Abstract
Aims
Tacrolimus (TAC) is one of the most successful immunosuppressive drugs in transplantation. Its pharmacokinetics (PK) and pharmacogenetics (PG) have been extensively studied, with many studies showing the influence of CYP3A5 on TAC metabolism and bioavailability. However, data concerning the functional significance of ABCB1 polymorphisms are uncertain due to inconsistent results. We evaluated the association between ABCB1 diplotypes, CYP3A5 polymorphisms and TAC disposition in a cohort of Brazilian transplant recipients.
Methods
Individuals were genotyped for the CYP3A5*3 allele and ABCB1 polymorphisms (2677G>A/T, 1236C>T, 3435C/T) using a TaqMan® PCR technique. Diplotypes were analyzed for correlation with the TAC dose-normalized ratio (Co : dose).
Results
We genotyped 108 Brazilian kidney recipients for CYP3A5 (11% CYP3A5*1/*1; 31% CYP3A5*1/*3 and 58% CYP3A5*3/*3) and ABCB1 haplotypes (42% CGC/CGC; 41% GCG/TTT and 17% TTT/TTT). Homozygous subjects for the CYP3A5*3 allele or carriers of the ABCB1 TTT/TTT diplotype showed a higher Co : dose ratio compared with wild type subjects [median (interquartile range) 130.2 (97.5–175.4) vs. 71.3 (45.6–109.0), P < 0.0001 and 151.8 (112.1–205.6) vs. 109.6 (58.1–132.9), P = 0.01, respectively]. When stratified for the CYP3A5*3 group, ABCB1 TTT/TTT individuals showed a higher Co : dose ratio compared with non-TTT/TTT individuals [167.8 (130.4–218.0) vs. 119.4 (100.2–166.3), P = 0.04]. Multivariate linear regression analysis showed that the effects of CYP3A5 polymorphisms and ABCB1 diplotypes remained significant after correction for confounding factors.
Conclusions
CYP3A5 is the major enzyme responsible for the marked interindividual variability in TAC PK, but it cannot be considered alone when predicting dose adjustment because ABCB1 diplotypes also affect TAC disposition, showing independent and additive effects on the TAC dose-normalized concentration.
Keywords: ABCB1 diplotype, cytochrome P450, kidney transplantation, pharmacogenetics, tacrolimus pharmacokinetics
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Tacrolimus disposition results largely from the actions of CYP3A5 enzymes and P-glycoprotein (PGP1). Several clinical studies have reported that homozygous carriers of the CYP3A5*3 allele require lower doses of tacrolimus to achieve similar target blood concentrations. In contrast, ABCB1 polymorphism studies have shown controversial results, and few studies have addressed the interaction between ABCB1 diplotype and tacrolimus (TAC) pharmacokinetics.
WHAT THIS STUDY ADDS
This study shows that individuals carrying the homozygote variant diplotype (TTT/TTT) present a higher TAC dose-normalized concentration. We also show that the effects of CYP3A5 polymorphism and ABCB1 diplotype on TAC dose-normalized concentration are independent and additive.
Introduction
The administration of the calcineurin inhibitor tacrolimus (TAC) is an important cornerstone of immunosuppressive therapy after kidney transplantation. Currently, TAC is one of the most successful immunosuppressive drugs in transplantation, but its clinical use is hampered by a narrow therapeutic window and large intra- and interindividual variability in pharmacokinetics (PK), which can result in either under-immunosuppression, leading to increased risk of allograft rejection, or over- immunosuppression and increased incidence of adverse effects, such as nephrotoxicity and neurotoxicity [1]. Consequently, therapeutic drug monitoring is currently regarded as a vital part of clinical practice in kidney transplantation.
TAC is metabolized by the cytochrome P450 (CYP) enzymes 3A4 and 3A5. The intrinsic clearance of TAC by CYP3A5 is approximately two-fold higher than that by CYP3A4, limiting the influence of this enzyme in TAC PK [2]. Tacrolimus is also a substrate of P-glycoprotein (PGP1), the product of the ATP-binding cassette transporter (ABCB1) gene, which acts as an efflux transporter that limits a drug's oral absorption [3–5]. The different degrees of intestinal and hepatic expression of CYP3A5, CYP3A4 and PGP1 regulate the absorption and hepatic clearance of TAC, leading to variable drug concentration within the systemic circulation and low oral bioavailability, both of which can influence drug efficacy or toxicity [5–7].
One of the causes that may lead to the differences in the functions of these proteins is the presence of genetic polymorphisms (SNPs) that may have functional effects on gene expression or protein structure. Several studies have shown an association between SNPs in genes encoding enzymes or transporters and differences in dosing, toxicity and drug interactions [8,9].
CYP3A5*3 is one of the most important SNPs for TAC disposition. Homozygous carriers of the CYP3A5*3 variant allele have higher TAC concentrations due to lower expression of CYP3A5 [8,10]. Individuals carrying the CYP3A5*1 allele, known as ‘CYP expressers’, exhibit 25–40% increased TAC clearance and two–three-fold lower dose-corrected trough TAC concentrations, suggesting the value of CYP3A5 genetic information in initial drug dosing [11].
The functional consequences of the most common and extensively studied ABCB1 polymorphisms (3435C>T, 1236C>T and 2677G>T/A) on TAC disposition are not completely understood and are still controversial [8,9]. The majority of the studies have failed to find an association between ABCB1 SNPs and TAC PK. Those that found an association suggested that the observed effect could, in fact, be related to CYP3A5 polymorphism [9,12–14]. The ABCB1 3435T variant allele occurs more frequently in conjunction with the CYP3A5*3 allele and also with the ABCB1 1236C>T and 2677G>T/A SNPs [15–17].
Individual SNPs may cause changes in gene expression or in protein function, either of which can have a physiological effect on the organism. However, SNPs are not inherited individually but in linkage disequilibrium, in which certain alleles of several contiguous polymorphisms are found together. The combined influence of SNPs may have stronger physiological effects than any single SNP alone [8,9]. Indeed, the ABCB1 1236T/2677T/3435T (TTT) variant haplotype significantly minimizes PGP1 activity (0–28% activity) compared with ABCB1 wild-type activity in transepithelial cells and is relatively frequent in several populations: approximately 32% of Caucasians, 5% of African-Americans, 27% of Asian-Americans and 35% of Mexican-Americans [17,18].
Similar to studies on ABCB1 genotype, most clinical studies have not detected any association between ABCB1 haplotypes and TAC PK [13,19,20], but others have indicated that the presence of three or more variant ABCB1 alleles is associated with lower dose-adjusted blood concentrations of TAC [14,16,21,22]. However, to the best of our knowledge, few studies have analyzed the effects of ABCB1 diplotypes (i.e. pairs of haplotypes) on TAC disposition and their interaction with the CYP3A5*3 genotype [21,23].
In this context, the aim of this study was to investigate the impact of ABCB1 diplotypes and CYP3A5 polymorphism on TAC dose-normalized concentration (Co : dose).
Methods
Subjects
This retrospective study was conducted on kidney transplant recipients at the Clinical Hospital of the Faculty of Medicine of Ribeirão Preto, Brazil. For the purpose of this study, 108 kidney recipients of any age and gender were included. Eligible patients were those with single kidney transplantation and administration of a TAC-based immunosuppressive regimen for at least 12 months [The study involves a period from 3 up to 12 months after transplant (follow-up period 9 months, 95% CI 8.9, 9.0)]. Patients receiving chronic therapy with drugs that could potentially interfere with the transport and metabolism of immunosuppressive drugs (e.g. macrolides, rifampicin, phenytoin, carbamazepine), those who underwent combined organ transplantations, those who had any known drug or alcohol addiction and those who started TAC immunosuppressive therapy more than 2 months after transplantation were excluded. The study was approved by the Institutional Review Board at the Clinical Hospital of the Faculty of Medicine of Ribeirão Preto (no. 13464/2010), and each included patient provided informed consent.
The initial TAC (Prograf®, Janssen-Cilag, Brasil) dosage was 0.05–0.2 mg kg−1 day−1 for all patients, and it was subsequently adjusted according to blood concentration or any toxicity sign. Target concentrations were 8–10 ng ml−1 during the first 3 months post-transplantation and were then adjusted to 3–7 ng ml−1 until the end of the first year. Tacrolimus was given twice a day in combination with sodium mycophenolate (720–1440 mg day−1) or mofetil (1–2 g day−1) and a tapering schedule of steroids (1000 mg i.v. methylprednisolone before the surgery and 0.5 mg kg−1 orally, which was progressively tapered to 5 mg once a day at 6 months after transplantation).
Data collection
TAC trough concentrations (Co, ng ml−1) were routinely obtained at steady-state from TAC whole blood concentrations measured immediately before the morning doses of TAC using an Abbott ARCHITECT TAC immunoassay (Abbott Diagnostics, IL, USA). Daily doses (mg kg−1) were retrieved from medical records from the third month up to 1 year post-transplantation. Dose-normalized TAC concentrations (Co : dose, ng ml−1 per mg day−1 per kg body weight) were calculated by dividing the drug trough concentration (Co, ng ml−1) by the daily dose adjusted for body weight (mg day−1 kg−1). Once the patients had more than one measurement in the study period, the median value was considered to represent Co : dose (median 5.0, range 4.0–8.0, number of blood samples collected/patient).
Genotype determination
Blood samples were drawn from each subject and immediately stored at −80°C until genotype analysis. Genomic DNA was isolated from peripheral blood leukocytes using a salting-out procedure [24]. Subjects were genotyped for the CYP3A5 SNP (rs776746) and the three major ABCB1 SNPs, 1236C>T (rs1128503), 2677 G > T/A (rs2032582) and 3435C>T (rs1045642). Genotypes were determined using a pre-designed TaqMan® Allele Discrimination assay (Applied Biosystems, Foster City, CA, USA): C_26201809_30 (CYP3A5 6986A>G), C_7586657_20 (ABCB1, 3435C>T), C_7586662_10 (ABCB1, 1236C>T), C_11711720D_40 (ABCB1, 2677G>A), and C_11711720C_30 (2677G>T).
TaqMan® polymerase chain reaction (PCR) amplification was performed in a total volume of 10 μl as follows: 30 ng of template DNA, 1× TaqMan® master mix and 1× drug metabolism assay. Fluorescence from PCR amplification was detected using a Roche LightCycler®480 and analyzed with the associated software version 1.5 (Roche Diagnostics GmbH, Mannheim, Germany). The PCR assay was carried out following the manufacturer's instructions.
Statistical analysis
The Power and Sample Calculation program (PS version 2.1.30, Vanderbilt University, USA) was used to estimate the number of individuals for this study. Santoro et al. reported a Co : dose value for the ABCB1 wild-type diplotype (CGC/CGC) of 1.92 ± 0.94 ng ml−1 mg−1. Considering an increase of 50% in Co : dose, a TTT variant haplotype frequency of 26%, a power of 0.80 and a type 1 error rate of 0.05, we calculated a sample size of 90 transplant recipients [23].
Clinical characteristics and pharmacokinetic data are expressed as median and interquartile range. Genotype groups were compared using non-parametric tests. The distribution of genotypes for each polymorphism was assessed for deviation from Hardy–Weinberg equilibrium, and differences in genotype frequency and in allele frequency between the groups were assessed using the χ2 test.
Linear regression analysis and non-linear fitting routines were performed to assess univariate relationships between variables (software JMP 5.0.1a; SAS Institute). In addition, a bivariate analysis was used to assess the potential confounding influence of each covariate on the association between TAC concentration, CYP3A5 polymorphism, and ABCB1 polymorphism. The final multivariate linear regression model considered TAC blood concentration as the dependent variable, whereas gender, age, corticoid dosage, haematocrit and CYP3A5 polymorphisms and ABCB1 diplotypes were used as independent variables. A value of P < 0.05 was considered statistically significant. Other statistical analyses were performed using GraphPad Prism version 5.1 for Windows (GraphPad Software, San Diego, CA, USA, [http://www.graphpad.com]). PHASE software version 2.1 was used to estimate the haplotype frequencies in each group.
Results
A total of 108 renal transplant recipients (48 women) with a median age of 52 (41–58) years at the time of transplantation were recruited between March and November 2011 and were included in this study. Demographic and clinical characteristics are listed in Table 1.
Table 1.
Clinical and demographic characteristics of renal transplant recipients up to 12 months post-tranplantation (n = 108)
| Parameters | Median (interquatile range) or number (%) |
|---|---|
| Co (ng ml−1) | 6.8 (5.3–10.0) |
| Dose (mg kg−1 day−1) | 0.07 (0.05–0.10) |
| Body weight (kg) | 64.8 (58.5–77.6) |
| Co : dose (ng ml−1 per mg day−1 per kg body weight) | 109.3 (70.3–145.9) |
| Serum creatinine (μmol l−1) | 124.2 (95.5–143.2) |
| Creatinine clearance* (ml min−1 1.73 m−2) | 55.7 (46.8−70.5) |
| Gender (male/female) | 60/48 |
| Receptor's age (years) | 52 (41–58) |
| Donor type (living/deceased) | 18/90 |
| Number of transplant (first/second1) | 95/13 |
| Induction therapy (n, %) | |
| Basiliximab usage | 65 (60.2) |
| ATG usage | 06 (5.6) |
| Duration of the dialysis before Tx (months) | 39 (24–70) |
| CMV infection (n, %) | 24 (22.2) |
| Cold ischaemia (h) | 25 (20–31) |
Creatinine clearance was estimated using MDRD equation. Data are expressed as median and interquartile ranges, or absolute numbers and percentiles. ATG, antithymocyte globulin; CMV, cytomegalovirus; Co, Tacrolimus trough concentration; Tx, transplant.
Allele, genotype and haplotype frequencies in transplanted subjects are shown in Tables 2 and 3. Allele frequencies did not deviate from Hardy–Weinberg equilibrium. The wild-type haplotype 1236C/2677G/3435C was the most common (49%), followed by the variant (TTT) haplotype (30%) (Table 3). Diplotype analysis showed 30 individuals carrying the wild-type CGC/CGC, 30 heterozygous CGC/TTT or TTT/CGC and 12 TTT/TTT. We observed that individuals carrying at least one wild-type haplotype, CGC/CGC or CGC/TTT, were responsible for the wild-type effect and thus were combined in a single group called non-TTT/TTT (n = 60), whereas individuals homozygous for the variant haplotype formed another group called TTT/TTT (n = 12).
Table 2.
Allelic frequency of 108 renal Tx recipients according to CYP3A5 and ABCB1 genotype
| Gene | SNP | Genotype frequency | Allelic frequency | HWE* |
|---|---|---|---|---|
| CYP3A5 | 6986A>G | AA = 0.11 | A = 0.26 | Yes |
| AG = 0.31 | G = 0.74 | |||
| GG = 0.58 | ||||
| ABCB1 | 1236C>T | CC = 0.38 | C = 0.60 | Yes |
| CT = 0.47 | T = 0.40 | |||
| TT = 0.15 | ||||
| 3435C>T | CC = 0.38 | C = 0.60 | Yes | |
| CT = 0.45 | T = 0.40 | |||
| TT = 0.17 | ||||
| 2677G>A/T | GG = 0.44 | G = 0.65 | Yes | |
| GT = 0.38 | T = 0.33 | |||
| GA = 0.03 | A = 0.02 | |||
| AT = 0.02 | ||||
| TT = 0.13 | ||||
| AA = 0.0 |
Hardy–Weinberg equilibrium (HWE): Chi-squaredb test P value <0.05.
Table 3.
Haplotype frequency of 108 renal transplant recipients according to ABCB1 SNPs
| Haplotype | SNP | Haplotype frequency | ||
|---|---|---|---|---|
| 1236C>T | 2677G/T>A | 3435C>T | ||
| CGC | C | G | C | 0.49 |
| TTT | T | T | T | 0.30 |
| CGT | C | G | T | 0.08 |
| TGC | T | G | C | 0.06 |
| TTC | T | T | C | 0.02 |
| CAC | C | A | C | 0.02 |
| TGT | T | G | T | 0.01 |
| CAT | C | A | T | 0.01 |
| CTT | C | T | T | 0.01 |
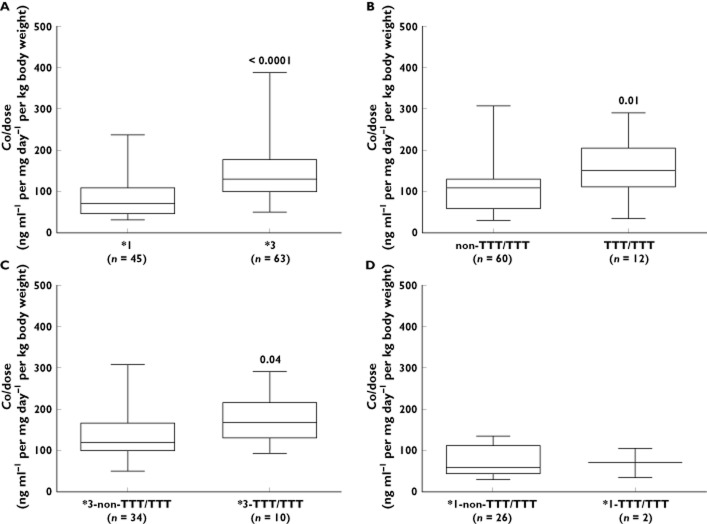
The presence of the CYP3A5*1 allele was strongly associated with lower dose-normalised concentration (Co : dose) up to 12 months post-transplant. Furthermore, we observed that CYP3A5*1 carriers required a higher TAC dose [0.10 mg kg−1 day−1 (0.07–0.12) vs. 0.06 mg kg−1 day−1 (0.04–0.08), P < 0.0001] but showed reduced blood TAC (Co) (Table 4, Figure 1A).
Table 4.
PK parameters of renal Tx recipients according to their CYP3A5 and ABCB1 genotypes up to 12 months post-transplant
| PK parameters | CYP3A5 *1 (n = 45) | CYP3A5*3 (n = 63) | P value |
|---|---|---|---|
| Co (ng ml−1) | 6.0 (4.7–8.2) | 7.3 (6.2–10.8) | 0.01* |
| Dose (mg kg−1 day−1) | 0.10 (0.07–0.12) | 0.06 (0.04–0.08) | <0.0001* |
| Co : dose† | 71.3 (45.6–109.0) | 130.2 (97.5–175.4) | <0.0001* |
| Non-TTT/TTT (n = 60) | TTT/TTT (n = 12) | P value | |
|---|---|---|---|
| Co (ng ml−1) | 6.7 (5.2–8.2) | 8.21 (6.3–11.2) | 0.08 |
| Dose (mg kg−1 day−1) | 0.07 (0.05–0.1) | 0.05 (0.04–0.08) | 0.16 |
| Co : dose† | 109.6 (58.1–132.9) | 151.8 (112.1–205.6) | 0.01* |
| (*3-non-TTT/TTT) (n = 34) | (*3 TTT/TTT) (n = 10) | P value | |
|---|---|---|---|
| Co (ng ml−1) | 7.20 (6.1–10.8) | 9.7 (6.3–11.3) | 0.37 |
| Dose (mg kg−1 day−1) | 0.06 (0.04–0.07) | 0.05 (0.04–0.06) | 0.29 |
| Co : dose† | 119.4 (100.2–166.3) | 167.8 (130.4–218.0) | 0.04* |
Statistically significant.
ng ml−1 per mg day−1 per kg body weight. Co, tacrolimus trough concentration; PK, pharmacokinetics; Tx, transplant.
Figure 1.
Tacrolimus dose-normalized concentration (Co : dose) up to 12 months post-transplantation of 108 renal transplant recipients according to their genotype. (A) Patients carrying CYP3A5*1 (n = 45) showed lower Co : dose when compared with patients homozygous CYP3A5*3 (n = 63), P < 0.0001. (B) Patients with non-TTT/TTT diplotype (n = 60) also showed lower Co :dose when compared with patients TTT/TTT (n = 12), P = 0.01. (C) Analysis considering CYP3A5 group stratified according to the ABCB1 genotype, patients carrying the *3-non-TTT/TTT diplotype (n = 34) showed lower Co : dose when compared with patients carrying *3-TTT/TTT diplotype (n = 10), P = 0.04. (D) Analysis considering CYP3A5 group stratified according to the ABCB1 genotype, patients carrying the *1-non-TTT/TTT diplotype (n = 26) showed higher Co : dose when compared with patients carrying *1-TTT/TTT diplotype (n = 2). The data, however, could neither be analyzed nor taken into consideration, due to the insufficient number of individuals. All data are expressed as median and percentiles
Diplotype analysis showed that patients carrying the homozygous diplotype variant (TTT/TTT) had a higher TAC dose-normalized concentration [151.8 ng ml−1 per mg day−1 per kg body weight (112.1–205.6) vs. 109.6 ng ml−1 per mg day−1 per kg body weight (58.1–132.9), P = 0.01) compared with individuals carrying the wild-type diplotype (non-TTT/TTT) (Table 4). On the other hand, no association was found between ABCB1 diplotype and either TAC dose requirement or TAC blood concentration.
When we restricted our analysis to CYP3A5 non-expressers (CYP3A5*3/*3) we found a higher Co : dose ratio in those patients carrying the ABCB1 variant diplotype (*3-TTT/TTT, n = 10) compared with *3-non-TTT/TTT (n = 34) [167.8 ng ml−1 per mg day−1 per kg body weight (130.4–218.0) vs. 119.4 ng ml−1 per mg day−1 per kg body weight (100.2–166.3), P = 0.04] (Table 4, Figure 1C).
Multivariate analysis showed that the effects of CYP3A5 polymorphisms and ABCB1 diplotypes remained significant even after adjusting the model for gender, age, haematocrit and corticoid dose (Table 5) and the effect was independent for each gene.
Table 5.
Effects of CYP3A5 genotypes and ABCB1diplotypes on tacrolimus dose-normalized concentration
| Source | Estimate | P value | |
|---|---|---|---|
| ABCB1 diplotypes (non-TTT/TTT) | −16.13 | 0.047* | |
| CYP3A5 (*1) | −30.73 | <0.001* | |
| Gender (male) | −4.97 | 0.413 | |
| Age (years) | 0.91 | 0.111 | |
| Corticoid dose (mg kg−1 day−1) | −149.71 | 0.348 | |
| Haematocrit (%) | −0.21 | 0.894 | |
| Model | |||
| R2 | 0.42 | ||
| RMSE | 48.66 | ||
Statistically significant. Were included in this model only carriers of *1 and *3 alleles for CYP3A5 and non-TTT/TTT and TTT/TTT diplotypes for ABCB1 (n = 72). R2, proportion of the variance around the mean of tacrolimus concentrations that is explained by the present model; RMSE, root mean square error. The final multivariate linear regression model was adjusted for gender, age, corticoid dose and haematocrit.
Discussion
Intestinal CYP3A and PGP1 are co-expressed and may act synergistically as a barrier to oral TAC absorption. Therefore, polymorphisms in these genes may play an important role in TAC bioavailability. SNPs in the CYP3A5 gene explain part of the between patient variability in the PK of TAC. However, the effects of ABCB1 gene polymorphisms are still unclear, as several contradictory results have been published [8]. Nonetheless, few studies have addressed the effect of the ABCB1 diplotype on TAC disposition and its interaction with the CYP3A5 genotype, and they have reported inconsistent results. Studies that performed haplotype analysis did not include all three ABCB1 SNPs in the same analysis, and others analyzed haplotypes rather than diplotypes [25–29].
The frequencies of SNPs and haplotypes in the CYP3A5 and ABCB1 genes, respectively, vary largely, so it is not uncommon to find some peculiarities in different populations. The Brazilian population is one of the most heterogeneous in the world, being a mixture of different ethnic groups, especially Europeans, Africans and Amerindians, which contributes to the formation of a multi-ethnic and highly admixed population [30,31]. As in other studies, we found the CYP3A5 to be an unusual gene in that the frequency of the variant allele (G) was higher (74%) than the wild-type (A) frequency. For the ABCB1 gene, the alleles were evenly distributed between the groups, with the wild-type allele having a slight higher frequency in European Caucasians and Black Africans. These results are consistent with frequencies recently reported in Brazilian and other populations and support the fact that the Brazilian population is special in terms of the distribution of genetic polymorphisms [23,32–35].
The frequency of CYP3A5 non-expressers and ABCB1 TTT/TTT is higher in White Brazilians than the frequency observed in European Caucasians, reflecting the admixture of Brazilian population. MacPhee et al. reported a lower Co : dose of TAC in Black kidney-transplanted patients, reflecting higher frequencies of CYP3A non-expressers in this ethnicity. Moreover, White patients carrying CYP3A5 non-expressers alleles showed TAC Co : dose similar to Black patients. Thus, they concluded that the main factor affecting TAC PK is related to White genetic background instead of ethnicity. These data support the clinical relevance of genotyping to CYP3A5 and PGP1 polymorphisms in populations with high frequency of non-expressers, like Brazilians, to avoid under-immunosuppression and risk of acute rejection of kidney transplant [36].
The presence of only one wild-type allele (*1) is enough to express CYP3A5 proteins, so individuals with at least one CYP3A5*1 allele have higher expression of these enzymes and therefore higher TAC metabolism and lower blood TAC concentration [23,27,37,38]. We found that participants with the CYP3A5*1 allele had significantly lower tacrolimus dose-normalized (Co : dose) post-transplantation compared with CYP3A5*3 homozygotes. Moreover, CYP3A5*1 carriers required a higher dose to reach the target concentration. Overall, the mean Co : dose ratio in CYP3A5 non-expressers (CYP3A5*3) was nearly two-fold higher even though their dose requirements were approximately half of those expressing this protein. These findings are consistent with previous studies and indicate lower metabolic capacity in patients with the variant allele [29,39,40]. Thervet et al., in a prospective clinical trial involving 280 renal transplant recipients, demonstrated that pretransplant TAC dose adaptation according to CYP3A5 genotype resulted in more rapid achievement of target Co and fewer dose modifications [11]. These findings suggest that the CYP3A5 genotype is an important factor in determining the dose requirement for TAC and that genotyping patients for this polymorphism may be useful in early prediction of the optimal dose.
The majority of studies reviewed by Staatz et al. found no evidence that ABCB1 polymorphisms play a significant role in TAC PK but suggested that the combined influence of all three ABCB1 polymorphisms could be more effective in predicting any influence than studying single SNPs alone [8]. Consistent with these findings, we found no association between TAC PK and ABCB1 polymorphisms individually (data not shown).
Patients carrying the wild-type ABCB1 haplotype (CGC) might have more active PGP1 pumps and would, therefore, extrude intracellular TAC more efficiently than individuals with the variant haplotype (TTT) [8]. Anglicheau et al. reported that TAC blood concentrations in renal transplant recipients carrying the variant TTT haplotype were significantly higher than those carrying the wild-type CGC [16]. In another study involving renal recipients, Roy et al. showed that patients wild-type for the ABCB1 haplotype had lower Co : dose compared with patients carrying the variant copy of this gene. However, none of these assays considered the individual's pair of haplotypes, which could result in even stronger differences [21]. Based on the three most clinically relevant exonic sites for the ABCB1 gene, we detected nine haplotypes. Two of them, the wild-type 1236C/2677G/3435C and the variant 1236T/2677T/3435T, accounted for 79% of the overall genetic variability, which is in agreement with reports of other groups in the Brazilian population [23,32]. In our comparison of TAC PK parameters, we found that patients carrying the variant ABCB1 diplotype (TTT/TTT) showed a higher Co : dose ratio than patients carrying the wild-type diplotype, which could support the hypothesis of increased absorption and reduced drug excretion due to lower functional activity of PGP1.
In the only other study that has analyzed ABCB1 diplotypes in the Brazilian population, Santoro et al., similar to our study, found a 71% increase in Co : dose in the TTT/TTT diplotype vs. non-TTT/TTT, but this difference was not statistically significant [23]. This could partially be because in their study, Co : dose was not normalized to dose per day or body weight, which could have resulted in higher sample variability. It is important to note that in our study, TAC blood target concentration were between 3 and 7 ng ml−1, and the observed trough concentration (Co) median was 6.8 (5.3–10.0) ng ml−1. A 71% increase in Co : dose, as we observed, is clinically relevant because it directly affects trough doses and could expose patients to TAC nephrotoxicity.
Authors who observed an association between ABCB1 genotype and/or haplotype and TAC PK suggested that the observed effect on TAC PK might, in fact, be the effect of CYP3A SNPs, as these have a major influence over TAC PK. Indeed, many studies have observed that the impact of ABCB1 SNPs is lost after eliminating the confounder CYP3A5 genotype [12–14,27]. To assess this hypothesis, we stratified CYP3A5 non-expressers (CYP3A5*3/*3 carriers), who lack CYP3A5 activity, into two groups according to their ABCB1 diplotype and observed that in the absence of CYP3A5, patients carrying the variant TTT/TTT diplotype (and thus having lower PGP1 activity) had a higher dose-normalized TAC concentration. This result shows that ABCB1 diplotype also plays a role in TAC PK and supports the hypothesis that the combination of multiple ABCB1 polymorphisms has a stronger effect on CNI disposition than any single polymorphism.
We also performed a multivariate analysis considering CYP3A5 polymorphism as a confounding variable and observed the maintenance of the ABCB1 genotype effect. Roy et al. had already considered this effect, studying 44 renal transplant recipients and analyzing the ABCB1 haplotype frequency in the CYP non-expressers group, but they did not detect a statistically significant effect and therefore did not include a diplotype analysis [21]. These results suggest that PGP1 expression could compensate for the lack of CYP3A5 expression and play a role in individuals expressing CYP3A5 enzymes. Thus, it is likely that these polymorphisms in the ABCB1 gene have an independent effect on TAC bioavailability.
In addition to genetic factors, clinical variables have been associated with TAC PK [28,41,42]. Corticoid drugs and TAC share the same metabolic pathway, so they could exhibit a drug–drug interaction in which corticoid induces CYP expression and consequently increasing TAC metabolism [43,44]. Several authors suggest that age affects metabolism in different ways, such as altering hepatic flow, CYP activity and liver size. Others showed that TAC binds to red blood cells and suggested that individuals showing high haematocrit could bind TAC more efficiently, reducing its metabolism [45]. To address these associations, we performed a multivariate regression analysis considering age, gender, corticoid dose and haematocrit as co-variables and observed that the effect of genetic variables on TAC PK remained even after considering them. These data are consistent with data from Cho et al. but do not support the findings in other populations, although none of these studies performed a diplotype analysis [28,39,41,42].
In conclusion, this study demonstrates that the CYP3A5*3 allele is highly associated with higher TAC bioavailability in renal transplant patients, confirming previous findings and supporting the idea that CYP3A5 is the major enzyme responsible for the marked interindividual variability in TAC PK. In addition, this study confirms that the combination of multiple ABCB1 polymorphisms has a stronger effect on CNI disposition. Finally, this study shows that the effects of CYP3A5 and ABCB1 on TAC bioavailability are independent and additive. The inclusion of CYP3A5 and ABCB1 genetic factors in an algorithm which takes into account clinical factors that may modify TAC PK could make it possible to calculate more precisely the initial TAC dosage in order to prevent drug toxicity and improve therapy towards individualized therapy.
Acknowledgments
We thank Mariana A. T de Oliveira and Renata H. C. Pocente for assisting with the PCR analysis, Juliana T. Abumansur for assisting with DNA sample preparation and Neuza L. de Almeida for help in including the patients.
We also would like to thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the financial support.
Competing Interests
All authors have completed the Unified Competing Interest form and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Laskow DA, Vincenti F, Neylan JF, Mendez R, Matas AJ. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation. 1996;62:900–905. doi: 10.1097/00007890-199610150-00005. [DOI] [PubMed] [Google Scholar]
- 2.Dai Y, Hebert MF, Isoherranen N, Davis CL, Marsh C, Shen DD, Thummel KE. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab Dispos. 2006;34:836–847. doi: 10.1124/dmd.105.008680. [DOI] [PubMed] [Google Scholar]
- 3.Kronbach T, Fischer V, Meyer UA. Cyclosporine metabolism in human liver: identification of a cytochrome P-450III gene family as the major cyclosporine-metabolizing enzyme explains interactions of cyclosporine with other drugs. Clin Pharmacol Ther. 1988;43:630–635. doi: 10.1038/clpt.1988.87. [DOI] [PubMed] [Google Scholar]
- 4.Sattler M, Guengerich FP, Yun CH, Christians U, Sewing KF. Cytochrome-P-450 3a enzymes are responsible for biotransformation of fk506 and rapamycin in man and rat. Drug Metab Dispos. 1992;20:753–761. [PubMed] [Google Scholar]
- 5.Saeki T, Ueda K, Tanigawara Y, Hori R, Komano T. Human P-glycoprotein transports cyclosporin A and FK506. J Biol Chem. 1993;268:6077–6080. [PubMed] [Google Scholar]
- 6.Zhang YC, Benet LZ. The gut as a barrier to drug absorption – combined role of cytochrome P450 3A and P-glycoprotein. Clin Pharmacokinet. 2001;40:159–168. doi: 10.2165/00003088-200140030-00002. [DOI] [PubMed] [Google Scholar]
- 7.Thervet E, Anglicheau D, Legendre C, Beaune P. Role of pharmacogenetics of immunosuppressive drugs in organ transplantation. Ther Drug Monit. 2008;30:143–150. doi: 10.1097/FTD.0b013e31816babef. [DOI] [PubMed] [Google Scholar]
- 8.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. Clin Pharmacokinet. 2010;49:141–175. doi: 10.2165/11317350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Knops N, Levtchenko E, van den Heuvel B, Kuypers D. From gut to kidney: transporting and metabolizing calcineurin-inhibitors in solid organ transplantation. Int J Pharm. 2013;452:14–35. doi: 10.1016/j.ijpharm.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 10.de Jonge H, Kuypers DR. Pharmacogenetics in solid organ transplantation: current status and future directions. Transplant Rev (Orlando) 2008;22:6–20. doi: 10.1016/j.trre.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Thervet E, Loriot MA, Barbier S, Buchler M, Ficheux M, Choukroun G, Toupance O, Touchard G, Alberti C, Le Pogamp P, Moulin B, Le Meur Y, Heng AE, Subra JF, Beaune P, Legendre C. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin Pharmacol Ther. 2010;87:721–726. doi: 10.1038/clpt.2010.17. [DOI] [PubMed] [Google Scholar]
- 12.Cheung CY, Op den Buijsch RA, Wong KM, Chan HW, Chau KF, Li CS, Leung KT, Kwan TH, de Vrie JE, Wijnen PA, van Dieijen-Visser MP, Bekers O. Influence of different allelic variants of the CYP3A and ABCB1 genes on the tacrolimus pharmacokinetic profile of Chinese renal transplant recipients. Pharmacogenomics. 2006;7:563–574. doi: 10.2217/14622416.7.4.563. [DOI] [PubMed] [Google Scholar]
- 13.Fredericks S, Moreton M, Reboux S, Carter ND, Goldberg L, Holt DW, MacPhee IA. Multidrug resistance gene-1 (MDR-1) haplotypes have a minor influence on tacrolimus dose requirements. Transplantation. 2006;82:705–708. doi: 10.1097/01.tp.0000234942.78716.c0. [DOI] [PubMed] [Google Scholar]
- 14.Loh PT, Lou HX, Zhao Y, Chin YM, Vathsala A. Significant impact of gene polymorphisms on tacrolimus but not cyclosporine dosing in Asian renal transplant recipients. Transplant Proc. 2008;40:1690–1695. doi: 10.1016/j.transproceed.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, Taylor A, Xie HG, McKinsey J, Zhou S, Lan LB, Schuetz JD, Schuetz EG, Wilkinson GR. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189–199. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- 16.Anglicheau D, Verstuyft C, Laurent-Puig P, Becquemont L, Schlageter MH, Cassinat B, Beaune P, Legendre C, Thervet E. Association of the multidrug resistance-1 gene single-nucleotide polymorphisms with the tacrolimus dose requirements in renal transplant recipients. J Am Soc Nephrol. 2003;14:1889–1896. doi: 10.1097/01.asn.0000073901.94759.36. [DOI] [PubMed] [Google Scholar]
- 17.Kroetz DL, Pauli-Magnus C, Hodges LM, Huang CC, Kawamoto M, Johns SJ, Stryke D, Ferrin TE, DeYoung J, Taylor T, Carlson EJ, Herskowitz I, Giacomini KM, Clark AG, Pharmacogenetics Membrane T. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics. 2003;13:481–494. doi: 10.1097/00008571-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Salama NN, Yang Z, Bui T, Ho RJY. MDR1 haplotypes significantly minimize intracellular uptake and transcellular P-gp substrate transport in recombinant LLC-PK1 cells. J Pharm Sci. 2006;95:2293–2308. doi: 10.1002/jps.20717. [DOI] [PubMed] [Google Scholar]
- 19.Haufroid V, Mourad M, Van Kerckhove V, Wawrzyniak J, De Meyer M, Eddour DC, Malaise J, Lison D, Squifflet JP, Wallemacq P. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics. 2004;14:147–154. doi: 10.1097/00008571-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Tsuchiya N, Satoh S, Tada H, Li Z, Ohyama C, Sato K, Suzuki T, Habuchi T, Kato T. Influence of CYP3A5 and MDR1 (ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplantation. 2004;78:1182–1187. doi: 10.1097/01.tp.0000137789.58694.b4. [DOI] [PubMed] [Google Scholar]
- 21.Roy JN, Barama A, Poirier C, Vinet B, Roger M. Cyp3A4, Cyp3A5, and MDR-1 genetic influences on tacrolimus pharmacokinetics in renal transplant recipients. Pharmacogenet Genomics. 2006;16:659–665. doi: 10.1097/01.fpc.0000220571.20961.dd. [DOI] [PubMed] [Google Scholar]
- 22.Chen JS, Li LS, Cheng DR, Ji SM, Sun QQ, Cheng Z, Wen JQ, Sha GZ, Liu ZH. Effect of CYP3A5 genotype on renal allograft recipients treated with tacrolimus. Transplant Proc. 2009;41:1557–1561. doi: 10.1016/j.transproceed.2009.01.097. [DOI] [PubMed] [Google Scholar]
- 23.Santoro A, Felipe CR, Tedesco-Silva H, Medina-Pestana JO, Struchiner CJ, Ojopi EB, Suarez-Kurtz G. Pharmacogenetics of calcineurin inhibitors in Brazilian renal transplant patients. Pharmacogenomics. 2011;12:1293–1303. doi: 10.2217/pgs.11.70. [DOI] [PubMed] [Google Scholar]
- 24.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Provenzani A, Notarbartolo M, Labbozzetta M, Poma P, Vizzini G, Salis P, Caccamo C, Bertani T, Palazzo U, Polidori P, Gridelli B, D'Alessandro N. Influence of CYP3A5 and ABCB1 gene polymorphisms and other factors on tacrolimus dosing in Caucasian liver and kidney transplant patients. Int J Mol Med. 2011;28:1093–1102. doi: 10.3892/ijmm.2011.794. [DOI] [PubMed] [Google Scholar]
- 26.Wu P, Ni X, Wang M, Xu X, Luo G, Jiang Y. Polymorphisms in CYP3A5*3 and MDR1, and haplotype modulate response to plasma levels of tacrolimus in Chinese renal transplant patients. Ann Transplant. 2011;16:54–60. [PubMed] [Google Scholar]
- 27.Gervasini G, Garcia M, Macias RM, Cubero JJ, Caravaca F, Benitez J. Impact of genetic polymorphisms on tacrolimus pharmacokinetics and the clinical outcome of renal transplantation. Transpl Int. 2012;25:471–480. doi: 10.1111/j.1432-2277.2012.01446.x. [DOI] [PubMed] [Google Scholar]
- 28.Stratta P, Quaglia M, Cena T, Antoniotti R, Fenoglio R, Menegotto A, Ferrante D, Genazzani A, Terrazzino S, Magnani C. The interactions of age, sex, body mass index, genetics, and steroid weight-based doses on tacrolimus dosing requirement after adult kidney transplantation. Eur J Clin Pharmacol. 2011;68:671–680. doi: 10.1007/s00228-011-1150-0. [DOI] [PubMed] [Google Scholar]
- 29.Yoon S-H, Cho J-H, Kwon O, Choi J-Y, Park S-H, Kim Y-L, Yoon Y-R, Won D-I, Kim C-D. CYP3A and ABCB1 genetic polymorphisms on the pharmacokinetics and pharmacodynamics of tacrolimus and its metabolites (M-I and M-III) Transplantation. 2013;95:828–834. doi: 10.1097/TP.0b013e31827eef57. [DOI] [PubMed] [Google Scholar]
- 30.Parra FC, Amado RC, Lambertucci JR, Rocha J, Antunes CM, Pena SD. Color and genomic ancestry in Brazilians. Proc Natl Acad Sci U S A. 2003;100:177–182. doi: 10.1073/pnas.0126614100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suarez-Kurtz G, Pena SD, Struchiner CJ, Hutz MH. Pharmacogenomic diversity among brazilians: influence of ancestry, self-reported color, and geographical origin. Front Pharmacol. 2012;3:191. doi: 10.3389/fphar.2012.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suarez-Kurtz G, Perini JA, Bastos-Rodrigues L, Pena SD, Struchiner C. Impact of population admixture on the distribution of the CYP3A5*3 polymorphism. Pharmacogenomics. 2007;8:1299–1306. doi: 10.2217/14622416.8.10.1299. [DOI] [PubMed] [Google Scholar]
- 33.Estrela RC, Ribeiro FS, Carvalho RS, Gregorio SP, Dias-Neto E, Struchiner CJ, Suarez-Kurtz G. Distribution of ABCB1 polymorphisms among Brazilians: impact of population admixture. Pharmacogenomics. 2008;9:267–276. doi: 10.2217/14622416.9.3.267. [DOI] [PubMed] [Google Scholar]
- 34.Ferreira PE, Veiga MI, Cavaco I, Martins JP, Andersson B, Mushin S, Ali AS, Bhattarai A, Ribeiro V, Bjorkman A, Gil JP. Polymorphism of antimalaria drug metabolizing, nuclear receptor, and drug transport genes among malaria patients in Zanzibar, East Africa. Ther Drug Monit. 2008;30:10–15. doi: 10.1097/FTD.0b013e31815e93c6. [DOI] [PubMed] [Google Scholar]
- 35.Turolo S, Tirelli AS, Ferraresso M, Ghio L, Belingheri M, Groppali E, Torresani E, Edefonti A. Frequencies and roles of CYP3A5, CYP3A4 and ABCB1 single nucleotide polymorphisms in Italian teenagers after kidney transplantation. Pharmacol Rep. 2010;62:1159–1169. doi: 10.1016/s1734-1140(10)70378-9. [DOI] [PubMed] [Google Scholar]
- 36.Macphee IA, Fredericks S, Tai T, Syrris P, Carter ND, Johnston A, Goldberg L, Holt DW. Tacrolimus pharmacogenetics: polymorphisms associated with expression of cytochrome p4503A5 and P-glycoprotein correlate with dose requirement. Transplantation. 2002;74:1486–1489. doi: 10.1097/00007890-200212150-00002. [DOI] [PubMed] [Google Scholar]
- 37.MacPhee IA, Fredericks S, Mohamed M, Moreton M, Carter ND, Johnston A, Goldberg L, Holt DW. Tacrolimus pharmacogenetics: the CYP3A5*1 allele predicts low dose-normalized tacrolimus blood concentrations in Whites and South Asians. Transplantation. 2005;79:499–502. doi: 10.1097/01.tp.0000151766.73249.12. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Li CJ, Zheng L, Zhang YJ, Jiang HX, Si-Tu B, Li ZH. Tacrolimus dosing in Chinese renal transplant recipients: a population-based pharmacogenetics study. Eur J Clin Pharmacol. 2011;67:787–795. doi: 10.1007/s00228-011-1010-y. [DOI] [PubMed] [Google Scholar]
- 39.Cho JH, Yoon YD, Park JY, Song EJ, Choi JY, Yoon SH, Park SH, Kim YL, Kim CD. Impact of cytochrome P450 3A and ATP-binding cassette subfamily B member 1 polymorphisms on tacrolimus dose-adjusted trough concentrations among korean renal transplant recipients. Transplant Proc. 2012;44:109–114. doi: 10.1016/j.transproceed.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Shi Y, Li Y, Tang J, Zhang J, Zou Y, Cai B, Wang L. Influence of CYP3A4, CYP3A5 and MDR-1 polymorphisms on tacrolimus pharmacokinetics and early renal dysfunction in liver transplant recipients. Gene. 2013;512:226–231. doi: 10.1016/j.gene.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 41.Kim JS, Aviles DH, Silverstein DM, LeBlanc PL, Vehaskari VM. Effect of age, ethnicity, and glucocorticoid use on tacrolimus pharmacokinetics in pediatric renal transplant patients. Pediatr Transplant. 2005;9:162–169. doi: 10.1111/j.1399-3046.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- 42.Ferraris JR, Argibay PF, Costa L, Jimenez G, Coccia PA, Ghezzi LF, Ferraris V, Belloso WH, Redal MA, Larriba JM. Influence of CYP3A5 polymorphism on tacrolimus maintenance doses and serum levels after renal transplantation: age dependency and pharmacological interaction with steroids. Pediatr Transplant. 2011;15:525–532. doi: 10.1111/j.1399-3046.2011.01513.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhao JY, Ikeguchi M, Eckersberg T, Kuo MT. Modulation of multidrug-resistance gene-expression by dexamethasone in cultured hepatoma-cells. Endocrinology. 1993;133:521–528. doi: 10.1210/endo.133.2.8102093. [DOI] [PubMed] [Google Scholar]
- 44.McCune JS, Hawke RL, LeCluyse EL, Gillenwater HH, Hamilton G, Ritchie J, Lindley C. In vivo and in vitro induction of human cytochrome P4503A4 by dexamethasone. Clin Pharmacol Ther. 2000;68:356–366. doi: 10.1067/mcp.2000.110215. [DOI] [PubMed] [Google Scholar]
- 45.Kim IW, Moon YJ, Ji E, Kim KI, Han N, Kim SJ, Shin WG, Ha J, Yoon JH, Lee HS, Oh JM. Clinical and genetic factors affecting tacrolimus trough levels and drug-related outcomes in Korean kidney transplant recipients. Eur J Clin Pharmacol. 2012;68:657–669. doi: 10.1007/s00228-011-1182-5. [DOI] [PubMed] [Google Scholar]