of severe to moderate pain for many years. In this review, we outline the structural and corresponding synthetic strategies that have been used to understand the structure–biological activity relationship in fentanyl-related compounds and derivatives and their biological activity profiles. We discuss how changes in the scaffold structure can change biological and pharmacological activities. Finally, recent efforts to design and synthesize novel multivalent ligands that act as mu and delta opioid receptors and NK-1 receptors are discussed.
Pain is a symptom accompanying most all diseases and different analgesics have been used to relieve pain. The International Association for the Study of Pain has defined pain “as an unpleasant sensory or emotional experience resulting from actual or potential tissue damage”.
In this review, we focus on the synthesis of compounds in the fentanyl series, the prototype of the 4-anilidopiperidine class of synthetic opioid analgesics. We outline the structural changes and corresponding synthetic strategies that have been used to understand the structure–biological activity relationship in fentanyl-related compounds and derivatives. Fentanyl is a synthetic μ-opioid agonist with a wide margin of safety, readily reversible minimal effects on the cardiovascular and respiratory systems, and its congeners are key structures for a family of most promising powerful synthetic opioid analgesics used for managing pain during and after surgery, to treat patients with severe pain, or sometimes to treat those with chronic pain.
Synthesis & biological activity of compounds in the fentanyl series
Pain is a protective phenomenon that accompanies many pathological conditions. However, in accomplishing signaling function, it can itself promote new health problems. Severe pain, which occurs as a result of trauma, surgery, myocardial infarction or other diseases is defined as acute pain. Chronic pain may be defined as pain that lasts beyond the term of painful stimulus and can continue indefinitely. Pain that is not curable by any known treatment is referred to as intractable pain [1]. The problem of pain alleviation is as old as humanity itself, and isolation of the oldest of the known analgesics, morphine from the opium poppy in 1804 by Friedrich Sertürner can be considered as the starting point for the development of the chemistry and pharmacology of pain.
However, despite research efforts involving the academic community and pharmaceutical industry devoted to pain research, it is necessary to confess that: “Biomedical science has greatly improved our understanding of pain in recent decades, but few novel molecular entities that address fundamentally new pain mechanisms have entered the clinic, despite dramatically increased pharmaceutical investment. Indeed, virtually all new analgesics approved over the past 25 years are derivatives or reformulations of opioids or aspirin-like drugs, existing drugs given for a new indication or older drugs given by a different route of administration” [2].
“59 drugs identified as analgesics were introduced from 1960 to 2009 and remain in use. Seven can be regarded as having novel molecular targets; however, only one, sumatriptan, was sufficiently effective to motivate the introduction of many similar drugs acting at the same target (triptans)” [3]. According to the same review [3], nine opioids have been introduced into the medicinal practice during the last five decades, and they are: pentazocine (1967), fentanyl (1968), butorphanol (1978), nalbuphine (1979), buprenorphine (1981), sufentanil (1984), alfentanil (1986), tramadol (1995) and remifentanil (1996) (Supplementary Figure 1S).
It is easy to notice that the two major ligand scaffolds are equally divided between morphine and fentanyl congeners. Four of them (pentazocine, butorphanol, nalbuphine and buprenorphine) are just more or less simplified or complicated modifications of morphine. The second “magnificent four” are compounds of the 4-anilidopiperidine series. Fentanyl, sufentanil, alfentanil and remifentanil represent modifications of fentanyl itself. Tramadol, considered as an opioid analgesic, is a compound belonging to the 3-amino-1-phenylpropan-1-ol series. It is a very weak μ-opioid receptor agonist, which at the same time induces serotonin release, inhibits noradrenaline reuptake and remotely could be considered as a very simplified morphine derivative (Supplementary Information 1).
Fentanyl was developed for parenteral administration. Due to a fast first-pass metabolism, oral administration is not available. A multitude of novel delivery systems and different approaches for fentanyl delivery have been developed in revent decades, including a transdermal system (patch) for long-term treatment of chronic pain [4,5]; fentanyl buccal tablets, which permit direct absorption of the drug through the oral mucosa providing rapid-onset analgesia for breakthrough pain, has been approved mainly for persistent pain of cancer patients [6–8]; and oral, transmucosal fentanyl, an intranasal fentanyl spray with a quicker onset [9,10]. Thousands of papers are published on fentanyl chemistry and reviewed in [11–14]. Its pharmacology reviewed in [15–23] and history in [24,25]. Information of fentanyl and its congeners is included in books and reviews on opioid analgesics [26–34], and also in corresponding chapters of popular textbooks of medicinal chemistry [35–40].
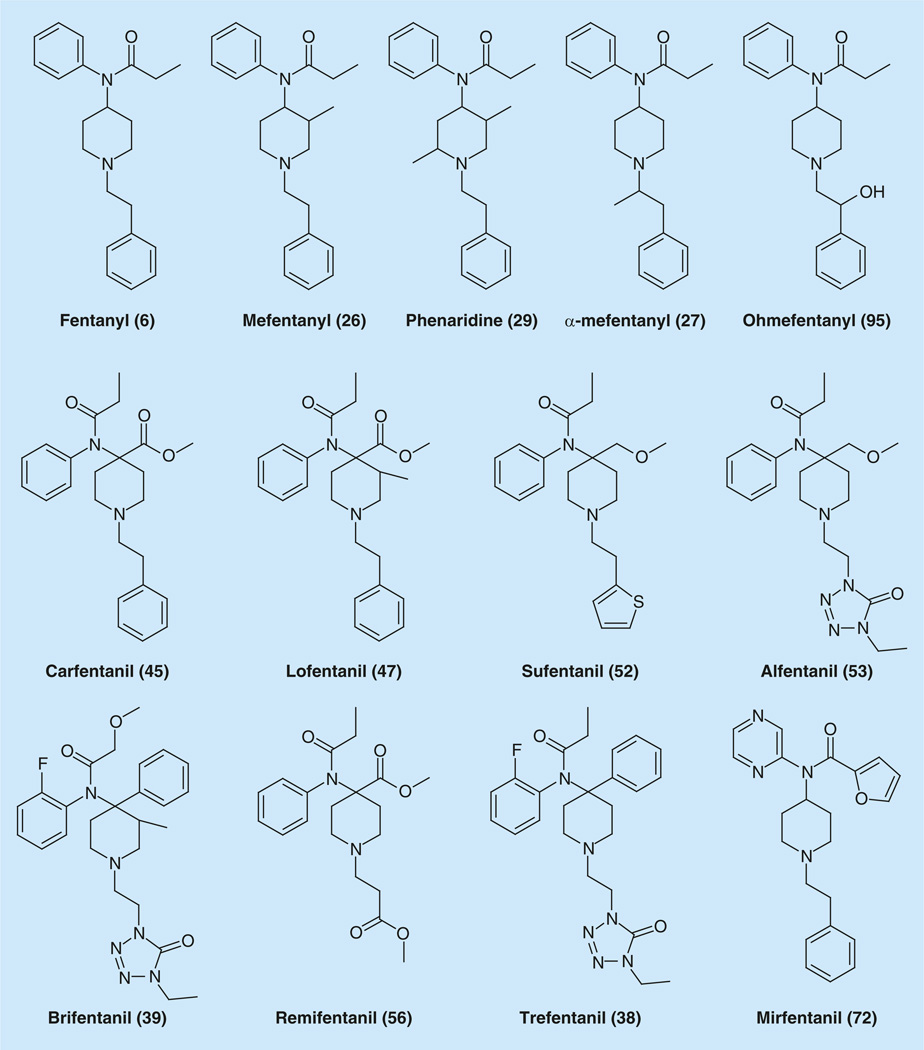
Fentanyl, invented more than 40 years ago, remains the mainstay of anesthesiologists throughout the world. The story of fentanyl began in the late 1950s and the beginning of the 1960s, when Paul Janssen (1926–2003) [41–44], one of the greatest contemporary scientists and most productive medicinal chemists in the world, creator of approximately 80 drugs, began looking for new chemical entities with significant analgesic activities in the pethidine (meperidine) series [45–48]. He probably was influenced by papers of the series dealing with pethidine containing anilide and phenylalkyl moieties [48] and did a series of brilliant observations in their structure–activity relationships, which lead him and an army of his followers all over the world to the invention of fentanyl and its numerous derivatives (Figure 1) [49–53]. As mentioned above, fentanyl (6), sufentanil (52), alfentanil (53) and remifentanil (56) became everyday ‘tailor made’ analgesics in medicinal practice [53].
Figure 1.
Fentanyl and major fentanyl analogs.
Fentanyl is an extremely powerful analgesic, 50–100-times more potent than morphine. Its LD50 is of 3.1 mg/kg in rats and 0.03 mg/kg in monkeys. The LD50 in humans is unknown. The safety margin (LD50/lowest ED50) is ~280 [54]. Just 0.1 mg of fentanyl is approximately equivalent to 10 mg of morphine and 75 mg of pethidine in analgesic activity. After the subcutaneous administration of 0.1 mg/kg of fentanyl to mice, maximum activity occurred at 10–15 min. The analgesic effect of fentanyl lasts no longer than 30 min after injection. For comparison, the peak activity of morphine occurred at 45 min after injection of 20 mg/kg of the. The duration of action at this dosage level of morphine appears to be greater than 1 h. The ED50 and 95% limits for fentanyl citrate injections is calculated to be 0.08 (0.045–0.142) mg/kg, while for morphine it is 15 (12–20) mg/kg [55]. Data on the relative analgesic potency of fentanyl and other analgesics on humans have been discussed [56,57]. Detailed fentanyl pharmacology is described in a few reviews [58–60].
The action of fentanyl is qualitatively similar to morphine. Cortical depression is minimal. Respiratory alterations may last longer than the analgesic effect. No significant cardiovascular effects were observed at usual therapeutic doses.
Fentanyl rapidly distributes with sequestration in fat and it extensively binds to human plasma proteins. It is metabolized mainly by the liver and is excreted via the kidney. Elimination half-life varies from 6 to 32 h. Action starts almost immediately with intravenous administration and after 7–8 min with intramuscular dosing. The peak effect that the drug achieves is observed in 5–15 min following intravenous injection. Duration of the analgesic effect is 1–2 h on intramuscular administration. So it has a faster onset of action but a shorter duration of action than morphine.
Fentanyl acts addictively with other opioids and depressants. In cases with prolonged use tolerance may be developed and correspondingly minimal effective dose may be increased. Physical dependence develops over a few days. Possible adverse effects include respiratory depression, bradycardia, nausea and vomiting, and some muscle, especially chest wall rigidity [58–60].
Fentanyl acts preferentially on μ receptors [61–66]. The opioid activity of fentanyl was evaluated according to comprehensive protocols which showed that it is more potent than morphine in the guinea pig ileum (GPI) and mouse vas deferens (MVD) assays. In the GPI, it has an IC50 of 3.45 ± 0.45 × 10−9 M compared with 3.31 ± 0.94 × 10−8 M for morphine. The IC50 of fentanyl in the MVD test is 9.45 ± 4.05 × 10−9 M while that for morphine was 1.94 ± 0.34 × 10−7 M [62]. The literature data show that all of fentanyl profile compounds of the 4-anilidopiperidin series are highly μ-selective, but could also produce affinity for δ- and κ-opiate receptors. Moreover, it seems that as their μ affinity increases, their selectivity decreases [64]. In general, all the compounds of the series tested had high affinity for the μ-binding site, low affinity for the δ-site and were almost inactive at the κ-site [65,66].
Biotransformation of fentanyl was most pronounced in the liver [67]. The main degradation pathway in vivo is oxidative dealkylation leading to the formation of the main metabolites, phenylacetic acid and norfentanyl [68].
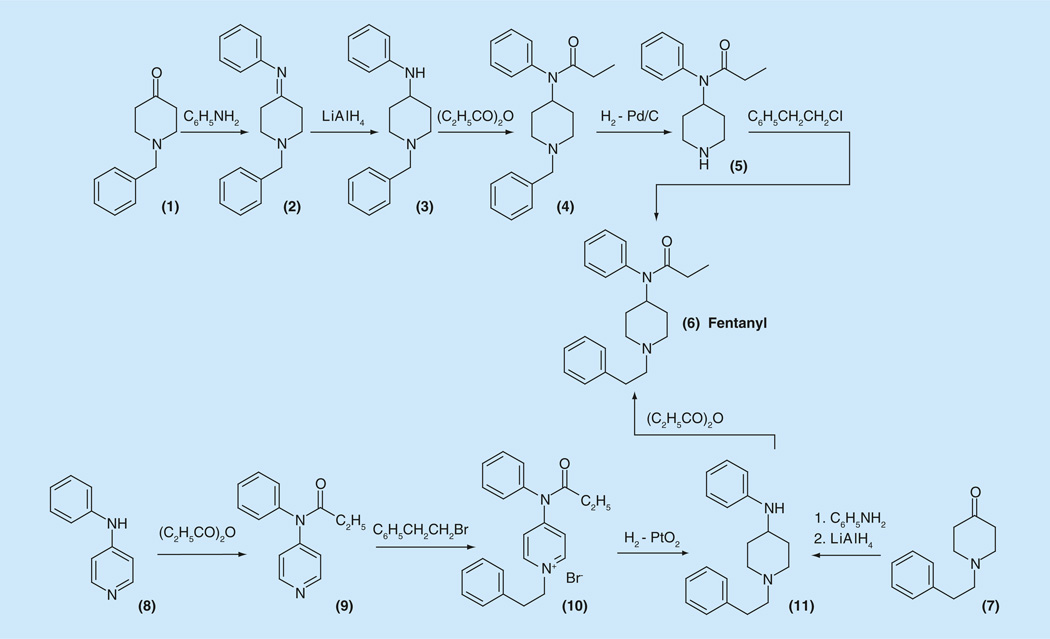
The first synthesis of fentanyl (6) was proposed by PAJ Janssen, starting with 1-benzypiperidin-4-one (1), which was condensed with aniline to give the corresponding Schiff base (2) (Figure 2). The double bond in the obtained imine (2) was reduced with lithium aluminum hydride, and the resulting 1-benzyl-4-anilinopiperidine (3) was acylated using propionic anhydride. The resulting 1-benzyl-4-N-propinoyl-anilinopiperidine (4) underwent debenzylation, using standard H2-Pd/C conditions, to give 4-N-propanoylanilinopiperidine or norfentanyl (5), which was then N-alkylated by 2-phenylethylchloride (or tosylate) to give the desired fentanyl (6) [49–53].
Figure 2.
Approaches to the synthesis of fentanyl.
Later, a shorter modified synthesis by the same scheme was proposed, starting directly from 1- (2-phenethyl)piperidin-4-one (7) (Figure 2) [69]. Another approach, which included a step of hydrogenation of pyridinium salt (10) has been developed [70]. Fentanyl (6) was prepared starting from 4-anilinopyridine (8), which on propionylation gave 4-N-propinoylanilidopyridine (9). The last was alkylated with 2-phenylethylbromide to give pyridinium salt (10), hydrogenation of which over PtO2 gave known 4-N-anilinopiperidine derivative (11), which was propionlated to the desired fentanyl (6) (Figure 2) [70].
The analgesic potency of fentanyl is approximately 300-times higher than that of morphine in the tail withdrawal test in rats. It has been enhanced up to 10,000-times (carfentanil; 45) that of morphine by making a series of minor, but very sensitive changes to the fentanyl structure. A huge amount of work had been carried our and published.
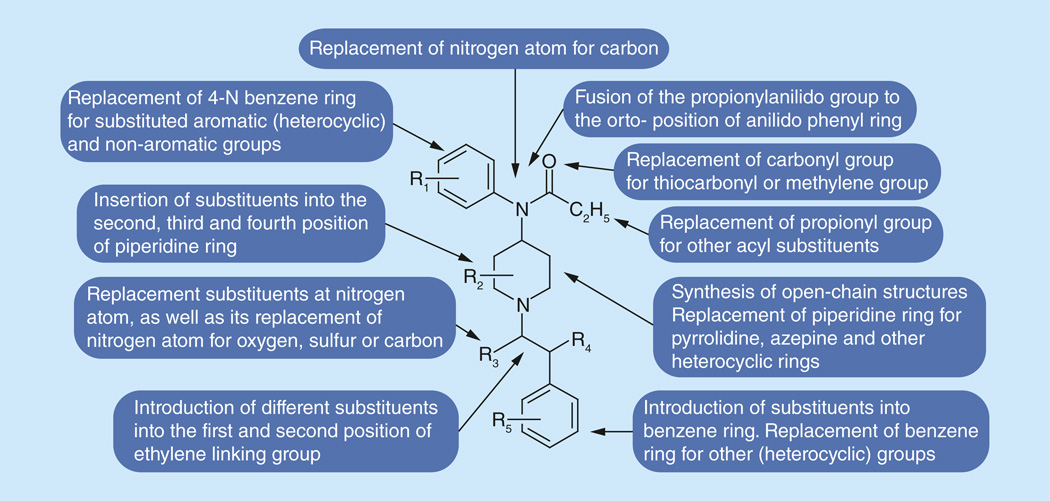
The main changes of practical meaning include replacement of piperidine ring for pyrrolidine or azepine rings, as well as synthesis of open chain compounds; replacement of phenyl group in the phenethyl- part of molecule for some aromatic heterocyles, mainly for thiophene and tetrazole; insertion of carbomethoxy-or methoxymethyl- into the forth position of piperidine ring; changes had been done in the 4-anilino- part of fentanyl molecule, mainly via replacement of hydrogen atoms in aromatic ring for fluorine atoms, or replacement of whole benzene ring for an aromatic heterocycle; additional methyl groups have been inserted into different positions of the piperidine ring; a lot of work had been done on replacement of propionyl-group in the 4-anilido-fragment for several other acyl groups, which gave a novel series of extremely potent analgesics with unusually high safety margins and already has become and will continue to remain the milestone for a series of analogous powerful compounds.
The possible ways for the creation of a series of novel potent analgesics with unusually high safety margin have been demonstrated with the fentanyl core structure becoming a milestone for a series of analogs powerful compounds (Figure 3).
Figure 3.
Modifications of the structure of fentanyl.
Numerous fentanyl analogs have been synthesized since 1964, including acyclic open chain compounds which have been shown to be strong analgesics [48]. One of them, diampromide (1S) which produces effects similar to other opioid analgesics, and is around the same potency as morphine with an ED50 of 4 mg/kg [71,72], another is 2,3-seco-fentanyl (2S) whose central-analgesic activity was found to be 40-times lower than fentanyl, but still 5–6-times higher than that of morphine (Supplementary Information 2)[73].
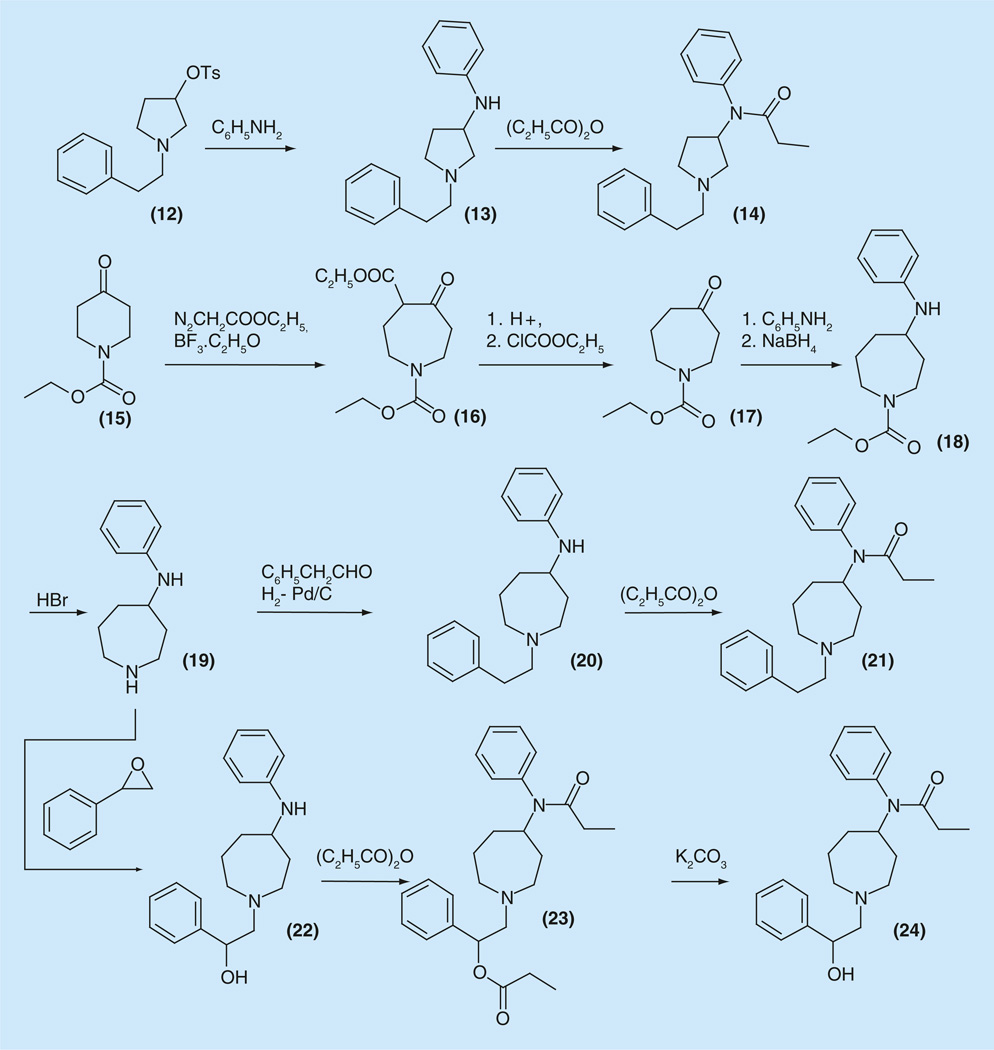
The preparation and analgesic properties of a series of fentanyl analogs with piperidine ring contraction or expansion, replacing it for pyrrolidine (14) or perhydroazepines (21) was described. The compound (14) was prepared by the nucleophilic replacement of the tosyl- or bromo- groups in compounds such as 3-tosyl-1-phenethylpyrrolidine (12) with aniline to get 3-anilinopyrrolidine derivative (13). Further acylation with different acid chlorides gave compounds with significant analgesic activity. The most active compounds were those in which the pyrrolidine N-substituent was phenethyl, O-methoxy-phenoxyethyl, or benzoylethyl. Compound 14 was found to be the most active with ED50 2 mg/kg (Figure 4) [74].
Figure 4.
Synthesis of perhydroazepine analogs of fentanyl.
The synthesis and analgesic properties of 4-(propanani1ido)perhydroazepines (21) also was described (Figure 4) [75,76]. The desired perhydroazepine derivative (21) was prepared via ring homologation of 1-carbethoxy-4-piperidinone (15) with ethyl diazoacetate and boron trif luoride to provide the β-keto ester (16), which after hydrolysis and decarboxylation in refluxing HCl, followed by carbamoylation with ethyl chloroformate, yielded perhydroazepinone (17) (Figure 4). Condensation of the obtained (17) with aniline, followed by NaBH4 reduction, provided 1-carbethoxy-4-anilinoperhydroazepine (18), which was decarbamoylated in refluxing 48% HBr to give (19). The appropriate 1-phenethyl substituent was incorporated by reductive alkylation of the first position of the perhydroazepine using as a carbonyl component phenylacetaldehyde to give 1-substituted 4-anilinoperhydroazepine derivative (20). The target compound (21) was obtained by propionylation of the last.
The 1-methyl, 1-benzyl, as well as 1-allyl and 1-(cyclopropylmethyl) derivatives also were prepared via direct alkylation of 19. Compound 21 was the most active among this series with ED50 2 mg/kg [75,76].
Either piperidine ring expansion (21) or ring contraction (14) of fentanyl significantly decreases analgesic activity of obtained entities. The most active among synthesized compounds were 14 and 21, which are N-phenethyl entities with 150- to 200-fold less potency than the corresponding piperidine homolog fentanyl (6).
Treatment of 19 with styrene oxide and following the transformations 22• → 24 afforded the new derivative 24, which, in the tail-flick assay had greater analgesic activity than other reported members of this series (Figure 4) [76].
A series of conformationally restricted, semirigid analogs of fentanyl as N-substituted propionylananilidonortropanes (3S) [77,78], nor-granatane (4S) [79], azabicyclo[2.2.2]octanes (5S) and (6S) [80,81] were synthesized, and stereochemically characterized. The scheme of synthesis of these compounds is practically the same as described above and has been started with appropriate ketones.
The 3-β-(chair conformation) of 3S displayed greater analgesic activity (ED50 0.047 mg/kg) in mice than the respective 3-a-isomer. [77]. 9-phenethyl-3-α-(N-tolylamido)norgranatane (4S) showed an ED50 value of 100 mg/kg [79]. The most potent fentanyl analogs of the isomeric azabicyclo[2.2.2]octane derivatives (5S, 6S) pair had a potency 600-times less than that of fentanyl (Supplementary Information 3) [80,81].
Insertion of methyl substituent in different positions of the fentanyl structure
The next series of changes in fentanyl structure involves insertion of different substituents into the fentanyl structure. And these substituents could be either simple as alkyl or aryl groups, or functional groups.
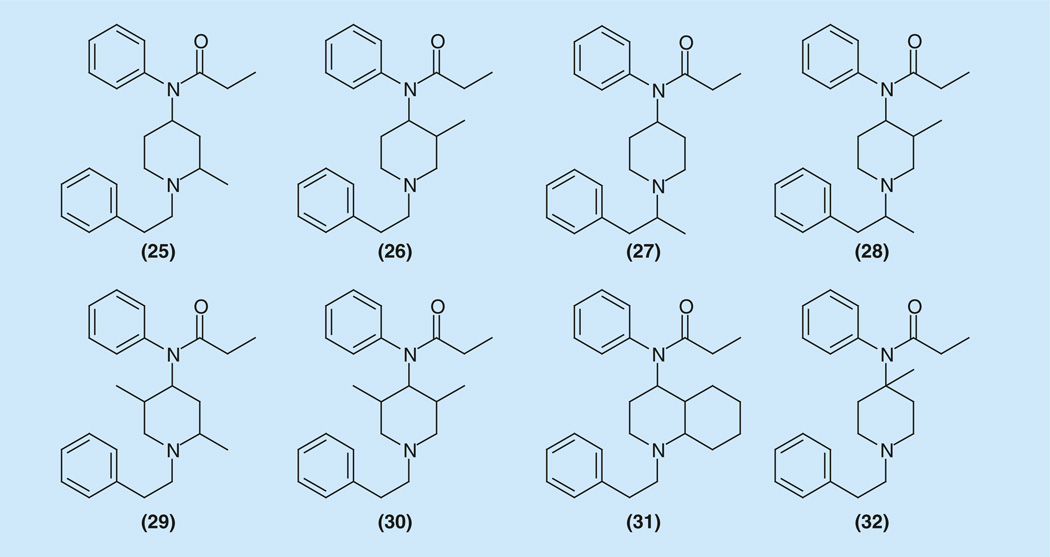
To check the effect of methyl substitution on the piperidine ring on analgesic activity of the 2-methyl-, 3-methyl-, 4-methyl-, 2,5-dimethyland 3,5-dimethyl- fentanyl derivatives were prepared (Figure 5).
Figure 5.
Methyl-substituted fentanyls.
2-methyl-fentanyl (25) possessing analgesic activity in the rat with an ED50 of 0.665 mg/kg [82] was proposed to be prepared from 2-methylpyridine N-oxide (7S) (Supplementary Information 4). The synthesis of 3-methyl-fentanyl (26) using the same approach was patented [83]. In the same paper [82], the preparation of 3-methyl-(26) and 2.5-dimethyl- (29) fentanyl derivatives using the same synthetic route is described, and ED50 values of 0.04 and 0.803 mg/kg, respectively were reported. Obtained data indicate that only 3-methylation has a major effect for enhancing analgesic potency, whereas 2-methyl or 2,5-dimethyl substitution is detrimental to analgesic activity. However, comparison with data obtained by other groups suggested this data may not be correct [84–88].
This article [82] was followed with another with a different approach to the synthesis of 3-methylfentanyl derivatives [89]. This method started with 3-methyl-4-oxopiperidinecarboxylate and afford afforded the end products cis-(+)-(26) as (3R,4S), cis-(−)-26) as (3S,4R), trans-(+)-(26) as (3S,4S), trans-(−)-(26) as (3R,4R) – mefentanyl (26) (Supplementary Information 5).
The cis-(+)-N-(3-methyl-l-(2-phenylethyl)-4-piperidyl]-N-phenylpropan-amide (26) is an extremely potent analgesic agent with an ED50 value of 0.00058 mg/kg, which is up to 6700-times higher than that of morphine. It has a fast onset of action, a shorter duration of action, and a high safety margin. Its cis-(−) counterpart is 120-times less potent than the cis-(+).
Isomeric α-methylfentanyl (27), obtained by the same synthetic method and containing an additional methyl group in the side chain in the a position to the basic nitrogen, also displayed high activity (ED50 = 0.0085 mg/kg) close to that of fentanyl (ED50 = 0.011 mg/kg). Insertion of two methyl groups simultaneously (28) in this position led to enhancement of activity depending on orientation of the 3-methyl substitution in the compounds with ED50 values from 0.011 for the (+)-cis, to 0.00075 mg/kg for the (−)-cis- isomers [89].
Another method for the synthesis of cis- and trans-3-alkylfentanyl analogs via alkylation of cyclohexylimine derivative of 1-(2-phenethyl) piperidin-4-ones was developed (Supplementary Information 5 & Supplementary Figure 7S) [90]. Except for the known (±)-cis-3-methylfentanyl and the novel (±)-cis-3-ethylfentanyl, the others were inactive or less active than fentanyl itself. The synthesis of 2.5-dimethyl-fentanyl – phenaridine (29) – started with 2-methylhex-5-en-3-yn-2-ol was described (Supplementary Information 6) [84,85].
Phenaridine as a mixture of isomers is slightly superior to fentanyl in potency and duration of action. Three isomers have been separated chromatographically and investigated by NMR spectroscopy which revealed that the isomers differ in orientation of the methyl groups in the piperidine ring. In the first one (45% content of the obtained isomeric mixture) the methyl groups oriented as 2-equatorial-5-axial, in the second (40% content) both are equatorial, and in the third one (15%), both methyl groups are oriented axial [86–88]. Absolute configuration of isomers by x-ray investigations was determined [91,92]. The duration of analgesic effects of the separated isomers was 125, 105, and 165 min for the respective isomers. The overall analgesic activity was comparable to that of fentanyl. Phenaridine itself (mixture of isomers) has an ED50 value of 0.0048 mg/kg (rats, subcutaneous, tail flick test; duration of action 35 min) [88].
An original method of synthesis was proposed for the synthesis of 3.5-dimethyl-fentanyl analogs. The compounds obtained as diastereomers, were tested for analgesic activity on mouse hot plate test and the majority of the compounds displayed good activity. The most promising was compound (30) with an ED50 value of 0.0025 mg/kg (Supplementary Information 7) [91–94].
To our knowledge, 2.3-dimethyl-fentanyl is not described in the literature. Decahydroquinoline analog (31) and other derivatives of the same series were synthesized starting from the 4-oxodecahydroquinoline showed low analgesic activities (ED50 = 25–50 mg/kg) (Supplementary Information 7 & Supplementary Figure 10S) [95].
A simple and efficient synthesis of 4-methylfentanyl (32) implementing the Ritter reaction was to 4-methylpiperidin-4-ol was proposed recently (Supplementary Information 8) [96]. The potency of 4-methyl fentanyl (32) was found to have an ED50 value of 0.0028 mg/kg in rats, which is approximately four-times greater than that of fentanyl (ED50 = 0.0105 mg/kg), while the time peak and the duration of action are the same as those of fentanyl.
The potency of compoud 32 was found to have an ED50 value of 0.0028 mg/kg in rats, which is approximately four-times greater than that of fentanyl (ED50 0.0105 mg/kg), while the time peak and the duration of action are the same as those of fentanyl (Supplementary Information 8).
Insertion of substituents other than methyl into the different positions of the fentanyl structure
Any information concerning incorporation of aromatic groups into the second or third position of 4-anilidopiperidines has not been found in the literature.
The incorporation of a 4-phenyl group into 4-anilidopiperidines led to novel potent opioid analgesics with a favorable pharmacological profile. Variations in the analgesic efficacy in the series was dependent on the substituents on the piperidine nitrogen and the anilido phenyl group.
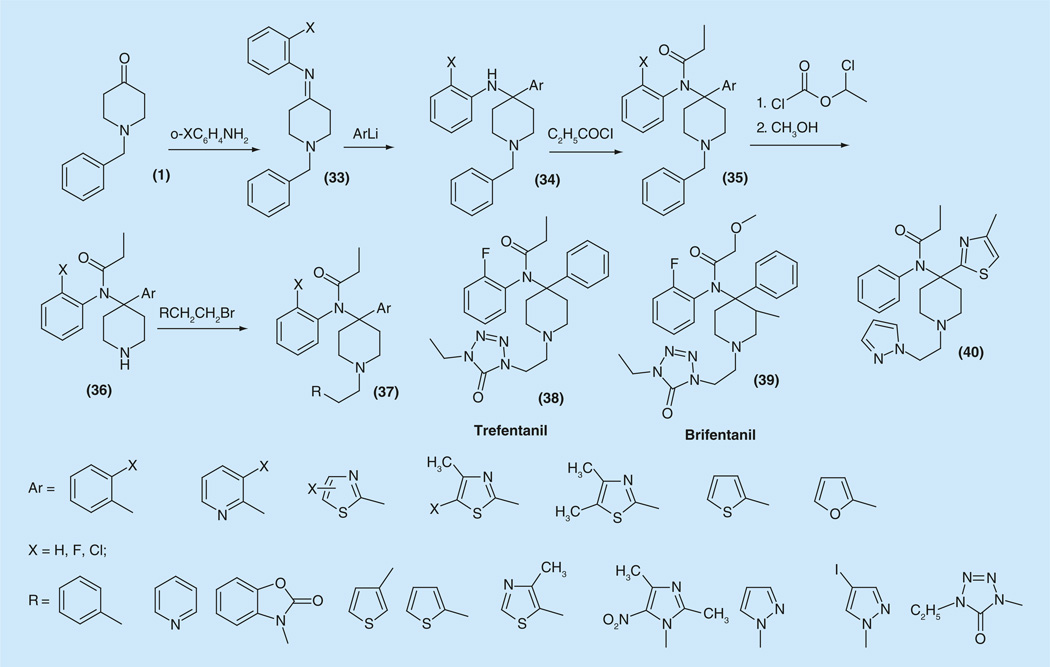
The synthesis, analgesic activity, and anesthetic properties of a series of 4-phenyl-4-anilido-piperidines and various heteroaryl substituents was carefully developed and described (Figure 6) [97].
Figure 6.
Synthesis of 4-aryl-fentanyl analogs.
Appropriately substituted anilines were reacted with l-benzyl-4-piperidone (1), to give imines (33). The aryl group was introduced into the fourth position of piperidine ring via reaction of the obtained imine with aryllithium to afford 4-aryl-4-anilinopiperidines (34). Propionylation of obtained diamines afforded amides (35). The debenzylation of (35) was achieved by reaction with 1-chloroethyl chloroformate followed by methanolysis. The secondary amines (36) were alkylated with arylethyl halides or tosylates to give a series of compounds (37). Among them, compounds such as trefentanil (38), brifentanil (39) and 40. Within this group 40 had the highest analgesic potency (ED50 = 0.047 mg/kg), shortest duration of action, rapid recovery of motor coordination following anesthesia doses, and greater cardiovascular and respiratory safety as compared with fentanyl and alfentanil [55]. A variety of other heterocyclic substitutions in both aromatic parts of fentanyl are proposed in patents [98,99]. Chemical modifications at the fourth position of the piperidine ring in fentanyl became an approach, which led to creation of the most powerful compounds of fentanyl series such as carfentanyl, sufentanil, alfentanil and others.
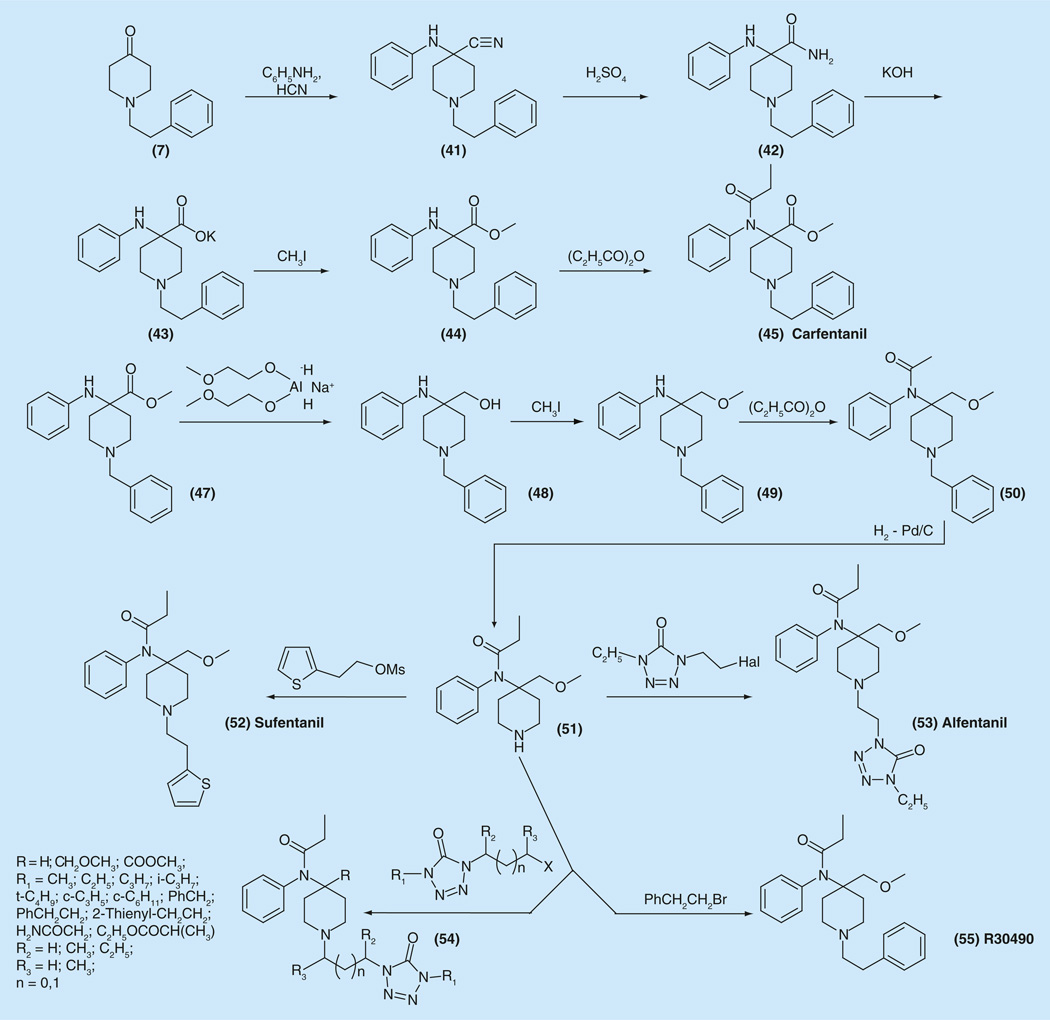
The synthesis of several derivatives of 4-arylamino-4-piperdinecarboxylic acids was reported (Figure 7). These acids are starting materials for the preparation of α-amino esters, ethers and ketones with strong analgesic activity [100,101]. A key step in the synthesis of all mentioned compounds became one of variations of the Strecker reaction, the interaction of imines, obtained from piperidin-4-ones and aromatic amines with hydrogen cyanide and is described in Figure 7. The aminonitrile 41 was synthesized via interaction piperidin-4-one (7), aniline and hydrogen cyanide. Hydrolysis of the obtained nitrile to corresponding acid was performed in two steps. Treatment of 41 with sulfuric acid gave amide 42, which was hydrolyzed further to acid 43 under basic conditions – using potassium hydroxide. Alkylation of the salt of obtained acid with methyl iodide gave aminoester 44, which finally was acylated with propionic anhydride to afford the desired carfentanil 45 [100,101], a highly potent and short-acting analgesic, (lowest ED50 = 0.00032 mg/kg) (10,000-times more potent than morphine) [100]. Carfentanil is intended only for animal use since its high potency makes it inappropriate for use in humans.
Figure 7.
Synthesis of carfentanyl, alfentanil sufentanil, R30490, and their analogs.
The relative potency and selectivity of carfentanil for the μ, κ, δ opioid receptors was determined in rat brain tissue homogenates. Carfentanil (45) was equipotent in displacing the μ and κ radioligands with IC50 values of 0.0006 and 0.0008 nM, respectively, while displacing the δ ligand with IC50 value of 0.75 nM. (Relative selectivity for μ/κ/δ ~1:1:1670) [102].
Introduction of an additional methyl group in the C-3 position of the piperidine ring resulted in another highly potent and long-acting compound lofentanil (47) (Figure 1), which is the longest acting compound in the series (more than 8 h) with an ED50 value of 0.0006 mg/kg [103].
Two very small changes in carfentanil structure, just reduction of carbonyl group in carboxy-function in the fourth position of piperidine ring transforming it to a methoxymethylene group and isosteric replacement of the phenyl ring at the phenethyl group with an heteroaromatic thienyl- and tetrazolyl rings led to two new analgesics with new properties, sufentanil (52) and alfentanil (53).
The synthesis of 4-(alkoxymethyl)piperidines was performed starting from (47) a benzyl analog of ether (44) [104]. Thus, 1-benzyl-4-phenylaminopiperidine-4-carboxylic acid methyl ester (47) was reduced with sodium bis-(2-methoxyethoxy) aluminium hydride (Red-Al) to give corresponding alcohol (48), sodium salt of which was selectively converted into methyl ether (49). The ether (49) was acylated with propionic anhydride to give (50). The latter was debenzylated via hydrogenation over Pd/C catalyst and the obtained amine (51) was alkylated with 2-(thiophen-2-yl)-ethyl mesylate to give desired (52) sufentanil. Sufentanil has a rapid onset of action, (ED50 = 0.00071 mg/kg, LD50 = 17.9 mg/kg), 4521-times more potent than morphine, and approximately five- to seven-times that of fentanyl at the time of peak effect. It has a relatively short duration of action comparable to that of fentanyl and an unusually high safety margin (LD50/lowest ED50 = 25,000).
N-alkylation of the same amine (51) with 1-(2-halogenyl)-4-ethyl-1H-tetrazol-5(4H)-one yielded another popular analgesic alfentanil (53).
The ED50 of alfentanil is 0.044 mg/kg. The safety margin in rats is 1080 (LD50 = 47.5 mg/kg i.v.). Alfentanil reaches its peak effect within 2 min after injection, and its duration of action is very short, 11 min in comparison with 30–35 min for pethidine and fentanyl, and 120 min for morphine [105,106].
As an analgesic in rats, alfentanil is 140-times more potent than pethidine and 72-times more potent than morphine. Alfentanil reaches its peak effect within 1 min after injection. Compared with fentanyl (6), alfentanil (53) reaches its peak effect is about four-times faster, but acts three-times shorter. While it gives less cardiovascular complications, it tends to give stronger respiratory depression. The detailed synthesis and structure–activity relationship of the series of compounds of the general formula (54) analogs of alfentanil (53) has been described [105]. N-alkylation of the amine (50) with phenethyl bromide gave R30490 (55), which has tenfold higher affinity than fentanyl for the μ receptor. R30490 is an excellent candidate to be a laboratory tool for probing determinants of μ-recognition and activation (Figure 7).
Analgesic activity of compounds (54) was evaluated on thermal tail withdrawal test in rats. It was found that these compounds with R = H are inactive at doses of 2.5 or 10 mg/kg. Maximal analgesic activity was found for compounds with R = COOCH3 (carfentanil analogs) with maximal analgesic activity when R1 represents lower alkyl groups. Compounds (53) with R = CH2OCH3 (sufentanil analogs) show the same activity profile as carfentanil analogs.
The ethylene group is the optimal bridge between the aromatic moiety and the nitrogen piperidine atom (R2 = R3 = H, n = 0). Duration of activity, in the series of carfentanil analogs when R1 = n-C3H7 and R1 = c-C3H5 is extremely short, while at R1 = CH3 and R1 = i-C3H7 compounds have an analgesic effect which last four-times longer. For sufentanil analogs (R4 = CH2OCH3) the duration of analgesic activity lasts between 11 and 20 min.
In an attempt to prepare novel analgesics in the fentanyl series, studies were initiated [107] to create new 1-(heterocyclyalkyl)-4-(propionanilido)-4-piperidinyl methyl esters and methylene methyl ethers where aromatic β-substituent (benzene, thiophene, tetrazole) at the first position of piperidine ring was replaced for a variety of other possible heterocyclic substituents.
The synthesized compounds were tested in the mouse hotplate test. Most of them exhibited an analgesia (ED50 < 1 mg/kg) superior to that of morphine. New interesting compounds like the pyrazolylethyl derivative (ED50 = 0.0099 mg/kg), phthalimidoethyl compounds with (ED50 = 0.056 mg/kg) and (ED50 = 0.119 mg/kg), were obtained, which exhibited appreciable μ-opioid receptor affinity, and were more potent and short-acting analgesics and less respiratory depressants than alfentanil. In addition, some of them showed a superior motor coordination following from full anesthetic doses in the rat rotarod test (Supplementary Information 9).
Summarizing above, introduction of additional substituents into the fourth position of the piperidine ring of fentanyl (6) such as a carbomethoxy group gave carfentanil (45), whereas introduction of a methoxymethyl group coupled with replacement of the phenyl ring of the phenethyl with a thienyl or tetrazolyl ring led to sufentanil (52) and alfentanil (53).
The next experiments with replacement of the phenyl ring of the phenethyl group in the first position of piperidine ring was substitution for a carbomethoxy group, which brought the discovery of the ultra-short acting powerful analgesic remifentanil (56) on the market on the market [108–111]. It is necessary to mention especially the great material on the synthesis of analogs of fentanyl summarized in [108].
A unique drug remifentanil (56) (Figure 1) with a high degree of analgesic potency (ED50 0.0044 mg/kg) and ultra-short duration of action (15 min.) became a clinically useful addition to the fentanyl family of analgesics [112,113]. Later, other data appeared in literature [114], according to which ED50 and 95% confidence limits of analgesic effect of remifentanil on mice were 0.73 (0.64–0.84) mg/kg and 0.19 (0.12–0.31) mg/kg, respectively in hot-plate and clam-tail tests. Analgesic effects of remifentanil on rat were 2.70 (1.15–6.34) mg/kg and 5.21 (2.11–12.85) mg/kg, respectively in formaldehyde and swing-tail methods. The analgesic action of remifentanil was the strongest at 1 min after intravenous injection. The action was weakened after 6 min., and disappeared after 12 min. Remifentanil (56) occupied its own place in the arsenal of opioid analgesics and described and discussed in many pharmacological reviews [115–124], including an originally titled review “Remifentanil: do we need another opioid?” [116]. Many modifications of the scheme of synthesis were proposed [125–129], among which is an interesting approach that applies the Ugi reaction for the synthesis of remifentanil (56) and carfentanil (45) (Supplementary Information 10) [129].
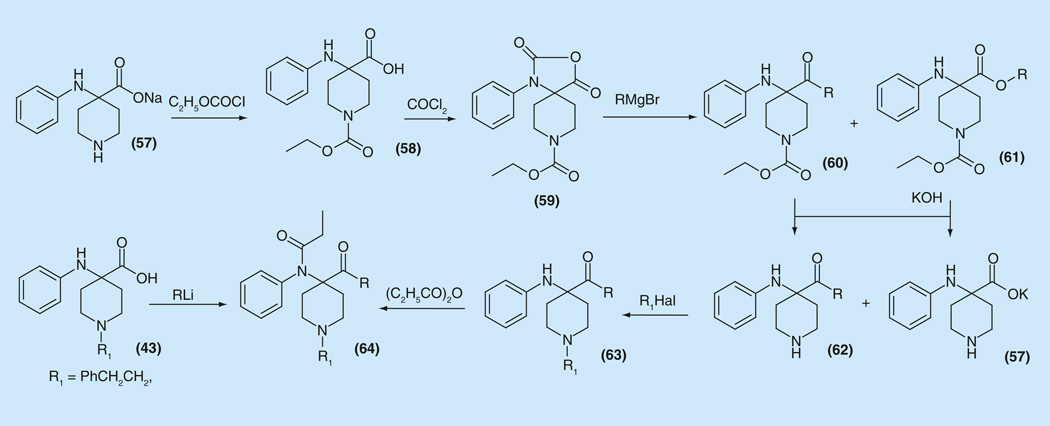
A number of pathways for the synthesis of acyl- substituents in the fourth position of piperidine ring were envisaged [100]. Acid 57 was used as starting materials for the preparation of α-amino ketones (64) via direct reaction with methyl- or butyl lithium (Figure 8).
Figure 8.
Alternative synthesis of 4-acyl- fentanyls from corresponding acids.
However, starting amino acids with other substituents on the piperidine nitrogen atom such as benzyl, methyl or hydrogen could not be used. For this reason the acid 57 salt was reacted with ethyl chloroformate to be transferred to 1-(ethoxycarbonyl)-4-(phenylamino)piperidine-4-carboxylic acid (58), which on interaction with phosgene gave oxazolidine-2,5-dione derivative (59). Then 58 was employed in a reaction with a Grignard reagent, which led to formation of two compounds, the desired ketone 60 and piperidin-edicarboxylate (61). Hydrolysis of the mixture gave easily separable ketone (62). Alkylation on the piperidine nitrogen atom gave the keto-derivative 63, subsequent acylation of which gave the target compound 64 with very good analgesic activity (ED50 = 0.00064–0.0013 mg/kg). For example, N-[4–acetyl-1-(2-phenylethyl)-4-piperidinyl]-N-phenylpropanamide (64 series) R1 = 1-(2-phenylethyl) (ED50 = 0.00064 mg/kg) was found to be 4900-times as potent as morphine.
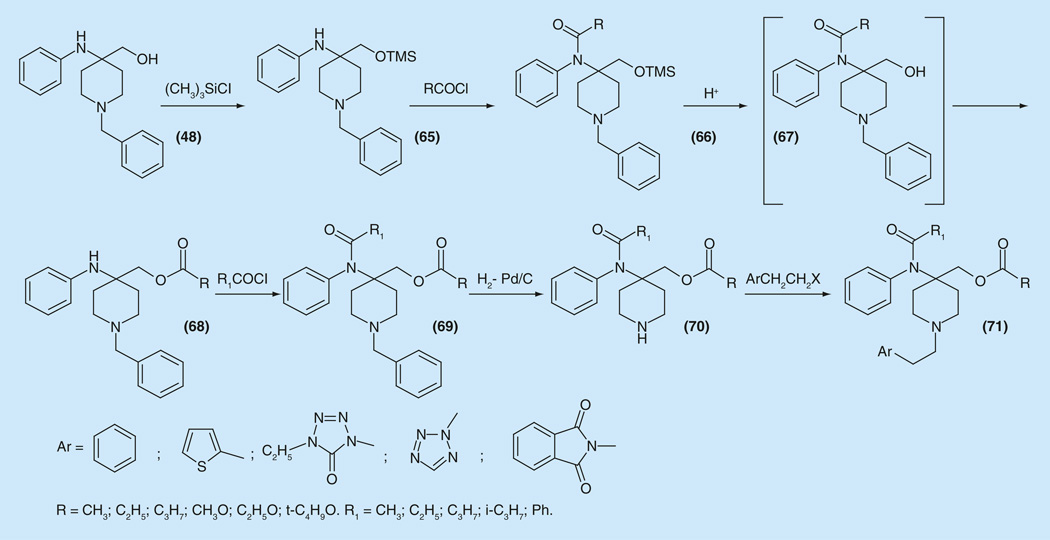
The synthesis and pharmacological evaluation of another class of 4,4-disubstituted fentanyl derivatives - 4-(acylamino)-4-[(acyloxy)-methyl] piperidines have been reported [130]. Many efforts to selectively acylate the hydroxy- group in 48 in the presence of the anilido nitrogen failed. The synthesis of target compounds was accomplished via protecting the hydroxyl group in 48 via formation of trimethylsilyl ether 65. After the acylation of anilido nitrogen in 65, attempts to unmask the hydroxy- group in 66 unexpectedly afforded product of intramolecular esterification 68. (During the hydrolysis of the O-trimethylsilyl ester acyl group migration took place). After a second acylation of the anilido nitrogen (69) followed by debenzylation (70) and further alkylation with arylethyl halogenides the desired products (71) were obtained [130]. The choice of the N-arylethyl substituent (phenyl, thienyl, tetrazol-on-il, phtalimidoil) was dictated by literature data (Figure 9).
Figure 9.
Synthesis of 4-acyloxymethyl- fentanyls.
Another set of analog compounds [130] was prepared by the condensation of aminoalkohol (48) with 1,l′-carbonyldiimidazole and the obtained adduct was transformed to series of carbonates (Supplementary Information 11).
Groups of fentanyl analogs with modified 4-anilido- fragment have been synthesized. p-F, I, and CH3 derivatives, the cyclohexyl analog, analogs with phenyl group of 4-anilido- fragment distanced from the fourth position of the piperidine ring have been described. Among compounds of that series all N-benzyl derivatives were inactive. All N-phenethyl analogs retained reasonable levels of activity less than that of fentanyl but more than that of morphine [131,132]. The traditional ortho-substituents (Cl, F, Me, MeO) [52,97,131,132] in the anilino- fragment of fentanyl do not act dramatically on the activity of the parent compound fentanyl. Significant changes were observed in two cases. Substitution with an -OH group brings more than a tenfold loss of activity. Introduction of a NO2- group causes more than a 1000-fold loss of activity, equal to removal of the N-propionyl group in fentanyl (Supplementary Information 12). Another research approach was based on modifications of the 4-anilido- fragment and its replacement by a variety of heterocycles [133].
Observations that have been done in this research allowed one to make important conclusions. One of them is substitution of the phenyl ring of the propionanilido group of fentanyl for heterocycles, resulted in a significant diminution of analgesic activity. The sole exception was the 2-pyridino derivative, which shows agonistic activity comparable with fentanyl. Another observation deserves much more attention. The compounds of the described series were also screened as opioid antagonists. The majority of these compounds (80%) were morphine antagonists, and they selectively antagonized respiratory depression. The compounds with the 2-pyridinyl, 4-pyridinyl and 2-pyrimidinyl rings were found to be inactive as antagonists. Variations of an acyl- chain within the amido- substructure of 4-(heteroani1ido)-piperidines likely play a larger role. Among the compounds, bearing a methoxymethyl chain attached to the amide carbonyl group opioid antagonists were not found. In contrast, 2- or 3-fury1 compounds were antagonists. Moreover, most furan-containing compounds selectively reverse respiratory depression. Two compounds that were antagonists, displayed different antagonistic profiles. 4-ethyl-2-pyridinyl compound resembled naloxone in inhibiting both morphine-induced analgesia and respiratory depression, while 2-pyrazine derivative mirfentanil (72) (Figure 1) (ED50 = 0.07 mg/kg, rat tail-flick test), inhibited morphine analgesia slightly, but completely reversed respiratory depression. These findings are difficult to explain. Another important observation was that antagonistic activity remains even in the absence of a 4-(heteroani1ido) substituent, but when the furan group was removed, there was no activity (Supplementary Information 13) [133].
Making parallel comparisons of chemical structures of opioid agonists, it is possible to conclude that practically every chemical class of compounds with opioid-agonist activity has a structurally similar opioid-antagonist compound. Agonist-antagonist transformation in these cases takes place as a result of small changes in the structure of the agonist. The only exceptions, where the corresponding change for agonist-antagonist transformations has not been found are the compounds of the fentanyl series.
These structurally unique fentanyl analogs provide a new gate into the area of creation of new powerful opioid antagonists [133,134]. The possibility of creation of opioid antagonists in the fentanyl has been patented [135].
Other fentanyl analogs in which the benzene ring of the propioanilido group was changed to a heterocyclic substituent, particularly for phenylpyrazole group also have been described [136–139]. Obtained compounds showed more potent analgesic properties than morphine, but less than fentanyl with longer duration of action.
It was proposed that imidazoline receptor agonists combined with opioid agonists could produce antinociceptive synergy. Thus fentanyl derivatives that incorporate guanidinium and 2-aminoimidazolinium groups were designed and synthesized, which incorporate both μ-opioid and I2-imidazoline receptor pharmacophores [140,141]. This publication was likely the first attempt for creation of bivalent ligands based on fentanyl as the μ- component. Binding assays indicate that guanidinium compounds are still potent μ-opioid ligands similar to fentanyl, but display moderate analgesic properties in vivo. The results for the I2-imidazoline receptor are less significant and the obtained compounds showed only low affinity (Supplementary Information 14).
Coming back to the role of substituents in the piperidine ring, here we will focus on the groups other than aliphatic or aromatic. Attempts to synthesize 3-methoxy- and 3-carbmethoxy- analogs of fentanyl should be noted. 3-methoxy-fentanyl analog has been prepared by traditional scheme (ketone-imine-amine-amide sequence) starting from 1-(2-phenylethyl)-piperidine-4-one (7), which was oxidized to give 3-hydroxy-piperidine-4-one dimethyl ketal, which was methylated to give 3-methoxy-piperidine-4-one ketal, converted to 3-methoxy-piperidine-4-one, which was further subjected to transformation to 3-methoxy-fentanyl. The effective dose (ED50 = 0.00064 mg/kg) was obtained for the cis-isomer of synthesized compound [142].
Using the regular scheme for the synthesis, starting from 3-methoxy-N-benzylpiperidine-4-one and separating on different stages cis- and trans- isomers, led to the synthesis of a plethora of highly active analgesics (ED50 = 0.00046–0.0019 mg/kg) (Supplementary Information 15).
The synthesis of 3-carbomethoxy fentanyl or iso-carfentanil has been accomplished starting from 3-carbomethoxy-1-(2-phenylethyl)-piperidine-4-one. Both (±) cis- (carbmethoxy group orientated axial – ED50 = 0.023 mg/kg) and (±) trans- (carbmethoxy group orientated equatorial – ED50 = 0.1 mg/kg) isomers of separated 3-carbomethoxy-fentanyl revealed significant but substantially reduced potency compared with fentanyl (ED50 = 0.011 mg/kg) (Supplementary Information 16) [143,144].
Attempts to functionalize the second position of fentanyl have been made. For example, (2R)-1-phenethyl-4-(N-phenylpropionamido) piperidine-2-carboxamide has been synthesized, which was 110- and 450-times less potent than fentanyl in the GPI and MVD assays, respectively (Supplementary Information 17) [145]. Other types of functionalization of the second position of the piperidine ring have been examined and lactam analogs of fentanyl were synthesized [146,147], but pharmacological studies were not given (Supplementary Information 14).
Conformationally restricted analogs of fentanyl
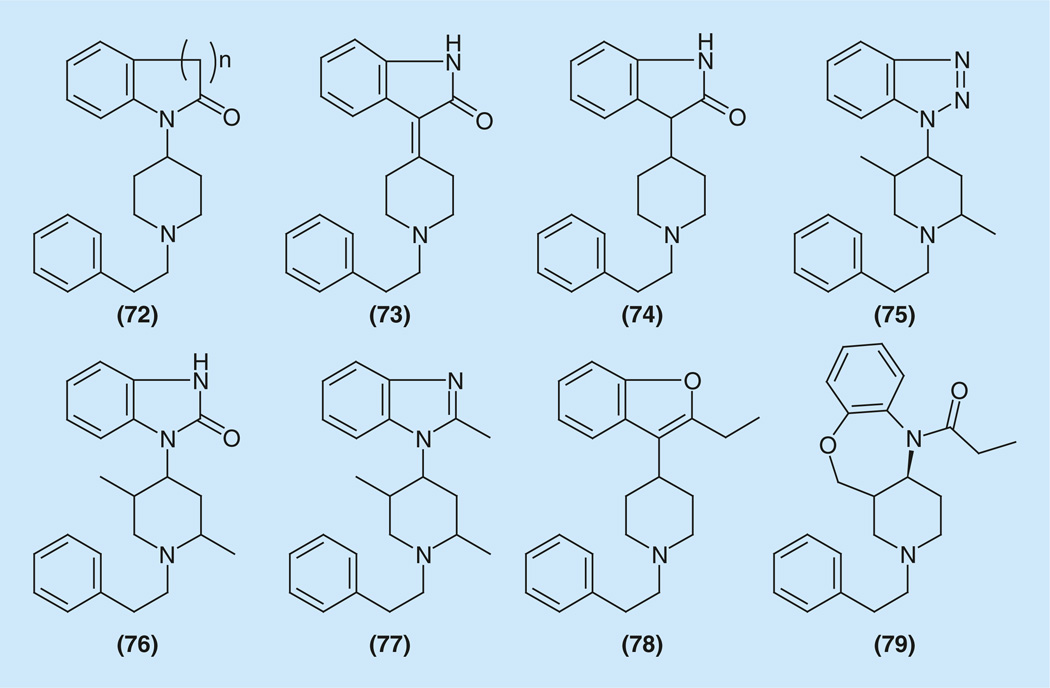
A number of ‘ring-closed’ analogs of fentanyl (72–79) were prepared (Figure 10). The first publication is probably that which described (4-piperidinyl)-2-indolinones (n = 1) and quinolinones (n = 2) (72). The synthetic approaches are very simple (Supplementary Information 18) [148–155]. The affinities are much less than that of fentanyl. From 500- to 800-times, until a complete loss of analgesic activity. In attempts to synthesize bivalent analgesics another series of ‘ring-closed’ analogs of fentanyl that combined fentanyl and indometacine structures some (4-piperidinyl)-indoles have been synthesized [152].
Figure 10.
Examples of ‘ring-closed’ analogs of fentanyl.
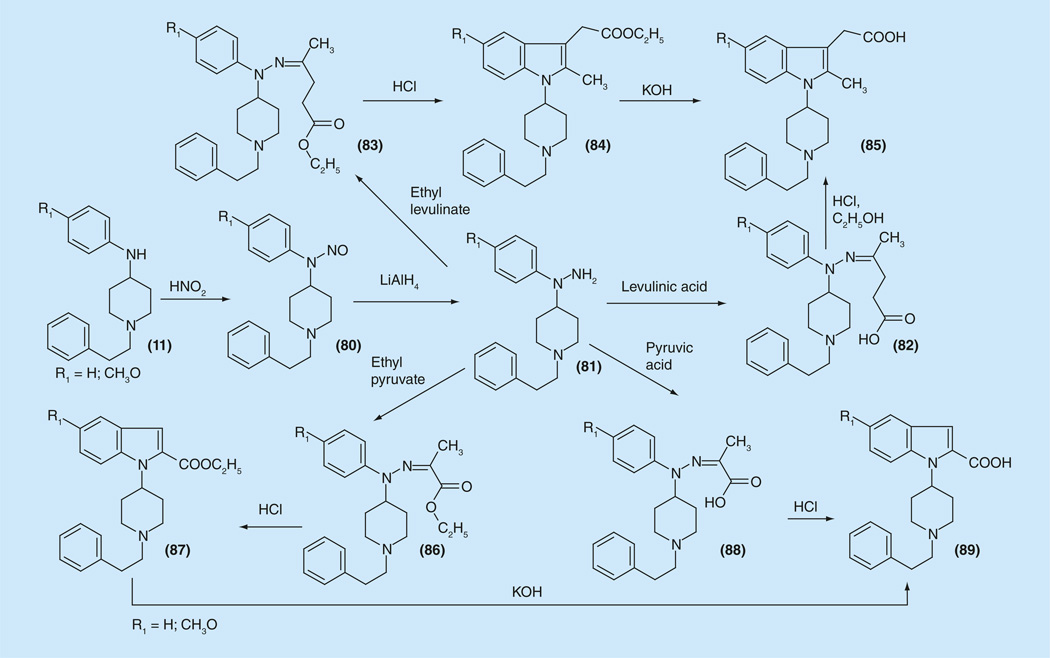
For creation of this series N-(piperidin-4-yl)-N-phenyl-hydrazines (81) were synthesized (Figure 11) starting from 4-anilinopiperidines (11), which were nitrosylated to the N-nitroso compounds (80) and then hydrogenated to the desired 4-piperidylphenylhydrazines (81). Then they were reacted with levulinic acid or its esters and the hydrazones 82 and 83 underwent Fishertype reactions, which led to indole derivatives, a series of indometacine analogs 84 and 85.
Figure 11.
One more example of ‘ring-closed’ analogs of fentanyl – indolylpiperidines.
Docking experiments of obtained molecules to their corresponding receptors were performed that predicted high COX-2 inhibitor activity compounds equal to indometacine itself and opioid activity two- to three-times higher than that of the fentanyl. However biological assays data were not consistent with the results from the molecular modeling. The synthesized compounds did not show any significant analgesic activity in the entire series [155]. Another series of indolylpiperidines 87 and 89 have been synthesized from phenylhydrazones 86 and 88 obtained from the same 4-piperidylphenylhydrazines (81). These compounds also did not show any significant analgesic activity for the entire series (Figure 11) [Vardanyan RS, Unpublished Data].
It is well known that for the most active representatives of the fentanyl series the aniline phenyl is perpendicular to the plane of the amide function [156,157]. For the whole series of ‘ring-closed’ compounds the perpendicular conformation of the anilido- moiety is excluded [150]. It can be suggested that these prepared compounds will bind poorly to opiate receptors, and that is the main reason for the absence of analgesic activity.
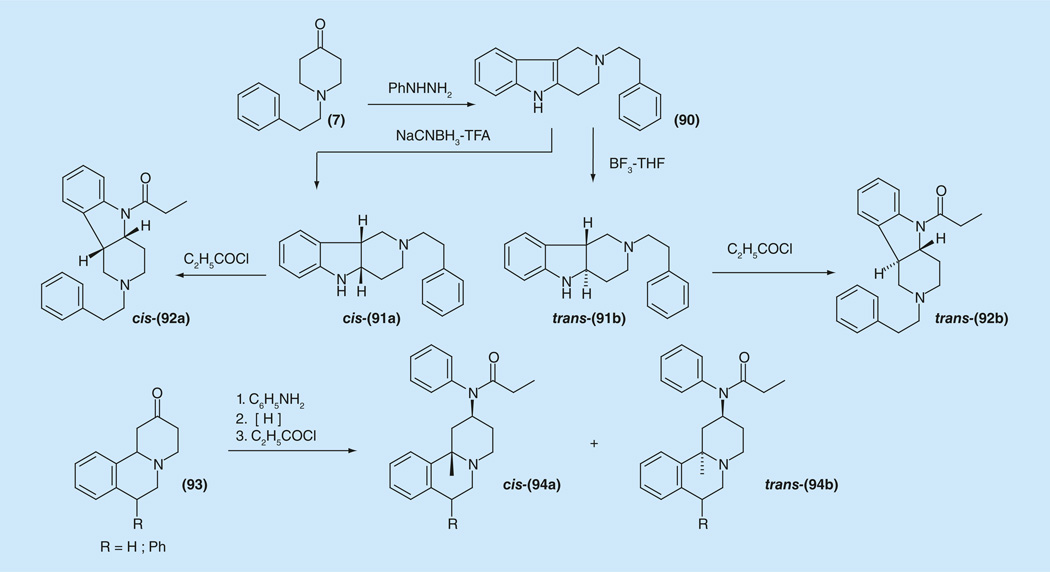
There is another series of ‘semi-rigid’ compounds where conformational mobility of pharmacophore groups is restricted and the first representatives are the pyridoindoles. These compounds have been synthesized from tetrahydro-γ-carboline (90), which was synthesized by Fischer condensation of piperidine-4-one (7) with phenylhydrazine [158,159]. Compounds 92a and 92b were inactive in the mouse phenylquinone writhing test at doses of up to 130 mg/kg (Figure 12 & Supplementary Information 18).
Figure 12.
Synthesis of series of pyridoindoles a benzo[a]-quinolizidine derivatives of fentanyl.
Another series of conformationally restricted analogs of fentanyl, of propionanilidobenzo[a]-quinolizidine derivatives 94a and 94b (Figure 12), were synthesized starting from hexahydro-pyridoisoquinolin-2-ones (93) and tested for analgesic activity and affinity for the opiate receptor of rat brain. These compounds displayed very weak analgesic activity (Figure 12) [158,159]. In addition, for the above discussed ‘semi-rigid’ derivatives it might be concluded that the analgesic activity in the fentanyl series does not ‘tolerate’ rigidity of whole molecule and is highly dependent on stereochemical factors.
Substituents in the side chain in the α- & β- positions of the basic nitrogen
Insertion of a methyl group in the N-substituent in the α- position to the basic nitrogen, also displayed high activity close to that of fentanyl, as mentioned above [89]. According to the first fentanyl patent [52] compound with methyl group in the β- position to the basic nitrogen has no advantages on fentanyl itself. Insertion of hydroxyl group in the β- position to the basic nitrogen in general enhances the potency of compounds of these series. But the most impressive results were obtained with the synthesis of ohmefentanyl (95), one of the most potent opioids known (Figure 1 & Supplementary Figure 26S). Ohmefentanyl had an ED50 value of 0.0002 mg/kg. For the four isolated stereoisomers of ohmefentanyl - (±)-cis-N-[l-(2-hydroxy-2-phenylethyl)-3-methyl-4-piperidyl]-N phenylpropanamides: ED50 values are: 0.0001 mg/kg for the 95a (2S,3R,4S) isomer; 0.0013 mg/kg for the 95b (2R,3R,4S) isomer; 0.08 mg/kg for the 95c (2R,3S,4R) isomer and 2.1 mg/kg for the 95d (2S,3S,4R) isomer.
The binding studies showed that the highest affinity and selectivity for the μ receptors were isomers 95b and 95c. At the same time, obtained data on the mouse vas deferens test showed potencies in the order 95a > 95b > 95c > 95d with concentrations in the fentomolar range. According to the mouse data, isomer 95a was 21,000-times more potent than 95d. Isomers 95b and 95c had similar opiate activities in vivo. The potency of 95a was from 20,000- to 50,000-times higher than that of morphine, which makes this isomer one of the most potent opiates known (Supplementary Information 19) [160,161].
Substituents in the aryl group in the 1-(2-arylethyl) moiety of fentanyl
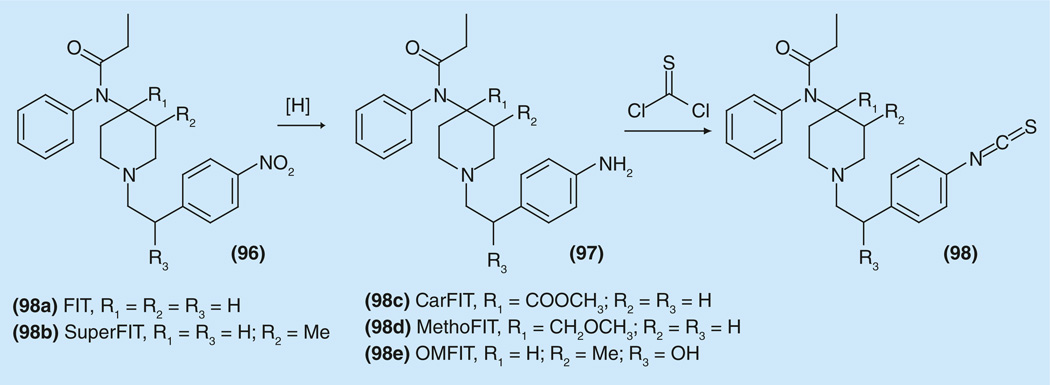
For variations of the character and substituents of the aryl group in the 1-(2-arylethyl) moiety in the fentanyl family, interesting changes took place when an isothiocyanate group was inserted into the phenyl group with the goal to synthesize irreversible ligands for opioid receptors [162]. The synthesis of the alkylating fentanyl derivatives was performed (Figure 13) [163,164].
Figure 13.
Synthesis of isothiocyanate derivatives of fentanyl.
The analgesic activity and opioid receptor binding affinities were examined for the isothiocyanate fentanyl (98a), 3-methylfentanyl (98b), carfentanil (98c), 4-methoxymethylfentanyl (98d), and ohmefentanyl (98e). In vivo antinociceptive activity was examined using the mouse hot plate test, and selectivity for opioid receptors was detected using binding assays. Fentanyl (ED50 not found), carfentanil (ED50 = 0.00041 mg/kg), and 4-methoxymethylfentanyl (ED50 = 0.00078 mg/kg), showed ED50 lower than those of their parent compounds. 3-methylfentanyl (ED50 = 0.33 mg/kg), and ohmefentanyl (ED50 = 0.0018 mg/kg) were stronger than the parent compounds. However the selectivity of all these compounds for δ receptors increased [163–165].
Replacement of anilino- moiety for benzylamino group
In continuation of studies devoted to involvement of imidazoline binding site in the modulation of analgesia, studies on creation of hybrid (bivalent) molecules bearing potent opioid and I2–imidazoline binding site ligands [140,141] has been continued and another sensitive change in fentanyl structure has been done. The phenyl moiety in anilino- fragment has been moved to one methylene group to give attractive compounds with a propionbenzylamino group [166]. The obtained compound showed no affinity to I2-imidazoline binding site (Ki > 10,000 nM), but displayed surprisingly high μ-opioid binding (Ki = 0.0098 nM) (on membranes). This observation brought to another study, synthesis and investigation of its ‘carba’ -analogs. Compound devoid nitrogen atom in piperidine ring of fentanyl was synthesized and showed 25-fold reduced receptor binding affinities as compared with piperidine nitrogen containing but, surprisingly it retained its opioid agonist activity [167]. Moreover, in the same paper it was shown that the propionbenzylamino fentanyl analog has not only high μ-opioid binding (Ki = 0.448 nM), but also had high κ receptor binding affinity (Ki = 0.536 nM) (Supplementary Information 20).
Replacement of 2-arylethylsubstituents at piperidine ring
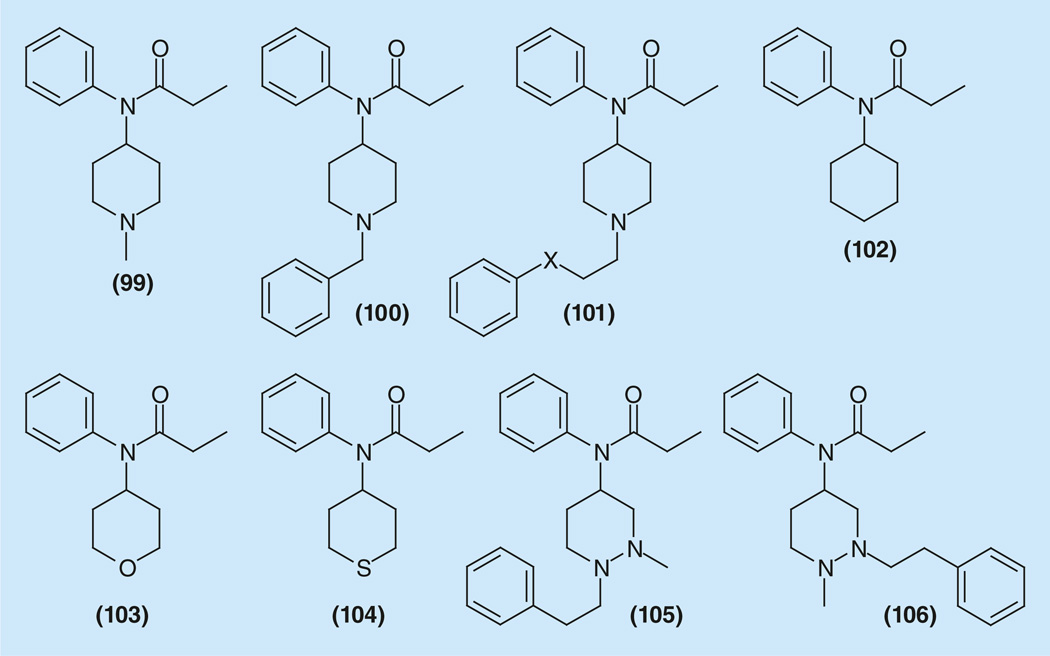
Replacement of 2-arylethylsubstituents at the piperidine ring nitrogen atom, resulted in sensitive changes of activity. Thus, N-methyl analog 99 is inactive at 100 mg/kg. N-benzyl analog 100 had greatly diminished activity (ED50 = 10–45 mg/kg) as compared with its N-phenethyl analog Fentanyl (7) (ED50 = 0.01 mg/kg) [11,168]. It is very important to mention, that this is not typical for the 4-phenylpiperidine series to which fentanyl is usually identified. Moreover, N-allyl substitution on the piperidine ring does not confer antagonist activity as in the case of morphinan derivatives. N-(1-propyl), N-(1-(2-phenoxyethyl)-, N-(1-(3-phenoxypropyl)- and N-(1-(2-cyanoethyl)-4-piperidinyl)-propionanilides (101), displayed activity similar to fentanyl [11,168,169]. Replacement of the nitrogen atom for oxygen, sulfur or carbon (102–104) resulted in loss of activity [Vardanyan RS, Unpublished Data]. Addition of a nitrogen atom into the second position of piperidine ring (105–106) also resulted in disappearance of activity (Figure 14) [170]. Replacement of amide carbonyl oxygen for sulfur via interaction of fentanyl with phosphorus pentasulfide preserves strong analgesic activity for N-(1-(2-phenylethyl) series of compounds [171].
Figure 14.
Replacement of 2-arylethylsubstituents at piperidine ring.
Bivalent ligands based on fentanyl
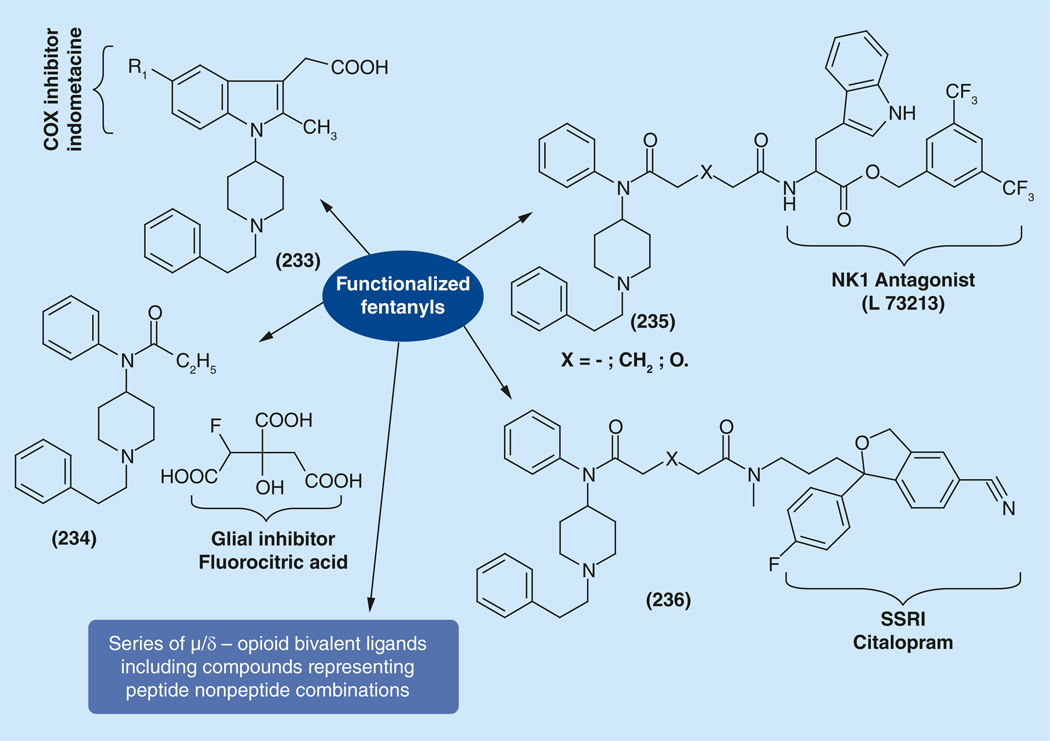
It is clear that activity at a single receptor is often insufficient and recent research, including in the pain area, is starting to be focused on ligands that have multiple activities. Multitargets (multivalent ligand) are believed to be ‘smarter’ than traditional drugs that primarily target a single receptor [172–178]. Attempts for creation of bivalent ligands based on fentanyl as a carrier of μ-opioid activity have been examined recently. The general idea of these studies is presented in Figure 15. The fundamental chemistry behind the work is focused on the creation of a plethora of new functionalized fentanyls able to undergo coupling reactions for the synthesis of bivalent ligands including peptide/non-peptide compounds. This approach gives the possibility to create a wide variety of μ-opioid/adjuvant analgesics bivalent ligands.
Figure 15.
Bivalent ligands based on fentanyl.
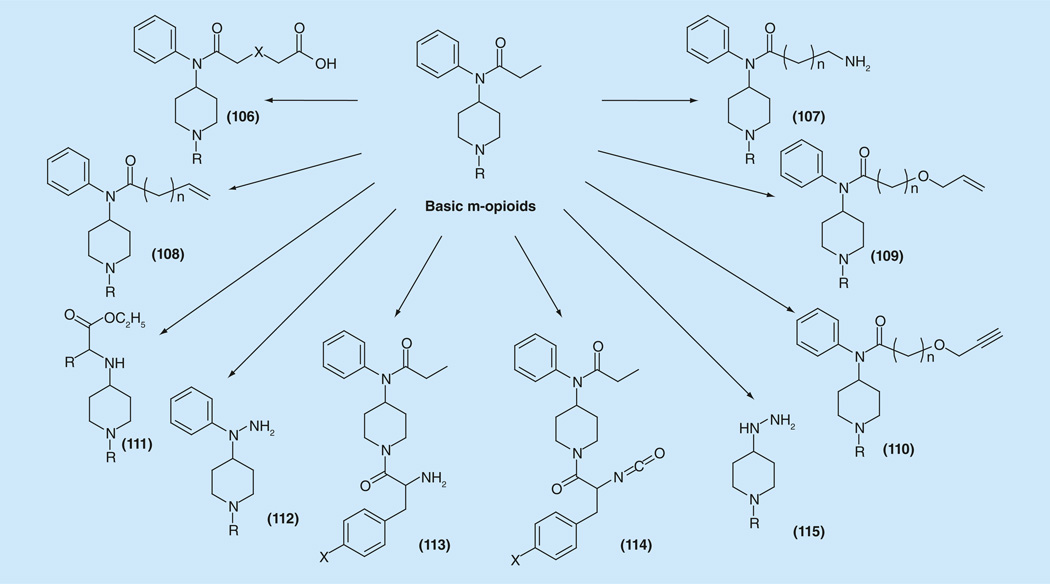
Newly synthesized types of functionalized μ-opioid derivatives for creation of bivalent ligands are presented in Figure 16. Among them are carboxy- (106) [179] and amino-fentanyl [180] derivatives (107), able to be involved in coupling reactions with amino acids and peptides, first of all intended for the synthesis of peptide/non-peptide compounds. Among them are ene-derivatives (108) [153], able to undergo a whole plethora of alkene chemistry and initially intended for thiol-ene ‘click’ reactions with cysteine derivatives. Allyloxy- and propargyl-oxy derivatives (109,110) [153], are synthesized also for their use in the many possibilities of double and triple bond reactions, and first of all in ‘click’ reactions. Amino acid derivatives (112) [154] were prepared for further synthesis of 1-(piperidin-4-yl)piperazine congeners. Phenylhydrazines (112) [152] and hydrazines (115) [181] for the synthesis of propionylhydrazide analogs of fentanyl and variety of hetercyclizations to give indole, pyrazole and pyridazine derivatives. Finally, phenylalanine and isocyanato- derivatives (113, 114) [153] have been synthesized for ‘parallel transfer’ of adjuvant ligands from the propionyl- part of fentanyl to the 1-(2-phenethyl)-moiety.
Figure 16.
Functionalized fentanyls for the synthesis of bivalent ligands.
The variety of newly created bivalent ligands based using the above mentioned functionalized fentanyls are presented in Figure 17. Synthesis of possible bivalent fentanyl/indomathacine (μ-agonist/COX inhibitor) compounds (84,85,87,89) was described above [152].
Figure 17.
Newly created bivalent ligands based on fentanyl.
We hypothesized that a bifunctional investigational tool could be compounds, pharmaceutical salts composed of μ-agonists and glial inhibitors (116), which could be prepared by an interaction of any opioid agonist as an organic base, in this case, fentanyl, with the classical glial inhibitor, fluorocitric acid, as an acid.
A possible advantage of this approach would be that after dissociation, both counterions will be able to act simultaneously and independently at their respective targets. However, since fluorocitric acid is not available commercially, we developed a large-scale method for the preparative synthesis of fluorocitric acid and its corresponding fentanyl salt [182]. The obtained compound was too toxic to continue studies in this direction.
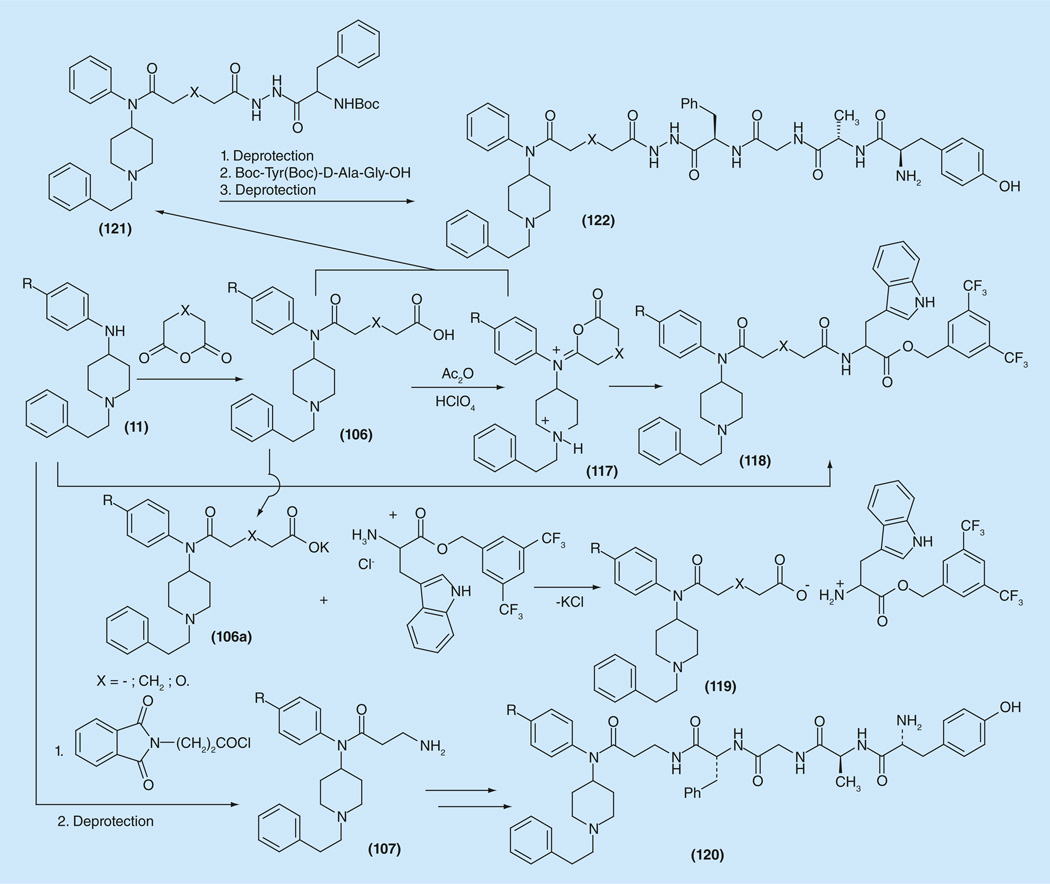
Attempts to synthesize μ-agonist/NK1 antagonist bivalent compounds (118) combining fentanyl and well known NK1 antagonist (L-732138), by replacing the acetyl moiety in it for carboxy-fentanyl moiety have been realized, starting from carboxy-fentanyl derivatives (106) as the opiod part and tryptophan 3,5-bis(trifluoromethyl) benzyl ester as the NK1 antagonists part.
The large-scale synthesis of carboxy-fentanyls (106), started from reaction of 1-phenethyl-N-phenylpiperidin-4-amines (11) and appropriate acid anhydride. ‘Peptide chemistry’ methods with the carbodiimide activation of carboxyl function were successfully used for the synthesis of covalently bonded fentanyl/L-732138 pair (Figure 18) [179]. Another ‘universal method’ for creation of substituted carboxy-fentanyls was developed based on the idea of ‘isoimidium perchlorate chemistry’. The isoimidium perchlorates (117) were readily prepared from 106, successfully reacted with l-tryptophan 3,5-bis(trifluoromethyl)benzyl ester and gave the desired 118. This method can be implemented for creation of any bivalent μ-/adjuvant analgesics with any other adjuvant containing any nucleophile group. The idea of ionic pair μ-agonist/NK1 antagonist compounds (119) has been realized in this case. The potassium salts (106a) were simply mixed with l-tryptophan 3,5-bis(trif luoromethyl)benzyl ether hydrochloride followed by removal of the resulting KCl.
Figure 18.
Attempts to synthesize fentanyl/adjuvant bivalent compounds.
The biological studies indicate that the synthesized functionalized fentanyls (233) dramatically lose analgesic activity, which probably can be explained with the appearance of the ‘bulky’ carboxyl group with negative charge in the structure, and which is more or less restored by distancing of the carboxyl group, as well as by replacement of charged groups for covalently bonded derivatives. At the same time the behavior of tryptophan 3,5-bis(trifluoromethyl) benzyl ester does not change much. The data obtained indicate that both ionic and covalently linked bivalent ligands compete with high affinity for [3H] Substance P binding sites in recombinant CHO cells expressing the human NK1 receptor. Bivalent ligands with covalent linkage between the two pharmacophors exhibit reduced affinities, relative to the parent opioid (fentanyl) at the rat MOR and for [3H]DAMGO binding sites with very low affinities. In functional assays, all compounds, in which opioid and NK1 pharmacophores are bound covalently or by ionic bonds showed NK1 antagonism in vitro with no NK1 agonist activity up to 1 uM [179].
Combinations of μ-opioid agonists with δ-opioid modulators are one of the most interesting and contradictory areas of analgesic studies. Some authors affirm that d-agonists increase antinociceptive responses to μ-agonists [183–185]. Others propose that “opioid compounds with mixed μ-agonist/δ-antagonist properties are expected to be analgesics with low propensity to produce tolerance and dependence” [186]. A third group write that “…μ-agonist signaling can be enhanced by cotreatment with δ- selective ligands including δ- selective antagonists” [187]. Another group suggests that “μ-δ receptor interactions lead to profound modulation of μ receptor” [188]. Finally, it is stated that “there is not only a differential localization of the δ- and μ- in dorsal root ganglion cells, but also that these differences underlie qualitatively distinct pain relieving profiles” [189]. Interestingly, biphalin, a dimeric encephalin analog with both potent μ- and δ- agonist activity was shown to have highly potent analgesic activity and greatly reduced μ-opioid ligand toxicities and reduced tolerance [190].
One of the early attempts for creation of μ-agonists/δ-agonists bivalent ligands with the fentanyl moiety was done via coupling aminofentanyl derivative 107 with known δ-receptor ligands from the enkephalin series [Tyr-D-Ala-Gly-Phe] to give compound 120 with good opioid affinity (1 nM at both δ- and μ- receptors) and bioactivity (34.9 nM in MVD and 42 nM in the GPI/LMMP bioassays) (Figure 18) [180].
Another approach was carried out by implementing possibilities of carboxy-fentanyls (106) or the corresponding isoimidium salts (117) for linking different pharmacophores. In this case the dligand, a Tyr-D-Ala-Gly-Phe from the enkephalin series was bonded to a fentanyl ligand via a hydrazine bond, as had been done in biphalin. For that purpose 106 or 117 were condensed with protected phenylalanine hydrazide to give (121), followed with coupling with [Boc-Tyr(Boc)-d-Ala-Gly-OH] and final deprotection. Compounds (122) showed practically the same bioactivity as (120) in the range (48–69 nM in MVD and 42–44 nM in GPI/LMMP bioassays) [153].
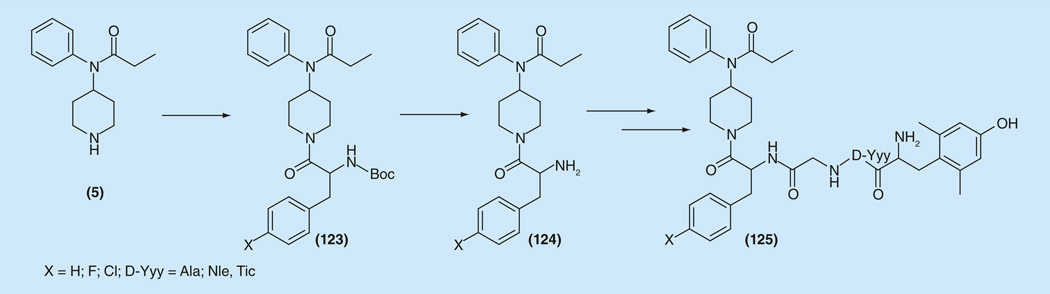
A dramatic 100-fold increase in activity happened when the δ-ligand Dmt-substituted enkephalin-like structure Dmt-D-Yyy-Gly-Phe was ‘parallel transferred’ from the propionyl- part of fentanyl to the ‘modified’ 1-(2-phenethyl)-moiety. First attempts in this direction have been done by replacing the phenethyl group of fentanyl with several aromatic ring containing aminoacids [191,192]. Obtained compounds showed broad (47 nM to 76 μM) and μ-selective activities. For the synthesis of possible μ/δ compounds (Figure 19) amine (5) was coupled with protected phenylalanine or its p-F(Cl)-substituted analogs to give (123), which after deprotection were stepwise transferred to desired bivalent ligands (125) [193,194]. Compounds with X = H and D-Yyy-Ala showed potent binding affinities (0.4 nM) at μ and δreceptors with increased hydrophobicity (aLogP = 2.96), and potent agonist activities in the MVD (1.8 nM) and GPI (8.5 nM) assays [193]. Ligands with X = F, D-Yyy-Nle showed potent subnanomolar opioid agonist activity (IC50 values of 0.37 and 0.26 nM in the MVD and GPI assays, respectively) with excellent efficacy (EC50 = 0.07 nM, Emax = 48% at hDOR; EC50 = 0.29 nM, Emax = 98% at rMOR) at both receptors. Binding at the μ receptor was subnanomolar (Ki = 0.02 nM), and the Emax at the rMORin the [35S]GTP-γ-S binding assay was 98%. These results suggest that halogen substitution (X = F, Cl) increases cell permeability and enhances potency. The effects are more pronounced for the μ receptor, and produce very well balanced (IC50 d/IC50 μ) mixed agonist activities for both receptors, respectively [194].
Figure 19.
One other attempt to synthesize fentanyl/δ-agonist bivalent compounds.
Conclusion
Some general structure–activity relationships for the fentanyl class of compounds can be summarized as follows:
-
▪
All piperidine compounds carrying the 4-N-anilinopiperidine group, with the exception of N-methyl derivatives, bind with high affinity to μ-receptors;
-
▪
The highest activity among 4-N-anilinopiperidines are 1–2(arylethyl) derivatives. (These two main conclusions clearly illustrate that the 4-anilidopiperidine and 1-[2(arylethyl)] moieties are the main players in displaying high μ-affinity and activity;
-
▪
The presence and nature of the ring substituents modulate the affinity and μ-selectivity, and at the same time modulate affinity at δ- and κ-receptors;
-
▪
Methyl substitution at the third position of the piperidine ring causes a sharp increase of μ-affinity and activity;
-
▪
Axial 4-carboxymethyl- or 4-methoxymethyl substituents sharply increase μ-affinity and activity in this series;
-
▪
Methyl substitution at the second position of the piperidine ring does not cause serious changes in activity;
-
▪
Attempts to rigidize any part of the general fentanyl structure causes sharp decrease of μ-affinity and activity;
-
▪
Fentanyls display great variations in enantio-specific binding;
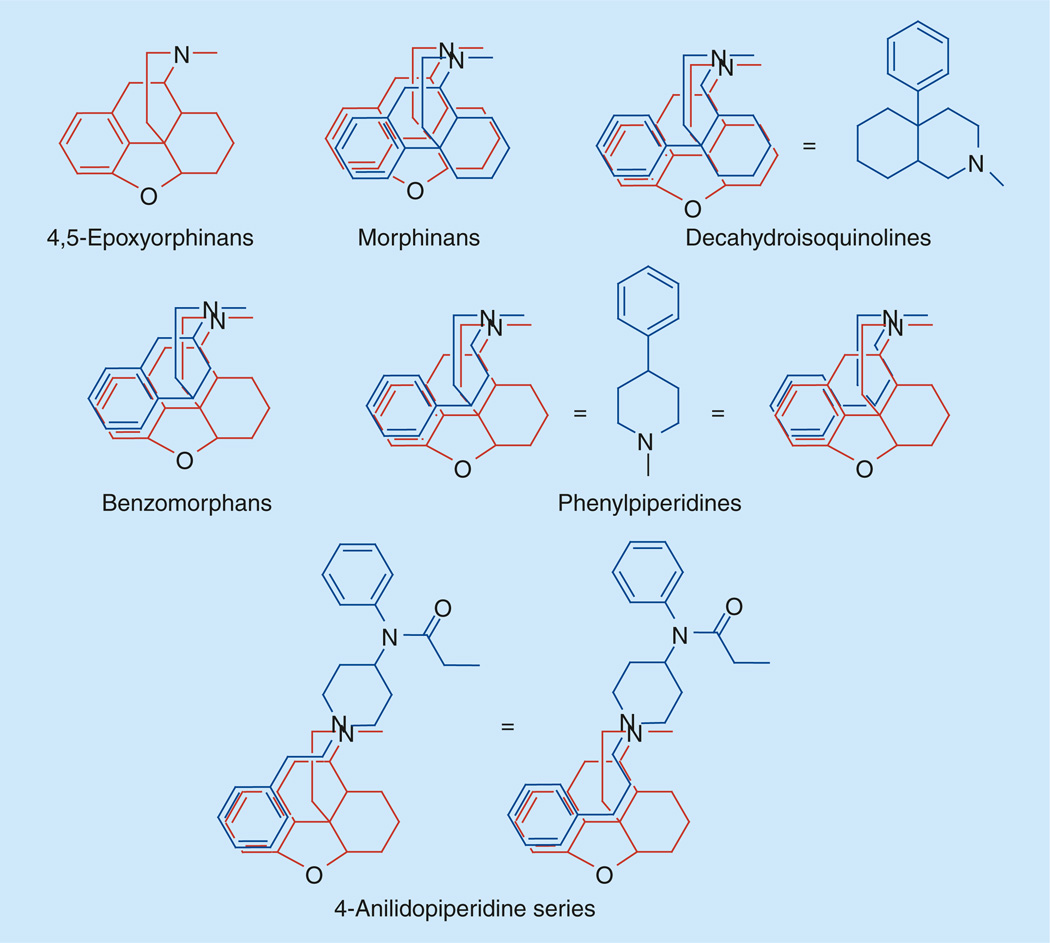
The fentanyl series is not related to the 4-phenylpiperidine class of opioids. Likely binding of all classes of opioids could be explained by a general arylethyl moiety present in all of them (Figure 20);
-
▪
There are still infinite unexamined possibilities to create new opioid analgesics mentioned in Figure 17 and clearly visible in Figure 20.
Figure 20.
Phenylethyl moiety present in all classes of opioids.
A large number of fentanyl analogs have been synthesized and structure activity relationships revealed, but the knowledge of their action is still incomplete and many questions remain unanswered. In general, the order of activity at receptor parallels that of binding affinity. However, the comparison of ED50 values, for μ- analgesics with their IC50 and Kd numbers at the corresponding binding site does not always correlate with experimental data. How to explain, why fentanyl (6), methylfentanyl (42), sufentanil (88), lofentanil (82) are more potent than it would be suggested from obtained binding values. Another example, the Kd of ohmefentanyl for the μ- site is approximately 5–10-times less than that of morphine, but it is nearly 7000-times more potent than morphine in analgesic tests. There is another contradiction, absence of correlation between lipid solubility and analgesic effect [195,196]. Another observation is that in general fentanyls are compounds with the highest affinity for the μ-receptor and the most potency at that receptor. However not all fentanyl derivatives are highly μ- selective. They also produce actions through δ- and κ- receptors. Lofentanil (82) is the least and R30490 (90), are the most μ-selective compounds, among fentanyl series. Although their structures are very close, and affinities at the μ-receptor are similar, R30490 displays much lower δ- and κ-affinities than lofentanil.
Structurally very, close carfentanil (81) and lofentanil (82) bind with high affinity to both μ- and d- receptors, but with varying selectivity [197]. Another interesting observation is that although a large number of compounds with high affinity at the μ- receptor were synthesized, no antagonist has been found that can match fentanyls in affinity.
A number of computational studies on the conformations of fentanyl and its analogs have been reported [198–203], and detailed investigation of the receptor-ligand interactions responsible for their selectivity and the m-receptor high affinity binding have been reported during the last decade [202,204].
It was shown that all the compounds of the 1–2(phenylethyl)-4-N-anilidopiperidines series occupy the same place on the receptor, which is located between the helices TM3 to TM7. “The ligand molecule is parallel to the transmembrane helices, with the N-phenylpropanamide group pointing to the extracellular region, and the N-phenethyl group placed deep in the pocket. The N-phenethyl group is in the gauche conformation, positioning the phenyl group in the region between TM6 and TM7” [205].
Future perspective
Many novel analogs and derivatives of fentanyl have been developed over the past 50 years. Although series of ‘tailor-made’ analgesics of fentanyl series are already inculcated in medicinal practice, there still are many possibilities for future development of fentanyl-related compounds with novel biological activity profiles. Of particular interest would be ligands with mixed and/or balanced mu and delta opioid receptor activities, including compounds that are mu receptor agonists and delta receptor antagonists. In addition, fentanyl-related compounds that are multivalent and address the changes in the expressed genome in pain pathways which lead to prolonged and neuropathic pain might be designed and evaluated for their application to prolonged and neuropathic pain states.
Supplementary Material
Executive summary.
-
▪
Excellent synthetic methods have been developed for the synthesis of fentanyl and numerous analogs and derivatives which are highly potent mu opioid receptor agonists.
-
▪
There still are many possibilities for future development of fentanyl-related compounds with novel biological activity profiles. In particular, ligands with mixed mu and delta opioid receptor activities, multivalent derivatives for use in cases of prolonged and neuropathic pain states.
-
▪
A plethora of opioid agonists of fentanyl series exists on the market, but there is not a structurally-corresponding antagonistic ‘pair’. For decades, it has been an evident gap in our knowledge about specific structural change, which could make possible the transformation of powerful opioid agonist properties of compounds of fentanyl series into powerful antagonists.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.future-science.com/doi/full/10.4155/FMC.13.215
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Woolf CJ, Borsook D, Koltzenburg M. Mechanism-based classifications of pain and analgesic drug discovery. In: Bountra C, Munglani R, Schmidt WK, editors. Pain: Current Understanding, Emerging Therapies and Novel Approaches to Drug Discovery. NY, USA: Marcel Dekker; 2003. pp. 1–8. [Google Scholar]
- 2.Woodcock J, Witter J, Dionne RA. Stimulating the development of mechanism-based, individualized pain therapies. Nat. Rev. Drug Disc. 2007;6(9):703–710. doi: 10.1038/nrd2335. [DOI] [PubMed] [Google Scholar]
- 3.Kissin I. The Development of new analgesics over the past 50 years: a lack of real breakthrough drugs. Anesth. Analg. 2010;110(3):780–789. doi: 10.1213/ANE.0b013e3181cde882. [DOI] [PubMed] [Google Scholar]
- 4.Grape S, Schug SA, Lauer S, Schug BS. Formulations of fentanyl for the management of pain. Drugs. 2010;70(1):57–72. doi: 10.2165/11531740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Nelson L, Schwaner R. Transdermal fentanyl: pharmacology and toxicology. J. Med. Toxicol. 2009;5(4):230–241. doi: 10.1007/BF03178274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeal W, Benfield P. Transdermal fentanyl: a review of its pharmacological properties and therapeutic efficacy in pain control. Drugs. 1997;53(1):109–138. doi: 10.2165/00003495-199753010-00011. [DOI] [PubMed] [Google Scholar]
- 7.Darwish M, Messina J. Clinical pharmacology of buccal tablet for the treatment of breakthrough pain. Exp. Rev. Clin. Pharmacol. 2008;1(1):39–47. doi: 10.1586/17512433.1.1.39. [DOI] [PubMed] [Google Scholar]
- 8.Taylor DR. Fentanyl buccal tablet: rapid relief from breakthrough pain. Exp. Opin. Pharmacother. 2007;8(17):3043–3051. doi: 10.1517/14656566.8.17.3043. [DOI] [PubMed] [Google Scholar]
- 9.Davis MP. Fentanyl for breakthrough pain: a systematic review. Exp. Rev. Neurotherapeut. 2011;11(8):1197–1216. doi: 10.1586/ern.11.63. [DOI] [PubMed] [Google Scholar]
- 10.Mystakidou K, Panagiotou I, Gouliamos A. Fentanyl nasal spray for the treatment of cancer pain. Exp. Opin. Pharmacother. 2011;12(10):1653–1659. doi: 10.1517/14656566.2011.585637. [DOI] [PubMed] [Google Scholar]
- 11.Casy AF, Hassan MM, Simmonds B, Staniforth D. Structure-activity relations in analgesics based on 4-anilinopiperidine. J. Pharm. Pharmacol. 1969;21(7):434–440. doi: 10.1111/j.2042-7158.1969.tb08284.x. [DOI] [PubMed] [Google Scholar]
- 12.Bagley JR, Kudzma LV, Lalinde NL, et al. Evolution of the 4-anilidopiperidine class of opioid analgesics. Med. Res. Rev. 1991;11(4):403–436. doi: 10.1002/med.2610110404. [DOI] [PubMed] [Google Scholar]
- 13.Vuckovic S, Prostran M, Ivanovic M, et al. Fentanyl analogs: structure-activityrelationship study. Curr. Med. Chem. 2009;16(19):2468–2474. doi: 10.2174/092986709788682074. [DOI] [PubMed] [Google Scholar]
- 14.Yadav P, Chauhan JS, Ganesan K, et al. Synthetic methodology and structure activity relationship study of N-[1-(2- phenylethyl)-piperidin-4-yl]-propionamides. Pharm. Sinica. 2010;1(3):126–139. [Google Scholar]
- 15.Yaksh TL, Noueihed RY, Durant PAC. Studies of the pharmacology and pathology of intrathecally administered 4-anilinopiperidine analogs and morphine in the rat and cat. Anesthesiol. 1986;64(1):54–66. doi: 10.1097/00000542-198601000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Planas E. Fentanyl pharmacological characteristics. Dolor. 2000;15(1):7–12. [Google Scholar]
- 17.Poklis A. Fentanyl: a review for clinical and analytical toxicologist. J. Tox. Clin. Tox. 1995;33(5):439–447. doi: 10.3109/15563659509013752. [DOI] [PubMed] [Google Scholar]
- 18.Andrews CJH, Prys-Roberts C. Fentanyl - a review. Clinics Anaesthesiol. 1983;1(1):97–122. [Google Scholar]
- 19.Mather LE. Clinical pharmacokinetics of fentanyl and its newer derivatives. Clin. Pharmacokinet. 1983;8(5):422–446. doi: 10.2165/00003088-198308050-00004. [DOI] [PubMed] [Google Scholar]
- 20.Massey J. Stop the pain: fentanyl is a viable alternative to morphine. JEMS. 2011;36(8):54–57. doi: 10.1016/S0197-2510(11)70206-6. [DOI] [PubMed] [Google Scholar]
- 21.Pasero C. Fentanyl for acute pain management. J. Perianesth. Nurs. 2005;20(4):279–84. doi: 10.1016/j.jopan.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Peng PW, Sandler AN. A review of the use of fentanyl analgesia in the management of acute pain in adults. Anesthesiol. 1999;90(2):576–599. doi: 10.1097/00000542-199902000-00034. [DOI] [PubMed] [Google Scholar]
- 23.Grass JA. Fentanyl: clinical use as postoperative analgesic-epidural/intrathecal route. J. Pain Symptom Manage. 1992;7(7):419–430. doi: 10.1016/0885-3924(92)90022-a. [DOI] [PubMed] [Google Scholar]
- 24.Stanley TH. Fentanyl. J. Pain Symptom Manage. 2005;29(5S):S67–S71. doi: 10.1016/j.jpainsymman.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Stanley TH. The history and development of the fentanyl series. J. Pain Symptom Manage. 1992;7(3 Suppl.):S3–S7. doi: 10.1016/0885-3924(92)90047-l. [DOI] [PubMed] [Google Scholar]
- 26.Casy AF, Parfitt RT. Opioid Analgesics: Chemistry and Receptors. NY, USA: Springer; 1986. [Google Scholar]
- 27.Lenz GR, Evans SM, Walters DE, Hopfinger AJ. Opiates. MA, USA: Academic Press; 1986. [Google Scholar]
- 28.Lednicer D. Central Analgetics - Chemistry and Pharmacology of Drugs, A Series of Monographs Vol. 1. NY, USA: Wiley-Interscience; 1982. [Google Scholar]
- 29.Buschmann H, Christoph T, Friderichs E, Maul C. Analgesics: From Chemistry and Pharmacology to Clinical Application. Weinheim, Germany: Wiley-VCH; 2002. [Google Scholar]
- 30.DeStevens G. Analgetics - Medicinal Chemistry, A Series of Monographs, Vol. 5. NY, USA: Academic Press; 1965. [Google Scholar]
- 31.Hellerbach J, Schnider O, Besendorf H, Pellmont B. Synthetic Analgesics, Part IIA: Morphinans, Organic Chemistry. Vol. VIII. London, UK: Pergamon Press; 1966. [Google Scholar]
- 32.Nathan B, Eddy NB, May EL. Synthetic Analgesics, Part IIB: Benzomorphans - International Series of Monographs on Organic Chemistry Vol. 8. London, UK: Pergamon Press; 1966. [Google Scholar]
- 33.Janssen PAJ. Synthetic Analgesics, Part I: Diphenylpropylamines - International Series of Monographs on Organic Chemistry Vol. 3. London, UK: Pergamon Press; 1960. [Google Scholar]
- 34.Sawynok J, Cowan A. Novel Aspects of Pain Management: Opioids and Beyond. NY, USA: Wiley-Liss; 1999. [Google Scholar]
- 35.Lemke TH, Williams DA, Roche VF, Zito SW. Foye’s Principles of Medicinal Chemistry. PA, USA: Lippincott Williams and Wilkins, WoltersKluwer Health; 2013. [Google Scholar]
- 36.Abraham DJ, Rotella DP. Burger’s Medicinal Chemistry and Drug Discovery 7th Edition. NJ, USA: John Wiley and Sons, Inc; 2010. [Google Scholar]
- 37.Teiggle DJ, Taylor JB. Comprehensive Medicinal Chemistry II. London, UK: Elsevier Science; 2006. [Google Scholar]
- 38.Thomas G. Fundamentals of Medicinal Chemistry. NJ, USA: Wiley-Blackwell; 2003. [Google Scholar]
- 39.Vardanyan RS, Hruby VJ. Synthesis of Essential Drugs. Amsterdam, The Netherlands: Elsevier; 2006. [Google Scholar]
- 40.Block J, Beale JM. Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry 11th Edition. PA, USA: Lippincott Williams and Wilkins; 2003. [Google Scholar]
- 41.Galemmo RA, Jr, Janssens FE, Lewi PJ, et al. In Memoriam: Dr Paul AJ Janssen (1926-003) J. Med. Chem. 2005;48(6):1686. [Google Scholar]
- 42.Black J. A personal perspective on Dr Paul Janssen. J. Med. Chem. 2005;48(6):1687–1688. doi: 10.1021/jm040195b. [DOI] [PubMed] [Google Scholar]
- 43.Van Gestel S, Schuermans V. Thirty-three years of drug discovery and research with Dr Paul Janssen. Drug Dev. Res. 1986;8(1-4):1–13. [Google Scholar]
- 44.Stanley TH, Egan TD, Van Aken H. A tribute to Dr Paul AJ Janssen: entrepreneur extraordinaire, innovative scientist, and significant contributor to anesthesiology. Anesth. Analgesia. 2008;106(2):451–462. doi: 10.1213/ane.0b013e3181605add. [DOI] [PubMed] [Google Scholar]
- 45.Janssen PAJ, Jageneau AH, Demoen PJA, et al. Compounds related to Pethidine. I. Mannich bases derived from norpethidine and acetophenones. J. Med. Pharm. Chem. 1959;1:105–120. doi: 10.1021/jm50002a008. [DOI] [PubMed] [Google Scholar]
- 46.Janssen PAJ, Jageneau AHM, Demoen PJA, et al. Compounds related to pethidine. II. Mannich bases derived from various esters of 4-carboxy-4-phenylpiperidine and acetophenones. J. Med. Pharm. Chem. 1959;1:309–317. doi: 10.1021/jm50005a002. [DOI] [PubMed] [Google Scholar]
- 47.Janssen PAJ, Jageneau AH, Demoen PJA, et al. Compounds related to pethidine. III. Basic ketones derived from norpethidine. J. Med. Pharm. Chem. 1960;2:271–280. doi: 10.1021/jm50010a003. [DOI] [PubMed] [Google Scholar]
- 48.Wright WB, Jr, Brabander HJ, Hardy RA., Jr Synthetic analgesics. III. Basic anilides and carbanilates containing the phenylalkyl moiety. J. Org. Chem. 1961;26:485–490. [Google Scholar]
- 49.Janssen PAJ, Gardocki JF. US3141823. 1964 [Google Scholar]
- 50.Janssen PAJ. FR M2430. 1964 [Google Scholar]
- 51.Janssen PA, Niemegeers CJ, Dony JG. The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawl reflex in rats. Arzneimittelforschung. 1963;13:502–507. [PubMed] [Google Scholar]
- 52.Janssen PAJ. US3164600. 1965 [Google Scholar]
- 53.Janssen PAJ. The development of new synthetic narcotics. In: Estafanous FG, editor. Opioids in Anesthesia II. MA, USA: Butterworth- Heinemann; 1983. pp. 37–44. [Google Scholar]
- 54.Van Bever WFM, Niemegeers CJE, Schellekens KHL, Janssen PAJ. N-4- substituted 1-(2-arylethyl)-4-piperidinyl-N-phenylpropanamides, a novel series of extremely potent analgesics with unusually high safety margin. Arzneimittelforschung. 1976;26(8):1548–1551. [PubMed] [Google Scholar]
- 55.Gardocki JF, Yelnosky J. Some of the pharmacologic actions of fentanyl citrate and droperidol. Toxicol. Appl. Pharmacol. 1964;6(1):48–62. doi: 10.1016/0041-008x(64)90021-3. [DOI] [PubMed] [Google Scholar]
- 56.Chrubasik J, Wust H, Schulte-Monting J, et al. Relative analgesic potency of epidural fentanyl, alfentanil, and morphine in treatment of postoperative pain. Anestheziol. 1988;68(6):929–933. doi: 10.1097/00000542-198806000-00016. [DOI] [PubMed] [Google Scholar]
- 57.Reynolds L, Rauck R, Webster L, et al. Relative analgesic potency of fentanyl and sufentanil during intermediate-term infusions in patients after long-term opioid treatment for chronic pain. Pain. 2004;110(1-2):182–188. doi: 10.1016/j.pain.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 58.Planas E. Fentanyl pharmacological characteristics. Dolor. 2000;15(1):7–12. [Google Scholar]
- 59.Barutell C, Ribera MV, Martinez P, et al. Fentanyl. Dolor. 2004;19(2):98–104. [Google Scholar]
- 60.Andrews CJH, Prys-Roberts C. Fentanyl - a review. Clin. Anaesthesiol. 1983;1(1):97–122. [Google Scholar]
- 61.Schulz R, Wuster M, Rubini P, Herz A. Functional opiate receptors in the guinea-pig ileum: their differentiation by means of selective tolerance development. J. Pharmacol. Exp. Ther. 1981;219:547–550. [PubMed] [Google Scholar]
- 62.Mohamed YH, Essawi MYH, Portoghese PS. Synthesis and evaluation of 1- and 2-substituted fentanyl analogs for opioid activity. J. Med. Chem. 1983;26(3):348–352. doi: 10.1021/jm00357a007. [DOI] [PubMed] [Google Scholar]
- 63.Magnan J, Paterson SJ, Tavani A, Kosterlitz W. The binding spectrum of narcotic analgesic drugs with different agonist and antagonist properties. Arch. Pharmacol. 1982;319(3):197–205. doi: 10.1007/BF00495865. [DOI] [PubMed] [Google Scholar]
- 64.Maguire P, Tsai N, Kamal J, et al. Pharmacological profiles of fentanyl analogs at μ, δ and κ opiate receptors. Eur. J. Pharmacol. 1992;213(2):219–225. doi: 10.1016/0014-2999(92)90685-w. [DOI] [PubMed] [Google Scholar]
- 65.Jin WQ, Paterson SJ, Kosterlitz HW, Casy AF. Interactions of some 4-anilino-piperidines and 4-phenylpiperidines with the μ-, δ- and κ-binding sites. Life Sci. 1983;33(Suppl. 1):251–253. doi: 10.1016/0024-3205(83)90490-3. [DOI] [PubMed] [Google Scholar]
- 66.Boas RA, Villiger JW. Clinical actions of fentanyl and buprenorphine. The significance of receptor binding. Brit. J. Anaesth. 1985;57(2):192–196. doi: 10.1093/bja/57.2.192. [DOI] [PubMed] [Google Scholar]
- 67.Lehmann KA, Weski C, Hunger L. Biotransformation of fentanyl. II. Acute drug interactions in rats and men. Anaesthesist. 1982;31(5):221–227. [PubMed] [Google Scholar]
- 68.Lehmann KA, Hunger L, Brandt K, Daub D. Biotransformation of fentanyl. III. Effect of chronic drug administration on distribution, metabolism and excretion in the rat. Anaesthesist. 1983;32(4):165–173. [PubMed] [Google Scholar]
- 69.Jonczyk A, Jawdosiuk M, Makosza M, Czyzewski J. Search for a new method for synthesis of the analgesic agent “Fentanyl”. Przeml. Chem. 1978;57(3):131–134. [Google Scholar]
- 70.Zee S-H, Wang W-K. A new process for the synthesis of fentanyl. J. Chin. Chem. Soc. 1980;27(4):147–149. [Google Scholar]
- 71.Casy AF, Hassan MMA. Analgesically active basic anilides: stereospecificity and structure of the basic group. J. Pharm. Pharmacol. 1967;19(1):17–24. doi: 10.1111/j.2042-7158.1967.tb07988.x. [DOI] [PubMed] [Google Scholar]
- 72.Wright WB, Jr, Hardy RA., Jr Synthetic analgesics. IV. Synthesis of enantiomers of basic anilides containing the phenalkyl moiety. J. Med. Chem. 1963;6:128–130. doi: 10.1021/jm00338a009. [DOI] [PubMed] [Google Scholar]
- 73.Ivanovic MD, Micovic IV, Vuckovic S, et al. The synthesis and pharmacological evaluation of (±)-2,3-seco-fentanyl analogs. J. Serb. Chem. Soc. 2004;69(11):955–968. [Google Scholar]
- 74.Helsley GC, Lunsford CD, Welstead WJ, Jr, et al. Synthesis and analgetic activity of some 1-substituted 3-pyrrolidinylanilides and dihydrobenz-oxazinones. J. Med. Chem. 1969;12(4):583–586. doi: 10.1021/jm00304a005. [DOI] [PubMed] [Google Scholar]
- 75.Finney ZG, Riley TN. 4-Anilidopiperidine analgesics. 3. 1-Substituted 4-(propananilido)-perhydroazepines as ringexpanded analogs. J. Med. Chem. 1980;23(8):895–899. doi: 10.1021/jm00182a016. [DOI] [PubMed] [Google Scholar]
- 76.DeRuiter J, Andurkar S, Riley TN, et al. Investigation of the synthesis and analgesic activity of 1-substituted 4-(propananilido) perhydroazepines. J. Het. Chem. 1992;29(4):779–786. [Google Scholar]
- 77.Bagley JR, Riley TN. Synthesis and conformational analysis of isomeric 3-propananilidotropanes. J. Het. Chem. 1977;14(4):599–602. [Google Scholar]
- 78.Riley TN, Bagley Jr. 4-Anilidopiperidine analgesics. 2. A study of the conformational aspects of the analgesic activity of the 4-anilidopiperidines utilizing isomeric N-substituted 3-(propananilido)nor-tropane analogs. J. Med. Chem. 1979;22(10):1167–1171. doi: 10.1021/jm00196a004. [DOI] [PubMed] [Google Scholar]
- 79.Fernandez MJ, Huertas RM, Galvez E, et al. Synthesis, and structural, conformational and pharmacological studies of new fentanyl derivatives of the norgranatane system. J. Chem. Soc. Perkin Trans. 1992;24:687–695. [Google Scholar]
- 80.Law S-J, Lewis DH, Borne RF. Synthesis and stereochemical analysis of isomeric N-substituted 5- and 6-propanilido-2- azabicyclo[2.2.2]octanes. J. Het. Chem. 1978;15(2):273–280. [Google Scholar]
- 81.Borne RF, Law S-J, Kapeghian JC, Masten LW. Evaluation of 2-azabicyclo[ 2.2.2]octane analogs of 4-anilidopiperidine analgesics. J. Pharm. Sci. 1980;69(9):1104–1106. doi: 10.1002/jps.2600690934. [DOI] [PubMed] [Google Scholar]
- 82.Riley TN, Hale DB, Wilson MC. 4-Anilidopiperidine analgesics. I. Synthesis and analgesic activity of certain ringmethylated-1-substituted 4-propananilidopiperidines. J. Pharmaceut. Sci. 1973;62(6):983–986. doi: 10.1002/jps.2600620627. [DOI] [PubMed] [Google Scholar]
- 83.Riley TN, Hale DB. US3923992. 1975 [Google Scholar]
- 84.Vartanyan SA, Vartanyan RS, Zhamagortsyan VN, et al. SU736583. 1985 [Google Scholar]
- 85.Vartanian RS, Airapetyan GK, Markaryan EA, et al. SU1100848. 1984 [Google Scholar]
- 86.Vartanyan RS, Martirosyan VO, Vartanyan SA, et al. Synthesis and analgesic activity of 4-anilides of 1-substituted 2,5-dimethylpiperidines. Khim. Farm. Zh. 1989;23(5):562–565. [Google Scholar]
- 87.Karapetyan AA, Struchkov YuT, Timofeeva TV, et al. Structure and activity of phenaridine stereoisomers. Khim. Farm. Zh. 1989;23(5):565–572. [Google Scholar]
- 88.Vartanyan RS, Martirosyan VO, Vartanyan SA, et al. Stereochemistry and biological properties of the new narcotic analgesic phenaridine. Khim. Farm. Zh. 1989;23(5):573–578. [Google Scholar]
- 89.Van Bever WFM, Niemegeers CJE, Janssen PAJ. Synthetic analgesics. Synthesis and pharmacology of the diastereoisomers of N-[3-methyl-1-(2- phenylethyl)-4-piperidyl]-N-phenylpropanamide and N-[3-methyl-1-(1-methyl- 2-phenylethyl)-4-piperidyl]-N-phenylpropanamide. J. Med. Chem. 1974;17(10):1047–1051. doi: 10.1021/jm00256a003. [DOI] [PubMed] [Google Scholar]
- 90.Ivanovic MD, Micovic IV, Vuckovic S, et al. The synthesis and preliminary pharmacological evaluation of the racemic cis and trans 3-alkylfentanyl analogs. J. Serb. Chem. Soc. 2004;69(7):511–526. [Google Scholar]
- 91.Karapetyan HA, Struchkov Yu T, Martirosyan VO, et al. The structure of analgesics of the 4-anilinopiperidine series. III. Structure of 1(e)-(2-phenethyl)-2(e),5(e)- dimethyl-4(e)-(N-propionylanilino) piperidine bisulfate. Zh. Strukt. Kh. 1990;31(2):141–145. [Google Scholar]
- 92.Karapetyan AA, Timofeyeva TV, Struchkov YuT, Martirosyan VO. Conformation of the 4-(propionylanilino) pharmacophore in stereoisomers of the 2,5-dimethyl derivative of phentanyl (phenaridine) Khim. Farm. Zh. 1992;26(9-10):25–28. [Google Scholar]
- 93.Dzhingozyan VK, Martirosyan VO, Karapetyan AA, et al. Effect of 2-methyl substituent on the conformation of 1-(2-phenylethyl) pharmacophore in 4-propanoyl(phenyl)piperidines. Khim. Farm. Zh. 1996;30(8):40–42. [Google Scholar]
- 94.Lalinde NL, Moliterni J, Spencer HK. US4939161. 1990 doi: 10.1021/jm00172a032. [DOI] [PubMed] [Google Scholar]
- 95.Prost M. DE2656678. 1977 [Google Scholar]
- 96.Micovic IV, Ivanovic MD, Vuckovic SM, et al. The synthesis and preliminary pharmacological evaluation of 4-methyl fentanyl. Bioorg. Med Chem. Lett. 2000;10(17):2011–2014. doi: 10.1016/s0960-894x(00)00394-2. [DOI] [PubMed] [Google Scholar]
- 97.Kudzma LV, Severnak SA, Benvenga MJ, et al. 4-phenyl- and 4-heteroaryl-4-anilidopiperidines. A novel class of analgesic and anesthetic agents. J. Med. Chem. 1989;32(12):2534–2542. doi: 10.1021/jm00132a007. [DOI] [PubMed] [Google Scholar]
- 98.Lin B-S, Kudzma LV, Spencer HK. US4791120. 1988 [Google Scholar]
- 99.Kudzma LV, Spencer HK. US4801615. 1989 [Google Scholar]
- 100.Van Daele PG, De Bruyn MF, Boey JM, et al. Synthetic analgesics: N-(1-[2-arylethyl]-4- substituted 4-piperidinyl) N-arylalkanamides. Arzneimittelforschung. 1976;26(8):1521–1531. doi: 10.1002/chin.197646236. [DOI] [PubMed] [Google Scholar]
- 101.Janssen PAJ, Van Daele GHP. US4179569. 1979 [Google Scholar]
- 102.Thompson RG, Menking D, Valdes JJ. Opiate receptor binding properties of carfentanil. Chem. Res. Dev. Eng. Cent. Report. 1987 [Google Scholar]
- 103.Van Bever WFM, Niemegeers CJE, Schellekens KHL, Janssen PAJ. N-4- substituted 1-(2-arylethyl)-4-piperidinyl-N-phenylpropanamides, a novel series of extremely potent analgesics with unusually high safety margin. Arzneimittelforschung. 1976;26(8):1548–1551. [PubMed] [Google Scholar]
- 104.Niemegeers CJ, Schellekens KH, Van Bever WF, Janssen PA. Sufentanil, a very potent and extremely safe intravenous morphine-like compound in mice, rats and dogs. Arzneimittelforschung. 1976;26(8):1551–1556. [PubMed] [Google Scholar]
- 105.Janssens F, Torremans J, Janssen PAJ. Synthetic 1,4-disubstituted 1,4-dihydro-5H-tetrazol- 5-one derivatives of fentanyl: alfentanil (R 39209), a potent, extremely short-acting narcotic analgesic. J. Med. Chem. 1986;29(11):2290–2297. doi: 10.1021/jm00161a027. [DOI] [PubMed] [Google Scholar]
- 106.Niemegeers CJE, Janssen PAJ. Alfentanil (R 39 209) - a particularly short-acting intravenous narcotic analgesic in rats. Drug Dev. Res. 1981;1(1):83–88. [Google Scholar]
- 107.Bagley JR, Thomas SA, Rudo FG, et al. New 1-(heterocyclylalkyl)-4-(propionanilido)-4- piperidinyl methyl ester and methylene methyl ether analgesics. J. Med. Chem. 1991;34(2):827–841. doi: 10.1021/jm00106a051. [DOI] [PubMed] [Google Scholar]
- 108.Janssen PAJ, Van Daele GHP. US4179569. 1979 [Google Scholar]
- 109.Kiricojevic VD, Ivanovic MD, Micovic IV, et al. An optimized synthesis of a key pharmaceutical intermediate: methyl 4-[(1-oxopropyl) phenylamino]piperidine-4- carboxylate. J. Serb. Chem. Soc. 2002;67(12):793–802. [Google Scholar]
- 110.Srimurugan S, Murugan K, Chen C. A facile method for preparation of [2H3]-sufentanil and its metabolites. Chem. Pharm. Bull. 2009;57(12):1421–1424. doi: 10.1248/cpb.57.1421. [DOI] [PubMed] [Google Scholar]
- 111.Henriksen G, Platzer S, Marton J, et al. Syntheses, biological evaluation, and molecular modeling of 18F-labeled 4-anilidopiperidines as μ-opioid receptor imaging agents. J. Med. Chem. 2005;48(24):7720–7732. doi: 10.1021/jm0507274. [DOI] [PubMed] [Google Scholar]
- 112.Feldman PL, James MK, Brackeen MF, et al. Design, synthesis, and pharmacological evaluation of ultrashort- to long-acting opioid analgesics. J. Med. Chem. 1991;34(7):2202–2206. doi: 10.1021/jm00111a041. [DOI] [PubMed] [Google Scholar]
- 113.Feldman PL, James MK, Brackeen MF, Johnson MR, Leighton H. EP383.579. 1990 [Google Scholar]
- 114.Cui Y, Pan L, Ning Y, Zhang K, Zheng J. Analgesic effect and time-effect relation of remifentanil. Zhongguo Yaowu Yilaixing Zazhi. 2003;12(4):268–269. 283. [Google Scholar]
- 115.Nora FS, Fortis E, Aparecida F. Remifentanil: do we need another opioid? Rev. Bras. Anestesiol. 2001;51(2):146–159. [Google Scholar]
- 116.Battershill AJ, Keating GM. Remifentanil: a review of its analgesic and sedative use in the intensive care unit. Drugs. 2006;66(3):365–385. doi: 10.2165/00003495-200666030-00013. [DOI] [PubMed] [Google Scholar]
- 117.Scott LJ, Perry CM. Remifentanil. A review of its use during the induction and maintenance of general anaesthesia. Drugs. 2005;65(13):1793–1823. doi: 10.2165/00003495-200565130-00007. [DOI] [PubMed] [Google Scholar]
- 118.Beers R, Camporesi E. Remifentanil update: clinical science and utility. CNS Drugs. 2004;52(6):1095–1104. doi: 10.2165/00023210-200418150-00004. [DOI] [PubMed] [Google Scholar]
- 119.Wilhelm W, Wrobel M, Kreuer S, Larsen R. Remifentanil. An update. Anaesthesist. 2003;52(6):473–494. doi: 10.1007/s00101-003-0540-9. [DOI] [PubMed] [Google Scholar]
- 120.Rosow CE. An overview of remifentanil. Anesth. Analg. 1999;89(4 Suppl):S1–S3. doi: 10.1097/00000539-199910001-00001. [DOI] [PubMed] [Google Scholar]
- 121.Peacock JE. Remifentanil clinical studies. Drugs Today. 1997;33(9):619–626. [Google Scholar]
- 122.Patel SS, Spencer CM. Remifentanil. Drugs. 1996;52(3):417–427. doi: 10.2165/00003495-199652030-00009. [DOI] [PubMed] [Google Scholar]
- 123.Buerkle HDS, Van Aken H. Remifentanil: a novel, short-acting, μ-opioid. Anesth. Analg. 1996;83(3):646–651. doi: 10.1097/00000539-199609000-00038. [DOI] [PubMed] [Google Scholar]
- 124.James MK. Remifentanil and anesthesia for the future. Exp. Opin. Invest. Drugs. 1994;3(4):331–340. [Google Scholar]
- 125.Floegel O, Weigl U. WO2010000282. 2010 [Google Scholar]
- 126.Cheng BK-M, Halvachs RE. WO2008066708. 2008 [Google Scholar]
- 127.Cheng B. WO2008045192. 2008 [Google Scholar]
- 128.Jacob M, Killgore JK. WO2001040184. 2001 [Google Scholar]
- 129.Malaquin S, Jida M, Gesquiere J-C, et al. Ugi reaction for the synthesis of 4-aminopiperidine-4-carboxylic acid derivatives. Application to the synthesis of carfentanil and remifentanil. Tetr. Lett. 2010;51(22):2983–2985. [Google Scholar]
- 130.Colapret JA, Diamantidis G, Spencer HK, et al. Synthesis and pharmacological evaluation of 4,4-disubstituted piperidines. J. Med. Chem. 1989;32(5):968–974. doi: 10.1021/jm00125a008. [DOI] [PubMed] [Google Scholar]
- 131.Casy AF, Huckstep MR. Structure-activity studies of fentanyl. J. Pharm. Pharmacol. 1988;40(9):605–608. doi: 10.1111/j.2042-7158.1988.tb05318.x. [DOI] [PubMed] [Google Scholar]
- 132.Peters D, Eriksen BL, Munro GN, Oestergaard E. WO2009077584. 2009 [Google Scholar]
- 133.Bagley JR, Wynn RL, Rudo FG, et al. New 4-(heteroanilido)piperidines, structurally related to the pure opioid agonist fentanyl, with agonist and/or antagonist properties. J. Med. Chem. 1989;32:663–671. doi: 10.1021/jm00123a028. [DOI] [PubMed] [Google Scholar]
- 134.Wynn RL, Bagley JR, Spencer HK, Spaulding TC. Evaluation of the morphine reversal actions and antinociceptive activity of a new class of opiate antagonists structurally related to fentanyl. Drug Develop. Res. 1991;22(2):189–195. [Google Scholar]
- 135.Vartanian RS, Vartanian SA, Martirosyan VO, et al. SU1139125. 1984 [Google Scholar]
- 136.Jagerovic N, Cano C, Elguero J, et al. Longacting fentanyl analogs: synthesis and pharmacology of N-(1-phenylpyrazolyl)-N- (1-phenylalkyl-4-piperidyl)propanamides. Bioorg. Med. Chem. 2002;10(3):817–827. doi: 10.1016/s0968-0896(01)00345-5. [DOI] [PubMed] [Google Scholar]
- 137.Giron R, Abalo R, Goicoechea C, et al. Synthesis and opioid activity of new fentanyl analogs. Life Sci. 2002;71(9):1023–3104. doi: 10.1016/s0024-3205(02)01798-8. [DOI] [PubMed] [Google Scholar]
- 138.Jimeno ML, Alkorta I, Cano C, et al. Fentanyl and its analog N-(1-phenylpyrazol-3-yl)-N- [1-(2-phenylethyl)-4-piperidyl]propanamide: 1H- and 13C-NMR spectroscopy, x-ray crystallography, and theoretical calculations. Chem. Pharm. Bull. 2003;51(8):929–934. doi: 10.1248/cpb.51.929. [DOI] [PubMed] [Google Scholar]
- 139.Goicoechea C, Sanchez E, Cano C, et al. Analgesic activity and pharmacological characterization of N-[1-phenylpyrazol-3-yl]-N-[1-(2-phenethyl)-4-piperidyl] propenamide, a new opioid agonist acting peripherally. Eur. J. Pharmacol. 2008;595(1-3):22–29. doi: 10.1016/j.ejphar.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 140.Montero A, Goya P, Jagerovic N, et al. Guanidinium and aminoimidazolinium derivatives of N-(4-piperidyl)propanamides as potential ligands for μ opioid and I2- imidazoline receptors: synthesis and pharmacological screening. Bioorg. Med. Chem. 2002;10(4):1009–1018. doi: 10.1016/s0968-0896(01)00356-x. [DOI] [PubMed] [Google Scholar]
- 141.Dardonville C, Jagerovic N, Callado LF, Meana JJ. Fentanyl derivatives bearing aliphatic alkaneguanidinium moieties: a new series of hybrid molecules with significant binding affinity for mu-opioid receptors and I2-imidazoline binding sites. Bioorg. Med. Chem. Lett. 2004;14(2):491–493. doi: 10.1016/j.bmcl.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 142.Lalinde NL, Moliterni J, Spencer HK. US4994471. 1991 [Google Scholar]
- 143.Micovic IV, Ivanovic MD, Vuckovic S, et al. 3-Carbomethoxy fentanyl: synthesis, pharmacology and conformational analysis. Het. Comm. 1998;4(2):171–179. [Google Scholar]
- 144.Vuckovic S, Prostran M, Ivanovic M, et al. Antinociceptive activity of novel fentanyl analog iso-carfentanyl in rats. Jpn. J. Pharmacol. 2000;84:188–195. doi: 10.1254/jjp.84.188. [DOI] [PubMed] [Google Scholar]
- 145.Essawi MYH, Portoghese PS. Synthesis and evaluation of 1- and 2-substituted fentanyl analogs for opioid activity. J. Med. Chem. 1983;26(3):348–352. doi: 10.1021/jm00357a007. [DOI] [PubMed] [Google Scholar]
- 146.Micovic IV, Roglic GM, Ivanovic MD, et al. The synthesis of lactam analogs of fentanyl. J. Chem. Soc. Perkin Trans. 1996;1(16):2041–2050. [Google Scholar]
- 147.Micovic IV, Roglic GM, Ivanovic MD, et al. The synthesis of 3,3-dimethylfentanyl and its lactam analog. J. Serb. Chem. Soc. 1996;61(10):849–857. [Google Scholar]
- 148.Klein W, Back W, Mutschler E. Potential analgesics. 3. 1-(4-piperidinyl)-2-indolinones and-3,4-dihydrocarbostyrils. Arch. Pharm. 1974;307(5):360–366. doi: 10.1002/ardp.19743070509. [DOI] [PubMed] [Google Scholar]
- 149.Walker GN, Smith RT, Weaver BN. Synthesis of new 3-(pyridylmethylene)-3-(pyridylmethyl)-3-(piperidylmethyl)-, and 3-(β-alkylaminoethyl)-2-indolinones. The reduction of isoindogenides, nitro compounds, and pyridines in a series of 2-indolinones. J. Med. Chem. 1965;8(5):626–637. [Google Scholar]
- 150.Lobbezoo MW, Soudijn W, Van Wijngaarden I. Opiate receptor interaction of compounds derived from or structurally related to fentanyl. J. Med. Chem. 1981;24(7):777–782. doi: 10.1021/jm00139a003. [DOI] [PubMed] [Google Scholar]
- 151.Vartanyan RS, Martirosyan VO, Vlasenko EV, Ovasapyan AA. Synthesis and biological activity of 1-substituted benzimidazole and benztriazole derivatives. Khim. Farm. Zh. 1982;16(8):947–951. [Google Scholar]
- 152.Kudzma LV, Evans SM, Turnbull SP, et al. Octahydro-1,2,3,4,4a,5,11,11a-pyrido3,4- c1,5benzoxazepines conformationally restricted fentanyl analogs. Bioorg. Med. Chem. Lett. 1995;5(11):1177–1182. [Google Scholar]
- 153.Van Dyke JW, Jr, Havera HJ, Johnson RD, et al. Cardiovascular activity of some substituted 2-aminobenzoquinolizines. J. Med. Chem. 1972;15(1):91–94. doi: 10.1021/jm00271a025. [DOI] [PubMed] [Google Scholar]
- 154.Maryanoff BE, McComsey DF, Taylor RJ, et al. Synthesis and stereochemistry of 7-phenyl-2-propionanilidobenzo[a] quinolizidine derivatives. Structural probes of fentanyl analgesics. J. Med. Chem. 1981;24(1):79–88. doi: 10.1021/jm00133a017. [DOI] [PubMed] [Google Scholar]
- 155.Vardanyan R, Vijay G, Nichol GS, et al. Synthesis and investigations of doublepharmacophore ligands for treatment of chronic and neuropathic pain. Bioorg. Med. Chem. 2009;17(14):5044–5053. doi: 10.1016/j.bmc.2009.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tollenaere JP, Moereels H, Raymaekers LA. Atlas of the Three-Dimensional Structure of Drugs, Vol. 1. Amsterdam, The Netherlands: Elsevier/North-Holland, Inc; 1979. [Google Scholar]
- 157.Koch MHJ, de Ranter CJ, Rolies M, Dideberg O. N-[4-(methoxymethyl)-1-(2-phenylethyl)- 4-piperidinyl]-N-phenylpropanamide. Acta Crystallogr. Sect. B. 1976;32:2529–2531. [Google Scholar]
- 158.Spickett W. Compounds affecting the central nervous system. II. Substituted 1,2,3,4-tetrahydropyrido [4,3-b] indoles. J. Med. Chem. 1966;9(3):436–438. doi: 10.1021/jm00321a051. [DOI] [PubMed] [Google Scholar]
- 159.Berger JG, Davidson F, Langford GE. Synthesis of some conformationally restricted analogs of fentanyl. J. Med. Chem. 1977;20(4):600–602. doi: 10.1021/jm00214a035. [DOI] [PubMed] [Google Scholar]
- 160.Wang Z-X, Zhu Y-C, Jin W-Q, et al. Stereoisomers of N-[1-(2-hydroxy-2- phenylethyl)-3-methyl-4-piperidyl]-N-phenylpropanamide: synthesis, stereochemistry, analgesic activity, and opioid receptor binding characteristic. J. Med. Chem. 1995;38(18):3652–3659. doi: 10.1021/jm00018a026. [DOI] [PubMed] [Google Scholar]
- 161.Brine GA, Stark PA, Liu JY, et al. Enantiomers of diastereomeric cis-N-[1-(2-hydroxy-2- phenylethyl)-3-methyl-4-piperidyl]-N-phenylpropanamides: synthesis, x-ray analysis, and biological activities. J. Med. Chem. 1995;38(9):1547–1557. doi: 10.1021/jm00009a015. [DOI] [PubMed] [Google Scholar]
- 162.Rice KC, Jacobson AE, Burke TR, Jr, et al. Irreversible ligands with high selectivity toward δ or μ opiate receptors. Science. 1983;220(4594):314–316. doi: 10.1126/science.6132444. [DOI] [PubMed] [Google Scholar]
- 163.Burke TR, Jr, Bajwa BS, Jacobson AE, et al. Probes for narcotic receptor mediated phenomena. 7. Synthesis and pharmacological properties of irreversible ligands specific for μ or δ opiate receptors. J. Med. Chem. 1984;27(12):1570–1574. doi: 10.1021/jm00378a008. [DOI] [PubMed] [Google Scholar]
- 164.Burke TR, Jr, Jacobson AE, Rice KC, et al. Probes for narcotic receptor mediated phenomena. 12 cis-(+)-3-methyl-fentanyl isothiocyanate, a potent site-directed acylating agent for δ opioid receptors. Synthesis, absolute configuration, and receptor enantioselectivity. J. Med. Chem. 1986;29(6):1087–1093. doi: 10.1021/jm00156a030. [DOI] [PubMed] [Google Scholar]
- 165.Chen B-Y, Jin W-Q, Chen Jie, et al. Analgesic activity and selectivity of isothiocyanate derivatives of fentanyl analogs for opioid receptor. Life Sci. 1999;65(15):1589–1595. doi: 10.1016/s0024-3205(99)00404-x. [DOI] [PubMed] [Google Scholar]
- 166.Dardonville C, Fernandez-Fernandez G, Gibbons S-L, et al. Synthesis and pharmacological studies of new hybrid derivatives of fentanyl active at the μ-opioid receptor and I2-imidazoline binding sites. Bioorg. Med. Chem. 2006;14(19):6570–6580. doi: 10.1016/j.bmc.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 167.Weltrowska G, Chung NN, Lemieux C, et al. “Carba”-analogs of fentanyl are opioid receptor agonists. J. Med. Chem. 2010;53(7):2875–2881. doi: 10.1021/jm9019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Srulevitch DB, Lien EJ. 4-phenylamidopiperidines: synthesis, pharmacological testing and SAR analysis. Acta Pharm. Jugoslavica. 1991;41(2):89–106. [Google Scholar]
- 169.Gupta PK, Yadav SK, Bhutia YD, et al. Synthesis and comparative bioefficacy of N-(1-phenethyl-4-piperidinyl)propionanilide (fentanyl) and its 1-substituted analogs in Swiss albino mice. Med. Chem. Res. 2013;22(8):3888–3896. [Google Scholar]
- 170.Vartanyan RS, Gyul’budagyan AL, Khanamiryan A Kh, et al. Synthesis of 1-phenethyl-2-methyl- and 1-methyl-2- phenethyl-4-(N-propionylanilino)hexahydro pyridazines. Arm. Khim. Zh. 1987;40(9):563–569. [Google Scholar]
- 171.Jilek J, Rajsner M, Valenta V, et al. Synthesis of piperidine derivatives as potential analgesic agents. Coll. Czech. Chem. Comm. 1990;55(7):1828–1853. [Google Scholar]
- 172.Morphy R, Rankovic Z. Designed multiple ligands, an emerging drug discovery paradigm. J. Med. Chem. 2005;48(21):6523–6543. doi: 10.1021/jm058225d. [DOI] [PubMed] [Google Scholar]
- 173.Dietis N, Guerrini R, Salvadori CG, et al. Simultaneous targeting of multiple opioid receptors: a strategy to improve side-effect profile. Br. J. Anaesth. 2009;103(1):38–49. doi: 10.1093/bja/aep129. [DOI] [PubMed] [Google Scholar]
- 174.Liu Z, Zhang J, Zhang A. Design of multivalent ligand targeting G-proteincoupled receptors. Curr. Pharm. Des. 2009;15(6):682–718. doi: 10.2174/138161209787315639. [DOI] [PubMed] [Google Scholar]
- 175.Schiller PW. Bi- or multifunctional opioid peptide drugs. Life Sci. 2010;86:598–603. doi: 10.1016/j.lfs.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shonberg J, Scammells PJ, Capuano B. design strategies for bivalent ligands targeting GPCRs. ChemMedChem. 2011;6:963–974. doi: 10.1002/cmdc.201100101. [DOI] [PubMed] [Google Scholar]
- 177.Balboni G, Salvadori S, Marczak ED, et al. Opioid biunctional ligands from morphine and the opioid pharmacophore Dmt-Tic. Eur. J. Med. Chem. 2011;46(2):799–803. doi: 10.1016/j.ejmech.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hruby VJ, Porreca F, Yamamura HI, et al. New paradigms and tools in drug design for pain and addiction. AAPS J. 2006;8(3):E450–E460. doi: 10.1208/aapsj080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.R, Kumirov VK, Nichol GS, et al. Synthesis and biological evaluation of new opioid agonist and neurokinin-1 antagonist bivalent ligands. Bioorg. Med. Chem. 2011;19(20):6135–6142. doi: 10.1016/j.bmc.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Petrov RR, Vardanyan RS, Lee YS, et al. Synthesis and evaluation of 3-aminopropionyl substituted fentanyl analogs for opioid activity. Bioorg. Med. Chem. Lett. 2006;16(18):4946–4950. doi: 10.1016/j.bmcl.2006.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nichol GS, Vardanyan R, Hruby VJ. Synthesis and crystallographic study of N′-(1- benzylpiperidin-4-yl)acetohydrazide. J. Chem. Crystallography. 2010;40(11):961–964. doi: 10.1007/s10870-010-9771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vardanyan R, Kumirov VK, Hruby VJ. Improved synthesis of d,l-fluoro-citric acid. J. Fluorine Chem. 2011;132(11):920–924. [Google Scholar]
- 183.Porreca F, Takemori AE, Sultana M, et al. Modulation of mu-mediated antinociception in the mouse involves opioid delta-2 receptors. J. Pharm. Exp. Therapeut. 1992;263(1):147–152. [PubMed] [Google Scholar]
- 184.Heyman JS, Vaught JL, Mosberg HI, et al. Modulation of mu-mediated antinociception by delta agonists in the mouse: selective potentiation of morphine and normorphine by [D-Pen2,D-Pen5]enkephalin. Eur. J. Pharm. 1989;165(1):1–10. doi: 10.1016/0014-2999(89)90764-4. [DOI] [PubMed] [Google Scholar]
- 185.Heyman JS, Jang Q, Rothman RB, et al. Modulation of μ-mediated antinociception by delta agonists: characterization with antagonists. Eur. J. Pharm. 1989;169(1):43–52. doi: 10.1016/0014-2999(89)90815-7. [DOI] [PubMed] [Google Scholar]
- 186.Schiller PW, Fundytus ME, Merovitz L, et al. The opioid μ agonist/d antagonist DIPPNH 2[y] produces a potent analgesic effect, no physical dependence, and less tolerance than morphine in rats. J. Med. Chem. 1999;42(18):3520–3526. doi: 10.1021/jm980724+. [DOI] [PubMed] [Google Scholar]
- 187.Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: β-arrestin2-mediated ERK activation by μ-d opioid receptor heterodimers. FASEB J. 2007;21(10):2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gomes I, Gupta A, Filipovska J, et al. A role for heterodimerization of μ and δ opiate receptors in enhancing morphine analgesia. Proc. Natl Acad. Sci. USA. 2004;101(14):5135–39. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Scherrer G, Imamachi N, Cao Y-Q, et al. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137(6):1148–59. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Horan PJ, Mattia A, Bilsky EJ, et al. Antinociceptive profile of biphalin, a dimeric enkephalin analog. J. Pharm. Exp. Ther. 1993;265(3):1446–1454. [PubMed] [Google Scholar]
- 191.Lee YS, Nyberg J, Moye S, et al. Understanding the structural requirements of 4-anilidopiperidine analogs for biological activities at μ and δ opioid receptors. Bioorg. Med. Chem. Lett. 2007;17(8):2161–2165. doi: 10.1016/j.bmcl.2007.01.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lee YS, Petrov R, Kulkarni V, et al. Development of μ/d opioid ligands: enkephalin analogs containing 4-anilidopiperidine moiety. Adv. Exp. Med. Biol. 2009;611:517–518. doi: 10.1007/978-0-387-73657-0_225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lee YS, Petrov R, Park CK, et al. Development of novel enkephalin analogs that have enhanced opioid activities at both μ and δ opioid receptors. J. Med. Chem. 2007;50(22):5528–5532. doi: 10.1021/jm061465o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lee YS, Kulkarani V, Cowell SM, et al. Development of potent μ and δ opioid agonists with high lipophilicity. J. Med. Chem. 2011;54(1):382–386. doi: 10.1021/jm100982d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xu X, Kim CH, Zhu YC, et al. (+)-cis-3- methylfentanyl and its analogs bind pseudoirreversibly to the mu opioid binding site: evidence for pseudoallosteric modulation. Neuropharmacology. 1991;30(5):455–462. doi: 10.1016/0028-3908(91)90006-w. [DOI] [PubMed] [Google Scholar]
- 196.Jin WQ, Xu H, Zhu YC, et al. Studies on synthesis and relationship between analgesic activity and receptor affinity for 3-methyl fentanyl derivatives. Sci. Sin. 1981;24:710–720. [PubMed] [Google Scholar]
- 197.Maguire P, Tsai N, Kamal J, et al. Pharmacological profiles of fentanyl analogs at μ δ and κ opiate receptors. Eur. J. Pharmacol. 1992;213(2):219–225. doi: 10.1016/0014-2999(92)90685-w. [DOI] [PubMed] [Google Scholar]
- 198.Tollenaere JP, Moereels H, van Loon M. On conformation analysis, molecular graphics, fentanyl and its derivatives. Prog. Drug Res. 1986;30:91–126. doi: 10.1007/978-3-0348-9311-4_3. [DOI] [PubMed] [Google Scholar]
- 199.Martins J, Andrews P. Conformation-activity relationships of opiate analgesics. J. Comput. Aided Mol. Des. 1987;1:53–72. doi: 10.1007/BF01680557. [DOI] [PubMed] [Google Scholar]
- 200.Cometta-Morini C, Loew GH. Development of a conformational search strategy for flexible ligands: a study of the potent m-selective opioid analgesic fentanyl. J. Comput. Aided Mol. Des. 1991;5:335–356. doi: 10.1007/BF00126667. [DOI] [PubMed] [Google Scholar]
- 201.Cometta-Morini C, Maguire PA, Loew GH. Molecular determinants of í receptor recognition for the fentanyl class of compounds. Mol. Pharmacol. 1992;41:185–196. [PubMed] [Google Scholar]
- 202.Brandt W, Barth A, Holtje H-D. A new consistent model explaining structure (conformation)-activity relationships of opiates with í-selectivity. Drug Des. Discov. 1993;10:257–283. [PubMed] [Google Scholar]
- 203.Rong S-B, Zhu Y-C, Jiang H-L, et al. Interaction models of 3-methylfentanyl derivatives with mu opioid receptors. Acta Pharmacol. Sin. 1997;18:128–132. [PubMed] [Google Scholar]
- 204.Subramanian GM, Paterlini G, Portoghese PS, et al. Molecular docking reveals a novel binding site model for fentanyl at the μ-opioid receptor. J. Med. Chem. 2000;43(3):381–391. doi: 10.1021/jm9903702. [DOI] [PubMed] [Google Scholar]
- 205.Dosen-Micovic L, Ivanovic M, Micovic V. Steric interactions and the activity of fentanyl analogs at the μ-opioid receptor. Bioorg. Med. Chem. 2006;14(9):2887–2895. doi: 10.1016/j.bmc.2005.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.