Abstract
Aims
To determine whether thiazolidinedione use is associated with a risk of bladder cancer.
Methods
We searched MEDLINE and EMBASE in June 2012 (with PubMed update to July 2013) and conducted meta-analysis on the overall risks of bladder cancer with pioglitazone or rosiglitazone and the risk with different categories of cumulative dose or duration of drug use.
Results
We screened 230 citations and included 18 studies, comprising five randomized controlled trials (RCTs) and 13 observational studies. Meta-analysis showed a significantly higher overall risk of bladder cancer with pioglitazone in RCTs [7878 participants; odds ratio (OR) 2.51, 95% confidence interval (CI) 1.09–5.80] and observational studies (>2.6 million patients; OR for ‘ever’ users vs. non-users 1.21, 95% CI 1.09–1.35). Subgroup analysis of observational studies by cumulative dose showed the risk of bladder cancer to be greatest with >28.0 g of pioglitazone (OR 1.64, 95% CI 1.28–2.12). A significantly increased risk was found with both 12–24 months (OR 1.41, 95% CI 1.16–1.71) and >24 months (OR 1.51, 95% CI 1.26–1.81) cumulative durations of pioglitazone exposure. No significant risk was seen with rosiglitazone in RCTs (OR 0.84, 95% CI 0.35–2.04) or ‘ever’ users vs. non-users in observational studies (OR 1.03, 95% CI 0.94–1.12); the evidence for any relationship between bladder cancer risk and rosiglitazone cumulative duration is limited and inconsistent. Direct comparison of pioglitazone to rosiglitazone ‘ever’ users yielded an OR of 1.25 (95% CI 0.91–1.72).
Conclusions
A modest but clinically significant increase in the risk of bladder cancer with pioglitazone was found, which appears to be related to cumulative dose and duration of exposure. We recommend that prescribers limit pioglitazone use to shorter durations.
Keywords: diabetes mellitus, meta-analysis, pioglitazone, rosiglitazone, thiazolidinedione, urinary bladder neoplasm
Introduction
The thiazolidinediones (TZDs), pioglitazone and rosiglitazone, are peroxisome proliferator-activated receptor γ (PPARγ) agonists that were developed as oral medications for the management of type 2 diabetes mellitus, although safety concerns have dogged both agents in recent years.
Bladder cancer is the fourth and 11th most common cancer type in men and women in developed countries, respectively [1], with an estimated combined 5 year relative survival rate range in the USA of 96.4–5.4% with increasing stage of disease at diagnosis [2]. The first signal for a possible risk of bladder cancer with pioglitazone arose from the preclinical rat carcinogenicity study included in its 1998/1999 licensing applications, which reported an increased incidence of hyperplasia (male and female) and malignant tumours (male) of the urinary bladder epithelium in rats administered pioglitazone [3–5]. Rosiglitazone alone was not associated with bladder cancer in 2 year nonclinical carcinogenicity studies reported in 2005 [6], although in 2008 it was shown to significantly promote bladder neoplasm incidence in rats pretreated with hydroxybutyl(butyl)nitrosamine (a urinary bladder specific carcinogen) [7].
The bladder cancers that arose following pioglitazone exposure occurred predominantly in male rats and were not observed in mice of either sex [8]. The mechanism for pioglitazone-induced male rat urinary bladder carcinogenesis is thus not thought to be due to direct PPARγ interaction, because little differential variation in PPARγ expression exists between the sexes/species [8]. The alternative ‘crystalluria’ hypothesis proposes that pioglitazone increases urinary solidification, resulting in chronic bladder irritation and carcinogenesis. Data supporting this hypothesis have been presented for rats [9,10], and it is known that male rats are more prone to develop urinary solids than female rats, mice or, indeed, primates (including humans) [8,9]. However, pioglitazone has also been observed to induce hyperplasia in the male rat urinary bladder despite suppression of urinary microcrystal formation through diet-induced urinary acidification [5,10,11], and so the summary of product characteristics for pioglitazone states that the relevance to humans of the tumourigenic findings in the male rat cannot be excluded [4].
Clinically, rosiglitazone has received little attention regarding bladder cancer risk in patients. This is in large part because the US Food and Drug Administration (FDA) imposed it under stringent prescribing restrictions [12] and the European Medicines Agency (EMA) suspended its marketing authorization in 2010 [13], following mounting concerns over its cardiovascular safety profile [14]. However, clinical evidence has accumulated to suggest an association between pioglitazone and bladder cancer. Consequently, the FDA issued a safety announcement in 2010 [15] and updated the drug labels of pioglitazone-containing medicines in 2011 to recommend that healthcare professionals avoid pioglitazone in patients with active bladder cancer and prescribe it with caution in those with a past history of bladder cancer [16]. The EMA Committee for Medicinal Products for Human Use went further following their own review in 2011, extending the new advised contraindications to include patients with previous bladder cancer or uninvestigated macroscopic haematuria, but continue to license pioglitazone [17,18]. As a result of one study [19], in 2011 France suspended the use of pioglitazone, and Germany and Luxembourg opted to recommend not starting new patients on pioglitazone [17].
However, since these decisions were made multiple further conflicting observational studies have been published. Previous meta-analyses have been undertaken, but none has assimilated all of the current available evidence [20–22]. The recent decision by India's ministry of health and family welfare department to revoke the ban it imposed on pioglitazone-containing compounds merely 6 weeks earlier highlights the need for an up-to-date systematic review of the present literature concerning pioglitazone and its potential risk of bladder cancer [23]. Furthermore, the FDA has recently voted to relax prescribing restrictions placed on rosiglitazone, making an investigation into its association with bladder cancer of clinical as well as mechanistic interest [24]. This present study has been conducted to determine, in a population of adult patients with type 2 diabetes mellitus: (i) does (a) pioglitazone ‘ever’ use or (b) rosiglitazone ‘ever’ use increase the risk of bladder cancer; and data permitting, (ii) is there a relationship between (a) cumulative dose or (b) cumulative duration of exposure and risk of bladder cancer with pioglitazone or, alternatively, rosiglitazone?
Methods
There were two independent reviewers involved in the process of study selection, data extraction and validity assessment throughout this review, with any discrepancies and disagreements being resolved through discussion amongst the team.
Inclusion criteria
Our inclusion criteria for randomized controlled trials (RCTs) were as follows: (i) parallel-group design of at least 52 weeks duration; (ii) prevention or treatment of diabetes mellitus; (iii) pioglitazone or rosiglitazone as the intervention vs. a control treatment, in which the comparison groups consisted of nonthiazolidinedione therapy or placebo; and (iv) outcome data (including explicit mention of zero events) on bladder cancer adverse events.
We also evaluated controlled observational studies (case–control or cohort) reporting the risk of bladder cancer with any pioglitazone or rosiglitazone exposure compared with those without such exposure. Eligible studies had to present an odds ratio (OR), relative risk (RR) or hazard ratio or sufficient data to enable us to calculate the OR.
Search strategy
An electronic search (MEDLINE and EMBASE) was carried out using OvidSP in June 2012, and we updated this through to July 2013 on a weekly basis using automated PubMed update notifications of newly published citations relating to thiazolidinediones and bladder cancer (see Supporting Information S1 for search terms). We examined the websites of the US FDA, the European regulatory authorities and clinical trial registers of pioglitazone and rosiglitazone [25,26]. The bibliographies of included studies and other existing systematic reviews were also used to identify relevant articles. We did not impose any restrictions on language or type of article.
Study selection
The initial screen involved checking titles and abstracts to exclude articles that clearly did not fulfil the inclusion criteria for relevant RCTs or observational studies evaluating the use of pioglitazone or rosiglitazone. Any potentially relevant reports were assessed further for eligibility after retrieving full-text versions.
Extraction of study characteristics and outcomes
Study characteristics and outcomes were extracted independently by pairs of reviewers from the team (CSK, YKL, RMT, CC-T and CAM). In order to avoid inadvertent double counting and data duplication, we matched regulatory reports and trial registry results to journal manuscripts based on sample size, duration, period of recruitment and intervention arms and we collated all the information together for each individual trial.
We used a standardized data-extraction format to record study characteristics, dose and frequency of interventions, and the mean age and sex of participants in the RCTs. The design and relevant data sources, study follow-up period, the number of study participants, mean age and sex and the crude and adjusted outcome results were recorded for the observational studies. Where available, we extracted data on bladder cancer outcomes according to different exposure categories, such as current use or past use, cumulative duration of use and cumulative dosage levels.
Any unclear items or discrepancies were resolved after rechecking against the source papers and discussion with a third reviewer. We aimed to contact authors if there were any areas that required clarification.
Assessment of risk of bias
We recorded information on blinding, allocation concealment, withdrawals and the loss to follow-up in RCTs. In accordance with recommendations on assessing adverse effects, we extracted information on participant selection, ascertainment of exposure and outcomes, as well as methods of addressing confounding in observational studies [27]. Studies deemed to be at high risk of bias were excluded from all meta-analyses.
We planned to generate funnel plots to assess the possibility of publication bias, provided that there were >10 studies available in a meta-analysis, with no evidence of substantial statistical heterogeneity [28].
Statistical analysis
We pooled trial data for binary outcomes based on Peto OR meta-analysis with Review Manager version 5.1.7 (Nordic Cochrane Center, Copenhagen, Denmark) [29]. For the observational studies, we conducted fixed-effect meta-analysis using the inverse variance method for pooled OR. We assumed similarity between the RR and OR because the adverse outcome of interest has low incidence [30].
We assessed statistical heterogeneity using the I2 statistic, with I2 > 50% indicating a substantial level of heterogeneity [31]. If substantial statistical heterogeneity was found, we planned to use random-effects modelling for the meta-analysis.
Prespecified subgroup analysis was performed by evaluating the effect of cumulative dose and duration of exposure.
The number needed to treat for harm and its 95% confidence interval (CI) with pioglitazone was calculated using Visual Rx, version 3.0 (Dr Chris Cates, University of London, UK) by applying the OR estimates to the control event rate in a large cohort study [32]. Herein, the number needed to treat for harm is the number of patients who need to be treated with pioglitazone for an additional patient to be harmed by an incident bladder cancer.
Results
We screened 230 citations overall. From our initial search, 14 studies met the inclusion criteria: five interventional [33–40] and nine observational [19,41–48]. Four further observational studies were selected for inclusion from weekly automated PubMed updates (up to July 2013) [49–52]. Figure 1 is a schematic diagram of the study selection process. Table 1 presents the main study characteristics of the five RCTs and risk-of-bias evaluations. Table 2 shows the main study characteristics and overall results of the 13 observational studies. Table 3 summarizes the meta-analysis results from the present study.
Figure 1.
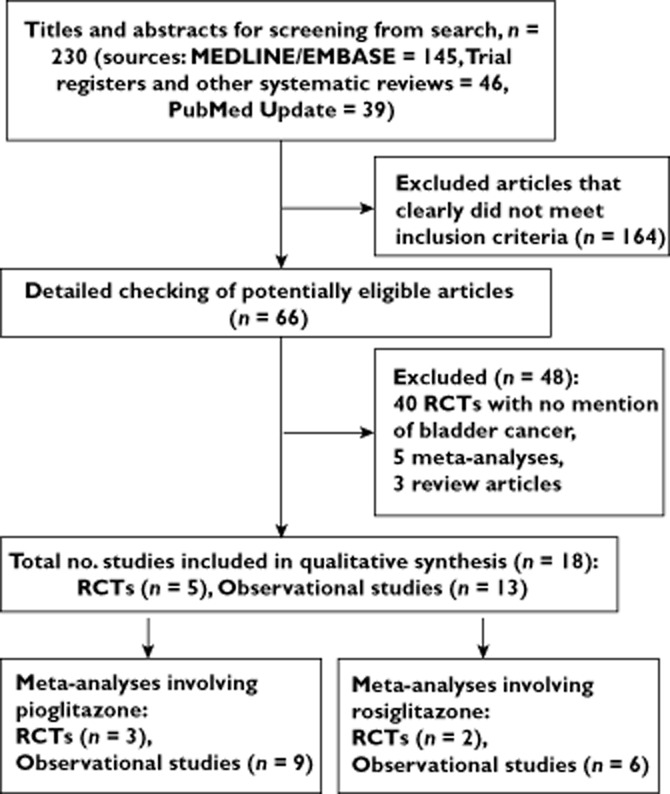
Flow diagram to show the process of study selection
Table 1.
Randomized controlled trial study characteristics and risk of bias assessment
| Losses (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study (first author, year) | Location | Study period | Follow-up (years) | Mean age (years) | Percentage male | Study size | Drug (no. of subjects) | Sequence generation | Allocation concealment | Monitoring of adverse events | Double blind | Withdrawal rates | Lost to follow-up |
| Pioglitazone | |||||||||||||
| Dormandy et al. (2005) [33], [34] | Europe | 2001–2005 | 3 | 62 | 66 | 5238 | Pioglitazone (2605) | Adequate | Adequate | Investigators collected AEs at every study visit. Trial records were evaluated to ensure that SAEs were reported. Serious events were checked against clinical notes | Yes | 427 (16.39) | 2 |
| Placebo (2633) | 438 (16.63) | 2 | |||||||||||
| Nissen et al. (2008) [35], [36] | Nortd and Soutd America | 2003–2006 | 1.5 | 60 | 67 | 543 | Pioglitazone (273) | Adequate | Adequate | Investigators reported listed adverse events | Yes | 92 | NA |
| Metformin (270) | 91 | NA | |||||||||||
| Tolman et al. (2009) [16], [37] | USA | 2000–2005 | 3 | 55 | 56 | 2097 | Pioglitazone (1051) | Adequate | Adequate | Adverse events were recorded at each study visit witdout adjudication | Yes | 649 | 116 |
| Glibenclamide (1046) | 641 | 114 | |||||||||||
| Rosiglitazone | |||||||||||||
| Home et al. (2009) [38], [40] | Europe and Australasia | 2001–2008 | 5.5 | 58 | 52 | 4447 | Rosiglitazone (2220) | Adequate | Adequate | AEs and SAEs were obtained for participants while on dual or triple oral tderapy, and SAEs tdereafter | No | NA | 60 |
| Metformin or sulfonylurea (2227) | NA | 67 | |||||||||||
| Kahn et al. (2006) [39], [40] | Europe and Nortd America | 2000–2006 | 4 | 56 | 58 | 4351 | Rosiglitazone (1456) | Adequate | Adequate | AEs were evaluated at study visit. Patients reported tde number of emergency room visits and hospitalizations, and any days when tdeir activity had been restricted | Yes | 558 (40.1) | 64 (4.6) |
| Sulfonylurea (1441) | 567 (42.4) | 69 (5.2) | |||||||||||
| Metformin (1454) | 545 (39.0) | 75 (5.4) | |||||||||||
For each randomized controlled trial, both the primary study publication and the source of the bladder cancer event data for the trial are cited. Abbreviations are as follows: AE, adverse events; NA, not available; SAE, serious adverse events.
Table 2.
Observational study characteristics and main results
| Risk of bladder cancer (95% CI)* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study (first author, year) | Country | Setting; database | Study period | Follow-up (years) | Mean age (years) | Percentage male | Cohort size | Drug comparison | Minimally/unadjusted | Fully adjusted | ||
| Cohort studies | ||||||||||||
| Fujimoto et al. (2012) [49] | Japan | Kitano Hospital | 2000–2011 | NR | NR | NR | 21 335 | P vs. no P | 1.75 (0.89–3.45) | – | ||
| Lewis et al. (2011) [41] | USA | Northern California; KPNC | 1997–2008 | P: median 9.3 No P: median 6.2 | 50% ≥60 | 53 | 193 099 | P vs. no P | 1.2 (0.9–1.5) | 1.2 (0.9–1.5) | ||
| Mamtani et al. (2012) [42] | UK | Population based; THIN | 2000–2010 | P: median 2.4 R: median 4.4 | 59% ≥60 | 57 | 28 514 | P vs. R | 1.16 (0.80–1.69) | 1.14 (0.79–1.66) | ||
| Neumann et al. (2012) [19] | France | Population based; PMSI, SNIIRAM | 2006–2009 | 3.1 | 47% ≥65 | 53 | 1 491 060 | P vs. no P | – | 1.22 (1.05–1.43) | ||
| R vs. no R | 1.08 (0.92–1.26) | |||||||||||
| Tseng (2011) [43] | Taiwan | Population based; NHI | 2003–2005 | Maximum 3 | NR | 50 | 998 947 | TZD vs. no TZD | – | 0.80 (0.34–1.90) | ||
| Tseng (2012) [44] | Taiwan | Population based; NHI | 2006–2009 | Maximum 4 | NR | NR | 54 928 | P vs. no P | 1.26 (0.67–2.39) | 1.31 (0.66–2.58) | ||
| Tseng (2013) [50] | Taiwan | Population based; NHI | 2006–2009 | Maximum 4 | 57% ≥60 | 51 | 885 236 | R vs. no R | 0.97 (0.87–1.08) | 0.98 (0.87–1.10) | ||
| Wei et al. (2013) [45] | UK | Population based; GPRD | 2001–2010 | P: mean 3.5 | 62 | 57 | 207 714 | P vs. no P | 0.99 (0.77–1.27) | 1.16 (0.83–1.62 | ||
| No P: mean 5.3 | ||||||||||||
| Case–control studies nested within a cohort | Cohort | Cases | Controls | |||||||||
| Azoulay et al. (2012) [46] | UK | Population based; GPRD | 1988–2009 | Mean 4.6 | 69 | 81 | 115 727 | 376 | 6699 | P vs. no TZD | 1.87 (1.13–3.09) | 1.83 (1.10–3.05) |
| 376 | 6699 | R vs. no TZD | 1.16 (0.79–1.69) | 1.14 (0.78–1.68) | ||||||||
| 201 | 632 | P vs. R | – | 1.60 (0.88–2.90) | ||||||||
| Chang et al. (2012) [47] | Taiwan | Population based; NHI | 2000–2007 | Median 7.9 | 71 | 67 | 606 583 | 1583 | 6308 | P vs. no P | 1.06 (0.82–1.37) | 0.95 (0.70–1.29) |
| 1583 | 6308 | R vs. no R | 1.11 (0.95–1.30) | 1.05 (0.81–1.36) | ||||||||
| Hsiao et al. (2013) [51] | Taiwan | Population based; NHI | 1997–2008 | Mean 3.6 | 66 | 68 | NR | 3412 | 17 060 | P vs. no P | 1.48 (1.23–1.78)† | 1.62 (0.92–2.86)‡ |
| 3412 | 17 060 | R vs. no R | 1.10 (0.97–1.25) † | 1.15 (0.67–1.98)‡ | ||||||||
| Case–control study | Cases | Controls | ||||||||||
| Song et al. (2012) [52] | Korea | Severance Hospital | 2005–2011 | NA | 69 | 84 | NA | 392 | 658 | P vs. no P | – | 2.09 (0.26–16.81) |
| Case/noncase study | Cases | Noncases | ||||||||||
| Piccinni et al. (2011) [48] | USA | FDA AERS | 2004–2009 | NA | Cases: 70 | NR | NA | 37 841 | 561 244 | P vs. no P | 4.30 (2.82–6.52) | – |
| 44 006 | 555 079 | R vs. no R | 0.38 (0.12–1.05) | |||||||||
Abbreviations are as follows: CI, confidence interval; FDA AERS, Food and Drug Administration Adverse Event Reporting System; GPRD, general practice research database; KPNC, Kaiser Permanente Northern California; NA, not applicable; NHI, national health insurance databases of Taiwan; NR, not reported; P, pioglitazone; PMSI, the hospital discharge database of France; R, rosiglitazone; SNIIRAM, the national health insurance information system of France; THIN, the health improvement network; TZD, thiazolidinedione.
Summary risk estimates used are as follows: hazard ratios [19,41,42,44,45,49,50], odds ratios [47,51,52], rate ratios [46], relative risks [43] and reporting odds ratios [48].
Unadjusted odds ratio results presented for Hsiao et al. [51] were calculated from raw values.
Adjusted odds ratios presented for Hsiao et al. [51] were calculated from pooling their adjusted results for current/recent/past drug user categories.
Table 3.
Summary table of meta-analysis results for risk of bladder cancer with pioglitazone or rosiglitazone
| Meta-analysis | Number of studies | Number of bladder cancer cases | Total number of patients pooled in meta-analysis | Type of meta-analysis | Odds ratio (95% confidence interval) | P value |
|---|---|---|---|---|---|---|
| Overall: use vs. no use of pioglitazone | ||||||
| Randomized controlled trials | 3 | 22 | 7878 | Fixed effect | 2.51 (1.09–5.80) | 0.03 |
| Observational studies | 8 | 9593 | 1 982 536 | Fixed effect | 1.21 (1.09–1.35) | 0.0005 |
| Pioglitazone: sensitivity analyses | ||||||
| Observational studies adjusting for smoking | 4 | 2417 | 408 185 | Fixed effect | 1.26 (1.05–1.52) | 0.01 |
| Observational studies adjusting for smoking or chronic obstructive pulmonary disease | 6 | 5994 | 483 585 | Fixed effect | 1.30 (1.09−1.54) | 0.003 |
| Pioglitazone: cumulative dose* | 3400 | 1 745 472 | – | |||
| <10.5 g | 4 | – | – | Fixed effect | 1.13 (0.94–1.35) | 0.20 |
| 10.5–28.0 g | 3 | – | – | Fixed effect | 1.22 (0.99–1.50) | 0.06 |
| >28.0 g | 3 | – | – | Fixed effect | 1.64 (1.28–2.12) | 0.0001 |
| Pioglitazone: cumulative duration* | 8395 | 1 773 835 | – | |||
| <12 months | 6 | – | – | Fixed effect | 1.10 (0.95−1.27) | 0.20 |
| 12–24 months | 5 | – | – | Fixed effect | 1.41 (1.16–1.71) | 0.0006 |
| >24 months | 5 | – | – | Fixed effect | 1.51 (1.26–1.81) | <0.00001 |
| Overall: use vs. no use of rosiglitazone | ||||||
| Randomized controlled trials | 2 | 21 | 8798 | Fixed effect | 0.84 (0.35–2.04) | 0.71 |
| Observational studies | 5 | 10 475 | 2 411 466 | Fixed effect | 1.03 (0.94–1.12) | 0.53 |
| Rosiglitazone: cumulative duration* | 4995 | 28 363 | – | |||
| <12 months | 2 | – | – | Random effects | 1.00 (0.86–1.17) | 0.96 |
| 12–24 months | 2 | – | – | Random effects | 1.53 (1.09–2.14) | 0.01 |
| >24 months | 2 | – | – | Random effects | 1.30 (0.56–3.04) | 0.54 |
| Pioglitazone vs. rosiglitazone use vs. no use | 2 | 182 | 29 356 | Fixed effect | 1.25 (0.91–1.72) | 0.16 |
The numbers provided for bladder cancer cases/total patients are composites; the individual number of bladder cancer cases/total patients for a given cumulative duration/dose will be less.
Interventional studies
There were three pioglitazone trials for which bladder cancer outcomes were found [33–37], with one trial where the data had to be sourced from the US product label [16,37]. Bladder malignancies from two rosiglitazone trials were specifically reported in a single publication that followed the original trial reports [38–40]. The pioglitazone trials were considered to be of high quality, as was one of the rosiglitazone trials with adequate randomization, allocation concealment, double blinding and reporting of withdrawals [39]; the second rosiglitazone trial was not double blinded and was considered to be at a moderate risk of bias [38] (Table 1).
Observational studies
The study designs of the 13 included observational studies were as follows: eight cohort [19,41–45,49,50]; four case–control [46,47,51,52], with three of these being case–control studies nested within cohorts [46,47,51]; and one case/noncase study (that calculated reporting OR based on disproportionality analysis of spontaneous adverse event reports) [48].
We viewed all of the observational studies to be susceptible to a variable degree of bias (Supporting Information S2). All the cohort and case–control studies are based on electronic administrative databases of healthcare use. With this methodology, regarding drug use we cannot be certain of patient consumption of medication and regarding bladder cancer occurrence and confounding factors, we cannot be certain of the validity of entered electronic diagnostic codes. From our 13 observational studies, three were excluded from meta-analysis [43,48,49]. Two were excluded because they were considered to be at a high risk of bias: (i) the case/noncase study [48], because it was conducted using disproportionality analysis (on relative frequencies of spontaneous reports) and does not provide risk data on numerical outcomes [53]; and (ii) one cohort study, because it failed to adjust for any potential confounders [49]. The third observational study excluded was a cohort study that reported results only for the generic thiazolidinedione drug class and did not provide results for individual thiazolidinediones [43].
Meta-analyses
Pioglitazone
The risk of bladder cancer with pioglitazone use vs. no use
The pooled sample size of the three RCTs was 7878 participants with 22 bladder cancers diagnosed [33–37], with a significant risk Peto OR 2.51 (95% CI 1.09–5.80, P = 0.03, I2 = 27%; Figure 2). The gross theoretical total of the eight eligible observational studies was 2 690 633 patients [19,41,44–47,51,52]. The pooled sample size for adjusted results, after accounting for nesting [46,47,51], was 1 982 536 patients with 9593 incident bladder cancers. The observational study summary result showed a lower magnitude but significant relationship between pioglitazone ‘ever’ use vs. no use and bladder cancer (OR 1.21, 95% CI 1.09–1.35, P = 0.0005, I2 = 0%; Figure 3).
Figure 2.
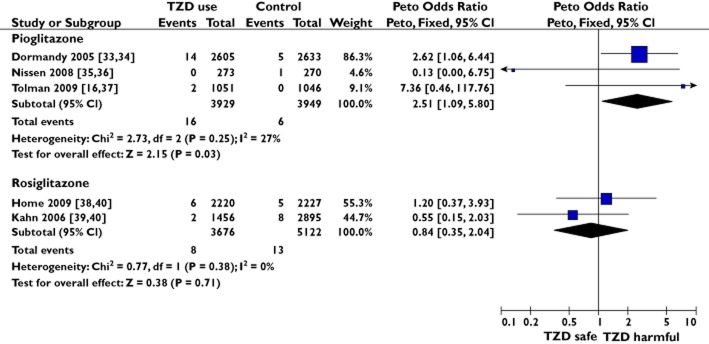
Meta-analyses of randomized controlled trials to show the risk of bladder cancer with pioglitazone or rosiglitazone
Figure 3.
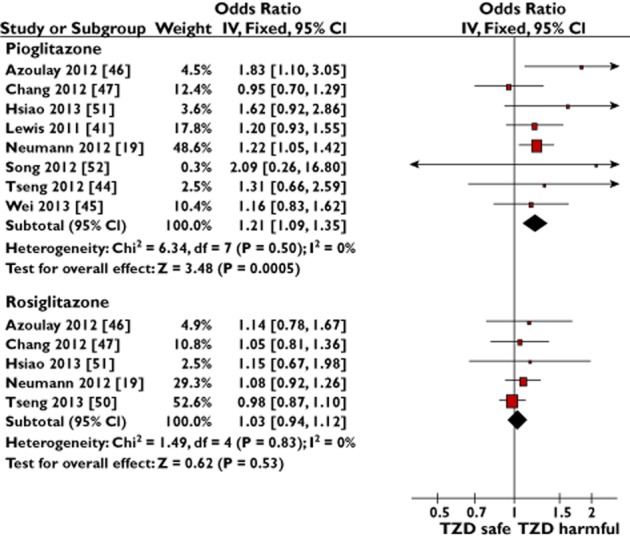
Meta-analyses of observational studies to show the adjusted risk of bladder cancer with ever use of pioglitazone or rosiglitazone
The risk of bladder cancer with increasing cumulative dose of pioglitazone use vs. no use
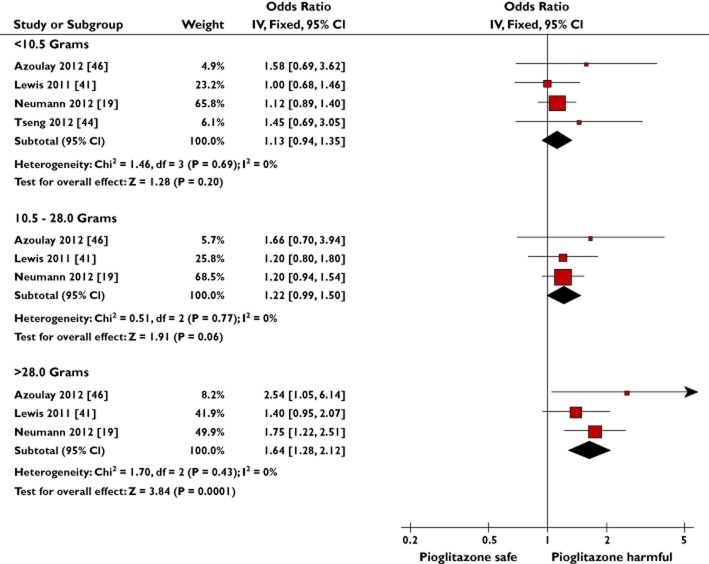
Five observational studies provided results for the risk of bladder cancer with different cumulative doses of pioglitazone exposure vs. no use [19,41,44,46,47] (Supporting Information S3); four were compatible for pooling [19,41,44,46]. The meta-analysis for cumulative dose showed a significant association between pioglitazone use and bladder cancer in the largest cumulative dose category (>28.0 g: OR 1.64, 95% CI 1.28–2.12, P = 0.0001, I2 = 0%), whereas the pooled OR for <10.5 g cumulative dose was 1.13 (95% CI 0.94–1.35, P = 0.20, I2 = 0%; Figure 4). In keeping with a dose–response effect, the intermediate cumulative dose exposure category (10.5–28.0 g) had an intermediate risk of borderline statistical significance (OR 1.22, 95% CI 0.99–1.50, P = 0.06, I2 = 0%). The test for subgroup differences found a significant difference in the associated risk seen with lower (<10.5 g) in comparison to higher (>28.0 g) cumulative dose (P = 0.02). No significant difference was found between the >28.0 and 10.5–28.0 g cumulative dose subgroups (P = 0.08). The observational study not compatible for pooling demonstrated a nonsignificant trend towards increased risk with increasing cumulative pioglitazone dosage [47].
Figure 4.
Meta-analysis of observational studies to show the adjusted risk of bladder cancer with increasing cumulative dose of pioglitazone
The risk of bladder cancer with increasing cumulative duration of pioglitazone use vs. no use
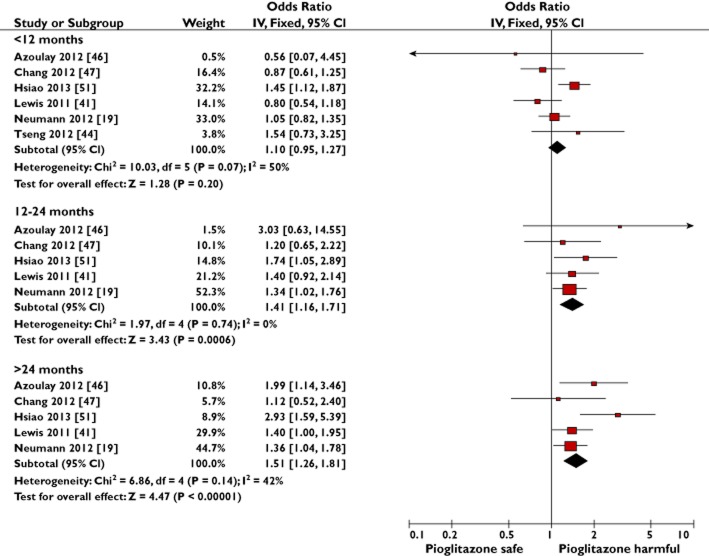
Six observational studies provided results for the risk of bladder cancer with different cumulative durations of pioglitazone exposure vs. no use [19,41,44,46,47,51]. All six were compatible for pooling, although one study first required its 2–3 and ≥3 year categories to be combined into one estimate by estimating the weighted average using the inverse variance method [47]. A significant association with bladder cancer for both the 12–24 and >24 month categories was seen, but the association was nonsignificant when pioglitazone treatment was for <12 months cumulative duration (Figure 5). The test for subgroup differences found a significant difference in the associated risk seen with shorter (<12 months) compared with longer (>24 months) durations (P = 0.007) and shorter (<12 months) compared with intermediate (12–24 months) durations (P = 0.05), but no significant difference was found between intermediate and longer durations of pioglitazone use (P = 0.6).
Figure 5.
Meta-analysis of observational studies to show the adjusted risk of bladder cancer with increasing cumulative duration of pioglitazone exposure
Sensitivity analyses
Smoking is the most important risk factor for bladder cancer [1]. In two sensitivity meta-analyses for the risk of bladder cancer with ‘ever’ use of pioglitazone vs. no use, we restricted the pooled observational studies to the following: (i) those that directly adjusted for smoking [41,45,46,52]; and (ii) those that directly adjusted for smoking [41,45,46,52] plus those that adjusted for chronic obstructive pulmonary disease [44,51], a surrogate marker for smoking (Supporting Information S4). In both cases, the risk of bladder cancer with pioglitazone use remained statistically significant and of equivalent magnitude to our primary pooled observational study pioglitazone result (Figure 3).
The number needed to treat with pioglitazone for harm
In order to estimate the number needed to treat for harm in a real-world population, we used an estimated baseline incidence rate for bladder cancer of 0.14% per 37.5 months follow-up from non-users of pioglitazone within the largest included observational study [19]. Table 4 shows the calculated numbers needed to treat for harm based on the pooled OR according to a range of pioglitazone exposures.
Table 4.
The number needed to treat for harm from pooled observational study populations with various exposures to pioglitazone
| Pioglitazone exposure | Odds ratio from meta-analysis (95% confidence interval) | Number needed to treat for harm* (95% confidence interval) |
|---|---|---|
| ‘Ever’ use | 1.21 (1.09–1.35) | 3408 (2045–7949) |
| >28.0 g cumulative dose | 1.64 (1.28–2.12) | 1119 (640–2556) |
| >24 months cumulative duration | 1.51 (1.26–1.81) | 1404 (885–2753) |
The number of patients who must be treated with pioglitazone, compared with an equal number not exposed to pioglitazone, to result in one additional case of bladder cancer in the pioglitazone treatment group.
Rosiglitazone
The risk of bladder cancer with rosiglitazone use vs. no use
The pooled sample characteristics of the two RCTs were 8798 participants with 21 bladder cancers [38–40], while for the five observational studies [19,46,47,50,51] the adjusted pooled results were from 2 411 466 participants with 10 475 bladder cancers. No clear association between rosiglitazone use and bladder cancer was seen with the pooled RCTs (Peto OR 0.84, 95% CI 0.35–2.04, P = 0.71, I2 = 0%; Figure 2) or the pooled observational studies (OR 1.03, 95% CI 0.94–1.12, P = 0.53, I2 = 0%; Figure 3).
The risk of bladder cancer with increasing cumulative duration of rosiglitazone use vs. no use
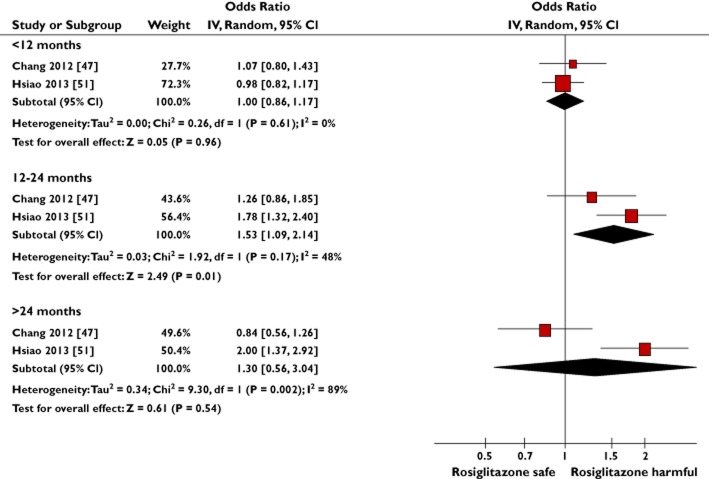
Four observational studies reported results for the risk of bladder cancer with different cumulative durations of rosiglitazone exposure vs. no use (Supporting Information S3) [46,47,50,51]; two studies were compatible for pooling [47,51]. Given the substantial heterogeneity detected in the cumulative duration >24 months subgroup (I2 = 89%), random effects meta-analysis was performed (Figure 6). Of the two studies that could not be pooled, neither reported a significant risk with different cumulative durations of rosiglitazone exposure [46,50]. We had no compatible data to conduct a pooled dose analysis for rosiglitazone, but of the three observational studies that provided results for cumulative dose, no significant risk was shown [46,47,50].
Figure 6.
Meta-analysis of observational studies to show the adjusted risk of bladder cancer with increasing cumulative duration of rosiglitazone exposure
The risk of bladder cancer with pioglitazone ‘ever’ users in comparison to rosiglitazone ‘ever’ users
Although the above analyses permit indirect comparisons between pioglitazone and rosiglitazone, we carred out a meta-analysis to contrast the two thiazolidinediones directly, after pooling the adjusted results of the two observational studies that report on the risk of bladder cancer with pioglitazone ‘ever’ use vs. rosiglitazone ‘ever’ use [42,46] (Supporting Information S5). The pooled sample size was 29 356 patients with 182 bladder cancer cases, and the summary risk estimate, whilst tending towards pioglitazone being harmful, was not statistically significant (OR 1.25, 95% CI 0.91–1.72, P = 0.16, I2 = 0%).
We did not conduct an asymmetry test for publication bias because there were <10 studies in any particular meta-analysis.
Discussion
In this study, we investigated the relationship between pioglitazone use, rosiglitazone use and incident bladder cancer. To summarize our results, we found the overall use of pioglitazone to be associated with a significant risk of bladder cancer in both the pooled estimate of RCTs (Figure 2) and the pooled estimate of observational studies (Figure 3). Importantly, we confirmed a cumulative dose and duration relationship between pioglitazone exposure and associated risk of bladder cancer (Figures 4 and 5). Unlike pioglitazone, we found no association between rosiglitazone ‘ever’ use and risk of incident bladder cancer (Figures 2 and 3). There is limited and inconsistent evidence on any relationship between risk of bladder cancer and cumulative duration of rosiglitazone exposure (Figure 6). There was a nonsignificant trend towards increased risk of bladder cancer in pioglitazone ‘ever’ users directly compared with rosiglitazone ‘ever’ users (Supporting Information S5).
Pioglitazone
Pioglitazone confers a risk of a number of established adverse drug reactions, including bone fractures (OR 2.23, 95% CI 1.65–3.01 in women) [54], oedema and congestive heart failure (OR 1.41, 95% CI 1.14–1.76) [55]. In comparison, the association between bladder cancer and ‘ever’ use of pioglitazone vs. no use from the pooled observational studies in this investigation was more modest (OR 1.21, 95% CI 1.09–1.35), whereas the risk magnitude appeared greater from the pooled RCTs (OR 2.51, 95% CI 1.09–5.80). However, it is inherently difficult to obtain precise estimates of rare events, and we note that the 95% CIs from the pooled RCT meta-analysis are relatively wide and encompass the 95% CI range of the pooled observational study meta-analysis. Of these two point estimates though, despite observational studies having less internal validity than RCTs, we consider the pioglitazone ‘ever’ use pooled observational study result (OR 1.21, 95% 1.09–1.35) to be closer to the underlying ‘true’ risk magnitude because, compared with the pooled RCT result, it is derived from a superior sample size from a wider population more representative of the general public, has a higher degree of statistical significance (0.0005 vs. 0.03, respectively) and negligible heterogeneity (0 vs. 27%, respectively).
We believe our finding that pioglitazone carries a significant but modest risk of human bladder cancer is credible for three reasons. Firstly, we observed consistent directions of effect between the pooled RCTs and pooled observational study results for pioglitazone ‘ever’ use (and rosiglitazone ‘ever’ use). Secondly, the results of our sensitivity meta-analyses were highly consistent with our primary pioglitazone pooled observational study risk estimate. Thirdly, we found a plausible ascending risk of bladder cancer associated with both cumulative dose and duration, with the greatest risk seen in those receiving >28.0 g dose (OR 1.64, 95% CI 1.28–2.12) or >24 months duration (OR 1.51, 95% CI 1.26–1.81) of pioglitazone therapy.
To aid clinical interpretation, we estimated the numbers needed to treat with pioglitazone for harm (an incident bladder cancer). Even with those at highest risk, the number needed to treat for harm was 1119 (95% CI 640–2556) and 1404 (95% CI 885–2753) for >28.0 g and >24 months cumulative pioglitazone exposures, respectively. By way of comparison, it has been previously estimated that the number of women with type 2 diabetes mellitus needed to treat with long-term thiazolidinedione therapy to result in a bone fracture within 1 year is between 21 and 55 patients, depending in part on the age of the women [54]. Therefore, although the risk of bladder cancer is of grave concern given its potential for morbidity and mortality, its occurrence is less frequent than other adverse effects. Nevertheless, the worldwide exposure to pioglitazone has been estimated to exceed 20 million patient-years [5] and so, hypothetically, using the conservative estimate of the basal rate of bladder cancer in patients with diabetes of 50/100 000 person-years [56] and our OR of 1.21, over 2000 additional bladder cancer cases may already be attributable to pioglitazone.
It has been suggested that pioglitazone is no longer required for management of type 2 diabetes in the face of its known adverse drug reactions, the accumulating evidence for its risk of bladder cancer and the newer antidiabetic oral medication classes with improved safety profiles that have been developed, such as dipeptidyl peptidase-4 inhibitors and sodium-glucose linked transporter 2 inhibitors [57]. Whilst we keenly anticipate these newer agents to benefit patient care increasingly, we are also aware that there are over 10 years of postmarketing data available for pioglitazone; therefore, despite its shortcomings, healthcare regulators, healthcare providers and patients know more about pioglitazone's risks of rare events than they do for the newer agents and so precautionary contraindications for pioglitazone therapy are already in place [16–18]. Both the EMA and the US FDA continue to view pioglitazone as possessing a positive benefit–risk profile, and following the expiry of its patent in 2012, several generic pioglitazone-containing compounds have been authorized [56]. Therefore, we envisage that in at least the short and medium terms, pioglitazone will retain its current clinical role. Within this clinical context, we consider our finding of an increased risk of bladder cancer with higher cumulative doses and durations of pioglitazone exposure to provide practical, useful pharmacovigilance information to healthcare providers. This information can facilitate the identification of patient subgroups most at risk of bladder cancer, in whom further pioglitazone therapy should be cautioned against and the use of alternative antidiabetic medications be encouraged.
Rosiglitazone
The ‘ever’ use of rosiglitazone was consistently shown not to be associated with the risk of bladder cancer in both the pooled RCT and pooled observational study analyses. We consider the rosiglitazone cumulative duration meta-analysis to be of interest but merely hypothesis generating. No robust conclusions can be drawn from it because it included only two studies with substantial heterogeneity, and the two observational studies that provided cumulative rosiglitazone duration data incompatible for pooling reported nonsignificant results [46,50]. On the contrary, one study has reported an increased risk of bladder cancer in patients commenced on rosiglitazone ≥5 years previously compared with patients who started rosiglitazone <1 year before [42].
Collectively though, our results suggest a pioglitazone-specific risk rather than a thiazolidinedione class effect, which may aid researchers investigating pioglitazone bladder carcinogenicity. Interestingly, whereas rosiglitazone is a specific PPARγ agonist, pioglitazone possesses partial PPARα as well as PPARγ agonism at therapeutic levels, imparting a pharmacological profile analogous to the dual PPARα/γ ‘glitazar’ compounds [11]. No glitazar has been approved for clinical use and several, notably including muraglitazar, were shown to induce rat urothelial bladder cancer in laboratory studies [6]. However, whilst muraglitazar-induced rat bladder carcinogenesis was ascribed to the seemingly rat-specific ‘crystalluria’ mechanism, other glitazars (e.g. aleglitazar) have not been associated with rodent bladder tumours [3] and, similar to pioglitazone, a bladder carcinogenic effect has been reported in rats administered naveglitazar without urolithiasis as an inciting event [58,59]. Therefore, although the dual PPARα/γ receptor agonism of pioglitazone may be contributory, the underlying mechanism of pioglitazone bladder carcinogenicity in humans is likely to be more complex.
Strengths and limitations
This study has some strengths. Although other meta-analyses have been published [20–22] (with only one featuring rosiglitazone [20]), to the best of the authors' knowledge this study represents the largest, most up-to-date and most comprehensive meta-analysis undertaken into the risk of bladder cancer associated with thiazolidinediones. This study incorporated a greater number of RCTs and observational studies, uniquely performed meta-analyses for both individual thiazolidinediones using pooled RCT and pooled observational study populations separately and stands out in providing a meta-analysis for pioglitazone using only pooled RCT data and for rosiglitazone using only pooled observational data. We uniquely carried out meta-analysis on the risk of bladder cancer associated with increasing cumulative durations of rosiglitazone therapy and, importantly, in all of our pioglitazone dose/duration meta-analyses, we tested for statistical differences between subgroups. This study is also original in conducting both sensitivity meta-analyses to address the potential confounding influence of smoking and a meta-analysis that directly compared pioglitazone with rosiglitazone use. Furthermore, the other studies included only one pioglitazone RCT [33], but unlike the present study, did not assimilate its updated bladder cancer safety data mentioned in a review of the study by the original authors [34], which importantly changed the study's association between pioglitazone and bladder cancer to statistically significant (estimated RR 2.83, 95% CI 1.02–7.85) [60,61].
There are limitations to the present study. At the level of the individual observational studies, different combinations of confounders were measured and no single study took account of all known bladder cancer risk factors [62]. At review level, firstly there is potential duplication of some participants where different included observational studies have used the same electronic database and overlapping follow-up periods (specifically, the UK General Practice Research Database [45,46] and the Taiwanese National Health Insurance databases [44,47,50,51]). However, the exact dates of these studies, their study designs, methods of participant selection and thiazolidinedione drug selection all varied (Table 2 and Supporting Information S2) and, with no evidence of complete overlap in populations, we considered it appropriate for these studies to contribute to the pooled results. Secondly, the heterogeneity of the observational study methodology entailed that for the pioglitazone cumulative dose and the rosiglitazone cumulative duration meta-analyses, not every study that provided results could be pooled, although we did still consider these incompatible results qualitatively. Finally, the systematic lack of an upper limit for the largest dose and duration categories prohibited a meaningful regression analysis.
Conclusion
The overall finding from this study is a small but significant association between adult pioglitazone use and bladder cancer. The risk of bladder cancer increases with both increasing cumulative dose and duration of pioglitazone exposure. No clear association between ‘ever’ use of rosiglitazone and bladder cancer was identified. There is limited and inconsistent evidence on any relationship between bladder cancer risk and cumulative duration of rosiglitazone exposure, although the possibility of an increased risk with longer durations cannot be excluded and, ideally, further investigations are warranted.
We consider that the appropriate application of our results relating to pioglitazone is to provide updated detailed information on the risk–benefit profile for those patients still eligible under the current regulatory guidance to receive pioglitazone therapy [16,17], particularly with regard to the potential risks of greater dose and duration of use. It would be prudent to avoid longer-term use of pioglitazone given that alternative oral antidiabetic medications are available.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Supporting Information S1
The search terms used for the primary search of this study and in the generation of the weekly automatic updates
Supporting Information S2
Observational study methodological characteristics and risk of bias assessment
Supporting Information S3
Comprehensive results summary for observational studies
Supporting Information S4
Sensitivity meta-analysis of observational studies to show the adjusted risk of bladder cancer with ever use of pioglitazone restricted to studies that: (i) control for smoking; (ii) control for smoking or chronic obstructive pulmonary disease status
Supporting Information S5
Meta-analysis of observational studies to show the adjusted risk of bladder cancer with ever use of pioglitazone compared with ever use of rosiglitazone
References
- 1.Burger M, Catto JWF, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C, Shariat S, Lotan Y. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatolovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) Bethesda, MD: National Cancer Institute; 2011. Available at http://seer.cancer.gov/csr/1975_2009_pops09/ (last accessed 2 October 2013) [Google Scholar]
- 3.Bortolini M, Wright MB, Bopst M, Balas B. Examining the safety of PPAR agonists - current trends and future prospects. Expert Opin Drug Saf. 2013;12:65–79. doi: 10.1517/14740338.2013.741585. [DOI] [PubMed] [Google Scholar]
- 4.European Medicines Agency. Actos: European public assessment report – Product Information. 2012. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000285/human_med_000624.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d124 (last accessed 12 October 2013)
- 5. Assessment report for Actos, Glustin, Competact, Glubrava, Tandemact. 2011. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000285/WC500126656.pdf (last accessed 12 October 2013)
- 6.El Hage J. Toxicity profile of peroxisome proliferator activated receptor (PPAR) agonists and preclinical safety profile for muraglitazar. 2005. Available at http://www.fda.gov/ohrms/dockets/ac/05/slides/2005-4169S2_02_02-FDA-ElHage.ppt (last accessed 12 October 2013)
- 7.Lubet RA, Fischer SM, Steele VE, Juliana MM, Desmond R, Grubbs CJ. Rosiglitazone, a PPAR gamma agonist: potent promoter of hydroxybutyl(butyl)nitrosamine-induced urinary bladder cancers. Int J Cancer. 2008;123:2254–2259. doi: 10.1002/ijc.23765. [DOI] [PubMed] [Google Scholar]
- 8.Cohen SM. Effects of PPARgamma and combined agonists on the urinary tract of rats and other species. Toxicol Sci. 2005;87:322–327. doi: 10.1093/toxsci/kfi266. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S, Arnold LL, Pennington KL, Kakiuchi-Kiyota S, Wei M, Wanibuchi H, Cohen SM. Effects of pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, on the urine and urothelium of the rat. Toxicol Sci. 2010;113:349–357. doi: 10.1093/toxsci/kfp256. [DOI] [PubMed] [Google Scholar]
- 10.Sato K, Awasaki Y, Kandori H, Tanakamaru Z-Y, Nagai H, Baron D, Yamamoto M. Suppressive effects of acid-forming diet against the tumorigenic potential of pioglitazone hydrochloride in the urinary bladder of male rats. Toxicol Appl Pharmacol. 2011;251:234–244. doi: 10.1016/j.taap.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Hillaire-Buys D, Faillie J-L, Montastruc J-L, Petit P. Stay vigilant: a glitazone (pioglitazone) can hide a glitazar! Eur J Clin Pharmacol. 2012;68:1681–1683. doi: 10.1007/s00228-012-1299-1. [DOI] [PubMed] [Google Scholar]
- 12. Press announcements – FDA significantly restricts access to the diabetes drug Avandia. 2010. [cited 2013 Oct 12]. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm226975.htm (last accessed 12 October 2013)
- 13. European Medicines Agency – news and events – European Medicines Agency recommends suspension of Avandia, Avandamet and Avaglim. 2010. [cited 12 Oct 2013]. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2010/09/news_detail_001119.jsp&mid=WC0b01ac058004d5c1 (last accessed 12 October 2013)
- 14.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, Kelman JA. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 15.Food and Drug Administration. FDA drug safety communication: ongoing safety review of Actos (pioglitazone) and potential increased risk of bladder cancer after two years exposure. 2010. Available at http://www.fda.gov/Drugs/DrugSafety/ucm226214.htm (last accessed 12 October 2013)
- 16.Food and Drug Administration. FDA drug safety communication: updated drug labels for pioglitazone-containing medicines. 2011. Available at http://www.fda.gov/Drugs/DrugSafety/ucm266555.htm (last accessed 12 October 2013)
- 17.European Medicines Agency Committee for Medicinal Products for Human Use. Questions and answers on the review of pioglitazone-containing medicines (Actos, Glustin, Competact, Glubrava and Tandemact). 2011. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2011/07/WC500109179.pdf (last accessed 12 October 2013)
- 18.European Medicines Agency. European Medicines Agency recommends new contra-indications and warnings for pioglitazone to reduce small increased risk of bladder cancer. 2011. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2011/07/news_detail_001311.jsp&mid=WC0b01ac058004d5cl (last accessed 12 October 2013)
- 19.Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, Allemand H. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012;55:1953–1962. doi: 10.1007/s00125-012-2538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colmers IN, Bowker SL, Majumdar SR, Johnson JA. Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: a meta-analysis. CMAJ. 2012;184:E675–683. doi: 10.1503/cmaj.112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Z, Shen Z, Lu Y, Zhong S, Xu C. Increased risk of bladder cancer with pioglitazone therapy in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract. 2012;98:159–163. doi: 10.1016/j.diabres.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Ferwana M, Firwana B, Hasan R, Al-Mallah MH, Kim S, Montori VM, Murad MH. Pioglitazone and risk of bladder cancer: a meta-analysis of controlled studies. Diabet Med. 2013;30:1026–1032. doi: 10.1111/dme.12144. [DOI] [PubMed] [Google Scholar]
- 23.Bhaumik S. Flip flop policy over pioglitazone licence causes media storm in India. BMJ. 2013;347:f4937–f4937. doi: 10.1136/bmj.f4937. [DOI] [PubMed] [Google Scholar]
- 24.Tucker ME. FDA panel advises easing restrictions on rosiglitazone. BMJ. 2013;346:f3769–f3769. doi: 10.1136/bmj.f3769. [DOI] [PubMed] [Google Scholar]
- 25.Glaxo SmithKline. Results summaries: rosiglitazone. n.d. Available at http://www.gsk-clinicalstudyregister.com/result_comp_list.jsp;jsessionid=25A02DE6B0E4CF1857C249D6EBF3250C?compound=rosiglitazone&studyType=All&phase=All&population=All&marketing=All (last accessed 16 October 2012)
- 26.US National Institutes of Health. ClinicalTrials.gov registry. n.d. Available at http://www.clinicaltrials.gov (last accessed 16 October 2012)
- 27.Loke YK, Price D, Herxheimer A. Chapter 14: adverse effects. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons; 2008. pp. 433–448. [Google Scholar]
- 28.Ioannidis JPA, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ. 2007;176:1091–1096. doi: 10.1503/cmaj.060410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26:53–77. doi: 10.1002/sim.2528. [DOI] [PubMed] [Google Scholar]
- 30.Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ. 1998;316:989–991. doi: 10.1136/bmj.316.7136.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deeks JJ, Higgins JP, Altman DG. Chapter 9: analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons; 2008. pp. 243–296. [Google Scholar]
- 32.Cates C. Dr Chris Cates EBM web site. 2008. Available at http://www.nntonline.net/visualrx/ (last accessed 2 October 2013)
- 33.Dormandy JA, Charbonnel B, Eckland DJA, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokán M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 34.Dormandy J, Bhattacharya M, van Troostenburg de Bruyn A-R. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf. 2009;32:187–202. doi: 10.2165/00002018-200932030-00002. [DOI] [PubMed] [Google Scholar]
- 35.Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A, Jure H, De Larochellière R, Staniloae CS, Mavromatis K, Saw J, Hu B, Lincoff AM, Tuzcu EM. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561–1573. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- 36.Takeda Global Research & Development Center Inc. Efficacy study of pioglitazone compared to glimepiride on coronary atherosclerotic disease progression in subjects with type 2 diabetes mellitus (PERISCOPE). 2012 Update. Available at http://clinicaltrials.gov/ct2/show/NCT00225277 (last accessed 2 October 2013)
- 37.Tolman KG, Freston JW, Kupfer S, Perez A. Liver safety in patients with type 2 diabetes treated with pioglitazone: results from a 3-year, randomized, comparator-controlled study in the US. Drug Saf. 2009;32:787–800. doi: 10.2165/11316510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 38.Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJV. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 39.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 40.Home PD, Kahn SE, Jones NP, Noronha D, Beck-Nielsen H, Viberti G. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia. 2010;53:1838–1845. doi: 10.1007/s00125-010-1804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP, Jr, Vaughn DJ, Nessel L, Selby J, Strom BL. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916–922. doi: 10.2337/dc10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mamtani R, Haynes K, Bilker WB, Vaughn DJ, Strom BL, Glanz K, Lewis JD. Association between longer therapy with thiazolidinediones and risk of bladder cancer: a cohort study. J Natl Cancer Inst. 2012;104:1411–1421. doi: 10.1093/jnci/djs328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tseng C-H. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia. 2011;54:2009–2015. doi: 10.1007/s00125-011-2171-z. [DOI] [PubMed] [Google Scholar]
- 44.Tseng C-H. Pioglitazone and bladder cancer: a population-based study of Taiwanese. Diabetes Care. 2012;35:278–280. doi: 10.2337/dc11-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei L, Macdonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254–259. doi: 10.1111/j.1365-2125.2012.04325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azoulay L, Yin H, Filion KB, Assayag J, Majdan A, Pollak MN, Suissa S. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ. 2012;344:e3645. doi: 10.1136/bmj.e3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang C-H, Lin J-W, Wu L-C, Lai M-S, Chuang L-M, Chan KA. Association of thiazolidinediones with liver cancer and colorectal cancer in type 2 diabetes mellitus. Hepatology. 2012;55:1462–1472. doi: 10.1002/hep.25509. [DOI] [PubMed] [Google Scholar]
- 48.Piccinni C, Motola D, Marchesini G, Poluzzi E. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011;34:1369–1371. doi: 10.2337/dc10-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujimoto K, Hamamoto Y, Honjo S, Kawasaki Y, Mori K, Tatsuoka H, Matsuoka A, Wada Y, Ikeda H, Fujikawa J, Koshiyama H. Possible link of pioglitazone with bladder cancer in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2013;99:e21–23. doi: 10.1016/j.diabres.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 50.Tseng C-H. Rosiglitazone is not associated with an increased risk of bladder cancer. Cancer Epidemiol. 2013;37:385–389. doi: 10.1016/j.canep.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 51.Hsiao F-Y, Hsieh P-H, Huang W-F, Tsai Y-W, Gau C-S. Risk of bladder cancer in diabetic patients treated with rosiglitazone or pioglitazone: a nested case–control study. Drug Saf. 2013;36:643–649. doi: 10.1007/s40264-013-0080-4. [DOI] [PubMed] [Google Scholar]
- 52.Song SO, Kim KJ, Lee B-W, Kang ES, Cha BS, Lee HC. The risk of bladder cancer in korean diabetic subjects treated with pioglitazone. Diabetes Metab J. 2012;36:371–378. doi: 10.4093/dmj.2012.36.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Boer A. When to publish measures of disproportionality derived from spontaneous reporting databases? Br J Clin Pharmacol. 2011;72:909–911. doi: 10.1111/j.1365-2125.2011.04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ. 2009;180:32–39. doi: 10.1503/cmaj.080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 56.Faillie J-L, Petit P, Montastruc J-L, Hillaire-Buys D. Scientific evidence and controversies about pioglitazone and bladder cancer: which lessons can be drawn? Drug Saf. 2013;36:693–707. doi: 10.1007/s40264-013-0086-y. [DOI] [PubMed] [Google Scholar]
- 57.Sinha B, Ghosal S. Pioglitazone – do we really need it to manage type 2 diabetes? Diabetes Metab Syndr. 2013;7:52–55. doi: 10.1016/j.dsx.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 58.Long GG, Reynolds VL, Lopez-Martinez A, Ryan TE, White SL, Eldridge SR. Urothelial carcinogenesis in the urinary bladder of rats treated with naveglitazar, a gamma-dominant PPAR alpha/gamma agonist: lack of evidence for urolithiasis as an inciting event. Toxicol Pathol. 2008;36:218–231. doi: 10.1177/0192623307311757. [DOI] [PubMed] [Google Scholar]
- 59.Long GG, Reynolds VL, Dochterman LW, Ryan TE. Neoplastic and non-neoplastic changes in F-344 rats treated with Naveglitazar, a gamma-dominant PPAR alpha/gamma agonist. Toxicol Pathol. 2009;37:741–753. doi: 10.1177/0192623309343775. [DOI] [PubMed] [Google Scholar]
- 60.Hillaire-Buys D, Faillie J-L, Montastruc J-L. Pioglitazone and bladder cancer. Lancet. 2011;378:1543–1544. doi: 10.1016/S0140-6736(11)61662-0. [DOI] [PubMed] [Google Scholar]
- 61.Singh S. Replies to Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: a meta-analysis. 2012. Available at http://www.cmaj.ca/content/184/12/E675.short/reply#cmaj_el_712534 (last accessed 23 November 2012)
- 62.Jacobs BL, Lee CT, Montie JE. Bladder cancer in 2010: how far have we come? CA Cancer J Clin. 2010;60:244–272. doi: 10.3322/caac.20077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
The search terms used for the primary search of this study and in the generation of the weekly automatic updates
Supporting Information S2
Observational study methodological characteristics and risk of bias assessment
Supporting Information S3
Comprehensive results summary for observational studies
Supporting Information S4
Sensitivity meta-analysis of observational studies to show the adjusted risk of bladder cancer with ever use of pioglitazone restricted to studies that: (i) control for smoking; (ii) control for smoking or chronic obstructive pulmonary disease status
Supporting Information S5
Meta-analysis of observational studies to show the adjusted risk of bladder cancer with ever use of pioglitazone compared with ever use of rosiglitazone