Abstract
Aims
This study investigated relevant pharmacodynamic and pharmacokinetic parameters during the transition from warfarin to rivaroxaban in healthy male subjects.
Methods
Ninety-six healthy men were randomized into the following three groups: warfarin [international normalized ratio (INR) 2.0–3.0] transitioned to rivaroxaban 20 mg once daily (od; group A); warfarin (INR 2.0–3.0) followed by placebo od (group B); and rivaroxaban alone 20 mg od (group C) for 4 days. Anti-factor Xa activity, inhibition of factor Xa activity, prothrombin time (PT), activated partial thromboplastin time, HepTest, prothrombinase-induced clotting time, factor VIIa activity, factor IIa activity, endogenous thrombin potential and pharmacokinetics were measured.
Results
An additive effect was observed on the PT and PT/INR during the initial transition period. The mean maximal prolongation of PT was 4.39-fold [coefficient of variation (CV) 18.03%; range 3.39–6.50] of the baseline value in group A, compared with 1.88-fold (CV 10.35%; range 1.53–2.21) in group B and 1.57-fold (CV 9.98%; range 1.37–2.09) in group C. Rivaroxaban had minimal influence on the PT/INR at trough levels. Inhibition of factor Xa activity, activated partial thromboplastin time and endogenous thrombin potential were also enhanced, but to a lesser extent. In contrast, the effects of rivaroxaban on anti-factor Xa activity, HepTest and prothrombinase-induced clotting time were not affected by pretreatment with warfarin.
Conclusions
Changes in pharmacodynamics during the transition from warfarin to rivaroxaban vary depending on the test used. A supra-additive effect on PT/INR is expected during the initial period of transition, but pretreatment with warfarin does not influence the effect of rivaroxaban on anti-factor Xa activity.
Keywords: international normalized ratio, pharmacodynamics, prothrombin time, rivaroxaban, therapy transition, warfarin
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Warfarin has a slow onset and offset of action, and the prothrombin time/international normalized ratio is used for dose titration.
Warfarin and rivaroxaban exert their anticoagulant effect via different mechanisms, but both drugs affect several coagulation tests.
The pharmacodynamic parameters may be affected during the transition from warfarin to rivaroxaban.
WHAT THIS STUDY ADDS
During transition from warfarin to rivaroxaban, a supra-additive effect was observed on the prothrombin time/international normalized ratio (particularly at peak rivaroxaban concentrations), but rivaroxaban had minimal influence at trough concentrations.
The effects of rivaroxaban on anti-factor Xa activity, HepTest and prothrombinase-induced clotting time were not affected by pretreatment with warfarin.
Introduction
Anticoagulants are indicated for the prevention and treatment of thromboembolic disorders. Parenteral anticoagulants (e.g. heparins) are used mostly as short-term therapies, whereas vitamin K antagonists (VKAs, e.g. warfarin) have, until recently, been the only oral agents available for long-term anticoagulant therapy. The anticoagulant effect of the VKAs is produced by interference with the cyclic interconversion of vitamin K and its 2,3 epoxide, leading to the hepatic production of coagulation factors II, VII, IX and X with reduced coagulant activity [1]. Although effective, the VKAs have a narrow therapeutic window, variable responses, and multiple food and drug interactions, which necessitate regular coagulation monitoring and dose titration. The anticoagulation intensity of the VKAs is determined by the prothrombin time (PT), expressed as the international normalized ratio (INR) [1]. Novel oral anticoagulants that directly target single coagulation factors (i.e. factor Xa or factor IIa) have been developed to overcome some of the limitations associated with the traditional anticoagulants, and some of these agents have already been approved for clinical use in specific indications. Owing to their mode of action, these novel agents also affect coagulation tests (such as PT) [2].
The PT is a global clotting test that can be used to assess the extrinsic pathway of the blood coagulation cascade; the test is sensitive for deficiencies in factors II, V, VII and X [3]. Rivaroxaban is an oral, direct factor Xa inhibitor. Dose-dependent prolongation of PT by rivaroxaban was observed in phase I studies in healthy subjects and phase II studies in the prevention of venous thromboembolism after major orthopaedic surgery of the hip or knee, when Neoplastin was used for the assay [4,5]. However, the PT responses vary markedly between reagents and, as with other direct factor Xa inhibitors, the INR system used for monitoring the VKAs does not correct for the variations observed with rivaroxaban when different PT reagents are used [6,7].
Rivaroxaban has been investigated extensively for the prevention and treatment of thromboembolic disorders, including thromboprophylaxis after major orthopaedic surgery [8–11], treatment of deep vein thrombosis and pulmonary embolism and prevention of recurrent venous thromboembolism [12,13], stroke prevention in patients with nonvalvular atrial fibrillation [14], and secondary prevention in patients with acute coronary syndrome [15]. In the phase III ROCKET AF study for stroke prevention in patients with nonvalvular atrial fibrillation, rivaroxaban 20 mg once daily (od; 15 mg for patients with creatinine clearance 30–49 ml min–1) was compared with INR-adjusted warfarin (target range 2.0–3.0). The study enrolled not only VKA-naïve patients, but also patients who were receiving warfarin and then transitioned to rivaroxaban. The transition took place when the INR was below 3.0 [14]. It is anticipated that, in clinical practice, some patients who require long-term anticoagulation and receive warfarin may be transitioned to rivaroxaban therapy. The main objective of the present study was to examine changes in a set of relevant pharmacodynamic parameters during the transition from warfarin to rivaroxaban. Such changes would be expected in similar transition settings (such as for some patients in ROCKET AF). This study was conducted in healthy individuals who were suitable for the required frequent study procedures, which might not be practical in an elderly patient population.
Methods
Participants and study design
This multicentre, randomized, placebo-controlled, parallel-group study enrolled 96 healthy men, aged 18–45 years, with a body mass index of 18–29 kg m–2. Randomization was carried out after a prestudy examination. The listed subjects were assigned to random numbers, starting with 01 in ascending order as they were listed. The treatment was determined by the randomization list. The study centres were as follows: ClinPharmCologne – MEDA Manufacturing GmbH, Köln, Germany; and Clinical Research Services Mönchengladbach GmbH, Mönchengladbach, Germany.
Variants of the enzyme that metabolizes warfarin, cytochrome P450 2C9, and of a key pharmacological target of warfarin, vitamin K epoxide reductase (VKORC1), contribute to differences in patients' responses to warfarin [16]. Therefore, genotyping was carried out to minimize potential genetic influence that may lead to changes in exposure in this phase I setting. Only individuals homozygous for the wild-type allele CYP2C9*1 and homozygous or heterozygous carriers of the C-allele at positions 6484 and 7566 of the VKORC1 gene, respectively, were randomized. A sample for the pharmacogenetic analysis was taken at a separate examination visit before the prestudy examination. Subjects were required to have a resting heart rate of 45–90 beats min–1, systolic blood pressure of 100–140 mmHg, diastolic blood pressure <85 mmHg and no relevant pathological changes in their electrocardiograms. Exclusion criteria included any clinically relevant condition or medical history that could affect the study results, such as known coagulation disorders (e.g. von Willebrand's disease, haemophilia), known disorders with increased risks of bleeding (e.g. periodontitis, haemorrhoids, acute gastritis, peptic ulcer) or a tendency to common causes of bleeding (e.g. nasal bleeding).
All subjects provided written, informed consent prior to enrolment. Study documentation was reviewed and approved by the Ethics Committee of the North Rhine Medical Council (Düsseldorf, Germany). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, the International Conference on Harmonisation, Good Clinical Practice guidelines and German drug law. The study was registered with EudraCT (2008-005540-16).
The study design involved three treatment groups (Figure 1). A randomization list was set up by Global Biostatistics, Bayer HealthCare (Wuppertal, Germany). In treatment group A, warfarin (Coumadin®; BMS, München, Germany) was administered in varying doses from day −6 to −1 (10 mg od or lower on days −6 and −5; and 2.5, 5, 10, 12.5 or 15 mg od from day −4 to −1, depending on INR levels), to achieve a steady state of warfarin (INR 2.0–3.0); rivaroxaban 20 mg od was given for 4 days (days 0–3) starting 24 h after warfarin was stopped. In treatment group B, the same warfarin regimens as treatment group A were used to achieve an INR of 2.0–3.0, followed by placebo od for 4 days (days 0–3) starting 24 h after warfarin was discontinued. The pretreatment period with warfarin could be prolonged for each individual until the target INR of 2.0–3.0 was reached. On day 5, 10 mg vitamin K (Konakion®; Hoffmann-La Roche, Basel, Switzerland) was administered to subjects receiving treatments A and B, prior to discharge. In treatment group C, rivaroxaban 20 mg od was given for 4 days (days 0–3) without prior warfarin treatment. The study design was single blinded for treatment groups A and B, and open label for treatment group C. In all treatment groups, the study drug was administered in the morning after a standard breakfast with ∼240 ml of water. The next meal was provided 4 h after dosing.
Figure 1.
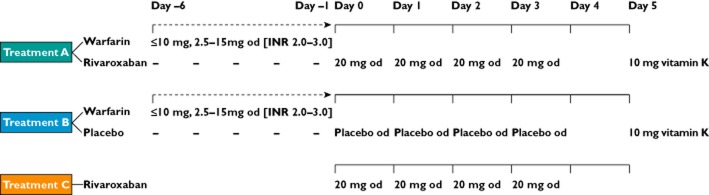
Study design and treatment groups. INR, international normalized ratio; od, once daily
Pharmacodynamics
Blood samples collected at predetermined intervals for pharmacodynamic analyses were centrifuged, and the plasma samples were frozen and stored at −15°C or lower until analysis at the Clinical Chemistry Laboratory and the Biomarker Laboratory of Bayer Pharma AG (Wuppertal, Germany) and Eurofins Medinet BV (Breda, The Netherlands). The following pharmacodynamic parameters were assessed: anti-factor Xa activity, inhibition of factor Xa activity, PT, activated partial thromboplastin time (aPTT), HepTest, prothrombinase-induced clotting time (PiCT), factor VIIa activity, factor IIa activity and endogenous thrombin potential (ETP).
Anti-factor Xa activity was determined using a spectrophotometric method based on a two-step assay procedure (Berichrom Heparin; Dade Behring, Marburg, Germany). A defined quantity of factor Xa was added to the plasma sample and incubated for 1 min at 37°C, allowing for inactivation of the exogenous factor Xa activity by inhibitors in the sample, followed by addition of a chromogenic substrate (Z-D-Leu-Gly-Arg-ANBA-methyl amide) and further incubation for 1 min at 37°C, to quantify the residual factor Xa activity. The absorbance was determined by spectrophotometry at 405 nm, and the amount of anti-factor Xa activity in the sample was read from a calibration curve produced from a set of calibration samples with known low-molecular-weight heparin concentrations. Inhibition of factor Xa activity, PT (using Neoplastin® as a reagent; Roche Diagnostics, Mannheim, Germany), aPTT and HepTest were determined as described previously [4]. The PiCT was performed as a one-step measurement (Pefakit PiCT reagent kit; Pentapharm Ltd, Potters Bar, UK) in accordance with the manufacturer's instructions.
Endogenous thrombin potential
Endogenous thrombin potential was measured using the calibrated automated thrombogram integrated ETP assay platform of Thrombinoscope (Maastricht, The Netherlands) in platelet-poor plasma samples after the addition of recombinant tissue factor (final concentration 1 pm). A fluorogenic thrombin substrate was added, and the formation of thrombin in the sample was quantified using a microtitre plate reader (FluoroScan; ThermoFisher Scientific, Schwerte, Germany). The amount of thrombin formed per time interval was determined from the slope of the fluorescence curve and quantified vs. the calibrators. The parameters determined were as follows: lag time; peak thrombin generation rate; time to peak thrombin generation rate; and area under the effect–time curve (AUC).
Factor VIIa and factor IIa activities
Factor VIIa and factor IIa activities were determined using optical coagulometric assays. Standard human plasma, control plasma samples, and factor II- and factor VII-deficient plasma were used in conjunction with the Dade Innovin Reagent kit (all obtained from Dade Behring, Marburg, Germany) in the thromboplastin time assay. The results were obtained using a reference curve generated with standard human plasma or pooled normal plasma mixed with the deficient plasma. Partial inhibition of the specific coagulation factor in the plasma samples to be analysed reduces its ability to compensate for the absence of the factor in the corresponding factor-deficient plasma included in the assay mixture, resulting in a prolonged thromboplastin assay time.
Pharmacokinetics
Blood samples were collected at predetermined intervals, and the plasma samples were stored at −15°C or lower until analysis at the DMPK Bioanalytics Laboratory of Bayer Pharma AG (Wuppertal, Germany). Concentrations of rivaroxaban in plasma were determined using validated high-performance liquid chromatography coupled with a tandem mass spectrometer (HPLC-MS/MS) after solid/liquid extraction [17]. The calibration range was 0.5 μg l–1 (the lower limit of quantification) to 500 μg l–1. The concentrations of the quality controls ranged from 1.35 to 398 μg l–1 and were determined with an accuracy of 97.9–100.4% and a precision of 2.5–5.3%. Plasma concentrations of R-warfarin and S-warfarin were determined by HPLC-MS/MS after solid/liquid extraction. The calibration range was 10 μg l–1 (lower limit of quantification) to 2000 μg l–1. The concentrations of the quality controls were 29.9–1615.0 μg l–1 for both R-warfarin and S-warfarin, which were determined with an accuracy of 92.4–109.6 or 91.3–109.2%, and a precision of 7.0–8.4 or 6.1–8.6%, for R-warfarin and S-warfarin, respectively. The pharmacokinetic parameters were calculated from the plasma concentration vs. time data using model-independent (compartment-free) methods. The parameters for rivaroxaban included the following: area under the plasma concentration–time curve from time 0 to 24 h (AUC(0–24)) and maximal plasma concentration (Cmax) after the first dose of rivaroxaban; AUC(0–24) and Cmax divided by dose per kilogram of bodyweight (AUC[0–24],norm and Cmax,norm, respectively); the time to reach Cmax (tmax) after the first dose; and terminal half-life (t1/2) after the last dose. The parameters for warfarin included t1/2 after the last dose for R- and S-warfarin and steady-state trough plasma concentrations at and after last dose.
Safety and tolerability
Adverse events were documented by questioning of the subjects by the investigators and by spontaneous reporting by the subjects. The incidence of abnormal findings in measurements for tolerability included vital parameters (such as heart rate and blood pressure), electrocardiogram findings and laboratory findings.
Statistical analyses
The sample size estimation was based on the maximal effect on PT relative to baseline. Data from a phase I pilot study on relative PT prolongation as well as intra-individual geometric coefficients of variation (CVs) from a pool of phase I studies were used for this calculation. Based on these, the mean maximal relative PT prolongation in the present study was expected to be 1.62, with a CV of 30%, and the aim was to achieve a maximal relative PT prolongation of ≤2-fold of the baseline value. Accordingly, 96 healthy men were enrolled into this study, of whom 84 were valid for the pharmacodynamic and pharmacokinetic analyses (28 per treatment group).
The statistical evaluation of pharmacodynamic parameters provided median changes from baseline per time point. Baseline was defined as the last measurement before the first warfarin dose in the warfarin followed by rivaroxaban group (A) and the warfarin followed by placebo group (B). In the rivaroxaban alone group (C), baseline was defined as the measurement immediately before first dose of rivaroxaban. The kinetic characteristics [including AUC from time 0 to the last data point (AUC(0–tn)) and maximal effect (Emax)] of PT prolongation were analysed in an explorative manner. For comparison of kinetics between the treatments, these characteristics were logarithmized and analysed using analysis of variance, including a treatment effect. Point estimates and exploratory one-sided 90% confidence intervals (CIs) for the treatment ratios were calculated by retransformation of the logarithmic results.
The pharmacokinetic characteristics of rivaroxaban, AUC(0–24) and Cmax after the first dose, and t1/2 of warfarin after the last dose were analysed assuming log-normally distributed data. The logarithms of these characteristics were analysed using analysis of variance, including a treatment effect. Based on these analyses, point estimates (least-squares means) and exploratory 95% CIs for the ratios were calculated by retransformation of the logarithmic data using the interindividual standard deviation of the analysis of variance.
Results
Study population
A total of 96 healthy men were enrolled into the study. The first visit occurred on 11 November 2008 and the last visit was on 10 November 2009; the study was completed on 9 April 2010. Thirteen subjects discontinued the study prematurely; among these, five did not receive any study medication, a further seven received only warfarin prior to discontinuation, and a single subject had elevated alanine aminotransferase levels after warfarin administration before the first dose of rivaroxaban. Subjects were valid for inclusion in the safety analysis (91 subjects) if they received at least one dose of study medication. Subjects were valid for pharmacokinetic and pharmacodynamic analyses if they received study medication and completed the pharmacokinetic and pharmacodynamic assessments without major protocol deviations. Of the 91 subjects included in the safety analysis, only 84 were valid for the pharmacokinetic and pharmacodynamic analyses (28 per treatment group). The baseline characteristics of the subjects are presented in Table 1.
Table 1.
Demographic characteristics of subjects
| Parameter | Warfarin/rivaroxaban (n = 28) | Warfarin/placebo (n = 28) | Rivaroxaban alone (n = 28) |
|---|---|---|---|
| Age (years) | |||
| Median (range) | 30.5 (19.0–44.0) | 30.0 (18.0–44.0) | 35.5 (18.0–45.0) |
| SD | 7.2 | 7.5 | 8.0 |
| Weight (kg) | |||
| Mean (range) | 80.7 (63.0–108.0) | 78.3 (53.0–97.0) | 80.9 (62.0–101.0) |
| SD | 9.4 | 10.9 | 10.8 |
| Height (cm) | |||
| Mean (range) | 180 (166.0–197.0) | 179.8 (162.0–193.0) | 180.5 (167.0–194.0) |
| SD | 7.5 | 7.1 | 7.0 |
| Body mass index (kg m−2) | |||
| Mean (range) | 24.9 (20.9–29.0) | 24.1 (19.5–29.0) | 24.8 (18.6–29.5) |
| SD | 2.2 | 2.8 | 2.9 |
Pharmacodynamics
Prothrombin time (expressed in seconds)
Treatment with rivaroxaban after pretreatment with warfarin (treatment A) showed a supra-additive effect on the prolongation of PT, compared with warfarin followed by placebo (treatment B) or rivaroxaban alone without pretreatment with warfarin (treatment C). In the warfarin followed by rivaroxaban group (A), the geometric mean Emax for PT was prolonged to 4.39-fold of baseline value (CV 18.03%; range 3.39–6.50), compared with 1.88-fold (CV 10.35%; range 1.53–2.21) in the warfarin followed by placebo group (B), and 1.57-fold (CV 9.98%; range 1.37–2.09) in the rivaroxaban alone group (C). The least-squares mean ratios were 2.79 (A/C) and 2.33 (A/B) for Emax, and 12.51 (A/C) and 2.30 (A/B) for baseline-adjusted AUC(0–24) (Table 2). At trough concentrations, rivaroxaban had minimal influence on PT. For example, at 24 h after the first dose of rivaroxaban (group A), the median PT was 1.56-fold (range 1.3–1.9) compared with 1.47-fold (range 1.2–1.8) in the warfarin followed by placebo group (B), and 1.02-fold (range 1.0–1.1) in the rivaroxaban alone group (C), relative to the respective baseline levels (see Figure 2A for median of ratio to baseline over time).
Table 2.
Treatment effects on prothrombin time
| Parameter | Warfarin/rivaroxaban (A) | Warfarin/placebo (B) | Rivaroxaban alone (C) | Test/reference | LS mean ratio (90% CI) |
|---|---|---|---|---|---|
| Emax (x-fold) | 4.39 | 1.88 | 1.57 | A/C | 2.79 (2.63–2.96) |
| A/B | 2.33 (2.20–2.47) | ||||
| Emax,BA (s) | 45.0 | 11.6 | 7.3 | A/C | 6.15 (5.60–6.76) |
| A/B | 3.88 (3.53–4.27) | ||||
| AUC(0–24) (x-fold h−1) | 55.35 | 37.89 | 20.41 | A/C | 2.71 (2.47–2.98) |
| A/B | 1.46 (1.33–1.60) | ||||
| AUCBA(0–24) (s h−1) | 413.41 | 179.94 | 33.06 | A/C | 12.51 (10.68–14.64) |
| A/B | 2.30 (1.96–2.69) |
Results are geometric means (n = 26–28). Abbreviations are as follows: AUC(0–24), area under the effect–time curve from time 0 to 24 h; BA, baseline adjusted; CI, confidence interval; Emax, maximal effect; LS, least-squares.
Figure 2.
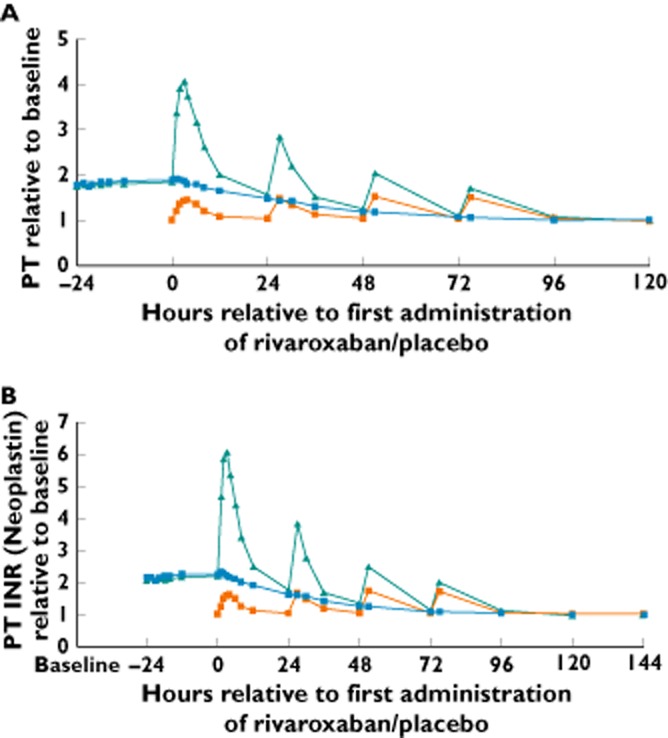
Prothrombin time (PT) expressed in seconds (A) and international normalized ratio (INR; B) after the administration of warfarin (INR 2.0–3.0) followed by rivaroxaban 20 mg once daily (od; treatment A), warfarin (INR 2.0–3.0) followed by placebo od (treatment B) and rivaroxaban alone 20 mg od (treatment C); relative prolongation is expressed as median ratio to baseline (n = 26–28).  , warfarin/rivaroxaban;
, warfarin/rivaroxaban;  , warfarin/placebo;
, warfarin/placebo;  , rivaroxaban alone
, rivaroxaban alone
Prothrombin time (expressed as international normalized ratio)
The PT determined as INR showed similar results to that expressed in seconds. Treatment with warfarin followed by rivaroxaban (group A) showed a supra-additive effect on INR, compared with treatment with warfarin followed by placebo (group B) and treatment with rivaroxaban alone (group C; Figure 2B). The geometric mean Emax for INR was prolonged to 6.65-fold (CV 23.39%; range 4.74–11.18) of baseline value with treatment A, 2.25-fold (CV 13.19%; range 1.72–2.77) with treatment B and 1.79-fold (CV 12.87%; range 1.51–2.58) with treatment C. However, at 24 h after rivaroxaban dosing (i.e. trough rivaroxaban concentrations), rivaroxaban had no residual or additional influence on the INR levels, either given alone or given after warfarin, on any of the treatment days. For example, the median absolute INR was 1.85 (range 1.53–2.31) at 24 h after the first dose of rivaroxaban in treatment group A, compared with 1.68 (range 1.34–2.13) in the warfarin followed by placebo group (B; see Figure 2B for median of ratio to baseline over time).
Inhibition of factor Xa activity
Treatment with warfarin followed by rivaroxaban (A) showed an additive effect on the inhibition of factor Xa activity, compared with treatment with warfarin followed by placebo (B) or rivaroxaban alone (C). The geometric mean Emax for inhibition of factor Xa was 76.0% for treatment A, 43.4% for treatment B and 49.7% for treatment C. However, at trough rivaroxaban concentrations, there was no additive effect of treatment with warfarin followed by rivaroxaban (group A) compared with warfarin followed by placebo (group B). At trough rivaroxaban concentration (prior to dosing on day 1), inhibition of factor Xa was 40.4% in the warfarin followed by rivaroxaban group (A) compared with 34.7% in the warfarin followed by placebo group (B). Treatment with rivaroxaban alone (group C) was associated with a residual 11.3% inhibition of factor Xa activity at 24 h after the first dose (see Figure 3A for median inhibition compared with baseline over time).
Figure 3.
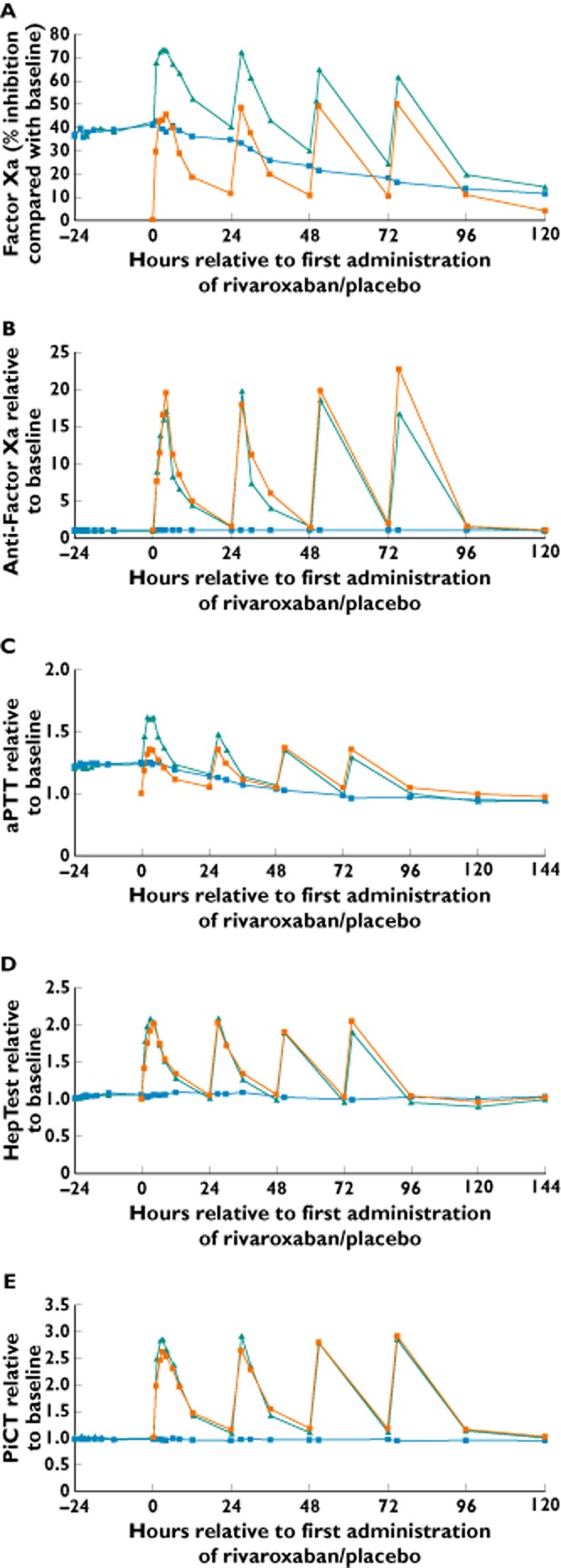
Effects of administration of warfarin [international normalized ratio (INR) 2.0–3.0] followed by rivaroxaban 20 mg once daily (od; treatment A), warfarin (INR 2.0–3.0) followed by placebo od (treatment B) and rivaroxaban alone 20 mg od (treatment C) on inhibition of factor Xa activity (A), anti-factor Xa activity (B), activated partial thromboplastin time (aPTT; C), HepTest (D) and prothrombinase-induced clotting time (PiCT; E; n = 26–28).  , warfarin/rivaroxaban;
, warfarin/rivaroxaban;  , warfarin/placebo;
, warfarin/placebo;  , rivaroxaban alone
, rivaroxaban alone
Anti-factor Xa activity
Treatment with warfarin and warfarin followed by placebo (group B) did not affect anti-factor Xa activity, in contrast to treatment with rivaroxaban (Figure 3B). The geometric mean Emax for anti-factor Xa activity was 15.83-fold of the baseline value for treatment with warfarin followed by rivaroxaban (A), 2.28-fold for warfarin followed by placebo (B) and 18.57-fold for treatment with rivaroxaban alone (C). The residual relative change expressed as the median of ratio to baseline values was 1.48 at 24 h after the first dose of rivaroxaban after warfarin (A), 1.00 in the warfarin followed by placebo group (B) and 1.50 in the rivaroxaban alone group (C; see Figure 3B for median of ratio to baseline over time).
Activated partial thromboplastin time
Treatment with warfarin followed by rivaroxaban (group A) showed a small, additive effect on the aPTT compared with treatment with warfarin followed by placebo (group B) or treatment with rivaroxaban alone (group C; Figure 3C). Relative to the respective baseline levels, the geometric mean Emax for aPTT was prolonged to 1.84-fold of baseline for treatment A, 1.30-fold for treatment B and 1.41-fold for treatment C. However, no relevant residual or additive effect was observed at trough concentrations of rivaroxaban on each of the treatment days, and the prolongations were small and similar between the treatment groups (see Figure 3C for median of ratio to baseline over time).
HepTest
Treatment with warfarin and warfarin followed by placebo (group B) did not affect HepTest (geometric mean Emax: 1.16-fold of baseline value). Treatment with warfarin followed by rivaroxaban (group A) resulted in HepTest prolongation; geometric mean Emax was prolonged up to 2.15-fold of baseline value, which was similar to that observed with the rivaroxaban alone group (C; geometric mean Emax: 2.01-fold of baseline value). There was no relevant additional effect observed after treatment with warfarin followed by rivaroxaban compared with treatment with rivaroxaban alone. At 24 h after rivaroxaban dosing, the values had returned to baseline levels on all days of rivaroxaban administration, and pretreatment with warfarin had no additional influence (see Figure 3D for median of ratio to baseline over time).
Prothrombinase-induced clotting time
Treatment with warfarin and warfarin followed by placebo did not affect PiCT. Treatment with warfarin followed by rivaroxaban (group A) was associated with a prolongation of PiCT up to 3.16-fold of baseline value compared with 1.14-fold of baseline after treatment with warfarin followed by placebo (group B) and 2.72-fold of baseline after treatment with rivaroxaban alone (group C). After 24 h and before the next rivaroxaban dose, PiCT had almost returned to baseline levels (1.08- to 1.14-fold of baseline value), and pretreatment with warfarin had no additional influence on the relative change induced by rivaroxaban (see Figure 3E for median of ratio to baseline over time).
Endogenous thrombin potential
Treatment with warfarin followed by rivaroxaban (group A) showed an additive effect on the reduction of ETP AUC and ETP peak and prolongation of lag time (see Figure 4 for median of ratio to baseline over time). For example, the ETP AUC was reduced to 24% of the baseline level after treatment with warfarin followed by rivaroxaban (A), compared with a reduction to 43% of baseline after warfarin followed by placebo (B) and to 61% of the baseline level after treatment with rivaroxaban alone (C). The ETP peak was reduced to 6, 46 and 17% of their baseline levels in treatment groups A, B and C, respectively. Marked prolongation of ETP time to peak was observed after treatment with warfarin followed by rivaroxaban (up to 3.55-fold of the baseline level) and after treatment with rivaroxaban alone (up to 3.84-fold of baseline); there was no additive effect for treatment with rivaroxaban after warfarin compared with treatment with rivaroxaban alone. Treatment with warfarin followed by placebo showed a small effect on ETP time to peak (up to 1.29-fold of baseline). All parameters, except for the AUC in the rivaroxaban alone group, did not return to pretreatment baseline values 24 h after rivaroxaban dosing (Figure 4).
Figure 4.
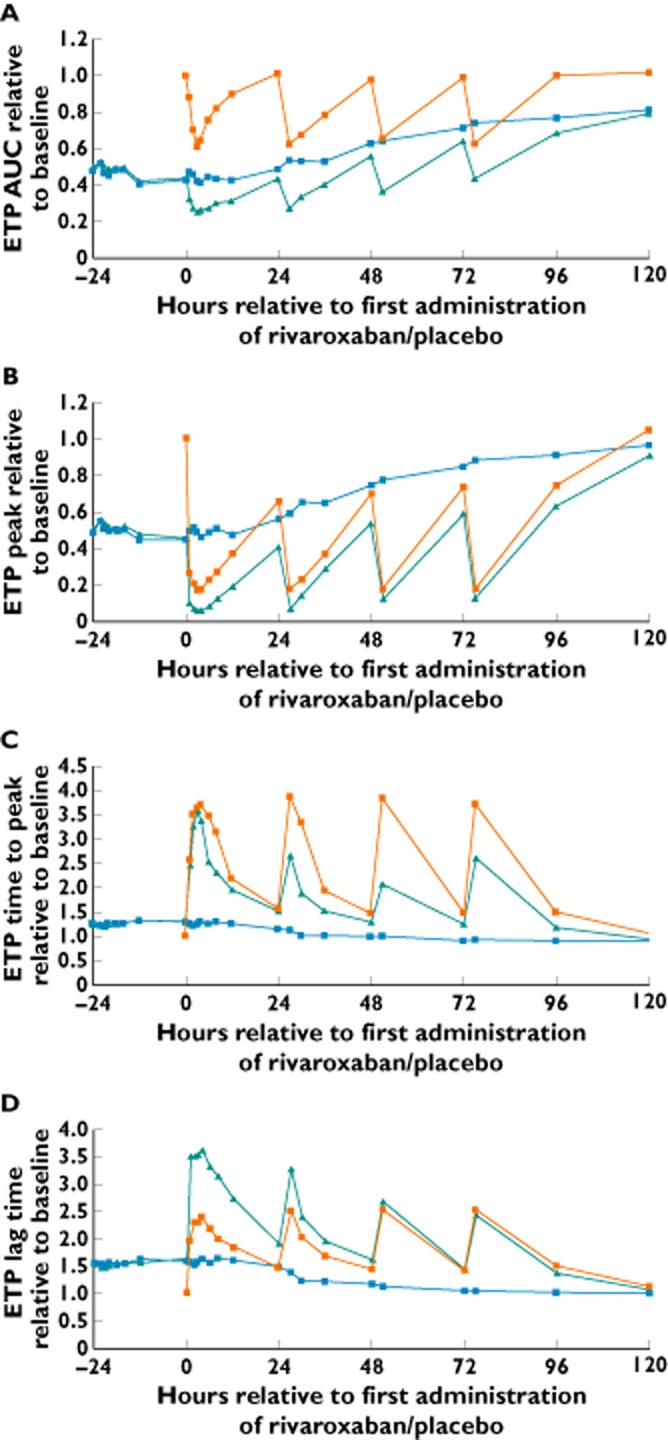
Effects of administration of warfarin [international normalized ratio (INR) 2.0–3.0] followed by rivaroxaban 20 mg once daily (od; treatment A), warfarin (INR 2.0–3.0) followed by placebo od (treatment B) and rivaroxaban alone 20 mg od (treatment C) on endogenous thrombin potential (ETP), as follows: area under the effect–time curve (AUC; A), peak (B), time to peak (C) and lag time (D). Results are relative change expressed as median ratio to baseline (n = 24–28).  , warfarin/rivaroxaban;
, warfarin/rivaroxaban;  , warfarin/placebo;
, warfarin/placebo;  , rivaroxaban alone
, rivaroxaban alone
Factor VIIa
Treatment with warfarin reduced factor VIIa to 0.12-fold of its baseline value, and levels then recovered gradually over the placebo-treatment period after warfarin (group B). Treatment with warfarin followed by rivaroxaban (group A) caused a maximal decrease of factor VIIa to 0.10-fold of its baseline value. Overall, factor VIIa activity recovered from warfarin-induced changes over the course of rivaroxaban treatment after warfarin. Treatment with rivaroxaban alone (group C) caused a small decrease in factor VIIa activity to 0.81-fold of baseline, but the values returned to baseline levels 24 h after rivaroxaban dosing (data not shown). However, because the assay does not measure factor VII activity directly (i.e. indirectly via a clotting time involving all factors downstream of factor VII), the effect observed with rivaroxaban may reflect an effect on the activity of factor X.
Factor IIa activity
Treatment with warfarin reduced factor IIa content to 0.36 of its baseline value; during the placebo phase after warfarin treatment (treatment B), factor IIa recovered gradually and baseline levels were almost reached 5 days after the last warfarin dose. Treatment with warfarin followed by rivaroxaban (group A) had no additional effect on factor IIa activity compared with treatment with warfarin followed by placebo (group B). Rivaroxaban alone (group C) did not affect factor IIa content (data not shown).
Pharmacokinetics
All parameters tested fulfilled the criteria for bioequivalence between treatments, and no pharmacokinetic interaction was observed between warfarin and rivaroxaban. The main pharmacokinetic parameters for rivaroxaban, AUC(0–24) and Cmax, were not affected by warfarin (treatment A) compared with rivaroxaban alone without pretreatment with warfarin (treatment C) after the first rivaroxaban dose on day 0. The main pharmacokinetic parameter for warfarin was the t1/2 for R- and S-warfarin after the last warfarin dose, comparing subsequent rivaroxaban with placebo after pretreatment with warfarin (treatment A vs. treatment B). No difference was observed between these two treatment groups; the 95% CIs constructed for the ratio for these two treatment groups were completely contained in the reference range (0.80–1.25) for bioequivalence (Table 3).
Table 3.
Pharmacokinetic parameters and comparison between treatment groups
| Parameter | Test group | Reference group | Test/reference: LS mean ratio (95% CI) | |
|---|---|---|---|---|
| Rivaroxaban | AUC(0–24) | Warfarin/rivaroxaban (A) | Rivaroxaban alone (C) | 0.95 (0.82–1.10) |
| Cmax | Warfarin/rivaroxaban (A) | Rivaroxaban alone (C) | 0.99 (0.85–1.16) | |
| R-Warfarin | t1/2 | Warfarin/rivaroxaban (A) | Warfarin/placebo (B) | 1.00 (0.88–1.15) |
| S-Warfarin | t1/2 | Warfarin/rivaroxaban (A) | Warfarin/placebo (B) | 0.96 (0.86–1.07) |
Results are geometric means (n = 28). Abbreviations are as follows: AUC(0–24), area under the plasma concentration–time curve from time 0 to 24 h; CI, confidence interval; Cmax, maximal drug concentration in plasma; LS, least-squares; t1/2, terminal half-life.
Safety and tolerability
Rivaroxaban was well tolerated in this study (i.e. in highly selected, healthy subjects). Drug-related adverse events occurred in 8% of the subjects during treatment with warfarin, in 21% of subjects receiving warfarin followed by rivaroxaban (treatment A), in no subjects receiving warfarin followed by placebo (treatment B) and in 11% of subjects after rivaroxaban alone (treatment C). Most adverse events were of mild intensity, a few were moderate and none was severe; all were resolved by the end of the study.
Discussion
Given that the mode of actions and the pharmacodynamics of warfarin and rivaroxaban are different, it is important to understand fully the impact on coagulation parameters of a transition from warfarin to rivaroxaban therapy. Therefore, the primary objective of this study was to investigate the changes in pharmacodynamic parameters during transitioning from warfarin at therapeutic anticoagulation level (INR 2.0–3.0) to rivaroxaban 20 mg od; a regimen that was used in the phase III ROCKET AF study [14] in patients with nonvalvular atrial fibrillation [a reduced dose (15 mg od) was used for patients with moderate renal impairment]. The dose of rivaroxaban selected in this study reflects the most common therapeutic dose in clinical settings when a transition from warfarin to rivaroxaban therapy may be indicated.
Guidelines recommend a therapeutic INR range of 2.0–3.0 for long-term anticoagulant therapy, such as for stroke prevention in patients with nonvalvular atrial fibrillation, and the treatment of deep vein thrombosis and pulmonary embolism (in combination with a parenteral anticoagulant during the initial treatment period) and long-term prevention of recurrent venous thromboembolism [18,19]. This target range is usually maintained with a daily warfarin dose between 2.5 and 10 mg. In the present study, warfarin was given for ∼6 days until the INR range was 2.0–3.0 before transitioning to rivaroxaban. Immediately before the transition from warfarin to rivaroxaban or placebo, median INR values were 2.2 in the warfarin followed by rivaroxaban group (treatment A) and 2.3 in the warfarin followed by placebo group (treatment B).
Given that PT is the most sensitive clotting assay for both rivaroxaban and warfarin, this assay displayed the largest degree of interaction, with supra-additive effects. Individual absolute INR values in the range of 4.19–10.29 were reached at 27 h after the last dose of warfarin and 3 h after the first dose of rivaroxaban. The INR values during the first 3 days after switching displayed time profiles known for rivaroxaban. The effect of rivaroxaban was added to the residual effect of warfarin on the INR profiles. The peak INR values declined over the first 3 days after switching from warfarin to rivaroxaban. The effects of warfarin followed by rivaroxaban were more than additive but not exponential compared with the effects of warfarin followed by placebo and rivaroxaban alone. As expected, the PT assay picked up the effects of both warfarin and rivaroxaban. However, when PT/INR was measured at the trough level of rivaroxaban, it was capable of detecting the warfarin effect with minimal impact from rivaroxaban, suggesting that if therapy transitioning from rivaroxaban to warfarin is required in certain circumstances, INR monitoring of the warfarin effect should be performed at the trough concentration of rivaroxaban during the co-administration period.
Inhibition of factor Xa activity, aPTT and ETP were also enhanced during the transition from warfarin to rivaroxaban in the present study; these assays are expected to be influenced by both agents. Combined effects were additive for inhibition of factor Xa activity, and a small additive effect was observed for ETP and aPTT. However, the rivaroxaban-induced changes recovered to baseline levels within 24 h, whereas recovery of warfarin-induced changes took ∼4 days after the last warfarin dose. In contrast, the effects of rivaroxaban on anti-factor Xa activity, HepTest and PiCT were not affected by pretreatment with warfarin; thus, these tests were capable of detecting the effects of rivaroxaban in the presence of warfarin. Treatment with rivaroxaban caused a small reduction of factor VIIa, but the effect was considered to be a result of the assay used. As expected, rivaroxaban had no relevant additional effect on warfarin-induced factor IIa activity.
In general, it was expected that all pharmacodynamic parameters would display different results depending on the assay set-up. All tests that rely on endogenous factor Xa were affected both by rivaroxaban and warfarin and would, therefore, show a pharmacodynamic interaction. This was the case for inhibition of factor Xa activity, PT (both in seconds and INR), aPTT and ETP. In contrast, assays where activated factor X is added to the sample such as anti-factor Xa, HepTest and PiCT assays, were capable of detecting the effect of rivaroxaban but not warfarin and were, therefore, able to detect the effect of rivaroxaban independent of warfarin during the switching period. Therefore, the effect depended on the types of tests used to measure the magnitude of the pharmacodynamic changes during the switching period from warfarin to rivaroxaban when supra-additive (INR), additive (inhibition of factor Xa, aPTT, ETP) or non-additive (anti-factor Xa activity, Heptest, PiCT) effects were observed. However, the pharmacokinetic parameters of rivaroxaban were not influenced by warfarin, and vice versa.
Studies have indicated that the anti-factor Xa chromogenic method is the best test for assessing rivaroxaban exposure when used in conjunction with rivaroxaban calibrators and controls [20], if a quantitative assessment of rivaroxaban exposure is required in certain clinical situations. It should be emphasized that, although rivaroxaban affects the PT/INR, this system is not suitable for assessing the anticoagulant intensity of rivaroxaban [6,7]. However, the conventional PT/INR is essential for monitoring the residual anticoagulant effect after the cessation of warfarin. As a result of the time frame required for newly produced fully functional coagulation factors, there is a delay in the recovery of coagulation activity after warfarin cessation. Conversely, if a transition from rivaroxaban to a VKA is planned, INR measurement of VKA activity using blood samples taken 24 h after rivaroxaban administration can be used as guidance for dose titration of the VKA, because the effect of rivaroxaban on the PT/INR is minimal at this time point.
Pretreatment with warfarin did not influence the pharmacokinetics of rivaroxaban after transitioning from warfarin (INR 2.0–3.0) to rivaroxaban 20 mg od. Pharmacokinetic parameters on the first day of rivaroxaban treatment after warfarin were similar to those for treatment with rivaroxaban alone. In addition, rivaroxaban did not influence the t1/2 of warfarin during transitioning from warfarin to rivaroxaban. Rivaroxaban was safe and well tolerated; however, this was a mechanistic study in healthy subjects and safety was not the main focus. One limitation of the present study is that rivaroxaban 20 mg od was administered for a period of only 4 days in highly selected, healthy subjects. The focus of the study was to investigate mechanistically the effects of warfarin and rivaroxaban on different coagulation tests during a transition period. The present study is not able to take into account for effects of age, comorbidities and concomitant medications which will most probably modify the individual response in a real-world patient population. Nevertheless, the data describe, in principle, which pharmacodynamic changes might be expected during transition from warfarin (INR 2.0–3.0) to rivaroxaban 20 mg od [21,22].
In summary, the results of this study demonstrate that the pharmacodynamic profiles during the transition from warfarin to rivaroxaban vary and depend on the type of test used. An expected supra-additive effect was observed on the prolongation of PT/INR during the initial transitioning period, with very limited influence of rivaroxaban on the PT/INR at 24 h after dosing. Based on these data, INR monitoring should be stopped once rivaroxaban is initiated during the transition from warfarin to rivaroxaban. Conversely, when switching from rivaroxaban to warfarin, INR monitoring of warfarin therapy should be performed at trough rivaroxaban levels during the co-administration period.
Acknowledgments
This study was funded by Bayer HealthCare AG. The authors would like to acknowledge Yong-Ling Liu, who provided editorial support with funding from Bayer HealthCare Pharmaceuticals and Janssen Scientific Affairs, LLC.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: DK, MB, WM and JK had editorial support from Yong-Ling Liu for the submitted work; DK, MB, WM and JK have been employees of Bayer HealthCare Pharmaceuticals in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines (8th Edition) Chest. 2008;133:160S–198. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and Factor Xa inhibitors in development. Clin Pharmacokinet. 2009;48:1–22. doi: 10.2165/0003088-200948010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Kamal AH, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc. 2007;82:864–873. doi: 10.4065/82.7.864. [DOI] [PubMed] [Google Scholar]
- 4.Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct Factor Xa inhibitor. Clin Pharmacol Ther. 2005;78:412–421. doi: 10.1016/j.clpt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Mueck W, Eriksson BI, Bauer KA, Borris L, Dahl OE, Fisher WD, Gent M, Haas S, Huisman MV, Kakkar AK, Kälebo P, Kwong LM, Misselwitz F, Turpie AGG. Population pharmacokinetics and pharmacodynamics of rivaroxaban – an oral, direct Factor Xa inhibitor – in patients undergoing major orthopaedic surgery. Clin Pharmacokinet. 2008;47:203–216. doi: 10.2165/00003088-200847030-00006. [DOI] [PubMed] [Google Scholar]
- 6.Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct Factor Xa inhibitors: Anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost. 2010;104:1263–1271. doi: 10.1160/TH10-05-0328. [DOI] [PubMed] [Google Scholar]
- 7.Lindhoff-Last E, Samama MM, Ortel TL, Weitz JI, Spiro TE. Assays for measuring rivaroxaban: their suitability and limitations. Ther Drug Monit. 2010;32:673–679. doi: 10.1097/FTD.0b013e3181f2f264. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W. RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 9.Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, Soglian AG, Pap AF, Misselwitz F, Haas S. RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008;372:31–39. doi: 10.1016/S0140-6736(08)60880-6. [DOI] [PubMed] [Google Scholar]
- 10.Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, Misselwitz F, Turpie AGG. RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776–2786. doi: 10.1056/NEJMoa076016. [DOI] [PubMed] [Google Scholar]
- 11.Turpie AGG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, Cushner FD, Lotke PA, Berkowitz SD, Bandel TJ, Benson A, Misselwitz F, Fisher WD. RECORD4 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009;373:1673–1680. doi: 10.1016/S0140-6736(09)60734-0. [DOI] [PubMed] [Google Scholar]
- 12.The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 13.The EINSTEIN–PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 14.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, Califf RM ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 15.Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CM. ATLAS ACS 2-TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]
- 16.Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e44S–e88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohde G. Determination of rivaroxaban – a novel, oral, direct Factor Xa inhibitor – in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;872:43–50. doi: 10.1016/j.jchromb.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th Edition) Chest. 2008;133:454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 19.Singer DE, Albers GW, Dalen JE, Fang MC, Go AS, Halperin JL, Lip GYH, Manning WJ American College of Chest Physicians. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians evidence-based clinical practice guidelines (8th Edition) Chest. 2008;133:546S–592. doi: 10.1378/chest.08-0678. [DOI] [PubMed] [Google Scholar]
- 20.Samama MM, Contant G, Spiro TE, Perzborn E, Guinet C, Gourmelin Y, Le Flem L, Rohde G, Martinoli JL. Evaluation of the anti-Factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost. 2012;107:379–387. doi: 10.1160/TH11-06-0391. [DOI] [PubMed] [Google Scholar]
- 21.Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood. 2012;119:3016–3023. doi: 10.1182/blood-2011-10-378950. [DOI] [PubMed] [Google Scholar]
- 22.van den Besselaar AM, Fogar P, Pengo V, Palareti G, Braham S, Moia M, Tripodi A. Biological variation of INR in stable patients on long-term anticoagulation with warfarin. Thromb Res. 2012;130:535–537. doi: 10.1016/j.thromres.2012.05.028. [DOI] [PubMed] [Google Scholar]


