Abstract
Pre-eclampsia is a serious multisystem disorder with diverse clinical manifestations. Although not causal, endothelial dysfunction and reduced nitric oxide bioavailability are likely to play an important role in the maternal and fetal pathophysiology of this condition. Lack of treatment modalities that can target the underlying pathophysiological changes and reverse the endothelial dysfunction frequently leads to iatrogenic preterm delivery of the fetus, causing neonatal morbidity and mortality, and the condition itself is associated with short- and longer term maternal morbidity and mortality. Drugs that target various components of the nitric oxide–soluble guanylyl cyclase pathway can help to increase NO bioavailability. The purpose of this review is to outline the current status of clinical research involving these therapeutic modalities in the context of pre-eclampsia, with the focus being on the following: nitric oxide donors, including organic nitrates and S-nitrosothiols; l-arginine, the endogenous precursor of NO; inhibitors of cyclic guanosine 3′,5′-monophosphate breakdown, including sildenafil; and other novel inhibitors of NO donor metabolism. The advantages and limitations of each modality are outlined, and scope for development into established therapeutic options for pre-eclampsia is explored.
Keywords: endothelial dysfunction, l-arginine, organic nitrates, pre-eclampsia, S-nitrosoglutathione, sildenafil
Introduction
Pre-eclampsia is a multisystem disorder that complicates 2–8% of pregnancies [1]. Its presentation can be highly variable but is usually characterized by hypertension and proteinuria after 20 weeks of gestation, in an otherwise normotensive woman [2]. Pre-eclampsia is a major cause of maternal mortality, accounting for 16% of the direct maternal deaths in developed countries [3], and can be associated with a wide spectrum of serious complications, including eclampsia, haemorrhagic stroke, haemolysis, elevated liver enzymes and low platelets (HELLP) syndrome, renal failure and pulmonary oedema.
Early onset pre-eclampsia is associated with significant perinatal morbidity and mortality. This is primarily due to the need for premature delivery, further complicated by the presence of intrauterine growth restriction. Despite recent improvements in survival rates at very early gestations, the incidence of long-term neurological complications and neurodevelopmental delay remains high [4]. In addition to the immediate complications of prematurity, over half of these extremely low-birthweight babies have neurodevelopmental impairment, and nearly one in 10 show signs of cerebral palsy at 18–22 months of age [5]. In addition, 10% of babies born before 26 weeks are affected by visual and/or hearing loss at 18 months [6]. The neurodevelopmental sequelae can persist into adolescence and adulthood, and cognitive impairment and school difficulties are seen in a significant proportion [7].
Pathophysiology of pre-eclampsia
The aetiology of pre-eclampsia is complex and not yet fully elucidated. The primary cause of pre-eclampsia is thought to be inadequate placentation [8]. The failure of trophoblast invasion and remodelling of the uterine spiral arteries leads to a high-resistance uterine circulation, causing a reduction in blood flow and placental ischaemia. The resultant reperfusion causes an increase in oxidative stress, leading to a widespread systemic inflammatory response [9] and alterations in angiogenic factor signalling. This, in turn, results in generalized endothelial dysfunction, which is thought to be central to the maternal manifestations of pre-eclampsia and may account for the diverse spectrum of its clinical complications [10,11], although direct evidence for this in humans is limited.
Vascular endothelium performs various haemostatic functions, many of which are mediated by nitric oxide (NO). Nitric oxide, originally identified as the endothelium-derived relaxing factor, is the predominant vasodilatory substance produced by the endothelium in response to mechanical and chemical stimuli. It is an autocrine and paracrine signalling molecule that is synthesized from l-arginine by a family of calcium–calmodulin-dependent enzymes called nitric oxide synthases (NOS); the most important one of which, in this context, is endothelial NOS (eNOS) (Figure 1). Nitric oxide causes relaxation of vascular smooth muscle cells by activating soluble guanylate cyclase (sGC), which in turn causes an increase in intracellular cyclic guanosine 3′,5′-monophosphate (cGMP) and activation of cGMP-dependent protein kinases.
Figure 1.
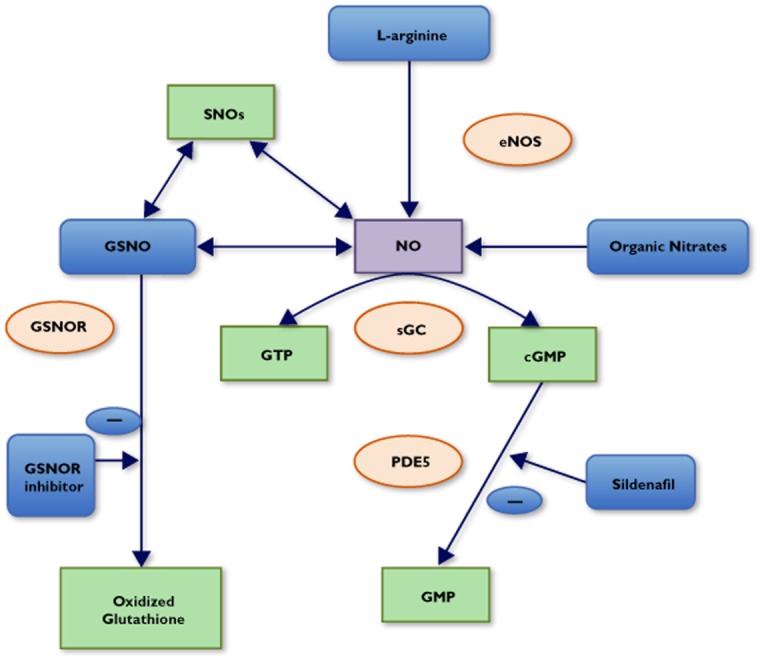
Overview of the mechanisms of action of NO donors and related drugs. Abbreviations are as follows: cGMP, cyclic guanosine 3′,5′-monophosphate; eNOS, endothelial nitric oxide synthase; GSNO, S-nitrosogluthione; GSNOR, S-nitrosoglutathione reductase; GTP, guanosine triphosphate; NO, nitric oxide; PDE5, phosphodiesterase 5; sGC, soluble guanylyl cyclase; SNOs, S-nitosothiols
A key feature of endothelial dysfunction in pre-eclampsia is a reduction in the bioavailability of NO. In turn, this is thought to lead to a rise in blood pressure due to a disturbance in the balance between vasodilator and vasoconstrictor influences on the vascular smooth muscle. Nitric oxide is also a potent inhibitor of platelet aggregation and activation by both cGMP-dependent and -independent mechanisms [12]. Increased aggregation and widespread systemic activaton of platelets is seen in women with this disorder [13]. The functions of NO also include inhibition of vascular smooth muscle cell proliferation [14] and inhibition of inflammatory cell activation [15].
In addition, NO can modulate protein function through S-nitrosylation, which may be of biological importance. Significant alterations to the placental S-nitroso-proteome are seen in pre-eclampsia compared with normotensive women [16]. S-Nitrosylation of endogenous thiols, the most abundant one of which is glutathione, produces a class of compounds called S-nitrosothiols (RSNO), which have a significant role in NO physiology, having a longer half-life than NO itself and acting as reservoirs of bioavailable NO.
An increase in the markers of oxidative stress is seen in women with pre-eclampsia [17,18] and small-for-gestational-age infants [18]. Angiotensinogen is predominantly in its more active oxidized form in women with pre-eclampsia, resulting in activation of the renin–angiotensin axis. This may partly explain the rise in blood pressure seen in these women [19]. A reduction in antioxidant factors was seen in some studies, which found lower levels of glutathione [17,20,21] and glutathione-to-haemoglobin ratios [20]. S-Glutathionylation of proteins has also been the focus of recent research. This post-translational modification is thought play a crucial role in the regulation of the intracellular redox state [21].
The levels of numerous pro-inflammatory and anti-angiogenic factors are raised in women with pre-eclampsia, including soluble Fms-like tyrosine kinase-1 (sFlt-1) [9]. Soluble Fms-like tyrosine kinase-1 is a circulating splice variant of the vascular endothelial growth factor receptor and binds to vascular endothelial growth factor and placental growth factor, hence reducing their bioavailability [22]. Serum levels of sFlt-1 are raised in women with pre-eclampsia, and correlate with the severity of the disease and proximity to its onset [23]. Endoglin is a transforming growth factor β1 and β3 coreceptor that is expressed on the surface of endothelial cells. Upregulation of endoglin in the placentae of pre-eclamptic pregnancies is associated with release of soluble endoglin (sEng) into the maternal circulation. Increased circulating sEng interferes with normal transforming growth factor β signalling [24]. Levels of sEng increase 2–3 months prior to the onset of clinical disease [15]. The increase in sFlt-1 and sEng levels in pre-eclampsia leads to a reduction in angiogenesis and downregulation of NO production [22,24].
Increasing knowledge about the pathophysiology of pre-eclampsia has not yet been translated into new treatment modalities for this condition [25]. The current management of severe pre-eclampsia involves treatment with antihypertensive medications, seizure prevention with magnesium sulphate and expediting delivery, sometimes at very early gestations. Therapeutic measures that focus on reversing the underlying endothelial dysfunction could potentially help to ameliorate the widespread systemic manifestations of this disorder. The aim of this review is to outline the current status of the various streams of pharmacological investigations, with the focus being on the restoration of the NO–sGC pathway (Table 1 ). Although oxidative stress has been investigated in the causation of pre-eclampsia, there is no proven benefit from traditional antioxidants [26]. This review also aims to explore the basis for the theoretical potential of endogenous antioxidants, such as glutathione, in the management of pre-eclampsia.
Table 1.
Summary of clinical studies involving NO donors, precursors and inhibitors of cGMP breakdown
| Study | Drug | Route | Number of participants | Study design | Study population | Results |
|---|---|---|---|---|---|---|
| Ramsay et al. (1994) [28] | GTN | Intravenous infusion | 15 | Nonrandomized study | Women with abnormal uterine artery Doppler indices at 24–26 weeks | Dose-dependent reduction in uterine artery diastolic blood flow. No effect on maternal cardiovascular parameters or fetal circulation |
| Grunewald et al. (1995) [29] | GTN | Intravenous infusion | 12 | Nonrandomized study | Women with severe pre-eclampsia | Significant reduction in maternal blood pressure (P < 0.01). Significant reduction in umbilical artery PI (P < 0.1); more pronounced decrease in participants with higher basal values. No significant change in uterine artery PI |
| Cetin et al. (2004) [30] | GTN | Intravenous infusion | 55 | Nonrandomized study | Twenty-four women with severe pre-eclampsia. Fifteen women with eclampsia. Sixteen women with HELLP syndrome | Significant reduction in systolic and diastolic BP in all groups (P < 0.05). No significant adverse effects to mother and fetus |
| Manzur-Verástegui et al. (2008) [31] | GTN vs. nifedipine | Intravenous infusion of GTN vs. sublingual nifedipine | 32 | Randomized, double- blind trial | Women with severe pre-eclampsia | Reduction in blood pressure was greater, faster and more reliable after GTN infusion vs. sublingual nifedipine. Rise in maternal heart rate occurred in both groups, twofold higher with nifedipine. No significant changes in fetal heart rate |
| Lees et al. (1998) [32] | GTN vs. placebo | Transdermal GTN patches (5 mg) vs. placebo patches for 10 weeks or until delivery | 40 | Randomized, double-blind, placebo-controlled trial | Women with abnormal uterine artery Doppler waveforms at 24–26 weeks | No significant difference in the rates of pre-eclampsia, growth restriction and preterm delivery. Significantly reduced risk of adverse events in the GTN group. No difference in maternal systolic and diastolic pressure, mean uterine artery RI or fetal umbilical or MCA PI |
| Picciolo et al. (2000) [33] | GTN vs. observation | Transdermal GTN patches (5 mg) worn from 16 to 38 weeks | 68 | Randomized study | Women <16 weeks with chronic hypertension, history of pre-eclampsia before 34 weeks or IUGR in previous pregnancies | No significant difference in rates of pre-eclampsia in the two groups. Rates of growth restriction, gestation at delivery, rates of caeserean section and premature delivery were similar between the two groups. Significant reduction in rate of bilateral uterine artery notching at 24 weeks in the GTN group (P < 0.05). No difference in umbilical artery and MCA PI |
| Cacciatore et al. (1998) [34] | GTN | Transdermal GTN patches (10 mg) worn for three consecutive days between 28 and 36 weeks | 17 | Nonrandomized study | Women with pre-eclampsia | Significant reduction in mean uterine artery RI and PI, reached maximum on last day of application. Return to normal of uterine artery RI and PI within 12 h of discontinuation of treatment. No significant change in umbilical artery or MCA RI or PI |
| Trapani et al. (2011) [35] | GTN | Transdermal GTN patches (50 mg, average dose 0.4 mg h−1) for 3 days | 30 | Nonrandomized study | Women with singleton pregnancies with severe pre-eclampsia and abnormal uterine and umbilical artery Doppler waveforms | Significant reduction in uterine and umbilical artery RI and PI (P < 0.001) on day 3 compared with day 1. Significant reduction in MAP (P < 0.05). No significant change in fetal MCA RI or PI |
| Luzi et al. (1999) [36] | GTN vs. placebo | Sublingual GTN 0.3 mg vs. placebo | 30 | Nonrandomized study | Ten women with mild pre-eclampsia. Ten women with threatened preterm labour. Ten healthy pregnant women (controls) ∼30 weeks gestation | Significant reduction in systolic and diastolic blood pressure in the pre-eclampsia group (P < 0.001). Significant reduction in uterine artery PI in both pre-eclampsia (P < 0.002) and threatened preterm labour group (P < 0.03); delta % significantly higher in the pre-eclampsia group. Significant decrease in umbilical artery PI in the pre-eclampsia group (P < 0.03). No change in fetal heart rate or fetal MCA PI |
| Thaler et al. (1999) [41] | ISDN vs. placebo | Sublingual ISDN 5 mg vs. placebo | 23 | Randomized, double-blind, placebo-controlled trial | Women with pregnancy-induced hypertension | Significant reduction in MAP (P < 0.0001) and increase in mean maternal heart rate (P < 0.0001) compared with placebo. Significant reduction in the mean S/D ratio of uterine (P < 0.0007) and umbilical arteries (P < 0.0001). Resolution of early diastolic notch in seven of 12 women |
| Nakatsuka et al. (2002) [42] | ISDN | Transdermal ISDN patches (range 4–30 days) | 12 | Nonrandomized study | Women with pre-eclampsia, oligohydramnios and raised uterine artery PI | Significant reduction in blood pressure. Significant reduction in uterine artery PI (P < 0.003). Significant reduction in uterine artery PI (P < 0.04). Approximately fourfold increase in size of amniotic fluid pockets |
| Martínez-Abundis et al. (2000) [43] | ISDN vs. placebo | Sublingual ISDN 5 mg (repeated on a second occasion) vs. placebo | 60 | Randomized, double-blind, placebo-controlled trial | Women with pre-eclampsia | ISDN was effective in reducing diastolic blood pressure to between 80 and 100 mmHg in 56.6% of women in 10 min and 96.6% in 40–60 min. No significant difference in fetal heart rate between the two groups |
| Thaler et al. (1996) [44] | ISDN | Sublingual ISDN 5 mg | 18 | Nonrandomized study | Women with low-risk pregnancies at 17–24 weeks | Significant reduction in MAP (P < 0.04). Significant increase in maternal heart rate (P < 0.01). Significant reduction in uterine and umbilical artery S/D (P < 0.001) |
| Makino et al. (1997) [45] | ISDN | Transdermal ISDN patch | 37 | Randomized, controlled trial | Eighteen women with pre-eclampsia at midgestation and 19 normotensive pregnant women | Significant reduction in umblilcal artery RI in pre-eclamptic women. No change in systemic blood pressure. No significant change in uterine artery RI |
| Groten et al. (2012) [46] | PETN | Oral PETN (Pentalong®) | 111 | Randomized, double-blind, placebo-controlled trial | Women with abnormal uterine artery Doppler waveforms (bilateral notch or RI > 0.7) at 19–24 weeks | Significant improvement in uteroplacental perfusion (Mean PI P < 0.01). Reduction in incidence of preterm birth <32 weeks, IUGR and pre-eclampsia. Improved outcomes in those women who developed pre-eclampsia. Four fetal losses, all in the placebo group |
| de Belder et al. (1994) [50] | GSNO | Intra-arterial infusion | 5 | Nonrandomized study | Health male volunteers | Reduction in ADP-induced platelet aggregation. Antiaggregatory effect at lowest dose only associated with a thershold rose in foream blood flow, indicating a preferential antiplatelet effect |
| Ramsay et al. (1995) [51] | GSNO | Intravenous infusion | 10 | Nonrandomized study | Healthy females of reproductive age | Significant reduction in ADP-induced platelet aggregation. No significant change in blood pressure or pulse rate |
| Langford et al. (1994) [52] | GSNO | Intracoronary infusion during PTCA | 13 | Nonrandomized study | Patients undergoing PTCA | Significant inhibiton of PTCA-induced increase in surface expression of P-selectin and glycoprotein IIb/IIIa. No change in blood pressure |
| Langford et al. (1996) [53] | GSNO vs. GTN | Intravenous infusion (at doses that caused not more than 10 mmHg fall in MAP) | 60 | Nonrandomized study | Twenty patients with acute myocardial infarction. Twenty patients with unstable angina. Twenty control volunteers without angina | Significant reduction in platelet P-selectin (P < 0.001) and glycoprotein IIb/IIIa (P < 0.05) expression after GSNO infusion. Significant reduction in platelet P-selectin (P < 0.02) and glycoprotein IIb/IIIa (P < 0.01) expression also after GTN infusion. The GSNO was better tolerated than the GTN |
| de Belder et al. (1995) [54] | GSNO | Intravenous infusion | 1 | Nonrandomized study | A 41-year-old woman in her second pregnancy at 38 weeks who developed HELLP syndrome and eclampsia immediately postpartum | Reduction in blood pressure and improvement in platelet count within 30 min of infusion, followed by complete recovery. |
| Lees et al. (1996) [55] | GSNO | Intravenous infusion | 10 | Nonrandomized study | Women with severe pre-eclampsia or pre-eclampsia with severe fetal compromise at 21–33 weeks gestation | Dose-dependent reduction in MAP (P < 0.005) and increase in maternal heart rate (P < 0.02). Significant reduction in mean uterine artery RI (P < 0.009). Significant reduction in platelet P-selectin expression (P < 0.01). No significant change in umbilical artery, fetal MCA or thoracic aorta PIs |
| T. Everett, I. Wilkinson, A. Mahendru, C. McEniery, S. Garner, A. Goodall and C. Lees (Addenbrookes Hospital, Cambridge University Hospitals NHS Foundation Trust, Cambridge, unpublished results) | GSNO | Intravenous infusion | 6 | Nonrandomized study | Women with early onset pre-eclampsia at 26–32 weeks | Significant fall in augmentation index at 30 μg min−1 of GSNO, without a significant fall in blood pressure (P < 0.0001). Significant reduction in platelet P-selectin expression (P = 0.03). Ratio of reduction in urinary PCR to pre-infusion PCR was significant (P = 0.02). Reduction in soluble endoglin of borderline significance (P = 0.06), no change in soluble Fms-like tyrosine kinase-1 levels. No change in maternal uterine artery and fetal Doppler PIs |
| Kaposzta et al. (2001) [58] | GSNO vs. l-arginine vs. placebo | Intravenous infusion | 42 | Randomized, double-blind, placebo-controlled trial | Patients undergoing carotid endarterectomy | Significant reduction in transcranial Doppler embolic signals in both the l-arginine and GSNO groups compared with control subjects (both P < 0.001). The reduction in Doppler embolic signals persisted at 24 h |
| Kaposzta et al. (2002) [59] | GSNO vs. placebo | Intravenous infusion | 20 | Randomized, double-blind, placebo-controlled trial | Patients with ≥50% internal carotid artery stenosis. Patients with three or more Doppler embolic signals recorded in a 30 min screening period | Significant reduction in transcranial Doppler embolic signals after GSNO infusion compared with placebo (P < 0.0001 at 0–3 h, P = 0.03 at 6 h and P < 0.0001 at 24 h). Effect on reduction in Doppler embolic signals persisted for 24 h |
| Facchinetti et al. (1999) [61] | l-Arginine | Intravenous infusion | 29 | Nonrandomized study | Twelve normotensive pregnant women. Seventeen women with pre-eclampsia | Significant reduction in maternal blood pressure in both groups, more pronounced in women with pre-eclampsia. Increase in serum nitrate levels in control subjects but not in women with pre-eclampsia. |
| Vadillo-Ortega et al. (2011) [62] | l-Arginine + antioxidants vs. antioxiants alone vs. placebo | Oral supplementation via medical food bars | 672 | Randomized, double-blind, placebo-controlled trial | Women with a history of pre-eclampsia in a previous pregnancy. Women with a first degree relative with a history of pre-eclampsia. 14–32 weeks gestation | Reduction in incidence of pre-eclampsia in the L-arginine + antioxidant supplements group compared with the other two groups (P < 0.001 compared with placebo) |
| Neri et al. (2010) [63] | l-Arginine vs. placebo | Oral supplementation | 80 | Randomized, double-blind, placebo-controlled trial | Pregnant women with mild chronic hypertension | No significant change in blood pressure after 10–12 weeks of treatment. Trend towards lower incidence of pre-eclampsia requiring delivery before 34 weeks and neonatal complications in the L-arginine group |
| Samangaya et al. (2009) [64] | Sildenafil vs. placebo | Oral administration | 35 | Randomized, double-blind, placebo-controlled trial | Women with pre-eclampsia at 24–34 weeks | No difference in time from randomization to delivery in the two groups |
Abbreviations are as follows: ADP, adenosine diphosphate; GSNO, S-nitrosoglutathione; GTN, glyceryltrinitate; HELLP syndrome, haemolysis, elevated liver enzymes and low platelet syndrome; ISDN, isosorbide dinitrate; IUGR, intrauterine growth restriction; MAP, mean arterial pressure; MCA, fetal middle cerebral artery; PETN, pentaerythryl tetranitrate; PI, pulsatility index; PTCA, percutaneous transluminal coronary angioplasty; RI, resistance index; S/D, ratio of peak systolic to end diastolic flow velocity.
Clinical studies and pharmacology of nitric oxide donors
Given that endothelial dysfunction and disruption of NO bioavailability are major contributors to the maternal manifestations of pre-eclampsia, supplementation with exogenous NO donors would be an apparently logical solution. The use of NO donors in cardiovascular diseases is well established, and evidence for their role in the prevention and treatment of pre-eclampsia is emerging. The classes of NO donors that have been studied in the context of pre-eclampsia include organic nitrates and S-nitrosothiols.
Organic nitrates
Organic nitrates have been used as vascular smooth muscle relaxants for over 100 years. The organic nitrates that have been studied in the context of pre-eclampsia include glyceryl trinitrate (GTN) or nitroglycerine and isosorbide dinitrate (ISDN) (Table 2).
Table 2.
Chemical structures and pharmacokinetics of glyceryl trinitrate, isosorbide dinitrate and S-nitrosoglutathione
| Nitric oxide donor | Chemical structure | Half life | Pharmacokinetics | Elimination |
|---|---|---|---|---|
| Glyceryl trinitrate | 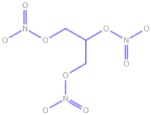 |
Estimated plasma half-life of 1–4 min | Well absorbed from gastrointestinal tract, mucosa and skin. Extensive first-pass metabolism via glucuronidation in the liver. Large volume of distribution of ∼200 l | Spontaneous hydrolysis in plasma into glyceryl dinitrate and glyceryl mononitrate. Metabolized by glucuronidation in the liver |
| Isosorbide dinitrate | 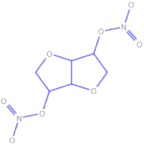 |
Plasma half-life of 1 h, increases after chronic dosing | Well absorbed from gastrointestinal tract, mucosa and skin. Variable bioavailability after oral administration due to extensive first-pass metabolism. Large volume of distribution of 2–4 l kg−1 | Hepatic metabolism via denitration and glucuronidation. Both 2- and 5-mononitrates are biologically active and have anti-anginal effect |
| S-Nitrosoglutathione | 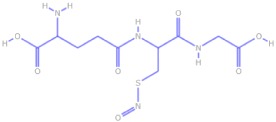 |
Unknown | Unknown | Multiple mechanisms of metabolism. Spontaneous release of NO moiety by exposure to light, heat and transition metals. Cell-mediated bioactivation by various enzymes. Reduction by endogenous thiols, which in turn undergo S-nitrosylation |
Glyceryl trinitrate
Glyceryl trinitrate is a widely used organic nitrate in clinical practice, particularly for the treatment of angina pectoris. It releases NO from its terminal nitrate group after enzyme-mediated bioactivation. Although the identity of the activating enzyme remains elusive, recent evidence has proposed mitochondrial aldehyde dehydrogenase as a likely candidate [27].
The use of GTN in women at risk for pre-eclampsia was first reported in 1994. A study of intravenous GTN infusion in 15 women with abnormal uterine artery Doppler velocimetry at 24–26 weeks gestation showed a dose-dependent reduction in uterine artery resistance without any effect on the maternal cardiovascular parameters or the fetal circulation [28]. Further studies, however, have shown a significant reduction in the maternal blood pressure [29–31] and umbilical artery resistance [29] without any significant adverse effects [31,32] after intravenous infusion of GTN.
Transdermal GTN patches have been the focus of various studies, for both prevention and management of pre-eclampsia and related disorders [32,33]. A randomized, placebo-controlled trial of low-dose transdermal GTN patches in women with abnormal uterine artery Doppler velocimetry at 24–26 weeks showed that although there was no change in the incidence of pre-eclampsia, growth restriction or preterm delivery, GTN increased the likelihood of a complication-free pregnancy, with a significant reduction in hazard ratio in the GTN-treated group. There was no effect on maternal cardiovascular parameters or on uterine and fetal Doppler velocimetry [32]. Studies of both transdermal [34,35] and sublingual GTN [36] in women affected by pre-eclampsia consistently showed a significant reduction in blood pressure and resistance in the uterine artery without an adverse effect on fetal Doppler parameters.
The evidence for the clinical use of GTN for the prevention or treatment of pre-eclampsia is, therefore, limited by the small numbers of women in the studies mentioned above, which were not powered to identify alterations in maternal or fetal outcomes. These studies have, nonetheless, highlighted the potential use of GTN as an antihypertensive agent in pre-eclampsia. However, it remains to be established whether GTN offers any competitive advantage over the existing treatment options in pre-eclampsia.
The major disadvantage of organic nitrates in general, and GTN in particular, is the development of tolerance upon continuous dosing, necessitating the requirement of regular ‘nitrate-free’ intervals. There are many theories regarding the molecular basis of tolerance, including increased oxidative stress and generation of superoxide anions, uncoupling of NOS leading to worsening of the underlying endothelial dysfunction, and plasma volume expansion due to fluid retention [37]. Continuous exposure to GTN may also reduce the activity of mitochondrial aldehyde dehydrogenase [27]. Paradoxically, the increase in oxidative stress and potentiation of endothelial dysfunction may also worsen the underlying disease process [38].
As previously noted, platelet activation plays a significant role in the aetiology of pre-eclampsia. Organic nitrates have not been found to have any significant in vivo antiplatelet effects [39].
The safety profile of these drugs is well established in the nonpregnant population. The side-effects observed with their use are not usually serious and include headache, flushing and dizziness, but can be severe enough to affect compliance, causing discontinuation of their use [40].
Isosorbide dinitrate
Isosorbide dintirate undergoes bioactivation by a similar mechanism to GTN, but has a longer half-life. A few small studies have demonstrated a reduction in maternal blood pressure [41–43] and resistance in the uterine arteries with the use of both transdermal and sublingual ISDN in women with pre-eclampsia [44,45]. However, it has the same disadvantages as GTN, including tolerance, worsening of the underlying endothelial dysfunction and lack of platelet effects at vasodilatory doses.
Other organic nitrates
A recent study demonstrated that pentaerythryl tetranitrate, a long-acting organic nitrate, improved uteroplacental perfusion in women at risk for pre-eclampsia. The frequency of pre-eclampsia, growth restriction and preterm births was also found to be lower in high-risk women who received pentaerythryl tetranitrate proplylaxis [46].
Sodium nitroprusside is a very potent nitrovasodilator used as an antihypertensive in nonpregnant patients for the treatment of hypertensive emergencies. It can cause profound hypotension, hence requiring very cautious dose titration. Another potential adverse effect of sodium nitroprusside is cyanide toxicity. The data for its use in pregnant patients is limited, with animal studies suggesting the risk of fetal toxicity.
S-Nitrosothiols
S-Nitrosothiols are a class of compounds that have an NO group attached to the thiol (RSH) moiety by a single chemical bond. S-Nitrosothiols release the NO moiety by a variety of mechanisms, such as exposure to light, heat and transition metals, in addition to bioactivation by various enzymes. As a result of this wide array of mechanisms of activation, S-nitrosothiols are not susceptible to tolerance [47]. Their NO moiety can be effectively transferred to endogenous thiols, which act as biological reservoirs of NO. This protects NO from being rapidly metabolized to nitrites in conditions of oxidative stress [38]. This permits a longer duration of action of S-nitrosothiols than can be expected from the short half-life of NO. S-Nitrosothiols can also transfer the NO moiety across plasma membranes by transnitrosation catalysed by protein disulphide isomerases [48]. The S-nitrosothiol that has been investigated in women with pre-eclampsia is S-nitrosoglutathione (GSNO).
S-nitrosoglutathione (Table 2) is an endogenous S-nitrosothiol that is found ubiquitously in tissues, in concentrations as high as 250 nm [49]. S-Nitrosoglutathione is not currently in clinical use, but has been the focus of research for its therapeutic role in a variety of conditions, including pre-eclampsia, cardiovascular and cerebrovascular disorders and cystic fibrosis. The effects of GSNO are tissue specific. A significant reduction in platelet aggregation in response to ADP [50,51] and activation via increased surface expression of P-selectin (an α-granule adhesion molecule) and glycoprotein IIb/IIIa (a fibrinogen receptor with increased expression on activated platelets) has been shown at doses that have only a minimal cardiovascular effect [52,53]. This has highlighted its therapeutic potential in conditions associated with platelet overactivation, which include pre-eclampsia.
The first reported use of GSNO in pre-eclampsia was in a severely thrombocytopaenic woman with postpartum HELLP syndrome in 1994 [54]. A rapid improvement in the patient's clinical condition and improvement in platelet count was seen with GSNO infusion. A study of GSNO infusion in 10 women with severe pre-eclampsia showed a significant dose-dependent reduction in blood pressure, uterine artery resistance and platelet activation. Despite a fall in blood pressure, there was no significant effect on the fetal circulation [55]. The reduction in blood pressure at doses that affect platelet activation was contrary to the findings in healthy volunteers in previous studies [50]. This was attributed to the reduction in endogenous production of NO in women with pre-eclampsia, leading to hypersensitivity of the NO-depleted vascular system to GSNO [55]. The use of GSNO in women with early onset pre-eclampsia has also been the focus of a recent dose-ranging study by our team, which showed a significant reduction in augmentation index (a biomarker of small vessel tone that is increased in pre-eclampsia [56]) and platelet activation, without any significant change in mean arterial pressure. A significant postinfusion reduction was observed in urinary protein-to-creatinine ratio in comparison to the pre-infusion value, and the reduction in sEng level approached significance. These studies, albeit small, indicate that GSNO may have a role in targeting the underlying endothelial dysfunction of pre-eclampsia. However, evidence for an effect of GSNO on clinical end-points, such as prolongation of pregnancy, is lacking. Further research is required to investigate the safety and efficacy of GSNO in women with pre-eclampsia and to elucidate effects on maternal and fetal outcomes.
S-Nitrosoglutathione is known to modulate cell signalling by post-translational S-nitrosylation and S-glutathionylation of redox-sensitive proteins [57]. As previously mentioned, an alteration in the redox state of various plasma proteins and the oxidative switch of angiotensinogen to its more active form have been shown in patients with pre-eclampsia. S-Nitrosylation of angiotensinogen by a S-nitrosothiol (S-nitroso-N-acetyl penicillamine) resulted in a shift towards its less active, reduced form [19]. It can, hence, be inferred that post-translational modification of proteins by GSNO may also target the underlying pathophysiological changes in pre-eclampsia.
Reduced levels of glutathione have been shown in women with pre-eclampsia [17,20,21]. It can hence be hypothesized that exogenous GSNO can replenish these antioxidant reserves of glutathione.
The therapeutic effects of GSNO, a molecule with a very short half-life, persisted up to 24 h after cessation of the infusion in two studies investigating the reduction of embolic signals from carotid plaque [58,59]. It is possible that this phenomenon is attributable to the post-translational modification reactions described above. It remains to be elucidated whether the changes in cardiovascular parameters, uteroplacental circulation or platelet function also persist in pre-eclamptic women after GSNO infusion.
S-Nitrosoglutathione has been found to have a favourable toxicity profile in animal toxicology studies using inhalational GSNO, with no biologically significant adverse effects seen [60].
Clinical studies and pharmacology of nitric oxide precursors
l-Arginine
l-Arginine acts as a precursor of NO and is converted into NO and l-citrulline by NOS, as described in the ‘Pathophysiology of pre-eclampsia’ section. It has been the focus of studies aimed at investigating its preventative role in women at high risk for developing pre-eclampsia. A study of intravenous infusion of l-arginine in pregnant women showed a significant reduction in blood pressure; an effect that was greater in women with pre-eclampsia [61]. Recently, the focus has been on the effects of l-arginine supplementation on the prevention of pre-eclampsia in high-risk women. A randomized, controlled trial showed that dietary supplementation with a combination of l-arginine and antioxidants was associated with a significant reduction in the incidence of pre-eclampsia, compared with antioxidants alone and placebo [62]. These results, however, have to be interpreted with caution, because the effects of l-arginine alone were not studied, and it is difficult to ascertain the relative contributions of l-arginine and antioxidants in reducing the incidence of pre-eclampsia. In addition, the prevalence of recurrent pre-eclampsia reported in the study population was very high (nearly 30%), hence raising a question about the generalizability of these results to other obstetric populations and low-risk women. Interestingly, a previous study that investigated the effects of l-arginine supplementation in women with chronic hypertension showed less need for antihypertensive medications and fewer maternal and neonatal complications, but no difference in the incidence of superimposed pre-eclampsia [63]. Given that l-arginine is a widely available food supplement, conclusive evidence about its beneficial effects could provide a feasible means of preventing pre-eclampsia. Further research is, hence, warranted into its role in reducing the incidence of pre-eclampsia in low-risk populations.
Clinical studies and pharmacology of inhibitors of cGMP breakdown
Phosphodiesterase inhibitors
Sildenafil citrate (SC), marketed as Viagra®, is a cGMP-specific phosphodiesterase inhibitor commonly used in the treatment of erectile dysfunction. It potentiates the action of NO downstream by inhibiting the degradation of cGMP. Sildenafil citrate was the focus of a randomized, placebo-controlled trial in 35 women with pre-eclampsia, which showed no significant difference in randomization-to-delivery interval. This indicated that treatment with SC did not prolong the pregnancy, although it was well tolerated and did not increase maternal or fetal morbidity or mortality [64]. Further in vivo studies of SC in rat models of pre-eclampsia have since shown a significant reduction in sFlt-1 and sEng [65], as well as an improvement in blood pressure, proteinuria and uteroplacental and fetal perfusion after treatment with SC [66]. However, the effects of SC on intrauterine growth restriction were found to be conflicting [67,68]. These studies indicate that SC may hold potential as a therapeutic option for pre-eclampsia; however, larger randomized, controlled trials are required to elucidate its role.
Clinical studies and pharmacology of inhibitors of NO donor metabolism
S-Nitrosoglutathione reductase inhibitors
S-Nitrosoglutathione is metabolized in vivo by GSNO reductase, an alcohol dehydrogenase that plays a central role in regulating the levels of endogenous GSNO. Small molecule inhibitors of this enzyme have recently been the focus of research. N6022 is a first-in-class compound that is a very potent, specific and fully reversible inhibitor of GSNO reductase [69]. It has been shown to improve endothelial function in vivo [70], and has been found to have an acceptable safety profile in animal toxicology studies [71]. N6022 is currently the focus of an early phase trial in humans for the treatment of asthma and cystic fibrosis. Hence, it is possible that GSNO reductase inhibitors hold potential to be studied in the context of pre-eclampsia, in conjunction with GSNO.
Clinical studies and pharmacology of other novel NO donors
The role of the antiplatelet agent aspirin in prevention of pre-eclampsia has been the focus of extensive research. In 1994, the CLASP trial found that although the reduction in the incidence of pre-eclampsia in women at risk was not significant, there was a trend towards a greater reduction in its incidence at earlier gestations. It was recommended that its use could be justified in women at risk of early onset pre-eclampsia requiring very preterm delivery [72]. Novel derivatives of aspirin that release NO have been investigated in the context of cardiovascular diseases, and have been found to have a more pronounced antithrombotic effect in vivo when compared with aspirin [73]. These nitroaspirins may hold potential in the prevention or treatment of pre-eclampsia in the future.
Diazeniumdiolates (NONOates) are compounds that decompose spontaneously at physiological pH to release two molar equivalents of NO, following first-order kinetics. The rate of NO release and therefore their biological effects are predictable. These compounds are currently the focus of research into treatment of certain cancers, but lack an established safety record, precluding their use in pregnancy.
Dinitrosyl iron complexes with glutathione have recently been studied in healthy volunteers for their antihypertensive effects and have been recommended for the second phase of clinical trials [74].
Conclusion
Pre-eclampsia is a serious condition with significant long-term consequences for both mother and baby. The mechanisms underlying the disease process are increasingly understood, but the discovery of a definitive cure has proved elusive. Glutathione and nitric oxide donors target the underlying molecular processes and have shown early potential, which may lead to them becoming valuable adjuncts to conventional management, which focuses largely on management of hypertension and seizure prevention.
As the most established nitric oxide donors, organic nitrates have been the obvious first line of investigation. However, the evidence for their effectiveness in the prevention and treatment of pre-eclampsia is currently limited. Their nonvascular pharmacological actions have been limited by hypotension, thus they offer little advantage over existing drugs. Also, given the known significant side-effects, including headache, patient compliance may be affected.
S-Nitrosoglutathione has also been infused in women with pre-eclampsia, but current data are limited to only a few small studies. Although theoretically GSNO is a tissue-selective NO donor, the limited evidence so far has shown it not only to target the endothelial dysfunction of pre-eclampsia, but also to reduce platelet aggregation and activation, whilst improving uteroplacental perfusion. It is likely that GSNO can replenish the reduced glutathione levels seen in women pre-eclampsia. In addition, the absence of any significant adverse effects contributes to its potential as a valuable therapeutic modality for pre-eclampsia. Further research is required to ascertain whether GSNO can alter major clinical end-points, such as prolongation of pregnancy, preventing delivery at very early gestations. It remains to be seen whether GSNO reductase inhibitors may also hold potential in the management of this condition.
Dietary supplementation with l-arginine and treatment with sildenafil citrate have been investigated in the prevention and treatment of pre-eclampsia, respectively. However, further studies are warranted before they can be employed in clinical practice. Novel NO donors, including nitroaspirins, are being investigated for their role in the treatment of various cardiovascular disorders, which may also pave the way for investigation of their use in the prevention or treatment of pre-eclampsia.
To conclude, altered nitric oxide physiology is a major factor in the aetiology of pre-eclampsia, and the delivery of exogenous NO is an attractive therapeutic option. Extensive research is, however, needed before NO donors can be incorporated into the existing treatment protocols for pre-eclampsia. There is currently very limited evidence for the preventative role of any of these drugs in women at risk of developing pre-eclampsia. Nonetheless, NO donors may hold potential for improving outcomes and reducing the burden of mortality and morbidity of women affected by pre-eclampsia, and their babies suffering growth restriction and preterm delivery as a result.
Conflict of Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work. C. C. Lees is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at the Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- 1.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). (Review) Hypertens Pregnancy. 2001;20:9–14. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- 3.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 4.Moore T, Hennessy EM, Myles J, Johnson SJ, Draper ES, Costeloe KL, Marlow N. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961. doi: 10.1136/bmj.e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hintz SR, Kendrick DE, Wilson-Costello DE, Das A, Bell EF, Vohr BR, Higgins RD NICHD Neonatal Research Network. Early-childhood neurodevelopmental outcomes are not improving for infants born at <25 weeks' gestational age. Pediatrics. 2011;127:62–70. doi: 10.1542/peds.2010-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, Simon NP, Wilson DC, Broyles S, Bauer CR, Delaney-Black V, Yolton KA, Fleisher BE, Papile LA, Kaplan MD. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 2000;105:1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 7.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. doi: 10.1016/S0140-6736(08)60136-1. Review. [DOI] [PubMed] [Google Scholar]
- 8.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 9.Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30(Suppl A):S38–42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243–1249. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 11.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 12.Crane MS, Rossi AG, Megson IL. A potential role for extracellular nitric oxide generation in cGMP-independent inhibition of human platelet aggregation: biochemical and pharmacological considerations. Br J Pharmacol. 2005;144:849–859. doi: 10.1038/sj.bjp.0706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konijnenberg A, Stokkers EW, van der Post JA, Schaap MC, Boer K, Bleker OP, Sturk A. Extensive platelet activation in preeclampsia compared with normal pregnancy: enhanced expression of cell adhesion molecules. Am J Obstet Gynecol. 1997;176:461–469. doi: 10.1016/s0002-9378(97)70516-7. [DOI] [PubMed] [Google Scholar]
- 14.Jeremy JY, Rowe D, Emsley AM, Newby AC. Nitric oxide and the proliferation of vascular smooth muscle cells. Cardiovasc Res. 1999;43:580–594. doi: 10.1016/s0008-6363(99)00171-6. [DOI] [PubMed] [Google Scholar]
- 15.Gaboury JP, Niu XF, Kubes P. Nitric oxide inhibits numerous features of mast cell-induced inflammation. Circulation. 1996;93:318–326. doi: 10.1161/01.cir.93.2.318. [DOI] [PubMed] [Google Scholar]
- 16.Zhang HH, Wang YP, Chen DB. Analysis of nitroso-proteomes in normotensive and severe preeclamptic human placentas. Biol Reprod. 2011;84:966–975. doi: 10.1095/biolreprod.110.090688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rani N, Dhingra R, Arya DS, Kalaivani M, Bhatla N, Kumar R. Role of oxidative stress markers and antioxidants in the placenta of preeclamptic patients. J Obstet Gynaecol Res. 2010;36:1189–1194. doi: 10.1111/j.1447-0756.2010.01303.x. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh TT, Chen SF, Lo LM, Li MJ, Yeh YL, Hung TH. The association between maternal oxidative stress at mid-gestation and subsequent pregnancy complications. Reprod Sci. 2012;19:505–512. doi: 10.1177/1933719111426601. [DOI] [PubMed] [Google Scholar]
- 19.Zhou A, Carrell RW, Murphy MP, Wei Z, Yan Y, Stanley PL, Stein PE, Broughton Pipkin F, Read RJ. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468:108–111. doi: 10.1038/nature09505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knapen MF, Mulder TP, Van Rooij IA, Peters WH, Steegers EA. Low whole blood glutathione levels in pregnancies complicated by preeclampsia or the hemolysis, elevated liver enzymes, low platelets syndrome. Obstet Gynecol. 1998;92:1012–1015. doi: 10.1016/s0029-7844(98)00333-0. [DOI] [PubMed] [Google Scholar]
- 21.Raijmakers MT, Zusterzeel PL, Steegers EA, Hectors MP, Demacker PN, Peters WH. Plasma thiol status in preeclampsia. Obstet Gynecol. 2000;95:180–184. doi: 10.1016/s0029-7844(99)00514-1. [DOI] [PubMed] [Google Scholar]
- 22.Sandrim VC, Palei AC, Metzger IF, Gomes VA, Cavalli RC, Tanus-Santos JE. Nitric oxide formation is inversely related to serum levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. Hypertension. 2008;52:402–407. doi: 10.1161/HYPERTENSIONAHA.108.115006. [DOI] [PubMed] [Google Scholar]
- 23.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 24.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA CPEP Study Group. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. Erratum in: N Engl J Med. 2006 Oct 26;355(17):1840. [DOI] [PubMed] [Google Scholar]
- 25.Everett TR, Wilkinson IB, Lees CC. Drug development in preeclampsia: a ‘no go’ area? J Matern Fetal Neonatal Med. 2012;25:50–52. doi: 10.3109/14767058.2011.557791. [DOI] [PubMed] [Google Scholar]
- 26.Rumbold A, Duley L, Crowther CA, Haslam RR. Antioxidants for preventing pre-eclampsia. Cochrane Database Syst Rev. 2008;(1) doi: 10.1002/14651858.CD004227.pub3. CD004227. doi: 10.1002/14651858.CD004227.pub3. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z, Zhang J, Stamler JS. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci U S A. 2002;99:8306–8311. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsay B, De Belder A, Campbell S, Moncada S, Martin JF. A nitric oxide donor improves uterine artery diastolic blood flow in normal early pregnancy and in women at high risk of pre-eclampsia. Eur J Clin Invest. 1994;24:76–78. doi: 10.1111/j.1365-2362.1994.tb02064.x. [DOI] [PubMed] [Google Scholar]
- 29.Grunewald C, Kublickas M, Carlström K, Lunell NO, Nisell H. Effects of nitroglycerin on the uterine and umbilical circulation in severe preeclampsia. Obstet Gynecol. 1995;86:600–604. doi: 10.1016/0029-7844(95)00197-y. [DOI] [PubMed] [Google Scholar]
- 30.Cetin A, Yurtcu N, Guvenal T, Imir AG, Duran B, Cetin M. The effect of glyceryl trinitrate on hypertension in women with severe preeclampsia, HELLP syndrome, and eclampsia. Hypertens Pregnancy. 2004;23:37–46. doi: 10.1081/PRG-120028280. [DOI] [PubMed] [Google Scholar]
- 31.Manzur-Verástegui S, Mandeville PB, Gordillo-Moscoso A, Hernández-Sierra JF, Rodríguez-Martínez M. Efficacy of nitroglycerine infusion versus sublingual nifedipine in severe pre-eclampsia: a randomized, triple-blind, controlled trial. Clin Exp Pharmacol Physiol. 2008;35:580–585. doi: 10.1111/j.1440-1681.2007.04838.x. [DOI] [PubMed] [Google Scholar]
- 32.Lees C, Valensise H, Black R, Harrington K, Byiers S, Romanini C, The CS. efficacy and fetal-maternal cardiovascular effects of transdermal glyceryl trinitrate in the prophylaxis of pre-eclampsia and its complications: a randomized double-blind placebo-controlled trial. Ultrasound Obstet Gynecol. 1998;12:334–338. doi: 10.1046/j.1469-0705.1998.12050334.x. [DOI] [PubMed] [Google Scholar]
- 33.Picciolo C, Roncaglia N, Neri I, Pasta F, Arreghini A, Facchinetti F. Nitric oxide in the prevention of pre-eclampsia. Prenat Neonatal Med. 2000;5:212–215. [: CN–00399568] [Google Scholar]
- 34.Cacciatore B, Halmesmäki E, Kaaja R, Teramo K, Ylikorkala O. Effects of transdermal nitroglycerin on impedance to flow in the uterine, umbilical, and fetal middle cerebral arteries in pregnancies complicated by preeclampsia and intrauterine growth retardation. Am J Obstet Gynecol. 1998;179:140–145. doi: 10.1016/s0002-9378(98)70264-9. [DOI] [PubMed] [Google Scholar]
- 35.Trapani A, Jr, Gonçalves LF, Pires MM. Transdermal nitroglycerin in patients with severe pre-eclampsia with placental insufficiency: effect on uterine, umbilical and fetal middle cerebral artery resistance indices. Ultrasound Obstet Gynecol. 2011;38:389–394. doi: 10.1002/uog.8983. [DOI] [PubMed] [Google Scholar]
- 36.Luzi G, Caserta G, Iammarino G, Clerici G, Di Renzo GC. Nitric oxide donors in pregnancy: fetomaternal hemodynamic effects induced in mild pre-eclampsia and threatened preterm labor. Ultrasound Obstet Gynecol. 1999;14:101–109. doi: 10.1046/j.1469-0705.1999.14020101.x. [DOI] [PubMed] [Google Scholar]
- 37.Gori T, Parker JD. Nitrate tolerance: a unifying hypothesis. Circulation. 2002;106:2510–2513. doi: 10.1161/01.cir.0000036743.07406.53. [DOI] [PubMed] [Google Scholar]
- 38.Miller MR, Megson IL. Recent developments in nitric oxide donor drugs. Br J Pharmacol. 2007;151:305–321. doi: 10.1038/sj.bjp.0707224. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bath PM, Pathansali R, Iddenden R, Bath FJ. The effect of transdermal glyceryl trinitrate, a nitric oxide donor, on blood pressure and platelet function in acute stroke. Cerebrovasc Dis. 2001;11:265–272. doi: 10.1159/000047649. [DOI] [PubMed] [Google Scholar]
- 40.Davis DG, Brown PM. Glyceryl trinitrate (GTN) patches are unsuitable in hypertensive pregnancy. Aust N Z J Obstet Gynaecol. 2001;41:474. doi: 10.1111/j.1479-828x.2001.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 41.Thaler I, Amit A, Kamil D, Itskovitz-Eldor J. The effect of isosorbide dinitrate on placental blood flow and maternal blood pressure in women with pregnancy induced hypertension. Am J Hypertens. 1999;12(4 Pt 1):341–347. doi: 10.1016/s0895-7061(98)00249-0. [DOI] [PubMed] [Google Scholar]
- 42.Nakatsuka M, Takata M, Tada K, Asagiri K, Habara T, Noguchi S, Kudo T. A long-term transdermal nitric oxide donor improves uteroplacental circulation in women with preeclampsia. J Ultrasound Med. 2002;21:831–836. doi: 10.7863/jum.2002.21.8.831. [DOI] [PubMed] [Google Scholar]
- 43.Martínez-Abundis E, González-Ortiz M, Hernández-Salazar F, Huerta-J-Lucas MT. Sublingual isosorbide dinitrate in the acute control of hypertension in patients with severe preeclampsia. Gynecol Obstet Invest. 2000;50:39–42. doi: 10.1159/000010278. [DOI] [PubMed] [Google Scholar]
- 44.Thaler I, Amit A, Jakobi P, Itskovitz-Eldor J. The effect of isosorbide dinitrate on uterine artery and umbilical artery flow velocity waveforms at mid-pregnancy. Obstet Gynecol. 1996;88:838–843. doi: 10.1016/0029-7844(96)00297-9. [DOI] [PubMed] [Google Scholar]
- 45.Makino Y, Izumi H, Makino I, Shirakawa K. The effect of nitric oxide on uterine and umbilical artery flow velocity waveform in pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 1997;73:139–143. doi: 10.1016/s0301-2115(97)02743-7. [DOI] [PubMed] [Google Scholar]
- 46.Groten T, Fitzgerald J, Lehmann T, Schneider U, Kähler C, Schleussner E. Reduction of preeclampsia related complications with the NO-donor penterythriltetranitrat (petn) in risk pregnancies – A prospective randomized double-blind placebo pilot study. Pregnancy Hypertens. 2012;2:181. doi: 10.1016/j.preghy.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Sayed N, Kim DD, Fioramonti X, Iwahashi T, Durán WN, Beuve A. Nitroglycerin-induced S-nitrosylation and desensitization of soluble guanylyl cyclase contribute to nitrate tolerance. Circ Res. 2008;103:606–614. doi: 10.1161/CIRCRESAHA.108.175133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zai A, Rudd MA, Scribner AW, Loscalzo J. Cell-surface protein disulfide isomerase catalyzes transnitrosation and regulates intracellular transfer of nitric oxide. J Clin Invest. 1999;103:393–399. doi: 10.1172/JCI4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, Feelisch M. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc Natl Acad Sci U S A. 2004;101:4308–4313. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Belder AJ, MacAllister R, Radomski MW, Moncada S, Vallance PJ. Effects of S-nitroso-glutathione in the human forearm circulation: evidence for selective inhibition of platelet activation. Cardiovasc Res. 1994;28:691–694. doi: 10.1093/cvr/28.5.691. [DOI] [PubMed] [Google Scholar]
- 51.Ramsay B, Radomski M, De Belder A, Martin JF, Lopez-Jaramillo P. Systemic effects of S-nitroso-glutathione in the human following intravenous infusion. Br J Clin Pharmacol. 1995;40:101–102. doi: 10.1111/j.1365-2125.1995.tb04545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langford EJ, Brown AS, Wainwright RJ, de Belder AJ, Thomas MR, Smith RE, Radomski MW, Martin JF, Moncada S. Inhibition of platelet activity by S-nitrosoglutathione during coronary angioplasty. Lancet. 1994;344:1458–1460. doi: 10.1016/s0140-6736(94)90287-9. [DOI] [PubMed] [Google Scholar]
- 53.Langford EJ, Wainwright RJ, Martin JF. Platelet activation in acute myocardial infarction and unstable angina is inhibited by nitric oxide donors. Arterioscler Thromb Vasc Biol. 1996;16:51–55. doi: 10.1161/01.atv.16.1.51. [DOI] [PubMed] [Google Scholar]
- 54.de Belder A, Lees C, Martin J, Moncada S, Campbell S. Treatment of HELLP syndrome with nitric oxide donor. Lancet. 1995;345:124–125. doi: 10.1016/s0140-6736(95)90088-8. [DOI] [PubMed] [Google Scholar]
- 55.Lees C, Langford E, Brown AS, de Belder A, Pickles A, Martin JF, Campbell S. The effects of S-nitrosoglutathione on platelet activation, hypertension, and uterine and fetal Doppler in severe preeclampsia. Obstet Gynecol. 1996;88:14–19. doi: 10.1016/0029-7844(96)00070-1. [DOI] [PubMed] [Google Scholar]
- 56.Hausvater A, Giannone T, Sandoval YH, Doonan RJ, Antonopoulos CN, Matsoukis IL, Petridou ET, Daskalopoulou SS. The association between preeclampsia and arterial stiffness. J Hypertens. 2012;30:17–33. doi: 10.1097/HJH.0b013e32834e4b0f. [DOI] [PubMed] [Google Scholar]
- 57.Martínez-Ruiz A, Lamas S. Signalling by NO-induced protein S-nitrosylation and S-glutathionylation: convergences and divergences. Cardiovasc Res. 2007;75:220–228. doi: 10.1016/j.cardiores.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 58.Kaposzta Z, Baskerville PA, Madge D, Fraser S, Martin JF, Markus HS. L-arginine and S-nitrosoglutathione reduce embolization in humans. Circulation. 2001;103:2371–2375. doi: 10.1161/01.cir.103.19.2371. [DOI] [PubMed] [Google Scholar]
- 59.Kaposzta Z, Martin JF, Markus HS. Switching off embolization from symptomatic carotid plaque using S-nitrosoglutathione. Circulation. 2002;105:1480–1484. doi: 10.1161/01.cir.0000012347.47001.97. [DOI] [PubMed] [Google Scholar]
- 60.Colagiovanni DB, Borkhataria D, Looker D, Schuler D, Bachmann C, Sagelsdorff P, Honarvar N, Rosenthal GJ. Preclinical 28-day inhalation toxicity assessment of s-nitrosoglutathione in beagle dogs and Wistar rats. Int J Toxicol. 2011;30:466–477. doi: 10.1177/1091581811412084. [DOI] [PubMed] [Google Scholar]
- 61.Facchinetti F, Longo M, Piccinini F, Neri I, Volpe A. L-arginine infusion reduces blood pressure in preeclamptic women through nitric oxide release. J Soc Gynecol Investig. 1999;6:202–207. doi: 10.1016/s1071-5576(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 62.Vadillo-Ortega F, Perichart-Perera O, Espino S, Avila-Vergara MA, Ibarra I, Ahued R, Godines M, Parry S, Macones G, Strauss JF. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: randomised controlled trial. BMJ. 2011;342:d2901. doi: 10.1136/bmj.d2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neri I, Monari F, Sgarbi L, Berardi A, Masellis G, Facchinetti F. L-arginine supplementation in women with chronic hypertension: impact on blood pressure and maternal and neonatal complications. J Matern Fetal Neonatal Med. 2010;23:1456–1460. doi: 10.3109/14767051003677962. [DOI] [PubMed] [Google Scholar]
- 64.Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, Baker PN. A randomised, double-blinded, placebo-controlled study of the phosphodiesterase type 5 inhibitor sildenafil for the treatment of preeclampsia. Hypertens Pregnancy. 2009;28:369–382. doi: 10.3109/10641950802601278. [DOI] [PubMed] [Google Scholar]
- 65.Ramesar SV, Mackraj I, Gathiram P, Moodley J. Sildenafil citrate decreases sFlt-1 and sEng in pregnant l-NAME treated Sprague-Dawley rats. Eur J Obstet Gynecol Reprod Biol. 2011;157:136–140. doi: 10.1016/j.ejogrb.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Herraiz S, Pellicer B, Serra V, Cauli O, Cortijo J, Felipo V, Pellicer A. Sildenafil citrate improves perinatal outcome in fetuses from pre-eclamptic rats. BJOG. 2012;119:1394–1402. doi: 10.1111/j.1471-0528.2012.03430.x. [DOI] [PubMed] [Google Scholar]
- 67.Stanley JL, Andersson IJ, Poudel R, Rueda-Clausen CF, Sibley CP, Davidge ST, Baker PN. Sildenafil citrate rescues fetal growth in the catechol-O-methyl transferase knockout mouse model. Hypertension. 2012;59:1021–1028. doi: 10.1161/HYPERTENSIONAHA.111.186270. [DOI] [PubMed] [Google Scholar]
- 68.Nassar AH, Masrouha KZ, Itani H, Nader KA, Usta IM. Effects of sildenafil in Nω-nitro-L-arginine methyl ester-induced intrauterine growth restriction in a rat model. Am J Perinatol. 2012;29:429–434. doi: 10.1055/s-0032-1304823. [DOI] [PubMed] [Google Scholar]
- 69.Green LS, Chun LE, Patton AK, Sun X, Rosenthal GJ, Richards JP. Mechanism of inhibition for N6022, a first-in-class drug targeting S-nitrosoglutathione reductase. Biochemistry. 2012;51:2157–2168. doi: 10.1021/bi201785u. [DOI] [PubMed] [Google Scholar]
- 70.Chen Q, Sievers RE, Varga M, Kharait S, Haddad DJ, Patton AK, Delany CS, Mutka SC, Blonder JP, Dubé GP, Rosenthal GJ, Springer ML. Pharmacological inhibition of S-nitrosoglutathione reductase improves endothelial vasodilatory function in rats in vivo. J Appl Physiol. 2013;114:752–760. doi: 10.1152/japplphysiol.01302.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colagiovanni DB, Drolet DW, Langlois-Forget E, Piché MP, Looker D, Rosenthal GJ. A nonclinical safety and pharmacokinetic evaluation of N6022: a first-in-class S-nitrosoglutathione reductase inhibitor for the treatment of asthma. Regul Toxicol Pharmacol. 2012;62:115–124. doi: 10.1016/j.yrtph.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 72.CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group. Lancet. 1994;343:619–629. [PubMed] [Google Scholar]
- 73.Momi S, Emerson M, Paul W, Leone M, Mezzasoma AM, Del Soldato P, Page CP, Gresele P. Prevention of pulmonary thromboembolism by NCX 4016, a nitric oxide-releasing aspirin. Eur J Pharmacol. 2000;397:177–185. doi: 10.1016/s0014-2999(00)00223-5. [DOI] [PubMed] [Google Scholar]
- 74.Chazov EI, Rodnenkov OV, Zorin AV, Lakomkin VL, Gramovich VV, Vyborov ON, Dragnev AG, Timoshin CA, Buryachkovskaya LI, Abramov AA, Massenko VP, Arzamastsev EV, Kapelko VI, Vanin AF. Hypotensive effect of Oxacom® containing a dinitrosyl iron complex with glutathione: animal studies and clinical trials on healthy volunteers. Nitric Oxide. 2012;26:148–156. doi: 10.1016/j.niox.2012.01.008. [DOI] [PubMed] [Google Scholar]


